Abstract
Background
Nearly ten years ago, we demonstrated that superoxide radical anion (O2−̣) reacts with the hydroethidine dye (HE, also known as dihydroethidium, DHE) to form a diagnostic marker product, 2-hydroxyethidium (2-OH-E+). This particular product is not derived from reacting HE with other biologically relevant oxidants (hydrogen peroxide, hydroxyl radical, or peroxynitrite). This discovery negated the longstanding view that O2−̣reacts with HE to form the other oxidation product, ethidium (E+). It became clear that due to the overlapping fluorescence spectra of E+ and 2-OH-E+, fluorescence-based techniques using the “red fluorescence” are not suitable for detecting and measuring O2−̣in cells using HE or other structurally analogous fluorogenic probes (MitoSOX™ Red or hydropropidine). However, using HPLC-based assays, 2-OH-E+ and analogous hydroxylated products can be easily detected and quickly separated from other oxidation products.
Scope of review
The principles discussed in this chapter are generally applicable in free radical biology and medicine, redox biology, and clinical and translational research. The assays developed here could be used to discover new and targeted inhibitors for various superoxide-producing enzymes, including NADPH oxidase (NOX) isoforms.
Major conclusions
HPLC-based approaches using site-specific HE-based fluorogenic probes are eminently suitable for monitoring O2−̣in intra-and extracellular compartments and in mitochondria. The use of fluorescence-microscopic methods should be avoided because of spectral overlapping characteristics of O2−̣-derived marker product and other, non-specific oxidized fluorescent products formed from these probes.
General significance
Methodologies and site-specific fluorescent probes described in this review can be suitably employed to delineate oxy radical dependent mechanisms in cells under physiological and pathological conditions.
Keywords: Superoxide radical anion, Hydroethidine, MitoSOX, Hydropropidine, 2-Hydroxyethidium, HPLC
1. Introduction
Ever since we published our first paper reporting that superoxide (O2−̣) reacts with hydroethidine (HE) to form a diagnostic marker product, 2-hydroxyethidium (2-OH-E+) [1], increasing number of researchers in the free radical biology and medicine field have successfully used this methodology to rigorously measure and assess the role of O2−̣ [2–7]. However, some investigators still employ only the fluorescence microscopy or related fluorescence-based techniques (intravital fluorescence microscopy, flow cytometry) when using HE for O2−̣detection. Recognizing that O2−̣reaction with HE does not yield ethidium (E+), some investigators interpreted the enhanced red fluorescence in their systems to increased formation of the superoxide-specific product, 2-OH-E+. In recent years, we have reported that the products formed from the reaction between mitochondria-targeted hydroethidine (Mito-HE or MitoSOX™ Red) and O2−̣are very similar to the products formed from HE reaction with O2−̣[8]. This implies that all of the limitations attributed to HE assay are also applicable to Mito-SOX™ Red, as well [9]. Yet investigators continue to use the MitoSOX-derived fluorescence as a measure of mitochondrial superoxide (mROS) in many areas of research including aging, neurodegeneration, and cancer [10]. More recently, we reported that a cell-impermeant fluorogenic probe, hydropropidine (HPr+), a positively-charged water-soluble analog of HE, reacts with extracellular O2−̣in exactly the same manner as do HE and MitoSOX [11]. For the first time, we showed that O2−̣reacts very similarly with three structurally analogous fluorogenic probes that localize in different cellular regions [11]. We used HPLC, MS and NMR techniques extensively to monitor and characterize products formed [11–13]. The purpose of this review is two-fold: First, to categorically demonstrate that the HPLC-based approach is the only viable method to understand the mechanism of oxidation of HE-based fluorogenic probes and detect intra- and extracellular superoxide, and second, to reiterate that HE- and MitoSOX-derived red fluorescence can’t be used to reliably monitor intracellular and mitochondrial O2−̣formation.
