Abstract
Children's early motor skills are critical for development across language, social, and cognitive domains, and warrant close examination. However, examiner-administered motor assessments are time consuming and expensive. Parent-report questionnaires offer an efficient alternative, but validity of parent report is unclear and only few motor questionnaires exist. In this report, we use cross-sectional and longitudinal data to investigate the validity of parent report in comparison to two examiner-administered measures (Mullen Scales of Early Learning, MSEL; Peabody Developmental Motor Scales, PDMS-2), and introduce a new parent-report measure called the Early Motor Questionnaire (EMQ). Results indicate strong correlations between parent report on the EMQ and a child's age, robust concurrent and predictive validity of parent report with both the MSEL and PDMS-2, and good test-re-test reliability of parent report on the EMQ. Together, our findings support the conclusion that parents provide dependable accounts of early motor and cognitive development.
Keywords: Motor Development, Parent report, Questionnaire, Autism
The motor system provides infants with a means of exploring and engaging with the world and is critical for several aspects of development (e.g., language, social interactions, and learning). Unfortunately, assessing motor skills via examiner-administered assessments is time consuming, expensive, and prone to underestimating a child's true ability due to a lack of performance at test. In contrast, parent-report measures are cost effective and draw on the extensive knowledge of a child's primary caregiver. However, the validity or parent report remains unclear as only few questionnaires on early motor development (during the first two years of life) exists and only a small number of studies have investigated the validity of parent report measures in the motor domain (e.g., Bodnarchuk & Eaton, 2004; Goldstein, 1985; Knobloch, Stevens, Malone, Ellison, & Risemberg, 1979). The present study introduces a new parent-questionnaire focusing on early motor development – the Early Motor Questionnaire (EMQ) – and investigates the concurrent and predictive validity of parent report in comparison to two examiner-administered assessments of early motor and cognitive development.
1.1. The role of early motor skills in development
Motor skills and abilities play a critical role for overall development during the first years and are predictive of later developmental outcomes. In particular, attainment of new motor skills open up opportunities for learning about the physical and social world (Bushnell & Boudreau, 1993; Gibson, 1988) and therefore have cascading effects on cognitive, social, and language development (Campos, et al., 2000; Iverson, 2010; Libertus & Needham, 2011). Embodied theories of development acknowledge the pivotal role of motor skills and motor experiences, especially early in life when children's bodies change rapidly and shape the dynamic interactions between child and environment (Needham & Libertus, 2011; Smith, 2005). Empirical support for embodied notions of development comes from studies showing that motor enrichment (i.e., ‘sticky mittens’) at age 3 months may facilitate concurrent motor and social development (Libertus & Needham, 2011). At the same time, motor deficits early in development have been associated with developmental delays or disorders. For example, children with motor delays have been reported to show increased rates of behavioral, affective, attentional, or social problems in later childhood (Gillberg & Kadesjo, 2003). Similarly, the presence of motor delays during the first four years of life has been found to predict later cognitive performance (in particular working memory and processing speed, Piek, Dawson, Smith, & Gasson, 2008). More recently, motor delays at age 6 months (i.e., head lag) have been associated with autism spectrum disorders (ASD) at age 36 months (Flanagan, Landa, Bhat, & Bauman, 2012).
Other prominent developmental theories also highlight the importance of early motor experiences. For example, Piaget's theory of development emphasizes the role of self-produced sensorimotor experiences for learning (Piaget, 1953). Similarly, Gibson's ecological theory of development suggests that the acquisition of new motor skills changes the perceived opportunities for actions on objects (Gibson, 1988; Gibson & Pick, 2000). Thus, motor experiences seem critical for learning, overall developmental trajectories across domains, and may even predict future learning delays or developmental disabilities. These findings highlight the potential value of an objective and reliable measure of early motor skills and emphasize the need for the development of such a measurement tool.
1.2. Motor-skill assessments
Motor skills can be assessed either via examiner-administered assessments or via parent-report measures. Both methods will be discussed in the following and each has its own benefits and limitations. For the current report, we use the term “standardized” to denote that a particular test has been standardized in the way its items are administered and has been normed to provide a standardized test score (in comparison to the normative-sample). Norm-referenced standard scores provide information about a child's performance relative to other children of the same age. While raw scores indicate how many items have been passed on a test, standardized scores compare performance to expected values at this age. Thus while raw scores increase with age, standardized scores are expected to remain relatively stable over time. However, if a child showed identical performance on a test at age 6 and at age 14 months, raw scores would not change but standard score would be lower for the second assessment since expectations at this age are that the child would pass more items.
1.2.1. Examiner-administered assessments of motor development
Standardized examiner-administered tests of motor development are widely used to identify developmental delays, to measure performance of particular skills, and to monitor progress of interventions. Administration is performed by a trained examiner using specific probes to create opportunities for objective, replicable observations presumed to be reflective of a child's abilities. A recent review of standardized developmental assessments identified the Bayley Scales of Infant Development (BSID-III, Bayley, 2006) and the Mullen Scales of Early Learning (MSEL, Mullen, 1995) as two of the most commonly used standardized developmental tests (Johnson & Marlow, 2006).The BSID-III assesses 3 sub-domains (cognitive, fine- and gross-motor, receptive and expressive language), is normed for ages 1-42 months, takes between 30-90 minutes to administer, and is one of the most widely used standardized developmental assessments (Bayley, 2006; Johnson & Marlow, 2006). However, due to its lengthy administration, the BSID-III is not commonly used in clinical populations such as in children with ASD – in this area the MSEL is the preferred assessment (e.g., Burns, King, & Spencer, 2013; Landa & Garrett-Mayer, 2006). The MSEL assesses 5 domains (Gross Motor, Fine Motor, Visual Reception, Receptive Language, Expressive Language), is normed for ages 0-68 months, and takes between 15-60 minutes to administer (Mullen, 1995). Another commonly used standardized assessment of early motor development is the Peabody Developmental Motor Scales (PDMS-2). The PDMS-2 assesses gross and fine motor development, is normed for ages 0-60 months, and takes 45-60 minutes to complete (Folio & Fewell, 2000). In contrast to the MSEL or BSID-III, the PDMS-2 focuses exclusively on motor development and offers a more in-depth assessment of this domain.
