Abstract
Background
Understanding the nature of environmental factors that contribute to behavioral health is critical for successful prevention strategies in individuals at-risk for psychiatric disorders. These factors are typically experiential in nature, such as stress and urbanicity, but nutrition, in particular dietary deficiency of omega-3 polyunsaturated fatty acids (n-3 PUFAs), has increasingly been implicated in the symptomatic onset of schizophrenia and mood disorders, which typically occurs during adolescence to early adulthood. Thus, adolescence may be the critical age range for the negative impact of diet as an environmental insult.
Methods
A rat model involving consecutive generations of n-3 PUFA deficiency was developed based on the assumption that dietary trends toward decreased consumption of these fats began four-five decades ago when the parents of current adolescents were born. Behavioral performance in a wide range of tasks, as well as markers of dopamine-related neurotransmission was compared in adolescents and adults fed n-3 PUFA adequate and deficient diets.
Results
In adolescents, dietary n-3 PUFA deficiency across consecutive generations produced a modality-selective and task-dependent impairment in cognitive and motivated behavior distinct from the deficits observed in adults. While this dietary deficiency affected expression of dopamine-related proteins in both age groups, in adolescents, but not adults, there was an increase in tyrosine hydroxylase expression that was selective to the dorsal striatum.
Conclusions
These data support a nutritional contribution to optimal cognitive and affective functioning in adolescents. Furthermore, they suggest that n-3 PUFA deficiency disrupts adolescent behaviors through enhanced dorsal striatal dopamine availability.
Keywords: Schizophrenia, Addiction, Anxiety, Nutrition, Fatty Acids, Cognition
INTRODUCTION
Environmental factors contribute to the etiology of mental illness (1, 2). Validating and understanding these factors will assist prevention in high-risk individuals. The main factors reported in this context are stress exposure and urbanicity (3, 4), but nutrition is gaining recognition as a potent environmental factor contributing to development of mental illness and symptom severity. Particularly, brain function is critically dependent on the intake of the so-called “essential” fatty acids that cannot be readily synthesized by the human body. Among these are omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs), which are required for brain development and maintaining optimal brain function (5–9).
In recent decades, dietary ratios of PUFAs in industrial societies (and increasingly in the third world) have dramatically shifted, resulting in an n-3 PUFA deficiency and disproportionately high n-6 to n-3 PUFA ratio (10–12). n-3 PUFA deficiency has been implicated in psychiatric disorders including schizophrenia (13, 14), depression (15,16), ADHD (17) and autism (18). For example, n-3 PUFA dietary supplementation significantly reduces the transition rates to psychosis in young individuals at high risk to developing schizophrenia (19). While animal studies have established the adverse effects of transient or maternal n-3 PUFA deficiency on adult behaviors (20–23), the symptomatic onset of major psychiatric illnesses, in particular schizophrenia and affective disorders, typically occurs during adolescence to early adulthood (24–26). Thus, this may be the critical age range for systematic intervention in at-risk individuals. Animal models of this dietary deficiency in adolescents that provide quantifiable behavioral measures are critical for understanding how n-3 PUFA deficiency influences overall behavioral health and symptoms of these illnesses.
To develop such a model, we reasoned that a “second generation” of deficient animals would best mimic the current state of human adolescent n-3 PUFA deficiency, given that dietary trends toward consumption of n-3 deficient fats began in the 1960’s and 1970’s when most parents of current adolescents were born. Thus, we investigated the impact of consecutive generations of dietary n-3 PUFA deficiency on a battery of cognitive and motivationally driven behaviors in adolescent rats. n-3 PUFA deficient adolescents displayed a wide range of behavior impairments, some of which suggested dopamine abnormalities. We therefore extended some of the behavioral measures to adult rats and assayed markers of dopamine-related neurotransmission in adolescents and adults. In adolescents, but not adults, we found an elevation of dopamine synthesis specific to the dorsal striatum. This is important given dopamine’s involvement in motivation and cognition (27–29), and recent reports of enhanced dorsal striatal dopamine availability in young individuals at high risk of developing schizophrenia (30, 31).
