Abstract
Knowledge about prenatal learning has been largely predicated on the observation that newborns appear to recognize the maternal voice. Few studies have examined the process underlying this phenomenon; that is, whether and how the fetus responds to maternal voice in situ. Fetal heart rate and motor activity were recorded at 36 weeks gestation (n = 69) while pregnant women read aloud from a neutral passage. Compared to a baseline period, fetuses responded with a decrease in motor activity in the 10-seconds following onset of maternal speech and a trend level decelerative heart rate response, consistent with an orienting response. Subsequent analyses revealed that the fetal response was modified by both maternal and fetal factors. Fetuses of women who were previously awake and talking (n = 40) showed an orienting response to onset of maternal reading aloud, while fetuses of mothers who had previously been resting and silent (n = 29) responded with elevated heart rate and increased movement. The magnitude of the fetal response was further dependent on baseline fetal heart rate variability such that largest response was demonstrated by fetuses with low variability of mothers who were previously resting and silent. Results indicate that fetal responsivity is affected by both maternal and fetal state and have implications for understanding fetal learning of the maternal voice under naturalistic conditions.
Keywords: fetal heart rate, fetal movement, maternal voice, auditory perception
1. Introduction
Neonates show preference for their mother’s voice just hours after birth (DeCasper & Spence, 1986; Fifer, 1987; Fifer & Moon, 1995; Moon et al., 1993; Spence & DeCasper, 1987; Granier-Deferre et al., 2011; Moon & Fifer, 2000). As evidenced originally by behavior during a non-nutritive sucking discrimination procedure (DeCasper & Fifer, 1980; Fifer, 1989), two dayold infants engage in more frequent sucking bursts to elicit an audio recording of their mother’s voice over that of another female (DeCasper & Fifer, 1980; Fifer, 1989). Newborn infants also show a physiological orienting response to the maternal voice, exhibiting heart rate decelerations (Ockleford et al., 1988) and fewer movements (Fernald, 1989) while listening to a recording of their mother’s voice versus a stranger female voice. Further, they prefer a low-pass filtered version of the maternal voice, designed to mimic prenatal speech sounds, over an unfiltered version (Fifer & Moon, 1995).
Preference for the maternal voice must be preceded by both fetal detection and learning through recurrent exposure. The fetal ear is well-equipped to detect auditory stimuli. Mechanisms of hearing are notably different for the fetus and neonate as oxygen diffusion by the placenta results in attenuation of the sensorineural threshold in utero relative to postnatal pulmonary oxygenation (Sohmer & Freeman, 1995), however, cochlear biomechanics are fully matured by near term (Granier-Deferre et al., 2011; Hepper & Shahidullah, 1994; Lecanuet & Schaal, 1996; Moon & Fifer, 2000). The human intrauterine environment provides a rich auditory environment as the fetus is exposed to sounds of the maternal cardiovascular, gastrointestinal, and respiratory systems and those generated by body movements. The maternal voice figures prominently on audio recordings of the uterine environment relative to background sounds. It is far less attenuated than other voices that emanate outside the uterus given that external transmission through the uterine wall is also coupled with internal vibrations of the maternal larynx and diaphragm (Busnel, 1979; Querleu et al., 1988). Spectral analysis indicates that the maternal voice has well preserved prosodic characteristics in utero (Spence & DeCasper, 1987), imparting information about rhythm and pitch contours (Moon et al., 2013). Thus, neonatal preference for the maternal voice is attributed to the uniquely multimodal sensory input during maternal speech (Moon & Fifer, 2000) and recurrent prenatal exposure (DeCasper et al., 1994; Gerhardt & Abrams, 2000) necessary for postnatal discrimination of the maternal voice versus others.
Two types of studies have evaluated the fetal response to the maternal voice as a basis for understanding prenatal learning. The first, more common, approach uses a recording of the maternal voice presented as an auditory stimulus via speakers placed near the maternal abdomen. Results are mixed. Two studies report an increase in fetal heart rate in response to the maternal voice relative to a stranger female voice (Kisilevsky et al., 2003; Kisilevsky et al., 2009), while two others show a significant decrease in fetal heart rate, consistent with the observed neonatal orienting response to the maternal voice (Fifer & Moon, 1994; Lecanuet et al., 2002). One study found that fetuses did not discriminate between their mother’s and a stranger’s voice played to them via audio recording (Hepper et al., 1993).
Presentation of the maternal voice from an external source does not capture the true nature of sound transmission to the fetus because it omits the acoustical complexity of the internally transmitted signal. Study of the spoken maternal voice in situ is uncommon, but is most germane to the underlying processes inherent to fetal detection of and attention to the maternal voice in the natural uterine environment. To our knowledge, there is only a single published study that relied on the maternal spoken voice to determine a fetal response. Hepper and colleagues (1993) evaluated ultrasound observed fetal motor activity in response to the maternal spoken voice in comparison to a presentation of a pre-recording of the maternal voice in a small sample (n = 10) of fetuses. Findings suggested 36 week old fetuses were capable of discrimination, as evidenced by a reduction in motor activity unique to onset of the maternal spoken voice (Hepper et al., 1993). Two other preliminary reports from conference proceedings document opposing fetal heart rate responses to the maternal spoken voice. One report found that fetuses exhibiting low heart rate variability and motor activity showed a heart rate decrease from baseline 5 seconds post onset of maternal spoken voice (Masakowski & Fifer, 1992), while the second reported a fetal heart rate acceleration (Lecanuet et al., 2002).