2. Redox similarities between HE, MitoSOX, HPr+
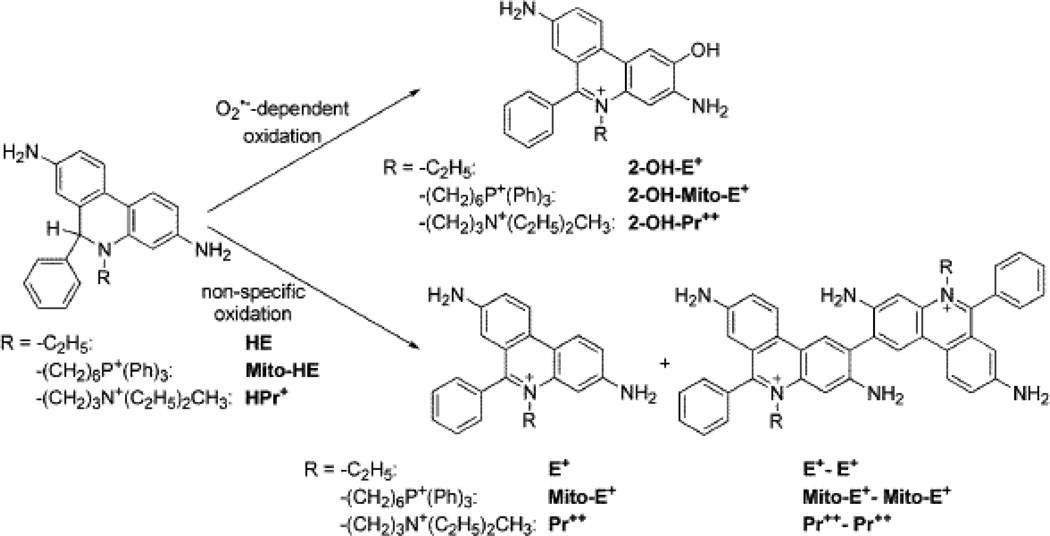
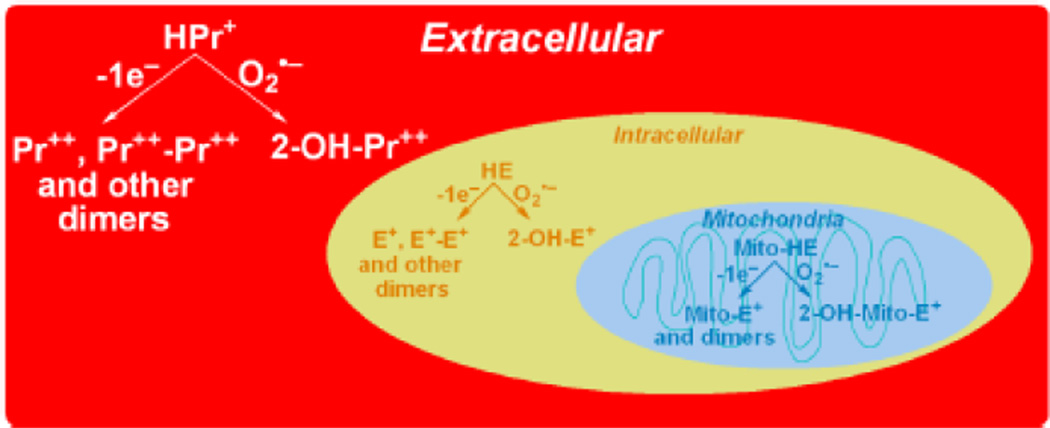
The reaction chemistry between O2−̣and reduced phenanthridinium compounds (HE, MitoSOX, and HPr+) is unique in that the corresponding hydroxylated products (2-OH-E+, 2-OH-Mito-E+, and 2-OH-Pr++) are formed as the only major products (Fig. 1). The actual mechanism presumably involves the formation of an intermediate radical cation that reacts with another molecule of O2−̣to ultimately form the 2-hydroxylated cationic product. Hydroxylation occurs specifically at the 2-position as evidenced by NMR characterization [11–14]. In a purely O2−̣generating system, no other products are formed. However, in the intracellular milieu, the presence of redox metal ions released from oxidative stress or heme proteins (cytochrome c) with peroxidase activity or other one-electron oxidants will easily oxidize these fluorogenic probes to several products, including the fluorescent phenanthridium cations (ethidium and analogs) and non-fluorescent or weakly fluorescent dimeric products (Figure 1). The enzymatic two-electron oxidation of HE to E+ has also been suggested [13]. In fact, hydroethidine has been originally developed as a vital stain, with the assumption that it is oxidized by intracellular oxidases [15]. As a result of non-specific oxidation process, the intracellular concentration of the fluorogenic probes will diminish, leading to lower efficiency of trapping of superoxide and decreased formation of O2−̣-specific diagnostic marker products. In addition to quantifying the oxidation products, HPLC measurements enable simultaneous assessments of the concentration of the fluorogenic probes, making data interpretation less tenuous. In some instances, the presence of a one-electron oxidizing system (e.g., peroxidases) may enhance formation of the superoxide specific product (e.g., 2-OH-E+), even without any change in superoxide flux [Michalski, Zielonka, Sikora & Kalyanaraman, unpublished data]. This is due to a much higher reactivity of HE radical cation with superoxide, as compared to the HE parent molecule. As a consequence, one-electron oxidation of HE or analog will result in a more effective competition with other pathways of O2−̣decay (e.g., dismutation by superoxide dismutases) leading to a net increase in hydroxylated product [9,14]. In this respect, HE resembles other probes for superoxide (e.g., luminol) which require “activation” by one-electron oxidation [16]. The one-electron oxidation of the probe will also lead to formation of the dimeric products (detectable by HPLC-based assays). Given the complexity of HE oxidative mechanisms in cells and the presence of efficient superoxide scavengers (e.g., SOD), 2-OH-E+ measurement by HPLC should not be considered as an absolute measure of intracellular O2−̣
Figure 1.