METHODS
Animals and Diets
Sprague-Dawley rats (Harlan, Frederick, MD) were pair-housed and maintained on a 12-hr light/dark cycle (lights on at 7pm) with ad libitum access to food and water. Females were mated with normal-chow fed males and placed on diets adequate (ADQ) or deficient (DEF) in n-3 PUFAs (Dyets Inc., Bethlehem, PA) (32–35). First generation (G1) rats were weaned at post-natal day (PND) 21, and males were tested on behavioral tasks. Adult female G1 rats also were mated and their litters comprised generation two (G2) rats, the males of which were subjected to the same behavioral tests. Brains of animals from both generations were analyzed for fatty acid lipids. Diets comprised comparable amounts of vitamins, minerals, basal fats and macronutrients (36), with the ADQ diet containing flaxseed oil as a precursor of the n-3 PUFA docosahexaenoic acid (DHA), which the DEF diet was lacking. Mild food restriction aimed at preserving motivation to seek a food reward while maintaining 85% of normal weight was employed as necessary during tests involving retrieval of sugar pellets. Animals underwent physical examinations at weaning (PND 21), early adolescence (PND 39–41) and adulthood (PND 70–73). All procedures were carried out in accordance with the National Institute of Health's Guide to the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Whole-Brain Lipid Extraction and Gas-Chromatography Analysis
Total lipids were extracted from half or whole brain by the Folch method (37) and reconstituted in chloroform/methanol. Formed fatty acid methyl esters (FAMEs) were extracted with heptane, dried and reconstituted in isooctane for gas-chromatography (GC) analysis using a flame ionization detector (34, 38). Peaks were identified by retention times of a FAME standard mix. Fatty acid concentrations (nmol/g brain wet weight) were calculated from GC peak areas against the standard.
Behavioral tests
General Considerations
We chose behavioral tasks relevant to cognitive and affective symptoms of psychiatric disorders that could be completed during the short rodent adolescent period. To alleviate individual variability due to seasonal variability and changes in test room environment, the breeding and testing of DEF and ADQ groups were performed concurrently.
Open Field Test
Adolescent and adult rats from both diets were allowed to explore an open field arena for 5 min (39). The cumulative time of center exploration and the total number of square crossings was scored from videos offline.
Novel Object Recognition Memory Test
Novel object recognition memory was assessed in the open field arena using two objects distinct in shape and color (40). Testing comprised of a 5 min acquisition phase (two identical objects) and a 5 min retention phase (an object identical to ones from acquisition phase and a novel object), separated by a 5 min retention interval. Discrimination index scores were calculated as ratios of time spent exploring the novel object to total time spent exploring both objects.
Instrumental Learning and Extinction
Animals were tested for instrumental learning and extinction during both adolescence (PND 29–46) and adulthood (PND 80–100) as described before (39). Rats were trained on a fixed-ratio schedule of 1 for 12 consecutive days in operant chambers fitted with 3 nose-poke holes and a food trough, by learning to poke for sucrose pellet reinforcement into the center nose-poke when illuminated. Each session lasted 99 trials or 30 min, whichever occurred first. After instrumental responses for reward were consistently performed, as previously defined (39), the stimulus-reward relationship was extinguished for 6 sessions. Outcome measures included the number of total trials completed, task-irrelevant pokes (nose pokes into left and right non-illuminated holes) and the latency for pellet retrieval (time from instrumental to food trough nose-poke).
T-maze Cognitive Set-Shifting
Set-shift testing was conducted at PND 38–40 for G2 adolescents or PND 90–100 for G2 adults in a Plexiglas maze with four arms that varied along two stimulus dimensions: brightness and texture (40, 41). For Set 1, rats were trained to a criterion performance level of 8 consecutive correct arm choices based on either brightness or texture. For Set 2, rats were tested based on the alternative discrimination strategy for 80 trials regardless of performance level. The number of trials required to reach criterion in both Sets and total test time was recorded. Perseverative responding on Set 2 was assessed as percent correct scores from either perseveration arms (PAs; i.e. start arms from which responding based on the correct contingency from Set 1 produced an incorrect response) or reinforcement arms (RAs; i.e. start arms from which responding based on the correct contingency from Set 1 produced a correct response).