To this end, other work has confirmed that the fetal response to speech and other sound stimuli varies by fetal state and/or the degree of fetal heart rate variability prior to stimulus application (Groome et al., 1994; Groome et al., 1999; Lecanuet et al., 1986; Lecanuet et al., 1989; Lecanuet et al., 1992; Zimmer et al., 1993). Specifically, fetuses stimulated when heart rate variability is low are more responsive relative to those stimulated when heart rate variability is higher. Although responsiveness depends, in part, on sound characteristics, they are more likely to startle in response to high intensity sounds (Groome et al, 1994) as well as show a decelerative orienting response when presented with low intensity sounds (Zimmer et al., 1993).
Thus, while it is clear that the maternal spoken voice should be detectable to the fetus little information is available on either whether or how the fetus responds to the spoken voice in situ. The goal of the current study is to describe the near-term fetal response, measured by changes in fetal heart rate and motor activity, to onset of spoken maternal voice while reading a neutral passage. This method provides a naturalistic context to maternal speech production and potential fetal response. Based on limited existing results that suggest that the maternal spoken voice evokes an ‘orienting’ movement response in the near term fetus (Hepper et al., 1993) we predict the fetal response will be consistent with mild orienting. Given that the fetal response to a presentation of the audiotaped maternal voice unfolds over tens of seconds (DeCasper et al., 1994), we expect the fetal response to have a relatively short latency, occurring within 30s of stimulus onset from when the mother begins to read aloud. Potential moderating influences of maternal and fetal parameters that might affect either the nature of the stimulus presented to the fetus (i.e., maternal state) or the propensity of the fetus to detect the stimulus (i.e., fetal heart rate variability level) were also evaluated.
2. Methods
2.1. Participants
Eligibility was restricted to normotensive, non-smoking women carrying a singleton fetus with uncomplicated pregnancies at the time of enrollment. Accurate dating of the pregnancy based on last menstrual period and early confirmation by ultrasound was required. Seventy-four women participated in maternal-fetal monitoring at 36 weeks gestation (M = 36.4 weeks GA, SD = 0.3). On average, participants were well educated (M years of education = 17.5 years, SD = 1.8) and mature (M age=32.4, SD= 4.5). The sample was 70% non-Hispanic white, 12% African American, and 18% Hispanic or Asian American. The majority of women were married (97%) and working outside the home at the time of data collection (91%). Most (64%) were expecting their first child. Fetuses under study were born at term (M = 39.6 weeks GA, SD = 1.1), of normal birthweight (M = 3333.2 grams, SD = 501.4) and had normal 5 min Apgar scores (M = 8.97, SD = 0.31). The study was approved by the university’s Institutional Review Board and women provided written, informed consent.
2.2. Procedure
Women visited the laboratory for fetal monitoring at 36 weeks gestation. To control for time of day and prandial effects, visits were scheduled at the same time of day (1:30 p.m.) and women were instructed to eat 1.5 hours prior to the start of data collection and not thereafter. A brief real-time ultrasound scan was conducted to determine fetal position as a consideration in placement of the Doppler transducer and provide photographs to parents. The read aloud procedure, which is the focus of this report, was done following a 50 minute undisturbed period of maternal-fetal monitoring that was part of a larger protocol unrelated to the maternal voice. During the initial 50 minute recording period, women completed several questions that were part of the larger protocol, and were then offered the opportunity to either remain awake for the duration of the baseline recording or to rest. Participants that stayed awake (n = 42, 58%) conversed informally with the investigators for the remainder of the period. For those that opted to rest (n = 29, 42%) the lights were dimmed and monitors quieted. These women rested with their eyes closed, exhibiting little to no movement and were silent for approximately 25 minutes (SD = 8.66 min).
The maternal reading aloud manipulation commenced after this 50 minute baseline recording. In the final 30 seconds of the baseline women who were resting were gently roused and the lights in the room returned to normal. No woman appeared to be deeply asleep or disoriented upon rousing. After concluding the baseline segment, there was a brief transition period where women remained in the same semi-recumbent position and fetal monitoring transducers were kept in place. Participants were instructed to read aloud from a neutral passage on nature (Annie Dillard, Pilgrim at Tinker Creek) for a 2 minute period. The reading period was audiotaped to encourage voice projection, but no further instruction was given for voice modulation. Participants were cued to begin and fetal monitoring resumed. Audio recordings of maternal reading were subsequently dually coded by two investigators for maternal animation and fluency using 3-point Likert-type scales; disputes were resolved by taking the mean of the two codes. Animation indexed maternal voice inflection and modulation, ranging from 1 (flat) to 3 (high inflection). Fluency indexed maternal voice fluidity and proficiency, ranging from 1(stammers or pauses while reading) to 3 (reads fluidly without interruption).
2.3. Fetal measures
Fetal data were collected using a Toitu MT320 fetal actocardiograph which detects fetal heart rate and movement using a single wide-array Doppler transducer positioned on the maternal abdomen. Continuous fetal heart rate and movement data were digitized from the output port of the monitor. Fetal heart rate (FHR) was detected and quantified using autocorrelation techniques, the standard method for antepartum fetal heart rate monitoring. The actograph detects fetal movements (FM) by preserving the remaining signal after bandpassing frequency components of the Doppler signal that are associated with FHR and maternal somatic activity. Reliability studies comparing actograph based versus ultrasound visualized fetal movements have found the performance of this monitor to be highly accurate in detecting both fetal motor activity and quiescence (Besinger & Johnson, 1989; DiPietro et al., 1999; Maeda et al., 1999).
Digitized fetal data were subsequently processed using customized software (GESTATE; James Long Company, Caroga Lake, NY). FHR data underwent error rejection procedures based on moving averages of acceptable values as needed. FM data represent raw voltage values generated from the actograph calibrated by multiplying by a conversion factor and scaled from 0 to 100 in arbitrary units (aus).