Examiner-administered assessments offer several benefits. First, the examiner is highly familiar with the assessment protocol and child development in general. Second, the probes and test stimuli are standardized and consistent across different examiners and sites, resulting in comparability of standard scores across age groups and settings. At the same time, examiner-administered assessments also have limitations. First, administration by a trained examiner is expensive and time consuming. Second, long assessments may fatigue the child and negatively impact test scores. Third, test scores depend heavily on the level of comfort and rapport between the child and examiner. And finally, examiner-administered assessments are not feasible for studies collecting data remotely (e.g., web surveys).
1.2.2. Parent-report measures of early motor development
Parent-completed developmental questionnaires (PCDQs) are structured tools providing access to parent's knowledge about their children (Easley, et al., 1996). PCDQs require minimal time for scoring and studies have estimated their costs to be only about $0.32 – $2.50 (Bricker & Squires, 1989; Bricker, Squires, Kaminski, & Mounts, 1988; Dobrez, et al., 2001). In contrast, examiner-administered assessments cost hundreds of dollars (Bricker & Squires, 1989; Bricker, et al., 1988; Dobrez, et al., 2001). PCDQs are utilized in all developmental domains including cognitive and language development (e.g., Fenson, et al., 2007; Rescorla & Alley, 2001; Sparrow, Balla, & Cicchetti, 1984), but relatively few PCDQs focus on early motor development (for a list of commonly used PCDQs see Duby, et al., 2006).
Some PCDQs that include measures of early motor development are the Minnesota Infant Development Inventory (MIDI, Creighton & Sauve, 1988; Ireton & Thwing, 1980), the Child Development Review – Parent Questionnaire (CDP-PQ, Ireton, 1996), the Child Development Inventory (CDI, Ireton & Glascoe, 1995), and the Ages & Stages Questionnaire (ASQ, Squires, Bricker, & Potter, 1997). Among these PCDQs, only the IDI and the ASQ cover early motor development during the first two years of life. The MIDI is designed for ages 0 – 18 months, includes 15 pass/fail items for gross motor and 15 pass/fail items for fine motor development, takes about 10 minutes to complete, and is arranged in a developmental sequence. The ASQ is a widely used developmental screening tool consisting of 11 questionnaires designed for different ages (total range of ASQ 4 – 48 months), it includes 6 items for gross motor and 6 items for fine motor development on each questionnaire, is scored on a 3-point scale, and takes about 15 minutes to complete. Thus, while there are some parent-report tools to measure early motor development, these PCDQs assess only a limited number of items (i.e., 30 items total on MIDI) or are non-continuous across development and therefore not as useful for research settings and longitudinal studies (i.e., ASQ uses different questionnaires for different ages).
Due to the retrospective nature of PCDQs, concerns regarding their validity remain (Long, 1992; Seifer, 2008) and parents may over- or underestimate their child's true abilities (especially in the motor domain; Bartlett & Piper, 1994). Nevertheless, several studies suggest that well designed and structured PCDQs may provide accurate information about a child's current developmental status (e.g., Bricker, et al., 1988; Faraone, Biederman, & Milberger, 1995; Glascoe & Dworkin, 1995; Knobloch, et al., 1979).
The validity of parent report on motor development has been investigated mostly in older children (Kennedy, Brown, & Chien, 2012; Kennedy, Brown, & Stagnitti, 2012; Wilson, Kaplan, Crawford, Campbell, & Dewey, 2000). In younger children, studies using daily diaries suggest that parents provide accurate accounts of their child's early motor skills (Bodnarchuk & Eaton, 2004; Ellis-Davies, Sakkalou, Fowler, Hilbrink, & Gattis, 2012). The validity of one-time motor-domain PCDQs remains largely unknown (c.f., Goldstein, 1985; Knobloch, et al., 1979).
1.3. The current study
In this report we examine concurrent and predictive validity of parent report on early motor development in 3- to 24-month-old children by comparing questionnaire to examiner-administered measures, and introduce a novel parent-report measure of early motor development called the Early Motor Questionnaire (EMQ). The EMQ is designed as a fast and easy to complete motor questionnaire, and is organized into 3 sections: Gross motor skills, fine motor skills, and perception-action integration skills. The EMQ is not a standardized assessment, but can be used in research settings, serve as a motor screener, or as complement to standardized measures of early motor development. In addition to assessing the validity of parent report, we examine the concurrent and predictive validity of the EMQ in comparison to the standardized MSEL and the PDMS-2.
2. Methods
2.1. Participants
Participants were 94 parent-child dyads with children aged between 3-24 months (M = 11.57 months, SD = 6.75 months, range 2.39 – 24.95 months) who enrolled in a longitudinal study on the early detection of ASD. Age was adjusted in 9 children who were born slightly prematurely (M = 35.22 weeks gestation, SD = 1.06). Children were recruited from 6 discrete age groups: 3, 6, 10, 14, 18, and 24 months. Detailed participant demographics for the entire sample, split by recruitment age group are shown in Table 1. About 59% of the children are younger siblings of a child with a confirmed diagnosis of ASD diagnosis (‘sib-As’) and are at heightened risk for developing ASD (Landa & Garrett-Mayer, 2006). EMQ and MSEL scores were available for all children. Additionally, 73 children completed the PDMS-2 (not completed at age 3 months, and missing for one 24-month-old). Children scored within the average range on the Gross and Fine Motor and Visual Reception scales of the MSEL and PDMS-2 (see standard scores in Table 2). Prior to participation, all parents provided oral and written informed consent. The Johns Hopkins Medical Institutions IRB approved all materials and procedures.