Western Blot Analysis
Western blot analysis was performed as described elsewhere (42–44). Brain regions of interest [prefrontal cortex (PFC), nucleus accumbens (NAc) and dorsal striatum (DS)] from adolescent and adult G2 rats were dissected on ice and homogenized in a buffer containing protease inhibitors. After adding Triton-X 100 (final concentration 1%), incubation and centrifugation, protein concentration levels were assessed in the supernatant using a spectrophotometer. Samples were separated by SDS-PAGE on 10% Tris-glycine polyacrylamide gels and transferred to nitrocellulose membranes using the Bio-Rad system. Membranes were then blocked in TBS buffer containing 5% dry milk, incubated with specific primary antibody, incubated with a horseradish peroxidase(HRP) - conjugated secondary antibody, then washed and protein bands were visualized using the Supersignal West Pico Pierce system (Thermo Scientific, Rockford, IL) and LabWorks software. Densitometric band analyses were accomplished using ImageJ NIH software. The protein contents of vesicular monoamine transporter 2 (VMAT2) and tyrosine hydroxylase (TH) were expressed as a ratio of tubulin band densities as a loading control. Primary antibodies against VMAT2, TH and α-tubulin were from Millipore (Billerica, MA) and Sigma-Aldrich (St. Louis, MO). Secondary antibodies conjugated with HRP were Protein A for VMAT2 and TH (GE Healthcare, Bickinghamshire, UK), and Goat Anti-Mouse IgG (H+L) for tubulin (Bio-Rad, Hercules, CA). Primary antibody concentrations included anti-VMAT2 (1:275), anti-TH (1:1,000) and anti-tubulin (1:1000), whereas secondary antibody concentrations for VMAT2 and TH were 1:4,000 and for tubulin, 1:10,000 respectively.
Data Analysis
Data for individual generations were analyzed using Student t-tests unless otherwise mentioned. Instrumental learning results were analyzed within each generation by two-way repeated measures ANOVA (Diet × Session, with Session as a repeated measure), followed by post hoc comparisons using individual Session t-tests. Results from Set 2 of the T-maze tests were analyzed using the Mann-Whitney test for nonparametric data because of the imposed ceiling of 80 trials. Significance was set at p < 0.05. The range of subject numbers for each figure is indicated in the figure legends.
RESULTS
General Health of Animals
General health observations were comparable between diet groups, such as grooming, hair, skin and eye conditions or food intake. Weight gain in a larger population of rats representative of groups used in this study (PND 21, 39–41, 70–73) corresponded to the growth chart of Harlan Sprague-Dawley rats (45) (Table 1).
Table 1.
Body weight gain throughout various developmental time points (weaning, PND 21; adolescence, PND 39–41; adulthood, PND 70–73) in both G1 and G2 rats from both n-3 ADQ and DEF dietary conditions. Data expressed as grams (mean ± SEM), N = number of animals / group.
| Weaning | Adolescence | Adulthood | |||||
|---|---|---|---|---|---|---|---|
| ADQ | DEF | ADQ | DEF | ADQ | DEF | ||
| G1 | Mean ± SEM | 60.7 ± 1.3 | 57.6 ± 1.1 | 164.8 ± 3.2 | 176.0 ± 3.9 | 348.0 ± 8.4 | 337.4 ± 5.6 |
| N | 24 | 30 | 14 | 13 | 17 | 20 | |
| G2 | Mean ± SEM | 61.0 ± 1.0 | 57.7 ± 0.9 | 157.1 ± 3.3 | 157.4 ± 3.0 | 357.8 ± 4.0 | 336.0 ± 4.8 |
| N | 59 | 55 | 17 | 15 | 21 | 18 | |
Whole-Brain Lipid Analysis
Dietary n-3 PUFA deficiency produced dramatic alterations in brain concentrations of fatty acids that were amplified in the subsequent generation (21) (Table 2). In addition to the expected reduction in DHAn-3, there was a compensatory increase in brain n-6 PUFAs.