2.4. Data analysis plan
The SAS MIXED procedure was used to estimate hierarchical linear models (HLM) to test change over time in fetal heart rate and fetal movement sampled in 200 ms intervals. HLM maximizes the repeated measures structure of the data, as observations nested within persons, accommodating the large number of data points indexed for each mother and fetus. This approach also offers a statistical framework to simultaneously estimate variance within and between persons, utilizing all available data.
To provide a comparable duration to the maternal read aloud manipulation, the last 2 min of data from the 50 minute baseline fetal monitoring were extracted as the baseline period, and average FHR and FM values were calculated. To evaluate the immediate fetal response to onset of maternal reading aloud, difference scores (deltas) were computed for FHR and FM values by subtracting the average baseline value from each data point of fetal measurement sampled from onset of reading aloud to 30 seconds post, separately for each subject. The use of deltas controls for individual differences in baseline FHR and FM than can affect the amplitude of stimulus-elicited changes in these parameters.
To explore the impact of maternal vocalization prior to the maternal read aloud stimulus on the fetal response, maternal state during baseline was dichotomized as awake/talking or resting/silent and entered at Level-2 in HLM models as a categorical predictor of fetal heart rate and fetal movement response. Fetal heart rate patterns during baseline were coded from fetal heart rate tracings by an investigator (JD) with significant training and experience in coding fetal heart rate patterns within the context of fetal state as first described by Dutch investigators (Nijhuis et al., 1982). Baseline periods were categorized as displaying low or high fetal heart rate variability, consistent with fetal states of IF or 2F, respectively. General linear modeling was applied to examine differences in the magnitude of the fetal heart rate and movement response by maternal state and fetal heart rate variability, with post hoc contrasts estimated to examine within group differences.
3. Results
3.1. Preliminary analyses
A preliminary examination of FHR and FM data revealed that five (6.8%) fetuses exhibited elevated heart rate and sustained high levels of movement at the end of the baseline period consistent with an active waking state. Since high fetal heart rate variability and motor activity interfere with stimulus detection and makes it difficult to discern a fetal response against this background, these cases were excluded from all analyses, resulting in a final sample of 69 fetuses (54% male).
Next, to rule out any impact of preparation for the read aloud protocol or change in maternal state for those previously resting, and following a previous study using a similar analytic approach to discern a fetal response to an event (Buss et al., 2009), the baseline interval was shifted to exclude the end of the data recording. On the basis of preliminary analysis revealing a fetal anticipatory response specific to fetuses of women who were awake and talking during the final 30 seconds, the 2 min baseline interval from 150-30 seconds was selected for analysis. For this group of fetuses, FHR significantly increased in the final 30 seconds (β = 0.53, SE = 0.17, t = 3.11, p < .01), resulting in a level difference in FHR at the conclusion of the baseline by maternal state (FHRawake = 147.0 bpm, FHRrest = 140.1 bpm; t = −2.19, p < .05). Prior to this, there was no difference in mean FHR by maternal state (Mawake = 140.7 bpm, Mrest = 140.0 bpm; t = 0.37, p = .71).
3.2. Fetal response to maternal spoken voice
Table 1 presents descriptive data for fetal heart rate and fetal movement during the 120 second unstructured baseline and 30 second reading aloud segments (n = 69). The continuous data streams of FHR and FM averaged across individuals are depicted in Figures 1a and 2a, respectively. Figures show data sampled once per second for clarity of presentation. To test the fetal FHR and FM response to the onset of maternal reading aloud, delta values representing the relative difference from the fetus’ average baseline were modeled from 0 to 30 s post onset of maternal reading aloud.
Table 1.
Descriptive values for fetal heart rate and fetal movement during the baseline and speech stimulus (N = 69)
| Baseline period (150 to 30 s) | Speech Stimulus Period | |||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Fetal heart rate (bpm) | 140.83 (7.66) | 124 to 158 | 141.95 (9.20) | 123 to 171 |
| Fetal movement (aus) | 4.56 (1.16) | 3.2 to 8.5 | 4.47 (1.19) | 3.3 to 9.9 |
Note. bpm = beats per minute; aus= arbitrary units.
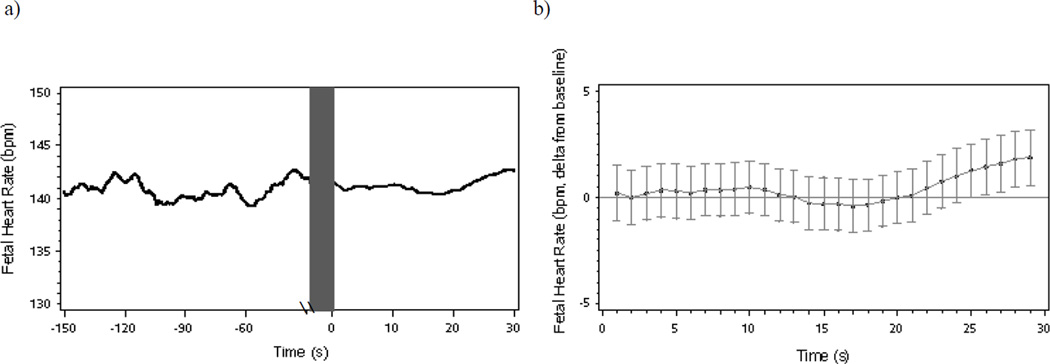
Figure 1.