Table 1. Sample Characteristics.
| N (% total) |
Age (SD) |
SES† (SD) |
Males (% group) |
Caucasian (% group) |
Sib-A (% group) |
|
|---|---|---|---|---|---|---|
| All ages | 94 (100%) |
11.57 (6.75) |
54.39 (10.64) |
60 (64%) |
75 (80%) |
55 (59%) |
| 3-month-olds | 20 (21%) |
3.08 (0.39) |
53.14 (12.21) |
12 (60%) |
13 (65%) |
16 (80%) |
| 6-month-olds | 16 (17%) |
6.43 (0.52) |
50.72 (11.63) |
10 (63%) |
12 (75%) |
4 (25%) |
| 10-month-olds | 17 (18%) |
10.43 (0.85) |
52.79 (11.80) |
9 (53%) |
16 (94%) |
7 (41%) |
| 14-month-olds | 16 (17%) |
14.72 (0.72) |
57.17 (7.64) |
11 (69%) |
14 (88%) |
10 (63%) |
| 18-month-olds | 16 (17%) |
18.30 (1.18) |
57.07 (6.92) |
10 (63%) |
14 (88%) |
11 (69%) |
| 24-month-olds | 9 (10%) |
24.10 (0.59) |
57.39 (12.23) |
8 (89%) |
6 (67%) |
7 (78%) |
Notes: Sib-A = younger siblings of a child with a confirmed ASD or PDD-NOS diagnosis.
= SES refers to Hollingshead socioeconomic score (Hollingshead, 1975), this score is derived from both parents' highest level of education and their occupation. Age is reported in months.
Table 2. Standardized Test Scores.
| PDMS-2 | MSEL | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| GMQ (SD) |
FMQ (SD) |
TMQ (SD) |
GM (SD) |
FM (SD) |
V R (SD) |
|
| All ages | 95.59 (11.42) |
101.89 (9.29) |
97.89 (10.38) |
48.69 (10.09) |
52.24 (8.35) |
54.13 (10.09) |
| 3-month-olds | --- | --- | --- | 48.65 (7.09) |
49.47 (4.91) |
56.94 (6.43) |
| 6-month-olds | 105.31 (7.98) |
106 (4.90) |
106 (6.88) |
50.50 (7.21) |
53.25 (7.39) |
54.19 (6.41) |
| 10-month-olds | 96.71 (8.43) |
107.94 (9.60) |
101.41 (8.14) |
45.77 (11.79) |
56.59 (10.82) |
56.65 (12.59) |
| 14-month-olds | 96.25 (8.92) |
102.44 (7.20) |
98.5 (7.29) |
52.63 (12.31) |
54.81 (6.88) |
53.50 (6.04) |
| 18-month-olds | 91.5 (10.74) |
97.38 (7.26) |
93.25 (8.93) |
49.44 (9.13) |
49.44 (8.34) |
48.19 (12.47) |
| 24-month-olds | 80.625 (10.49) |
88.75 (5.50) |
82.25 (7.81) |
42.79 (11.53) |
47.89 (8.43) |
55.67 (14.35) |
Notes: PDMS-2 = Peabody Developmental Motor Scales, 2nd edition; MSEL = Mullen Scales of Early Learning; GMQ = Gross Motor Quotient; FMQ = Fine Motor Quotient; TMQ = Total Motor Quotient; GM = Gross Motor scale; FM = Fine Motor scale; VR = Visual Reception scale. PDMS-2 scores have a mean of 100 and a standard deviation of 15. MSEL scores have a mean of 50 and a standard deviation of 10.
2.2. Measures
A trained Master's or Ph.D. level professional administered all standardized developmental assessments and a primary caregiver completed the EMQ. The examiner was blind to EMQ scores at the time of assessment. The MSEL was generally performed before the PDMS-2, and consequently the examiner was not blind to the child's performance on the MSEL while administering the PDMS-2.
2.2.1. Mullen Scales of Early Learning (MSEL)
The MSEL is a standardized developmental assessment with 5 Scales (Mullen, 1995): Gross Motor (GM; 35 items); Fine Motor (FM; 30 items); Visual Reception (VR; 33 items); Receptive Language (RL; 33 items); and Expressive Language (EL; 28 items). Standardization of the measure was completed on 1849 children. The MSEL is a shared measure given by researchers who contribute data to the Baby Siblings Research Consortium database (e.g., Ozonoff, et al., 2011), and by researchers in the British Autism Study of Infant Siblings (e.g., Gliga, Elsabbagh, Hudry, Charman, & Johnson, 2012). Also, it is widely used in research studies on developmental disabilities (Burns, et al., 2013). For the current report, the MSEL was scored as detailed in its assessment manual by obtaining raw scores for each of its scales. The MSEL also allows for calculation of an “Early Learning Composite” score, but no such overall score was used here.
2.2.2. Peabody Developmental Motor Scales (PDMS-2)
The PDMS-2 is a standardized motor assessment composed of 2 Scales (Folio & Fewell, 2000): Gross Motor (reflexes, stationary, locomotion, and object manipulation); and Fine Motor (grasping, and visual-manual integration). Scores are available as composite Gross Motor Quotient (GMQ) and Fine Motor Quotient (FMQ), and as an overall Total Motor Quotient (TMQ). Standardization of the measure was completed on 2003 children. The PDMS-2 is used in clinical and research settings (e.g., Provost, Heimerl, & Lopez, 2007; Provost, Lopez, & Heimerl, 2007). Further, the PDMS-2 has good reliability, and concurrent validity with the BSID-III (Connolly, McClune, & Gatlin, 2012). For the current report, the PDMS-2 was scored as detailed in its assessment manual by obtaining subtest raw scores and then combining these raw scores to calculate the composite GMQ and FMQ scales as standard scores.