Scheme showing the superoxide-specific hydroxylation and non-specific oxidation of hydroethidine and its analogs.
3. Overlapping fluorescence characteristics: 2-OH-E+ and E+ and MitoSOX products
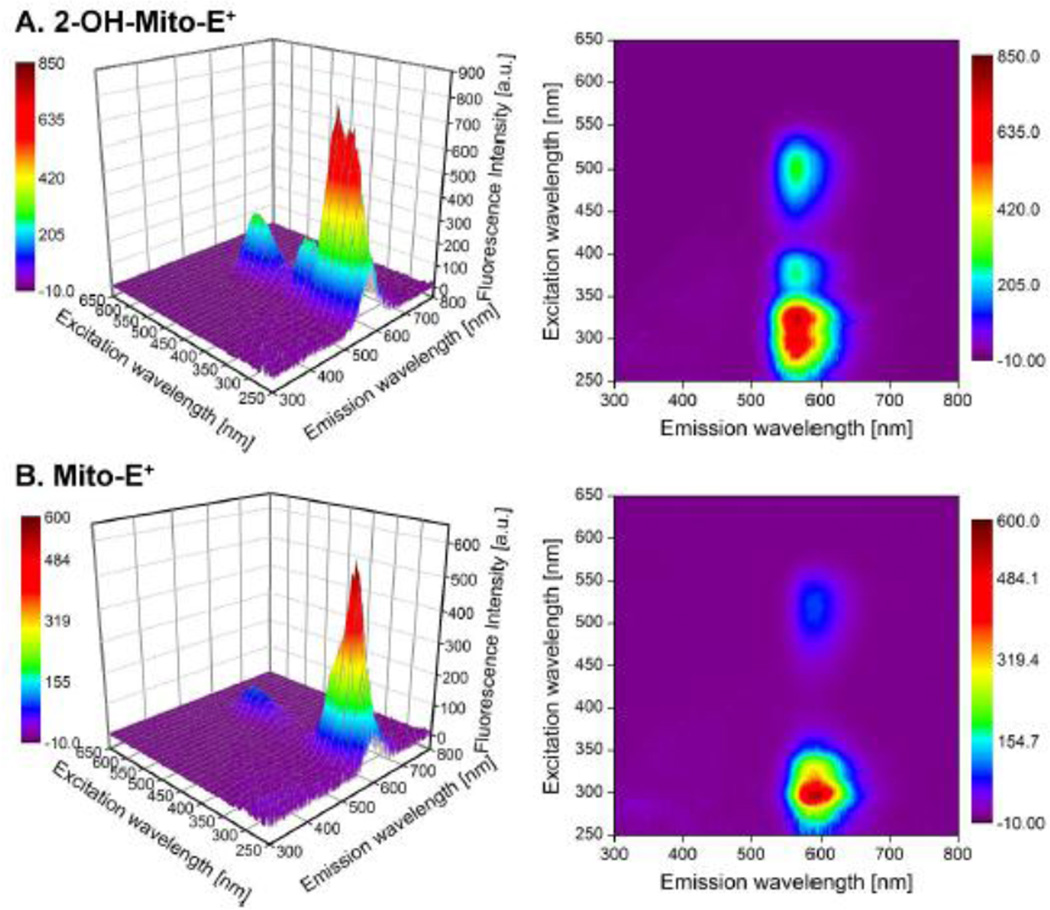
Fluorescence-based methods using HE have been used to detect O2−̣in various cellular milieu for decades [17]. The principles of fluorescence spectroscopy are still applicable in fluorescence microscopy and flow cytometry. We have reported that there exists significant fluorescence spectral overlap between 2-OH-E+ and E+. Therefore, the use of fluorescence-based techniques alone to measure O2−̣can be very misleading; in virtually all of the biological systems tested, both 2-OH-E+ and E+ were formed in differing ratios [9,12]. The intracellular E+ levels are often ca. ten-fold higher than those of 2-OH-E+, effectively masking the changes in the fluorescence intensity due to 2-OH-E+ [9,12]. The fluorescence quantum yields of 2-OH-E+ and E+ and their analogs formed from MitoSOX and HPr+ increase upon binding to DNA. As shown in Figure 2, both hydroxylated and non-hydroxylated oxidation products derived from MitoSOX (e.g., Mito-2-OH-E+ and Mito-E+) share a similar spectral property. The hydroxylated products (e.g., 2-OH-E+) display an additional excitation band, with a maximum between 350 and 400 nm, this feature becomes even more prominent in the presence of DNA [18]. This spectral feature was used to selectively monitor 2-OH-E+ and 2-OH-Mito-E+ [12]. However, the other fluorescent oxidation product (E+ or Mito-E+) will significantly contribute to the total fluorescence intensity, depending upon its intracellular levels. This situation is particularly grim with regard to the use of fluorescence techniques to primarily detect mitochondrial superoxide using the mitochondria-targeted HE analog, MitoSOX. The red fluorescence which is detected from cell and tissues incorporated with MitoSOX has often been equated to mitochondrial