Table 2.
Brain fatty acids alterations in two consecutive generations of rats fed n-3 PUFA adequate or deficient diets. DHA, docosahexaenoic acid; DPA, docosapentaenoic acid. Other fatty acids analyses revealed a significant increase in vaccenic acid (18:1n-9) in G2 DEF rats, and no changes in as oleic acid (18:1n-9) or eicosanoic acid (20:1n-9) in either generation of rats (data not shown). Data expressed as nmol/g brain wet weight (mean ± SEM, n = 9–10/group).
| Gen/Diet | Linoleic Acid (18:2n-6) |
Arachidonic Acid (20:4n-6) |
Adrenic Acid (22:4n-6) |
DPA (22:5n-6) |
DHA (22:6n-3) |
|---|---|---|---|---|---|
| G1 ADQ | 746.6 ± 11.6 | 9548.6 ± 108.0 | 2107.5 ± 21.8 | 382.7 ± 8.6 | 9042.8 ± 65.2 |
| G1 DEF | 691.7 ± 11.2** | 10870.5 ± 184.9*** |
2735.7 ± 43.4*** |
5953.5 ± 240.6*** |
3350.7 ± 90.0*** |
| G2 ADQ | 810.4 ± 19.4 | 9622.9 ± 73.9 | 2102.6 ± 13.4 | 316.9 ± 12.2 | 9256.1 ± 57.4 |
| G2 DEF | 702.6 ± 9.0*** | 10967.7 ± 62.6*** |
2894.6 ± 41.4***+ |
7231.0 ± 68.4***+ |
1646.0 ± 45.4***+ |
p < 0.05
p < 0.01
p < 0.001 compared with corresponding ADQ diet group within respective generation;
p < 0.001 compared with G1 DEF rats.
Behavior Assessment During Adolescence
Open Field Test
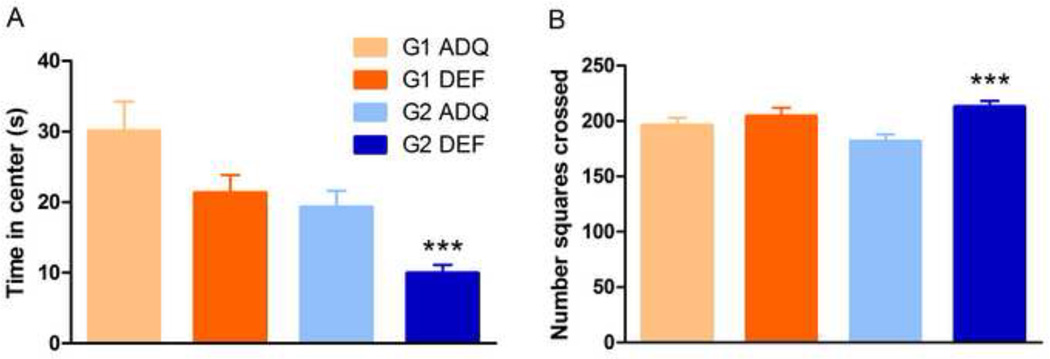
Open field center exploration was reduced in G2 DEF rats (t46=3.833, p<0.001, Figure 1A), whereas only a trend was observed in G1 DEF rats (t34=1.874, p=0.07) compared to ADQ counterparts. Similarly, there was a significant increase in total square crossings in G2 DEF rats (t46=3.684, p<0.001, Figure 1B), but not in G1 DEF animals. Thus, G2 and not G1 DEF rats were hyperactive and displayed more anxiety-related behavior. Center exploration also was different between the two ADQ groups, which could be due to normal variability in different cohorts of animals tested at different times (see “General Considerations” in Methods).
Figure 1.
Effects of generational n-3 fatty acid deficiency in the open field test. (A) G1 DEF rats displayed a trend toward less time spent in the center (p=0.07), while G2 DEF rats spent significantly less time in open area (*** p<0.001) compared to ADQ-fed animals. (B) There were no dietary effects on locomotion in G1 rats, whereas G2 DEF rats displayed hyperlocomotion seen as increased mean number of total square crossings during the 5 min test (*** p < 0.001). Data shown as mean ± SEM, n = 16–28/group.