2.2.3. Early Motor Questionnaire (EMQ)
The EMQ is a parent-report measure of early motor development organized around different ‘contexts’ a child encounters during everyday situations (e.g., sitting at a table, playing on the floor). The items included on the EMQ describe motor behaviors typically emerging within the first 2 years of life (0-24 months) and are similar to items included on the MSEL or other motor assessments. Other primary caregivers (e.g., a grandparent or a nanny) may also complete the EMQ. However, due to the age range assessed by the EMQ, it is not targeted towards teachers.
The EMQ uses a 5-point scale ranging from −2 to +2 to quantify parents' certainty. A behavior is rated −2 if the parent is sure the child does not show the behavior yet, and +2 if parent remembers a particular instance where the child exhibited the behavior in question. Further, the EMQ is divided into 3 sections, a Gross Motor section (GM: 49 items), a Fine Motor section (FM: 48 items), and a Perception-Action section (PA: 31 items). According to optional feedback provided by 49 parents (52% of the sample), completion of the EMQ takes about 17 minutes (M = 16.57, SD = 10.65). Examples of prototypic EMQ items are shown in Table 3. The full EMQ can be obtained from the first author.
Table 3. Sample EMQ Items.
| Gross Motor Scale | |
|---|---|
| When placed into a sitting position on the floor, your child is able to… | |
| A | … sit independently without support (hands lifted up). |
| B | … use hands and legs to scoot forward on his/her bottom? |
| C | … maintain a stable sitting position while turning head and torso to look around? |
| Fine Motor Scale | |
|---|---|
| When sitting on your lap or in a high chair while playing with toys, you notice your child is able to… | |
| A | … successfully hold on to a small object such as a ring or stick? |
| B | … reach for a toy with one hand by extending the arm and fingers? |
| C | … successfully grasp a toy with one hand following a reach? |
| Perception-Action Scale | |
|---|---|
| While lying on his/her back in a crib, baby gym, or on the floor, your child sometimes will… | |
| A | … turn the head all the way to one side (90°) to follow your face? |
| B | … notice his/her own hands and look at them for some time? |
| C | … swat at toys hanging from a baby gym or car seat? |
Notes: Parents respond to each item on a 5-point scale, ranging from −2 (Parent is sure child does not show behavior) to +2 (parent is sure child shows behavior and remembers particular instance).
2.3. Procedure
The EMQ was mailed to all families with the request to complete the questionnaire prior to their visit to our lab. Nineteen caregivers (25%) failed to complete the EMQ prior to MSEL and PDMS-2 administration and completed it at home following observation of these assessments. Caregivers also completed unrelated experimental and standardized assessments during their visit, but the current report focuses only on the EMQ, MSEL, and PDMS-2 data.
2.4. Data analysis
Correlation analyses were used to investigate concurrent and predictive validity of EMQ scores. In addition, partial correlation was used to control for factors that may influence parent report such as socio-economic status (SES, Hollingshead, 1975), ASD risk group (high-risk sib-As vs. low-risk controls), time of EMQ completion (before vs. after MSEL/PDMS-2 observation), and person completing the EMQ (mother vs. other). Potential influences of these variables include that parents with higher SES might have more knowledge about child development and have higher expectations regarding their child's abilities. Similarly, parents with a child with ASD might pay more attention to the development of their younger child and may be more knowledgeable about development in general. Controlling for these and other factors in our analyses allows for greater generalizability of our findings. Partial correlations also controlled for age when assessing concurrent validity, or for the time gap between assessments when assessing predictive validity. The mother completed the EMQ for 84 children, the father for 6 children, a grandmother for 1 child, and for 3 children it is unknown who competed the EMQ. All concurrent validity results are based on cross-sectional data, predictive validity data is based on longitudinal data.
EMQ scores were computed separately for the GM, FM, and PA domains. For 10 children (11%), EMQ data was incomplete due to missing values. Missing singleton values were replaced with scores of 0 (6 children). For multiple missing values in a row, the affected sub-scale was removed from analyses (4 children).
For the EMQ and MSEL, all analyses were performed using raw scores as EMQ sections can be matched directly onto Scales of the MSEL (PA section matches onto VR of MSEL). In contrast, EMQ sections do not match directly onto PDMS-2 Scales, instead the composite Gross Motor Quotient (GMQ) and Fine Motor Quotient (FMQ) are compared the GM and FM sections of the EMQ respectively. Since GMQ and FMQ are composite scores, we will use standard scores on these Scales to compare to the EMQ. Examining correlations between PDMS-2 standard scores and EMQ raw scores is statistically sound and this approach complements the analysis approach used with the MSEL.
3. Results
There were no gender differences on any of the three EMQ sections (GM: Mmale = 13.48, Mfemale = −3.71, p = .14; FM: Mmale = −5.13, Mfemale = −11.71, p = .43; PA: Mmale = 10.90, Mfemale = 8.76, p = .74). Similarly, there were no gender differences in corresponding domains of the MSEL (GM: Mmale = 15.38, Mfemale = 13.59, p = .23; FM: Mmale = 14.52, Mfemale = 13.74, p = .59; VR: Mmale = 15.75, Mfemale = 14.53, p = .45), or on the PDMS-2 (GMQ: Mmale = 112.37, Mfemale = 109.50, p = .67; FMQ: Mmale = 100.81, Mfemale = 102.31, p = .81). Therefore, data were collapsed across gender for all further analyses.
3.1. Correlations with age
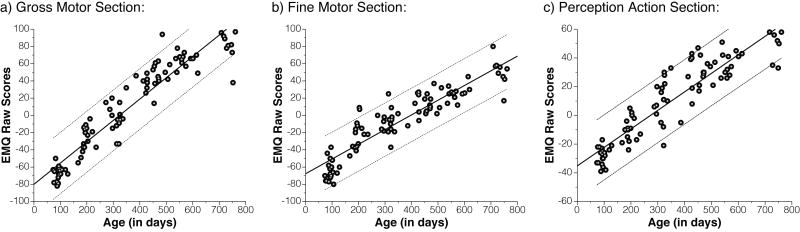
The relation between raw EMQ scores and age was investigated to determine whether parent ratings on the EMQ increase with age. Raw and partial correlation coefficients between EMQ scores and age were highly significant in all three domains (GM: r = .94, rPartial = .95; FM: r = .91, rPartial = .92; PA: r = .92, rPartial = .93, all ps < .01), suggesting that caregivers' responses on the EMQ are sensitive to the incremental changes in motor development over time (Figure 1).