superoxide [19]. Mitochondria are enriched with several heme proteins. Other dyes such as DCFH have been shown to undergo cytochrome c-mediated oxidation to a green fluorescent product [20,21]. The use of this dye to detect H2O2 was severely criticized because of its propensity to form the green fluorescent product in the presence of cytochrome c released from mitochondria [21]. We have shown that there was attenuated oxidation of MitoSOX in mitochondrial preparations (mitoplasts) depleted of cytochrome c, and there was substantial oxidation of MitoSOX in intact mitochondria, consistent with the high reactivity of both HE and MitoSOX with cytochrome c [13].
Figure 2.
The fluorescence excitation-emission matrix (FEEM) spectra of Mito-2-hydroxyethidium (superoxide-specific product of MitoSOX oxidation, upper panel) and Mito-E+ (non-specific oxidation product of MitoSOX, lower panels) in the presence of DNA in 10 mM Tris buffer (pH 7.4) containing EDTA (1 mM).
We, like other researchers, used both HE and MitoSOX-derived red fluorescence as a measure of intracellular and mitochondrial O2−̣and DCFH-derived fluorescence to monitor oxidative events [22]. While in case of DCFH, we attributed the green fluorescence derived from DCFH to iron (or heme iron) - mediated oxidation of the dye by H2O2 rather than to H2O2 alone, we presumed that HE-derived red fluorescence is reporting superoxide production [22]. With increasing knowledge of the chemical mechanisms of oxidation of HE and MitoSOX, we now realize that it was rather naïve to interpret the changes in fluorescence in such a simplistic viewpoint, and that it is necessary to perform detailed HPLC analyses to separate the various HE oxidation products and to fully understand the origin of red fluorescence. Recently, HE-derived red fluorescence was used to monitor iron-dependent intracellular oxidative events in neuronal cells [23]. We illustrate this point in the following section with recent examples.