Novel Object Recognition Memory Test
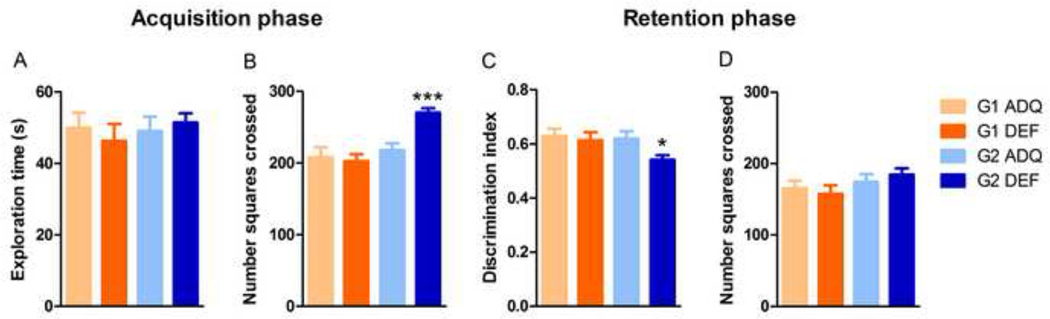
During the acquisition phase, no differences were observed in total time spent exploring objects in either G1 DEF or G2 DEF rats (Figure 2A). G2 DEF rats displayed higher square crossings than ADQ rats (t43=-4.716, p<0.001), consistent with open field data, suggesting they are hyperactive (Figure 2B). During the retention phase, G2 DEF rats spent significantly less time investigating the novel object, with no exploration changes toward the familiar object (t43=2.556, p<0.05, Figure 2C), suggesting short term recognition memory impairment. There were no differences in square crossings between ADQ and DEF rats in either G1 or G2 during the retention phase (Figure 2D). Note that G2 DEF rats were hyperactive during acquisition and not retention, likely because the environment was not as novel during the latter phase, suggesting that the hyperactivity of G2 DEF animals is exacerbated by novelty.
Figure 2.
Novel object recognition memory in consecutive generations of n-3 PUFA deficient animals. No differences were observed between groups in cumulative time spent exploring the identical objects during the 5 min acquisition phase (A), but G2 DEF rats displayed significantly higher square crossings than ADQ rats (*** p < 0.001) (B). During the 5 min retention test phase, both G1 groups and G2 ADQ group spent significantly more time exploring the novel object (discrimination index > 0.5), but G2 DEF rats did not differentiate between the novel and familiar objects compared to G2 ADQ rats (* p < 0.05) (C). There were no differences in locomotion assessed as mean square grid crossings (D). Data presented as mean ± SEM, n = 15–26/group. Significance set at p < 0.05.
Instrumental Learning
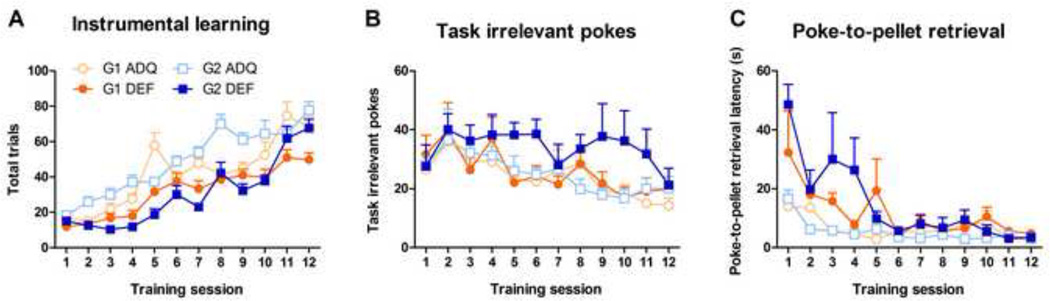
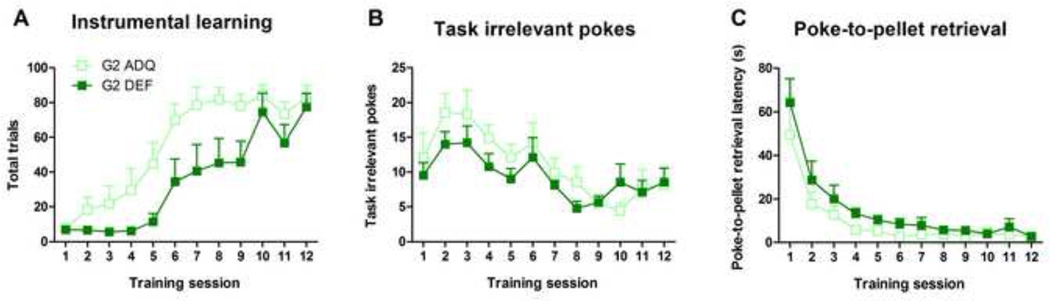
G2 DEF adolescent rats displayed significantly slower learning compared with their respective ADQ. counterparts in Sessions 2–10, as revealed by the average total number of trials per session (G2 – Diet: F(1,20)=31.75, p<0.001; Diet × Session interaction: F(11,220)=3.78, p<0.001, Figure 3A). The G1 DEF rats also displayed slower learning (G1 - Diet: F(1,21)=12.81, p<0.01; Diet × Session interaction: F(11,231)=2.45, p<0.01) but this difference was only significant in sessions 4, 5, 7,11 and 12. Other measures in this task indicated increased task-irrelevant pokes, but reduced