Figure 1.
Correlations between EMQ and age. All three EMQ sections correlate strongly with age. Solid and dashed lines show linear fit with 95% confidence intervals.
3.2. Concurrent validity
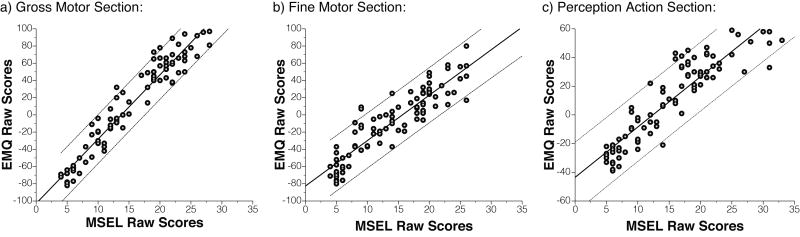
Concurrent validity assesses how well a test correlates with a previously established and validated measure (e.g., a gold standard). To establish the concurrent validity of parent report, EMQ scores were compared to scores from the examiner-administered MSEL and the PDMS-2. Raw and partial correlation coefficients between corresponding EMQ and MSEL sections were significant (GM: r = .97, rPartial = .67, both p < .01; FM: r = .91, p < .01, rPartial = .22, p = .04; PA/VR: r = .91, p < .01, rPartial = .27, p = .02), suggesting that parent report on the EMQ is predictive of MSEL scores above and beyond influences of age and other factors (Figure 2).
Figure 2.
Concurrent validity of EMQ with MSEL. The EMQ and MSEL correlate strongly with each other on corresponding sections (Perception Action section corresponds to Visual Reception scale on MSEL). Correlations remain strong after controlling for influences of age and other factors. Solid and dashed lines show linear fit with 95% confidence intervals.
Similarly, raw and partial correlation coefficients between EMQ raw scores and PDMS-2 standard scores were calculated. To our surprise, correlations between EMQ and PDMS-2 scores on corresponding sections were negative (GM-GMQ: r = −.45; FM-FMQ: r = −.43, both ps < .01). This negative relation seems to be caused by a negative correlation between the PDMS-2 and age in our sample (GMQ: r = −.61; FMQ: r = −.62, both ps < .01). The EMQ shows a positive relation with the PDMS-2 once effects of age are controlled for (GM-GMQ: rPartial = .47; FM-FMQ: rPartial = .40, both ps < .01). This significant positive relation confirms the concurrent validity of parent report on the EMQ using a second, motor-specific instrument.
The negative relation between age and PDMS-2 itself is noteworthy and remains even after controlling for influences from gender, SES, and ASD risk status (GMQ: rPartial = −.55; FMQ: rPartial = −.57, both ps < .01). This pattern may be a feature of our sample. By offering free developmental assessments, it is possible that the current study attracted a larger proportion of parents with concerns about their child's development and consequently more children may have developed mild delays as they grow older and consequently scored lower on the PDMS-2. A slight negative, although non-significant, relation was also observed between MSEL standard scores and age (all rs > −.08, ps > .11).1 At the same time, this negative relation could also be due to the structure of the PDMS-2 itself as negative relations between the PDMS-2 and the Bayley Scales of Infant Development II have been reported (Connolly, et al., 2006).
3.3. Predictive validity
Scores from a second examiner-administered assessment were available for 50 participants (53%) on the MSEL and for 45 participants (48%) on the PDMS-2. To determine predictive validity of parent report on the EMQ, raw and partial correlations were calculated between EMQ scores at time 1, and MSEL or PDMS-2 scores at time 2. The second visit occurred approximately 4.66 months (SD = 1.62) after the first visit.
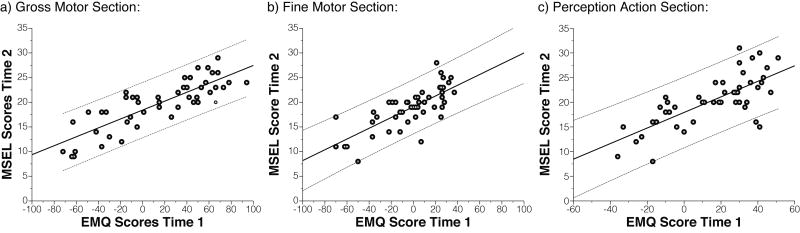
Raw and partial correlation coefficients between EMQ at time 1 and MSEL at time 2 were significant on all corresponding scales of the two instruments (GM: r = .83, rPartial= .89; FM: r = .75, rPartial= .83; PA/VR: r = .73, rPartial= .77, all ps < .01; see Figure 3). Similarly, raw and partial correlations between corresponding scales on the EMQ at time 1 and PDMS-2 at time 2 were significant (GM-GMQ: r = −.45, rPartial= −.56; FM-FMQ: r = −.39, rPartial= −.48, all ps < .01). Thus, parent reported EMQ scores show good predictive validity and were significantly correlated with examiner-derived scores on the MSEL or PDMS-2 obtained approximate 4.7 months later.
Figure 3.
Predictive validity or EMQ with MSEL. EMQ scores correlate strongly with corresponding MSEL scores obtained approximately 4.7 months later. Correlations remain strong after controlling for influences of the time-gap between assessments and other factors. Solid and dashed lines show linear fit with 95% confidence intervals.