4. Analyses of HE-derived fluorescence and hydroxylation/oxidation products of HE
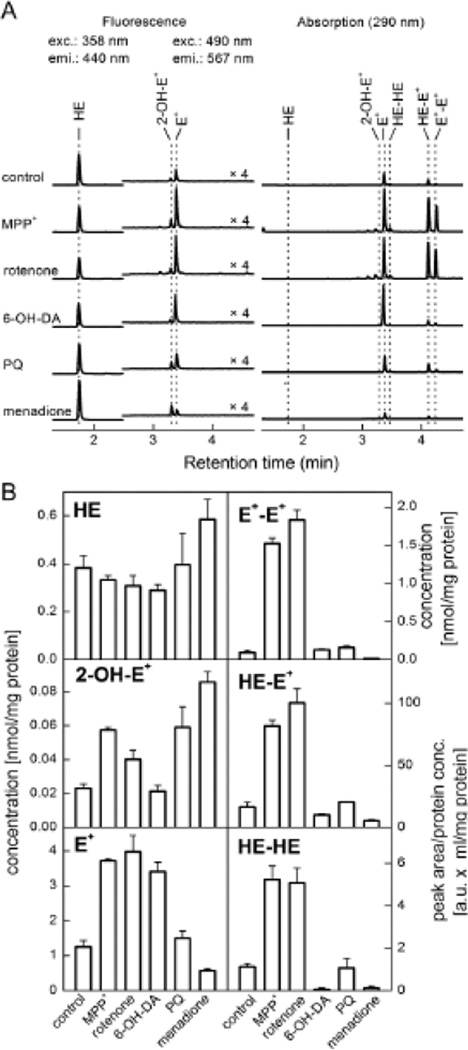
In a recent study, we measured by HPLC formation of HE-derived products (2-OH-E+, E+ and dimers) in dopaminergic N27 cells treated with MPP+, rotenone, 6-hydroxydopamine, paraquat, or menadione [24]. All of these agents have previously been suggested to stimulate intracellular O2−̣formation. As shown in Figure 3, MPP+, rotenone, and paraquat stimulated a slight but significant increase in 2-OH-E+, characteristic marker product of HE and O2−̣, but concomitantly, these agents (MPP+ and rotenone) also stimulated substantial increase in E+ and dimeric product formation (Fig. 3) indicative of a one-electron oxidation process [24]. As discussed above, this shows that an increased production of 2-OH-E+ may be attributable to a one-electron oxidation of the probe in the case of MPP+ and rotenone, but not with paraquat or menadione. The HPLC-based analysis provides additional information enabling more rigorous interpretation of the experimental data.
Figure 3.
(A) HPLC traces showing the formation of several oxidation products of HE formed from extracts of N27 cells treated with agents shown. (B) Intracellular levels or peak areas of various products derived from HE. (Adapted from ref. 23).
6-Hydroxydopamine, an agent that has been thought to enhance O2−̣formation, did not induce 2-OH-E+ formation, but only enhanced formation of non-specific oxidation products. Fluorescence microscopy experiments, however, showed increased red fluorescence under these and other conditions generating other one-electron oxidizing species [24,25]. In the absence of HPLC results, one would have interpreted the fluorescence results incorrectly and equated the increase in red fluorescence to enhanced O2−̣formation from all the agents and ignoring the additional oxidation pathway(s) of the probe. Clearly, these examples emphasize the ultimate need for HPLC analyses of products from HE, MitoSOX, as well as HPr+.
In order to best interpret intracellular HE oxidation and 2-OH-E+ formation, it is important to consider the stability of HE in cell culture medium and the factors affecting the transport of HE into cells. These aspects have been discussed in a previous review [9]. Other groups have used the ratio between 2-OH-E+ and HE as a quantitative parameter [26,27].