goal-directed behavior. For example, G2 DEF rats displayed a tendency to perform more task-irrelevant pokes during training (F(1,20)=4.05, p=0.06, Figure 3B) after animals had learned the task, especially in sessions 6 and 8 (p<0.05) with a trend in sessions 5, 9, 10 (p<0.07). There were no diet-related differences in task-irrelevant pokes in G1 rats. The average latency from instrumental poke to pellet retrieval was higher in G2 DEF rats (Diet: F(1,20)=12.28, p<0.01; Diet × Session interaction: F(11,220)=3.74, p<0.001), while in G1 DEF rats only a trend was observed: Diet (F(1,21)=3.96, p=0.06 (Figure 3C), with no Diet × Session interaction. No significant effects were observed during extinction (data not shown).
Figure 3.
Effects of generational n-3 fatty acid deficiency on instrumental learning performance and extinction during adolescence. G2 DEF adolescent rats displayed consistently slower learning measured by total trials compared with their ADQ counterparts (A), while G1 DEF adolescent rats performed poorer only on some sessions. G2 DEF adolescent rats also displayed a trend for higher task-irrelevant pokes (p=0.06) (B) and significantly higher latencies for pellet retrieval (C). Data expressed as mean ± SEM, n = 10–12/group. Significance set at p < 0.05.
T-Maze Cognitive Set-Shifting
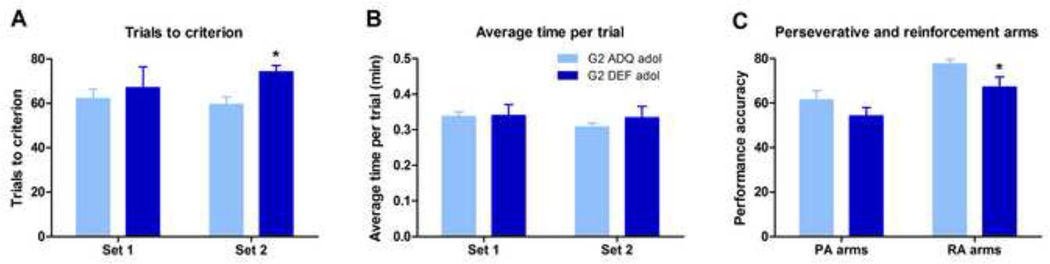
Adolescent G2 rats from both diets learned the initial discrimination (Set 1) comparably (Figure 4A). There were no differences for the average time per trial in Set 1 between ADQ and DEF-reared rats (Figure 4B). This is different than the slower learning rate observed in the instrumental learning task (above), suggesting that an operant task that includes many distracting elements (e.g., 3 nose holes, cue lights, food magazine) may be more susceptible to learning deficits than a maze, where the animal simply needs to run down an alley to receive a food reward. During the extradimensional set-shift (Set 2) portion of the T-maze test, however, G2 DEF adolescents rats required higher number of trials to reach criterion compared to ADQ rats (Mann-Whitney U=7, p<0.05, Figure 4A). No differences were detected between diet groups for the average time per trial in Set 2 (Figure 4B), or performance accuracy (percentage of correct responses) from PA arm starts (Figure 4C). However, G2 DEF adolescents displayed reduced performance accuracy from RA arm starts (t13=2.31, p<0.05, Figure 4C).