3.4. Test-retest reliability
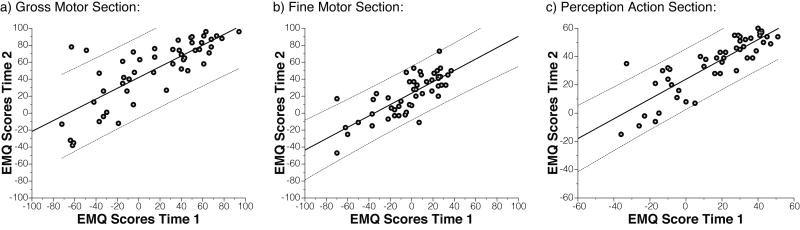
Longitudinal EMQ scores were available for 51 participants (54%). To determine the stability of repeated EMQ administrations, raw and partial correlations were calculated between EMQ scores at time 1 and at time 2. The second visit occurred approximately 4.69 months (SD = 1.60) after the first visit. Raw and partial correlation coefficients were significant for all three EMQ scales (GM: r = .78, rPartial= .80; FM: r = .77, rPartial= .79; PA: r = .85, rPartial= .88, all ps < .01; see Figure 4), indicating that parent report on the EMQ shows good test-retest reliability over a 4-5 month period.
Figure 4.
Test-retest reliability of EMQ over two time points obtained approximately 4.7 months apart. EMQ scores at time 1 correlate strongly with EMQ scores at time 2. Correlations remain strong after controlling for influences of the time-gap between assessments and other factors. Solid and dashed lines show linear fit with 95% confidence intervals.
4. Discussion
The results reported here answer the two research questions raised in our introduction. First, the results provide direct evidence for the validity of parent report by showing that parents provide accurate and reliable accounts of their children's early motor development when using the EMQ. And second, the results show promise for the EMQ as a reliable and valid measure or early motor development: EMQ scores increase linearly with age, show high concurrent validity with two separate examiner-administered measures (MSEL and PDMS-2), have good predictive validity with MSEL and PDMS-2 scores obtained nearly five months later, and have good test-re-test reliability. These findings are robust and remain significant even after controlling for influences of age, SES, ASD risk status, and other extraneous variables.
4.1. Validity of parent report on early motor development
This is the first study to compare both concurrent and predictive validity of parent report on early motor development with two standardized, examiner-administered measures of motor ability in the same children. Our results are in agreement other studies comparing parent report to examiner-administered assessments and add further support to the validity of well-designed parent-report measures (e.g., Bricker & Squires, 1989; Bricker, et al., 1988; Goldstein, 1985; Knobloch, et al., 1979; Wilson, et al., 2000).
Concurrent validity of parent report in the motor domain was assessed by Goldstein (1985) and Bordnarchuk and Eaton (2004). Goldstein (1985) compared parent report on the motor scale of the Vineland Adaptive Behavior Scales (VABS; Sparrow, et al., 1984) to the motor scale of the Bayley Scales of Infant Development (BSID, Bayley, 1969) in 12-month-olds infants who graduated from an intensive care nursery. Similar to our findings, Goldstein (1985) reported strong correlations (r = .86) between VABS and the BSID. Bodnarchuk and Eaton (2004) compared the examiner-administered Alberta Infant Motor Scales (AIMS, Piper, Pinnell, Darrah, Maguire, & Byrne, 1992) to a custom-designed parent- report version of the AIMS in 2.5- to 15.7-month-old children and report high concordance between parent report and the examiner-administered AIMS. However, the parent-report version of the AIMS used by Bodnarchuk and Eaton (2004) was structured as a daily diary and assessed only 12 gross motor milestones with dichotomous outcome classifications (present/absent).
Predictive validity of parent report was assessed by Knobloch and colleagues (1979) using the Parent Development Questionnaire (PDQ) completed at around 7 months of age and the Gesell Developmental and Neurologic Evaluation (GDNE) completed at around 10 months of age. Agreement between parent report on the PDQ and the GDNE was good, but the PDQ questionnaire allows open-ended answers and requires review and scoring by a developmental specialist.
Our results expand these previous findings by investigating concurrent and predictive validity in the same children, by explicitly accounting for influences of age and other factors, and by comparing parent report to two different examiner-administered measures (the MSEL and PDMS-2). Taken together, there is now good evidence supporting the validity of parent-report measures in the domain of early motor development. Consequently, parent report should receive more attention during diagnostic evaluations and during research assessments (Glascoe & Dworkin, 1995).
4.2. Applications
In addition to evaluating validity of parent-report measures, the current study introduces the EMQ. The items included on the EMQ are also commonly assessed on other standardized measures such as the MSEL. As such, the EMQ provides a new tool for clinicians and researchers alike to quickly assess early motor development, offering an alternative to lengthy and expensive in-lab or in-clinic assessments.
A key future application of the EMQ may be in longitudinal research projects. Repeated examiner-administered assessments in longitudinal studies are expensive and may lead to significant training effects over time. Spacing fewer assessments over a longer period of time addresses cost and training-effect issues, but may lead to sparse sampling and degradation of developmental trajectories (Adolph, Young, Robinson, & Gill-Alvarez, 2008). The EMQ can be used repeatedly without risks of training effects in the child (although ‘training effects’ may be present in the parent) and may even be used in conjunction with other measures, such as the MSEL, to fully evaluate motor development.
Moreover, the EMQ could be used as a first-step screening instrument to identify children who should receive further assessments. For example, children scoring low on the EMQ are also likely to score low on the MSEL. Finally, the EMQ can be used in situations where direct observation is not possible such as for online surveys or telephone-based studies, or as additional assessment in-between lab visits during large scale-longitudinal studies.
4.3. Limitations
The current report provides strong evidence for the validity of parent report. However, several limitations need to be considered with regard to the EMQ as a new assessment tool. First, the EMQ has not yet been standardized and age-equivalence scores are not yet available. Second, no item analysis has been conducted for the EMQ at this point because sample size (especially in individual age group) is relatively small in this preliminary report. Data from more participants and additional longitudinal data from existing participants are currently being collected and this question will be addressed in the future. Finally, parents knew their children would be assessed during this study and may have taken additional care in completing the EMQ.