5. Site-specific fluorogenic probes for O2−̣
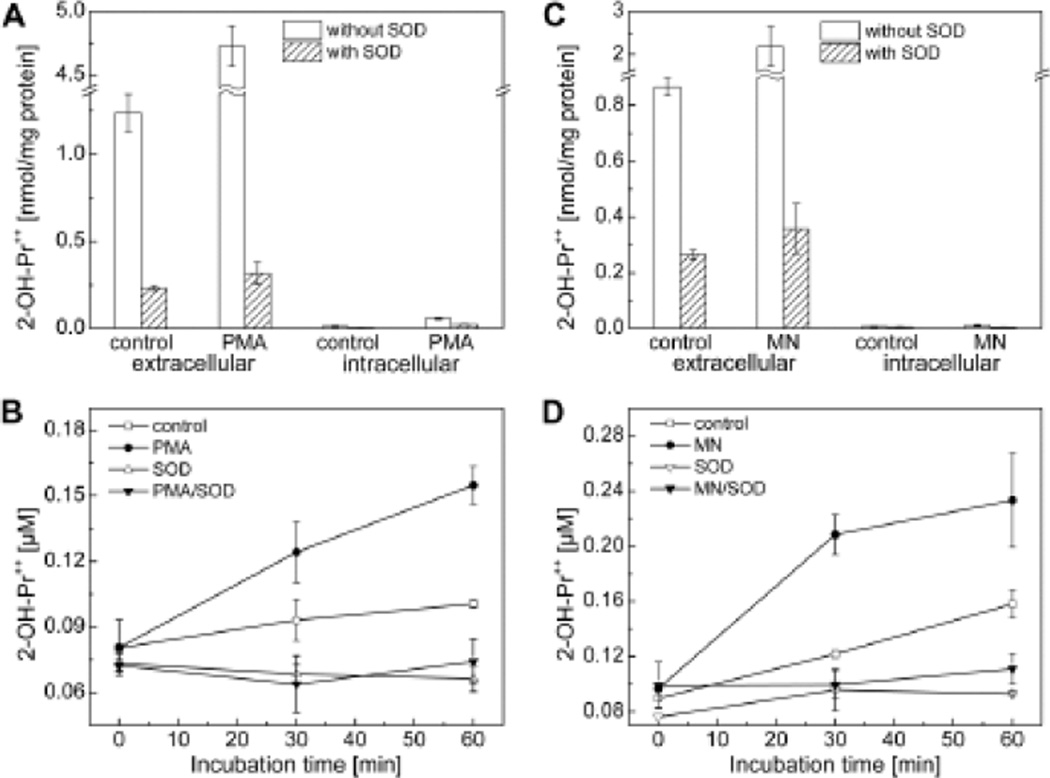
In addition to HE and MitoSOX, which shares similar redox chemistry, we recently developed a membrane-impermeant fluorogenic probe, hydropropidine (HPr+), a positively-charged, water-soluble analog of HE [11]. HPr+ is a two-electron reduction product of propidium iodide (Pr++ •2I-). In contrast to MitoSOX, wherein the positive charge is delocalized over three aromatic phenyl groups, the positive charge in HPr+ is highly localized on the nitrogen atom of the alkyl group which inhibits its cellular uptake. The reaction chemistry between HPr+ and O2−̣and other one-electron oxidants was shown to be similar to that of HE [11]. Superoxide reacts with HPr+ to form exclusively 2-OH-Pr++, and with one-electron oxidants (ferricyanide, hydroxyl radicals, peroxynitrite and peroxidase/H2O2) to form the propidium dication (Pr++) and several dimeric products (e.g., Pr++-Pr++). Because HPr+ is cell-impermeant, it will serve as an ideal probe for detecting extracellularly-generated or released O2−̣and one-electron oxidizing species [11]. Using two methods of stimulation of O2−̣generation in RAW 264.7 macrophages, we showed that SOD-inhibitable formation of 2-OH-Pr++ was stimulated in the extracellular compartment of macrophages treated with PMA or menadione. Under these conditions, negligible amount of 2-OH-Pr++ was detected in cell lysates (Fig. 4).
Figure 4.
Measurement of extracellular and intracellular formation of superoxide-specific products formed from HPr+ probe. (A–D) Macrophages were stimulated with either PMA or menadione to induce O2−̣generation. (Adapted from ref. 11).
6. Next generation probes in high-throughput screening of ROS and RNS
To date, three different reduced phenanthridinium-based compounds (HE, MitoSOX Red, and HPr+) have been developed that can track superoxide formation in extracellular, intracellular, and mitochondrial locations. Knowing their one-electron oxidation chemistry, reaction kinetics, and product profiles in the presence of O2−̣generating systems will undoubtedly enable global profiling of multiple oxidants in biological systems. With improved analytical techniques to rapidly detect products derived from fluorogenic probes, one can use these probes to monitor O2−̣and other oxidant formation using a high-throughput global profiling methodology [25].
Another caveat with Mito-SOX Red or other triphenylphosphonium-containing probes is that these probes could be localized in other intracellular compartments (with a more negative membrane potential) in addition to mitochondria, the primary site of accumulation. Thus, in order to obtain a complete understanding of the cytosolic and mitochondrial contribution to the HPLC signal, it is necessary to isolate and determine the concentration of Mito-SOX Red in the various cellular organelles.