(a) Mean fetal heart rate during a 120-second baseline period and 30-second maternal reading aloud period and (b) fetal heart rate changes (deltas and mean standard error) from baseline following onset of maternal reading aloud.
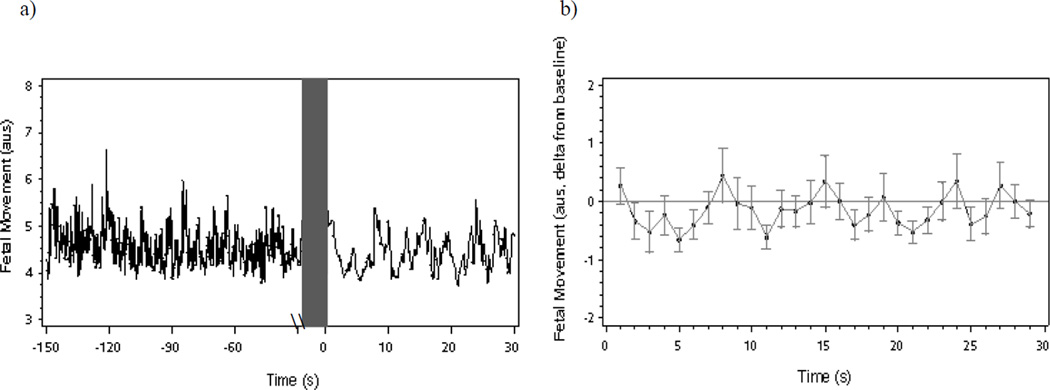
Figure 2.
(a) Mean fetal movement during a 120-second baseline period and 30-second maternal reading aloud period and (b) fetal movement changes (deltas and mean standard error) from baseline following onset of maternal reading aloud.
3.2.1. Fetal heart rate
In the full sample there was a trend level quadratic decrease in FHR following onset of the reading aloud period (β = 0.006, SE = 0.004, t = 1.69, p = .09) (see Figure 1b for delta FHR). The linear change was not significant (β = −0.16, SE = 0.13, t = −1.20, p = .23).
3.2.2. Fetal movement
For FM, both the linear and quadratic decline was significant, occurring with the first 8 seconds post onset of maternal reading aloud (linear: β = −0.53, SE = 0.19, t = −2.78, p < .01, quadratic: β = 0.059, SE = 0.022, t = 2.67, p < .01) (see Figure 2b for delta FM). The suppression of motor activity was transient, and not observed across the entire 30 s read aloud period (linear: β = −0.01, SE = 0.04, t = −0.32, p = .75, quadratic: β = 0.01, SE = 0.01, t = 0.37, p = .71).
3.3. Fetal response to maternal spoken voice as a function of maternal state during baseline
The read aloud period was applied following a baseline period with natural variation in maternal state. To determine whether this affected either the manner in which women read aloud or the fetal response analyses were conducted comparing those women who were awake and conversing during the 120 second baseline period (n = 40; 58%) to those who were resting and silent (n = 29; 42%).
3.3.1. Maternal state and maternal reading aloud
Group differences in maternal animation (M = 1.94, SD = 0.74) and fluency (M = 2.30, SD = 0.65) were evaluated to determine if maternal state during the baseline period impacted qualities of the maternal voice during the reading manipulation. There were no differences in maternal vocal animation (χ2 (2, 69) = 0.59, p = .75) or fluency (χ2 (2, 69) = 0.42, p = .81) by maternal state during baseline suggesting that rest prior to reading aloud did not diminish voice inflection or language fluidity.
3.3.2. Maternal state and fetal heart rate
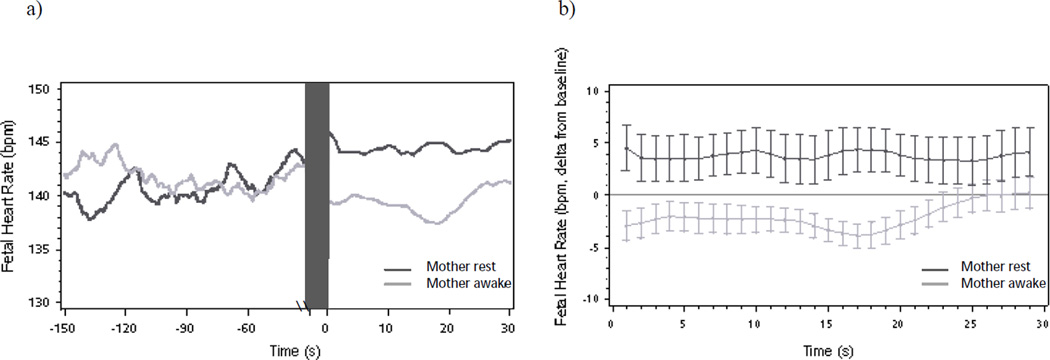
Next, maternal state was added to the hierarchical linear models of FHR and FM response as a dichotomous predictor modeled at Level-2. During the baseline period, there was no difference in mean FHR by maternal state (Mawake = 140.7 bpm, Mrest = 140.0 bpm; t = 0.37, p = .71). At onset of maternal reading aloud, fetuses of women who were resting and silent had higher FHR relative to fetuses of women who were awake and talking (β = 5.77, SE = 2.78, t = 2.07, p < .05) (see Figures 3a and 3b). As women continued to read, fetuses of women who were previously resting sustained elevated FHR in comparison to baseline levels (β = 4.16, SE = 2.12, t = 1.96, p = .05) that did not show quadratic change over time from onset to 30 seconds post (β = 0.0009, SE = 0.006, t = 0.16, p = .87). In comparison, there was a significant quadratic decline in FHR among fetuses who had pre-exposure to the maternal voice during baseline (β = 0.01, SE = 0.005, t = 2.10, p < .05). The linear change in FHR was non-significant for fetuses of both awake (β = −0.24, SE = 0.17, t = −1.40, p = .17) and resting (β = −0.04, SE = 0.20, t = −0.19, p = .85) maternal state groups.