To determine the clinical value of the EMQ it is necessary to establish its sensitivity to detect delayed motor development. Although children at heightened risk for ASD were included in our study, the majority of children performed within the normal range on standardized scores and no ASD outcome information is available at this time. Whether the EMQ can be used to identify early signs of motor delays or ASD will be investigated once outcome data becomes available for our sample.
5. Conclusion
Parents' ratings on the Early Motor Questionnaire show good concurrent and predictive validity in comparison to objective, standardized, examiner- administered motor development measures. These results suggest that parents can provide dependable reports of their child's early motor development, and that the newly introduced EMQ provides a reliable, valid, and inexpensive parent-report measure of children's early motor development. Future studies will be required to further scrutinize the EMQ and its psychometric profile.
Highlights.
Parent report on early motor development was compared to standardized assessments.
The Early Motor Questionnaire (EMQ) for ages 3-24 months is introduced.
Parent's report correlated strongly with examiner-administered assessments.
Parents provide reliable and valid information on early motor skills using the EMQ.
Acknowledgments
We would like to thank the children and their families for their generous participation in and commitment to this research. We also thank the research staff at the Kennedy Krieger Institute Center for Autism and Related Disorders. Financial support was provided by NIMH grant R01 MH59630 to RL, and by an Autism Science Foundation Post-doctoral Fellowship to KL.
Footnotes
Correlations between the MSEL and PDMS-2 are not reported here because the examiner always had access to the scores of the MSEL when administering the PDMS-2, leading to potentially inflated correlation scores.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolph KE, Young JW, Robinson SR, Gill-Alvarez F. What is the shape of developmental change? Psychological Review. 2008;115(3):527–543. doi: 10.1037/0033-295X.115.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D, Piper M. Mothers' Difficulty in Assessing the Motor Development of Their Infants Born Preterm: Implications for Intervention. Pediatric Physical Therapy. 1994;6(2):55–60. [Google Scholar]
- Bayley N. The Bayley Scales of Infant Development. New York: Psychological Corporation; 1969. [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development, third edition. San Antonio, TX: Harcourt Assessment, Inc; 2006. [Google Scholar]
- Bodnarchuk JL, Eaton WO. Can parent reports be trusted?: Validity of daily checklists of gross motor milestone attainment. Journal of Applied Developmental Psychology. 2004;25(4):481–490. [Google Scholar]
- Bricker D, Squires J. Low Cost System Using Parents to Monitor the Development of At-Risk Infants. Journal of Early Intervention. 1989;13(1):50–60. [Google Scholar]
- Bricker D, Squires J, Kaminski R, Mounts L. The Validity, Reliability, and Cost of a Parent-Completed Questionnaire System to Evaluate At-Risk Infants. Journal of Pediatric Psychology. 1988;13(1):55–68. doi: 10.1093/jpepsy/13.1.55. [DOI] [PubMed] [Google Scholar]
- Burns TG, King TZ, Spencer KS. Mullen Scales of Early Learning: The Utility in Assessing Children Diagnosed With Autism Spectrum Disorders, Cerebral Palsy, and Epilepsy. Applied Neuropsychology: Child. 2013;2(1):33–42. doi: 10.1080/21622965.2012.682852. [DOI] [PubMed] [Google Scholar]
- Bushnell EW, Boudreau JP. Motor Development and the Mind -the Potential Role of Motor Abilities as a Determinant of Aspects of Perceptual Development. Child Development. 1993;64(4):1005–1021. [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel Broadens the Mind. Infancy. 2000;1(2):149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Connolly BH, Dalton L, Smith JB, Lamberth NG, McCay B, Murphy W. Concurrent validity of the Bayley Scales of Infant Development II (BSID-II) Motor Scale and the Peabody Developmental Motor Scale II (PDMS-2) in 12-month-old infants. Pediatr Phys Ther. 2006;18(3):190–196. doi: 10.1097/01.pep.0000226746.57895.57. [DOI] [PubMed] [Google Scholar]
- Connolly BH, McClune NO, Gatlin R. Concurrent validity of the Bayley-III and the Peabody Developmental Motor Scale-2. Pediatric Physical Therapy. 2012;24(4):345–352. doi: 10.1097/PEP.0b013e318267c5cf. [DOI] [PubMed] [Google Scholar]
- Creighton DE, Sauve RS. The Minnesota Infant Development Inventory in the developmental screening of high-risk infants at eight months. Canadian Journal of Behavioural Science/Revue canadienne des sciences du comportement. 1988;20(4):424–433. [Google Scholar]
- Dobrez D, Sasso AL, Holl J, Shalowitz M, Leon S, Budetti P. Estimating the cost of developmental and behavioral screening of preschool children in general pediatric practice. Pediatrics. 2001;108(4):913–922. doi: 10.1542/peds.108.4.913. [DOI] [PubMed] [Google Scholar]
- Duby J, Lipkin P, Macias M, Wegner L, Duncan P, Hagan J, et al. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics. 2006;118(1):405–420. doi: 10.1542/peds.2006-1231. [DOI] [PubMed] [Google Scholar]
- Easley AM, Liptak GS, Bair L, Campbell T, Kaupang K, Strucker J. The Use of Parent-Completed Developmental Questionnaires by Physical Therapists and Physicians. Pediatric Physical Therapy. 1996;8(3):104–110. [Google Scholar]
- Ellis-Davies K, Sakkalou E, Fowler NC, Hilbrink EE, Gattis M. CUE: The continuous unified electronic diary method. Behav Res Methods. 2012 doi: 10.3758/s13428-012-0205-1. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Milberger S. How reliable are maternal reports of their children's psychopathology? One-year recall of psychiatric diagnoses of ADHD children. Journal of the American Academy of Child and Adolescence Psychiatry. 1995;34(8):1001–1008. doi: 10.1097/00004583-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Fenson L, Marchman V, Dale P, Reznick JS, Thal D, Bates E. MacArthur-Bates Communicative Development Inventories. 2nd. Baltimore, MD: Brookes Publishing Company; 2007. [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. The American Journal of Occupational Therapy. 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Folio MR, Fewell RR. Peabody developmental motor scales examiner's manual. 2nd. Austin, TX: Pro-Ed Inc; 2000. [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting and acquiring of knowledge. Annual Review of Psychology. 1988;39:1–41. [Google Scholar]