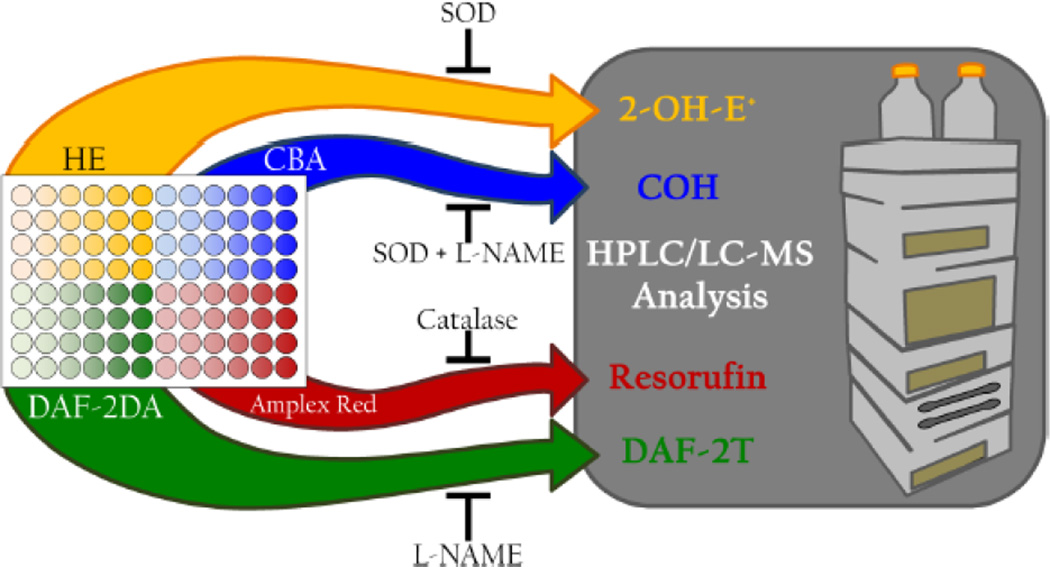
Recently, we described an experimental design for detecting and identifying multiple reactive oxygen and nitrogen species simultaneously in biological systems in response to a treatment of interest [25]. We used a 96-well plate to demonstrate the feasibility for developing a high-throughput global profiling methodology for detecting reactive oxygen and nitrogen species (Fig. 5). Initial screening of reactive oxygen and nitrogen species generated in cells in response to a treatment was performed using four different dyes (hydroethidine, coumarin-7-boronic acid, diaminofluorescein, and Amplex Red), in a 96-well plate by monitoring fluorescence intensity increase over time of incubation. Information obtained from the plate reader can be used to design more selective studies using HPLC or UHPLC to rigorously verify the identity of the formed species by determining the specific products formed from the fluorogenic probes. The investigator can substitute judiciously the various site-specific probes to simultaneously monitor intra- and extracellularly-formed superoxide and other one-electron oxidants in cellular systems. The site-specific detection of products derived from O2−̣and oxidant reactivity is illustrated (Fig. 6) using the extracellular, intracellular, and intramitochondria-localized fluorogenic probes. Other, non-HPLC-based methods (e.g., microcolumn hydrophobic chromatography) have been used to selectively measure 2-OH-E+ [28,29]. However, we believe that HPLC measurement of intracellular HE and other oxidation products (HE dimers) besides 2-OH-E+ will give a total profile of intracellular O2−̣and other one-electron oxidants. Future experiments should rigorously analyze the applicability and reliability of the FACS method [30] (in conjunction with HPLC verification) as an adjunct method for intracellular O2−̣detection.
Figure 5.
Experimental design suggested for simultaneously detecting multiple oxidants in a 96-well plate using the probes shown. (Adapted from ref. 24).
Figure 6.
Oxidant-dependent formation of different oxidation products from site-specific extra-, intracellular and mitochondria-targeted probes.
7. Conclusions
Understanding the redox chemistry of fluorogenic dyes is vital for furthering their cellular and biological applications with any degree of confidence. With the increased understanding of the reaction mechanism between superoxide and hydroethidine-based fluorescent dyes, we are now in a position to monitor unambiguously the formation of several oxidizing species, including superoxide. Recent developments of HPLC-based techniques enable rapid separation and detection of the specific marker products formed from these dyes. Given the ambiguity of the direct fluorescence measurements, HPLC based assays remain the only reliable approach to monitor and quantify specific oxidation products.