Figure 4.
n-3 polyunsaturated fatty acid generational deficiency induced cognitive set-shifting performance impairments in G2 adolescent rats. (A) Adolescent G2 DEF rats learned the initial discrimination during Set 1 at a rate comparable to ADQ rats, but required significantly more trials to reach criterion during the extradimensional set shift (Set 2). (B) No differences in average time per trial were detected between ADQ and DEF rats on either test day. (C) G2 DEF adolescent rats displayed comparable performance accuracy from PA arm starts; however they showed significantly reduced performance accuracy from RA arm starts. Data expressed as mean ± SEM, n = 6–9/group. Significance set at p < 0.05.
Select testing of G2 adult rats
Instrumental Learning and Extinction
A two-way repeated measures ANOVA for G2 adults revealed a significant overall Diet effect (F(1, 16)=7.59, p<0.05), with DEF animals displaying a slower learning curve than ADQ-fed rats, but not a Diet × Session interaction (Figure 5A). Unlike adolescents, there were no differences in task-irrelevant pokes (Figure 5B) or poke-to-pellet retrieval latency (Figure 5C).
Figure 5.
G2 DEF adult rats displayed a similar pattern of impaired instrumental learning compared with ADQ rats, such as adolescents (A), with no differences in other measures recorded (B–C). Data expressed as mean ± SEM, n = 9/group. Significance set at p < 0.05.
T-Maze Cognitive Set-Shifting
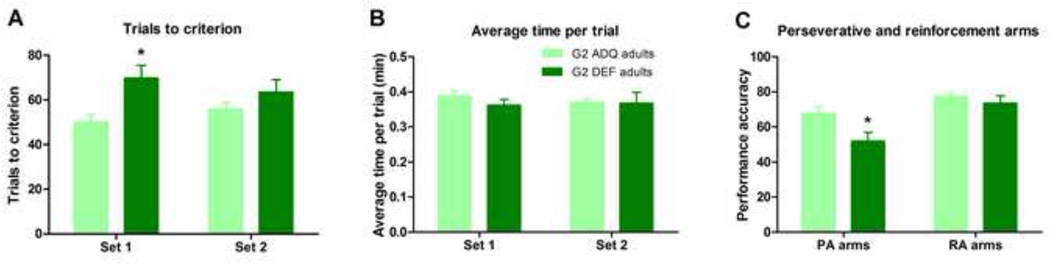
During Set 1, adult G2 DEF rats required significantly more trials to reach the criterion of 8 consecutive correct trials in comparison with ADQ-fed rats (t15=2.86, p<0.05, Figure 6A). There were no differences in the G2 adult rat group during Set 2 (Mann-Whitney U=29, p=n.s., Figure 6A). No differences in average time per trial were detected in G2 rats on either test day (Figure 6B). G2 DEF rats displayed reduced performance accuracy from PA arm starts compared with ADQ group on Set 2 (t15=2.52, p<0.05), but there were no differences in performance accuracy between groups from RA arm starts (Figure 6C).
Figure 6.
N-3 dietary generational deficiency induced cognitive learning impairments in G2 adult rats. (A) Adult G2 DEF rats exhibited learning impairments during the initial discrimination in the T-maze set-shifting test, but performed the extradimensional set-shift comparably to ADQ rats. (B) No differences in average time per trial were detected between ADQ and DEF rats on either test day. (C) G2 DEF adults displayed significantly reduced performance accuracy from PA arm starts on Set 2 but there were no performance accuracy differences from RA arms. Data expressed as mean ± SEM, n = 8–9/group. Significance set at p < 0.05.