Figure 3.
(a) Mean fetal heart rate and (b) fetal heart rate changes (deltas and mean standard error) from baseline following onset of maternal reading aloud as a function of maternal state during baseline. Note fetal orienting response to maternal reading aloud among fetuses of mothers’ previously awake, fetal heart rate elevation among fetuses of mothers resting and silent.
3.3.3 Maternal state and fetal movement
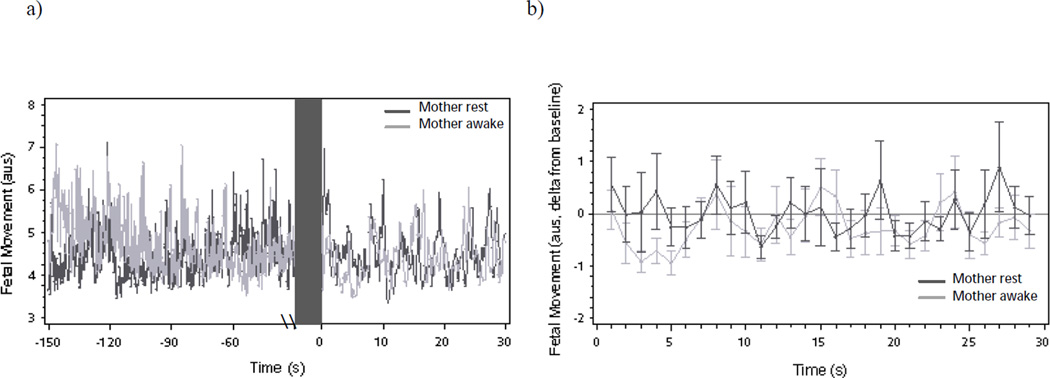
Similar to FHR, FM during the baseline did not differ by maternal state (Mawake = 4.52 a.u.s., Mrest = 4.30 a.u.s.; t = 0.95, p = .34). At onset of reading aloud, there was a significant spike in FM among fetuses of women who were previously resting and silent (β = 1.14, SE = 0.55, t = 2.07, p < .05) that was not observed among fetuses of women who were already awake and talking (β = 0.24, SE = 0.52, t = 0.47, p = .64) (see Figures 4a and 4b). In the seconds following, fetuses of women who had been awake and talking showed a significant quadratic suppression in FM relative to baseline (β = 0.07, SE = 0.03, t = 2.23, p < .05) not observed in fetuses of women who were previously resting (β = 0.05, SE = 0.03, t = 1.48, p = .15). The linear decline in FM was significant for fetuses of women who had been awake and talking (β = −0.53, SE = 0.25, t = −2.09, p < .05) and reached a trend level for fetuses of women who had been resting and silent (β = −0.54, SE = 0.30, t = −1.81, p = .08).
Figure 4.
(a) Mean fetal movement and (b) fetal movement changes (deltas and mean standard error) from baseline following onset of maternal reading aloud as a function of maternal state during baseline. Note level difference in movement following onset of reading aloud but no difference in motor activity over time by maternal state.
3.4. Fetal response to maternal speech as a function of fetal and maternal state during baseline
Twenty-one (30%) of the fetuses exhibited low heart rate variability during baseline with the remainder (51; 70%) in a state of high heart rate variability. Fetal state, as indicated by heart rate variability, was not contingent on maternal state (χ2 (1, 69) = 2.83, p = .09).
3.4.2. Maternal-fetal state and fetal heart rate
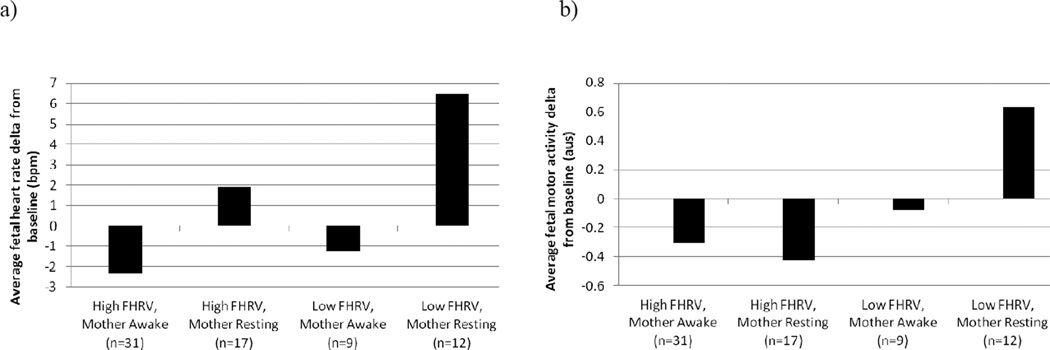
The FHR delta values previously computed for each data point were averaged across the 30 second read aloud period to generate an indicator of the overall magnitude of fetal response. Independently, fetal heart rate variability was not a significant predictor of the fetal response (F (1, 67) = 2.93, p = .09). However, as depicted in Figure 5a, there was a significant difference in the magnitude and direction of fetal heart rate response by maternal and fetal state (F (3, 65) = 3.34, p < .05). Contrast estimates showed that among fetuses with low heart rate variability, maternal rest prior to reading aloud induced a greater fetal response relative to maternal alertness and ongoing vocalizations (F (1, 67) = 4.22, p < .05). This was not the case for fetuses with high heart rate variability; the magnitude of the fetal response was not significantly different by maternal state (F (1, 67) = 2.01, p = .16). Relative to all other groups, fetuses with low heart rate variability of mothers who were resting and silent showed the largest heart rate response (F (1, 67) = 6.39, p < .05).