- Gibson EJ, Pick AD. An ecological approach to perceptual learning and development. New York: Oxford University Press; 2000. [Google Scholar]
- Gillberg C, Kadesjo B. Why bother about clumsiness? The implications of having developmental coordination disorder (DCD) Neural Plast. 2003;10(1–2):59–68. doi: 10.1155/NP.2003.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascoe FP, Dworkin PH. The role of parents in the detection of developmental and behavioral problems. Pediatrics. 1995;95(6):829–836. [PubMed] [Google Scholar]
- Gliga T, Elsabbagh M, Hudry K, Charman T, Johnson MH. Gaze following, gaze reading, and word learning in children at risk for autism. Child Development. 2012;83(3):926–938. doi: 10.1111/j.1467-8624.2012.01750.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ. Accuracy of parental report of infants' motor development. Perceptual and Motor Skills. 1985;61(2):378. doi: 10.2466/pms.1985.61.2.378. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Ireton H. The child development review: Monitoring children's development using parents' and pediatricians' observations. Infants and Young Children. 1996;9(1):42–52. [Google Scholar]
- Ireton H, Glascoe FP. Assessin Children's Development Using Parents' Reports: The Child Development Inventory. Clinical Pediatrics. 1995;34(5):248–255. doi: 10.1177/000992289503400504. [DOI] [PubMed] [Google Scholar]
- Ireton H, Thwing E. Minnesota Infant Development Inventory. Minneapolis: Behavior Science System; 1980. [Google Scholar]
- Iverson JM. Developing language in a developing body: the relationship between motor development and language development. Journal of Child Language. 2010;37(2):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Marlow N. Developmental screen or developmental testing? Early Human Development. 2006;82(3):173–183. doi: 10.1016/j.earlhumdev.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Brown T, Chien CW. Motor skill assessment of children: is there an association between performance-based, child-report, and parent-report measures of children's motor skills? Physical & Occupational Therapy in Pediatrics. 2012;32(2):196–209. doi: 10.3109/01942638.2011.631101. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Brown T, Stagnitti K. Top-down and bottom-up approaches to motor skill assessment of children: Are child-report and parent-report perceptions predictive of children's performance-based assessment results? Scandinavian Journal of Occupational Therapy. 2012 doi: 10.3109/11038128.2012.693944. [DOI] [PubMed] [Google Scholar]
- Knobloch H, Stevens F, Malone A, Ellison P, Risemberg H. The validity of parental reporting of infant development. Pediatrics. 1979;63(6):872–878. [PubMed] [Google Scholar]
- Landa RJ, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psycholgy and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Libertus K, Needham A. Reaching experience increases face preference in 3-month-old infants. Developmental Science. 2011;14(6):1355–1364. doi: 10.1111/j.1467-7687.2011.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TM. The Use of Parent Report Measures to Assess Infant Development. Pediatric Physical Therapy. 1992;4(2):74–77. [Google Scholar]
- Mullen EM. Mullen: Scales of Early Learning (AGS Edition) Circle Pines, MN: American Guideline Service Inc; 1995. [Google Scholar]
- Needham A, Libertus K. Embodiment in Early Development. Wiley Interdisciplinary Reviews: Cognitive Science. 2011;2:117–123. doi: 10.1002/wcs.109. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128(3):e488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The Origin of Intelligence in the Child. New York: Routledge & Kegan Paul Ltd; 1953. [Google Scholar]
- Piek JP, Dawson L, Smith LM, Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Human Movement Science. 2008;27(5):668–681. doi: 10.1016/j.humov.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Piper MC, Pinnell LE, Darrah J, Maguire T, Byrne PJ. Construction and validation of the Alberta Infant Motor Scale (AIMS) Canadian Journal of Public Health. 1992;83(Suppl 2):S46–50. [PubMed] [Google Scholar]
- Provost B, Heimerl S, Lopez BR. Levels of gross and fine motor development in young children with autism spectrum disorder. Physical & Occupational Therapy in Pediatrics. 2007;27(3):21–36. [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disordorders. 2007;37(2):321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Alley A. Validation of the language development survey (LDS): a parent report tool for identifying language delay in toddlers. J Speech Lang Hear Res. 2001;44(2):434–445. doi: 10.1044/1092-4388(2001/035). [DOI] [PubMed] [Google Scholar]
- Seifer R. Handbook of Research Methods in Developmental Science. Blackwell Publishing Ltd; 2008. Who Should Collect Our Data: Parents or Trained Observers? pp. 15–137. [Google Scholar]
- Smith LB. Cognition as a dynamic system: Principles from embodiment. Developmental Review. 2005;25(3–4):278–298. [Google Scholar]
- Sparrow S, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Squires J, Bricker D, Potter L. Revision of a Parent-Completed Developmental Screening Tool: Ages and Stages Questionnaires. Journal of Pediatric Psychology. 1997;22(3):313–328. doi: 10.1093/jpepsy/22.3.313. [DOI] [PubMed] [Google Scholar]
- Wilson BN, Kaplan BJ, Crawford SG, Campbell A, Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. American Journal of Occupational Therapy. 2000;54(5):484–493. doi: 10.5014/ajot.54.5.484. [DOI] [PubMed] [Google Scholar]