Highlights.
O2−̣reacts with HE, MitoSOX, and hydropropidine to form hydroxylated cations
One-electron oxidants react with these probes to form other oxidation products
Fluorescence microscopy is not amenable for unambiguous detection of superoxide
HPLC is the only unambiguous approach to measure O2−̣using HE-based probes
Abbreviations
- 2-OH-E+
2-hydroxyethidium
- E+
ethidium
- HE
hydroethidine
- HPr+
hydropropidine
- MitoSOX™ Red or Mito-HE
HE conjugated to TPP+ group
- TPP+
triphenylphosphonium cation
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 2.Dikalov S, Griendling KE, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgiou CD, Papapostolou I, Grintzalis K. Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils. Nat. Protoc. 2008;3:1679–1692. doi: 10.1038/nprot.2008.155. [DOI] [PubMed] [Google Scholar]
- 4.Palazzolo-Balance AM, Suquet C, Hurst JK. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry. 2007;46:3536–3548. doi: 10.1021/bi700123s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic. Biol. Med. 2010;49:545–558. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurindo FR, Fernandes DC, Santos CX. Assessment of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation products. Methods Enzymol. 2008;441:237–260. doi: 10.1016/S0076-6879(08)01213-5. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree MJ, Tatham AL, Al-Wekeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J. Biol. Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 8.Kalyanaraman B. Oxidative chemistry of fluorescent dyes: Implications in the detection of reactive oxygen and nitrogen species. Biochem. Soc. Trans. 2011;39:1221–1225. doi: 10.1042/BST0391221. [DOI] [PubMed] [Google Scholar]
- 9.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth”. Free Radic. Biol. Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J. Neurosci. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. Hydropropidine: A novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic. Biol. Med. 2013;43:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vásquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [Erratum: Proc. Natl. Acad. Sci. U.S.A. 102 (2005) 9086.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez OM, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radic. Biol. Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallop PM, Paz MA, Henson E, Latt SA. Dynamic Approaches to the delivery of reporter reagents into living cells. BioTechniques. 1984;1:32–36. [Google Scholar]
- 16.Zielonka J, Kalyanaraman B. Methods of Investigation of Selected Radical Oxygen/Nitrogen Species in Cell-free and Cellular Systems. In: Pantopoulos K, Schipper HM, editors. Principles of Free Radical Biomedicine. Volume 1. Nova Science Publishers; 2011. [Google Scholar]
- 17.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'dichlorofluorescein. J. Leukoc. Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 18.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 19.Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta. 2011;1813:1382–1394. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burkitt MJ, Wardman P. Cytochrome c is a potent catalyst of dichlorofluorescein oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem. Biophys. Res. Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 22.Kalivendi SV, Kotamraju S, Cunningham S, Shang T, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem. J. 2003;371:151–164. doi: 10.1042/BJ20021525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firdaus WJ, Wyttenbach A, Giuliano P, Kretz-Remy C, Currie RW, Arrigo AP. Huntington inclusion bodies are iron-dependent centers of oxidative events. FEBS J. 2006;273:5428–5441. doi: 10.1111/j.1742-4658.2006.05537.x. [DOI] [PubMed] [Google Scholar]
- 24.Dranka BP, Zielonka J, Kathansamy AG, Kalyanaraman B. Alterations in bioenergetics function induced by Parkinson’s disease mimetic compounds: Lack of correlation with superoxide generation. J. Neurochem. 2012;122:941–951. doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zielonka J, Zielonka M, Sikora A, Adamus J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J. Biol. Chem. 2011;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maghzal GJ, Stocker R. Improved analysis of hydroethidine and 2-hydroxyethidium by HPLC and electrochemical detection. Free Radic. Biol. Med. 2007;43:1095–1096. doi: 10.1016/j.freeradbiomed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Laurindo FR, Fernandes DC, Santos CX. Assessment of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation products. Methods Enzymol. 2008;441:237–260. doi: 10.1016/S0076-6879(08)01213-5. [DOI] [PubMed] [Google Scholar]
- 28.Georgiou CD, Papapostolou I, Grintzalis K. Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils. Nat. Protoc. 2008;3:1679–1692. doi: 10.1038/nprot.2008.155. [DOI] [PubMed] [Google Scholar]
- 29.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 30.Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]