Figure 5.
Differential (a) fetal heart rate and (b) fetal movement response by maternal-fetal state concordance during baseline.
3.4.2. Maternal-fetal state and fetal movement
The independent effect of fetal heart rate variability during baseline on average FM response (F (1, 67) = 2.86, p = .10) and the overall model predicting FM by combined maternal-fetal state were non-significant (F (3, 65) = 1.32, p = .27). The contrast of the motor response magnitude for fetuses with low heart rate variability of women who were resting compared to all other groups reached a trend level of significance (F (1, 67) = 3.24, p = .08), such that only this group showed an average increase in motor activity in response to the maternal spoken voice, consistent with a startle response (see Figure 5b).
4. Discussion
These results provide information on the naturalistic processes through which the fetus responds to the spoken maternal voice and confirm that the fetal capacity to detect and respond to the maternal voice originates prior to birth. Fetuses displayed an orienting response to the onset of maternal reading aloud characterized by a significant reduction in the amplitude of fetal movement, peaking at 5 seconds post onset and returning to baseline 2–3 seconds thereafter. There was also a trend for suppression of fetal heart rate during the 30 seconds after women began to read aloud. These findings are consistent with the single published study on a small sample which also reported a reduction in motor activity to the maternal spoken voice (Hepper et al., 1993). In addition, this is consistent with movement suppression observed in neonates (Fernald, 1989). The trend for a deceleration in fetal heart rate in response to maternal spoken voice is novel as all published work has relied on the presentation of maternal voice via audiotape. However, a similar orienting cardiac response has also been reported in response to an audio-recording of the maternal voice (Fifer & Moon, 1994; Ockleford et al., 1988).
The nature of the fetal response is qualified by the observation of the importance of what the pregnant woman was doing prior to the stimulus period. Because maternal state was not controlled during the baseline period, there was variation in the degree to which the read aloud protocol introduced a change to the fetal auditory milieu. The orienting response detected in the total sample was most pronounced in those fetuses of women who had been awake and talking during the baseline period. The experimental manipulation, in which women were asked to read unfamiliar prose, introduces a change in the nature of the maternal voice stimulus from normal discourse to a more formal and continuous speech pattern. Prosodic features of speech are altered during reading aloud such that projection and contours increase, disfluency diminishes or becomes absent, and stress or tension in the voice changes (Blaauw, 1994). Existing literature indicates that fetuses can detect changes in prosody. For example, fetuses react to an audio recording of a familiar rhyme to which they were repeatedly exposed during pregnancy with a decrease in heart rate as compared to a novel rhyme (DeCasper et al., 1994). Fetuses also detect temporal variations unique to speech streams compared to other sound stimuli, evidenced by a sustained attention response (i.e., maintained heart rate suppression of longer duration) following the presentation of speech relative to a transient response observed with a music melody of the same length (Granier-Deferre et al., 2011).
In contrast, fetuses of women who were resting and silent during the baseline but roused to engage in the read aloud activity showed a cardiac accelerative change and a transient spike in motor activity. This pattern of fetal heart rate and motor activity is consistent with a mild startle response (Lecanuet & Schaal, 1996). We suggest that, for this group, the experimental manipulation coincides with the onset of maternal vocalization as well as the transition from maternal rest to wakefulness and the corresponding intrauterine auditory changes, and as a result the fetus responds with a transitory accelerative response.
Fetal baseline state prior to the stimulus, approximated by heart rate variability level was independent of maternal state and further impacted the direction and magnitude of the fetal response. While we did not find support for an independent effect of fetal heart rate variability on heart rate and movement response, there was evidence to suggest an interactive effect of maternal-fetal state concordance. Fetuses with low heart rate variability of women who were also resting and quiet during baseline showed a significant elevation in their heart rate when mothers read aloud and a trend level increase in motor activity compared to other groups. With the addition of a maternal state change these fetuses may experience the greatest disruption in the sound environment. This corroborates past work which suggest fetuses in a state characterized by low heart rate variability are most likely to show a fetal response to speech sound stimuli. However, it is important to note that the strength of these findings and our ability to detect significant differences between other groups are limited by the small sample size of fetuses with low heart variability at the end of the baseline recording.
These results provide a more naturalistic evaluation of the fetal response to maternal voice than is provided by an experimental design in which the maternal voice is provided via a recording played by a speaker applied to the maternal abdomen. Prior work has demonstrated that the maternal spoken voice yields the least amount of attenuation since vocal vibrations are transmitted through the body and has well preserved prosodic characteristics (Busnel, 1979; Querleu et al., 1988). However, because our interpretation that fetuses respond to prosodic differences in maternal speech patterns was essentially an incidental one extrapolated from maternal state variation, it awaits confirmation by a more controlled design in which segments of maternal speech and silence are controlled and applied in varying order. While we were able to assess that maternal rest prior to reading aloud did not influence maternal animation or fluency while reading aloud, we were unable to control inherent variation in the volume of the maternal voice. Volume of the maternal voice impacts the quality and level of sound reaching the fetus (James, 2010) which may be different from the day to day maternal speech environment provided to the fetus. Finally, we did not have an audio recording of the baseline period to examine within group variation among the women awake and talking with respect to quality of the maternal voice while conversing prior to reading aloud. Nonetheless, these findings contribute to the small literature on fetal detection of and responses to the maternal spoken voice in situ.
4.1. Conclusions
In summary, the near term fetus has a mature auditory system that reliability detects and responds to sound (Bushnel & Granier-Deferre, 1983; Birnholz & Benacerraf, 1983; Querleu et al., 1988; Hepper & Shahidullah, 1994). The current findings highlight that not only is the maternal spoken voice a salient auditory stimulus for the fetus, but the near-term fetus shows an orienting response to induced changes in the maternal spoken voice. This suggests that over time and with recurrent exposure (DeCasper et al., 1994), prosodic variation in the maternal spoken voice shapes auditory learning in utero with implications for postnatal recognition of and preference for the maternal voice.
Highlights.
The near term fetus has a mature auditory system that reliability detects and responds to the maternal voice
Fetal response to the maternal spoken voice was dependent on maternal state prior to reading aloud
Fetuses responded with orienting when mothers were awake and talking prior to reading aloud
Fetuses showed a startle response when mothers were resting and silent during baseline
Fetuses with low heart rate variability of mothers who were at rest were the largest responders to maternal spoken voice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armitage S, Baldwin B, Vince M. Fetal sound environment of sheep. Science. 1980;208:1173–1174. doi: 10.1126/science.7375927. [DOI] [PubMed] [Google Scholar]
- Bench R. Sound transmission to the human fetus through the maternal abdominal wall. Journal of Genetic Psychology. 1968;113:1172–1174. doi: 10.1080/00221325.1968.10533811. [DOI] [PubMed] [Google Scholar]
- Besinger RE, Johnson TRB. Doppler recordings of fetal movement: Clinical correlation with real-time ultrasound. Obstetrics and Gynecology. 1989;74:277–280. [PubMed] [Google Scholar]
- Blaauw E. The contribution of prosodic boundary markers to the perceptual difference between read and spontaneous speech. Speech Communication. 1994;14:359–375. [Google Scholar]
- Birnholz JC, Benacerraf BR. The development of human fetal hearing. Science. 1983;222:516–518. doi: 10.1126/science.6623091. [DOI] [PubMed] [Google Scholar]
- Busnel M. Mesures intravaginales du niveau et des distorsions acoustiques de bruits maternals. Electrodiagnostic Therapie. 1979;16:142. [PubMed] [Google Scholar]
- Busnel MC, Granier-Deferre C. And what of fetal audition? In: Oliverio A, Zapella M, editors. The behavior of human infants. New York: Plenum; 1983. [Google Scholar]
- Buss C, Davis E, Class Q, Gierczak M, Pattillo C, Glynn L, et al. Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Human Development. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper A, Fifer W. Of human bonding: Newborns prefer their mothers' voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- DeCasper A, Lecanuet J, Busnel M, Granier-Deferre C, Maugeais R. Fetal reactions to recurrent maternal speech. Infant Behavior and Development. 1994;17:159–164. [Google Scholar]
- DeCasper A, Spence M. Prenatal maternal speech influences newborn's perception of speech sounds. Infant Behavior and Development. 1986;9:133–150. [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK. Fetal movement detection: Comparison of the toitu actograph with ultrasound from 20 weeks gestation. Journal of Maternal-Fetal Medicine. 1999;8:237–242. doi: 10.1002/(SICI)1520-6661(199911/12)8:6<237::AID-MFM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Fernald A. A cross-language study of prosodic modifications in mothers' and fathers' to preverbal infants. Journal of child language. 1989;16:477. doi: 10.1017/s0305000900010679. [DOI] [PubMed] [Google Scholar]
- Fifer W. Neonatal preference for mother's voice. In: Krasnagor, editor. Perinatal development: A psychobiological perspective. Academic Press, Inc.; 1987. pp. 111–124. Chapter 115. [Google Scholar]
- Fifer W. Psychobiology of newborn auditory preferences. Seminars in Perinatology. 1989;13:430–433. [PubMed] [Google Scholar]
- Fifer W, Moon C. The role of the mother's voice in the organization of brain function in the newborn. Acta Paediatrica. Supplement. 1994;397:86–93. doi: 10.1111/j.1651-2227.1994.tb13270.x. [DOI] [PubMed] [Google Scholar]
- Fifer WP, Moon CM. The effects of fetal experience with sound. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Lawrence Erlbaum Associates; 1995. pp. 351–366. [Google Scholar]
- Gerhardt K, Abrams R. Fetal exposures to sound and vibroacoustic stimulation. Journal of Perinatology. 2000;20:20–29. doi: 10.1038/sj.jp.7200446. [DOI] [PubMed] [Google Scholar]
- Graham F, Anthony B, Zeigler B. The orienting response and developmental processes. In: Siddle D, editor. Orienting and habitutation: Perspectives in human research. Exeter, England: John Wiley; 1983. pp. 371–430. [Google Scholar]
- Granier-Deferre C, Ribeiro A, Jacquet A, Bassereau S. Near-term fetuses process temporal features of speech. Developmental Science. 2011;14:336–352. doi: 10.1111/j.1467-7687.2010.00978.x. [DOI] [PubMed] [Google Scholar]
- Grimwade J, Walker D, Bartlett M, Gordon S, Wood C. Human fetal heart rate change adn movement in response to sound and vibration. American Journal of Obstetrics and Gynecology. 1971;109:86–90. doi: 10.1016/0002-9378(71)90839-8. [DOI] [PubMed] [Google Scholar]
- Groome L, Bentz L, Mooney D, Singh K, Collins H. Early heart rate responses of normal human term fetuses to vibroacoustic stimulation. American Journal of Perinatology. 1994;11:273–278. doi: 10.1055/s-2007-994590. [DOI] [PubMed] [Google Scholar]
- Groome L, Mooney D, Holland S, Smith L, Atterbury J, Dykman R. Behavioral state affects heart rate response to low-intensity sound in human fetuses. Early Human Development. 1999;54:39–54. doi: 10.1016/s0378-3782(98)00083-8. [DOI] [PubMed] [Google Scholar]
- Hepper P, Scott D, Shahidullah S. Newborn and fetal response to maternal voice. Journal of Reproductive and Infant Psychology. 1993;11:147–155. [Google Scholar]
- Hepper P, Shahidullah B. Development of fetal hearing. Archives of Disease in Childhood. 1994;71:F81–F87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. Fetal learning: A critical review. Infant and Child Development. 2010;19:45–54. [Google Scholar]
- Kisilevsky B, Hains S, Lee K, Xie X, Huang H, Ye H, et al. Effects of experience on fetal voice recognition. Psychological Science. 2003;14:220–224. doi: 10.1111/1467-9280.02435. [DOI] [PubMed] [Google Scholar]
- Kisilevsky B, Hains S, Brown C, Lee C, Cowperthwaite B, Stutzman S, et al. Fetal sensitivity to properties of maternal speech and language. Infant Behavior & Development. 2009;32:59–71. doi: 10.1016/j.infbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Lecanuet J-P, Granier-Deferre C, Cohen H, LeHouezec R, Busnel M-C. Fetal response to acoustic stimulation depend on heart rate variability pattern, stimulus intensity, and repetition. Early Human Development. 1986;13:269–283. doi: 10.1016/0378-3782(86)90061-7. [DOI] [PubMed] [Google Scholar]
- Lecanuet J. Differential fetal auditory reactiveness as a function of stimulus characteristics and state. Seminars in Perinatology. 1989;13:421–429. [PubMed] [Google Scholar]
- Lecanuet J, Granier-Deferre C, Jacquet A, Busnel M. Decelerative cardiac responsiveness to acoustical stimulation in the near term fetus. The Quarterly Journal of Experimental Psychology. 1992;44:279–303. doi: 10.1080/02724999208250616. [DOI] [PubMed] [Google Scholar]
- Lecanuet J, Schaal B. Fetal sensory competencies. European Journal of Obstetrics & Gynecologyand Reproductive Biology. 1996;68:1–23. doi: 10.1016/0301-2115(96)02509-2. [DOI] [PubMed] [Google Scholar]
- Lecanuet J, Manera S, Jacquet A. Fetal cardiac responses to maternal sentences, to playback of these sentences, and to their recordings by another woman's voice; Paper presented at the XIII International Conference on Infant Studies; Toronto, Ontario, Canada. 2002. [Google Scholar]
- Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by doppler actocardiogram and fetal b-mode imaging. Clinics in Perinatology. 1999;26:829–851. [PubMed] [Google Scholar]
- Masakowski Y, Fifer W. The effects of maternal speech on fetal behavior; Paper presented at the VIII International Conference on Infant Studies; Paris, France. 1992. [Google Scholar]
- Moon C, Cooper R, Fifer W. Two-day-olds prefer their native language. Infant Behavior and Development. 1993;16:495–500. [Google Scholar]
- Moon C, Fifer W. The fetus: Evidence of transnatal auditory learning. Journal of Perinatology. 2000;20:S36–S43. doi: 10.1038/sj.jp.7200448. [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Prechtl HFR, Martin CB, Bots RSG. Are there behavioural states in the human fetus? Early Human Development. 1982;6:47–65. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- Ockleford EM, Vince MA, Layton C, Reader MR. Responses of neonates to parents' and others' voices. Early Human Development. 1988;18:27–36. doi: 10.1016/0378-3782(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Querleu D, Renard X, Versyp F, Paris-Delrue L, Crepin G. Fetal hearing. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1988;29:191–212. doi: 10.1016/0028-2243(88)90030-5. [DOI] [PubMed] [Google Scholar]
- Shahidullah S, Hepper P. The developmental origins of fetal responsiveness to an acoustic stimulus. Journal of Reproductive and Infant Psychology. 1993;11:135–142. [Google Scholar]
- Sohmer H, Freeman S. Functional development of auditory sensitivity in the fetus and neonate. Journal of Basic and Clinical Physiology and Pharmacology. 1995;6:95–108. doi: 10.1515/jbcpp.1995.6.2.95. [DOI] [PubMed] [Google Scholar]
- Spence M, DeCasper A. Prenatal experience with low-frequency maternal-voice sounds influence neonatal perception of maternal voice samples. Infant Behavior and Development. 1987;10:133–142. [Google Scholar]
- Timor-Tritsch I. The effect of external stimuli on fetal behavior. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1986;21:321–329. doi: 10.1016/0028-2243(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Vince M, Billing A, Baldwin B, Toner J, Weller C. Maternal vocalisations and other sounds in the fetal lamb's sound environment. Early Human Development. 1985;11:179–190. doi: 10.1016/0378-3782(85)90105-7. [DOI] [PubMed] [Google Scholar]
- Walker D, Grimwade J, Wood C. Intrauterine noise: A component of the fetal environment. American Journal of Obstetrics and Gynecology. 1971;109:91–95. doi: 10.1016/0002-9378(71)90840-4. [DOI] [PubMed] [Google Scholar]
- Zimmer E, Fifer W, Kim Y, Rey H, Chao C, Myers M. Response of the premature fetus to stimulation by speech sounds. Early Human Development. 1993;33:207–215. doi: 10.1016/0378-3782(93)90147-m. [DOI] [PubMed] [Google Scholar]