Overall, these data suggest that set-shifting task impairments are different in adults vs. adolescents, with adults showing deficits in rule learning and adolescents in set-shifting ability.
Open field Test
Open field center exploration time was reduced in G2 DEF adult rats (DEF: 22±8 sec; ADQ: 43±3 sec, t12=2.24, p<0.05). A non-significant trend was observed in total number of square crossings in the two groups (DEF: 339+63; ADQ: 268 + 83, t12=-1.88, p=0.08).
Western Blot Analysis of dopamine-related proteins
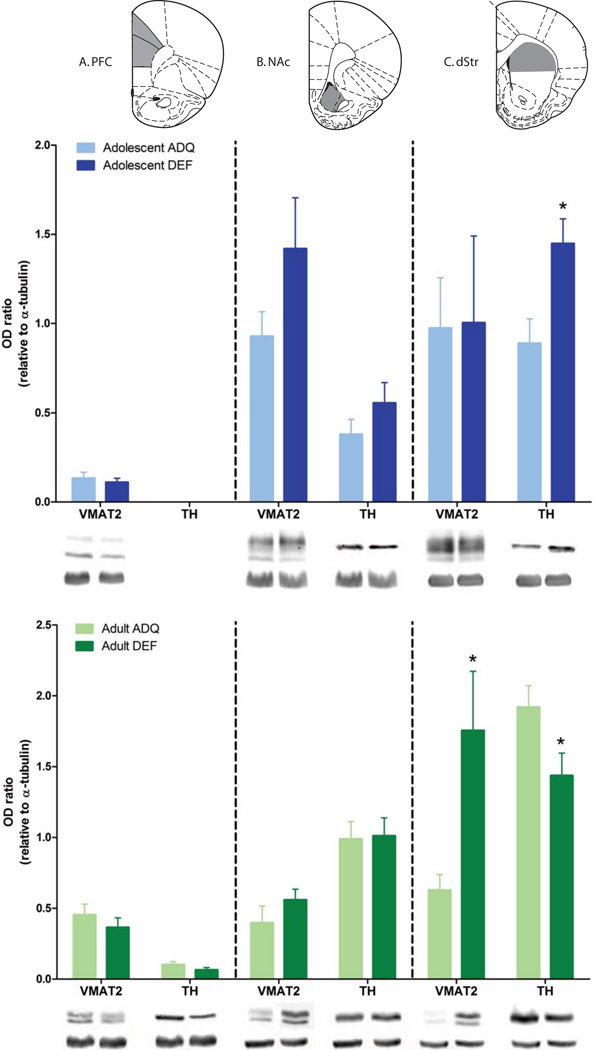
Behavioral impairments such as hyperactivity, instrumental learning and set-shifting deficits, may suggest suboptimal dopamine neurotransmission (47–49). Furthermore, previous studies in adult rodents (50–53) proposed a role for VMAT in striatum, frontal cortex or nucleus accumbens after maternal n-3 PUFA deficiency. Thus, we focused on G2 adolescent and adult subjects and measured VMAT2 and TH as an index of dopamine availability in these three key dopamine innervated areas (PFC, NAc and DS). Whereas no significant diet-related differences were observed in NAc or PFC, TH levels in DS of G2 DEF adolescent animals were elevated compared with ADQ rats, as shown in Figure 7 (t10=-2.88, p<0.05). In adult DS, there were significant elevations of VMAT2 expression (t14=2.611, p<0.05), as well as reductions of TH expression (t14=2.194, p<0.05) in G2 DEF rats (Figure 7C).
Figure 7.
N-3 PUFA generational deficiency changed markers of dopamine neurotransmission in dorsal striatum (dStr) of G2 DEF adolescent and adult rats compared to the ADQ-fed group. Protein levels of VMAT2 and TH in G2 DEF rats, adolescents or adults, were not different than those in ADQ rats in prefrontal cortex (PFC) (Panel A, top and bottom) and nucleus accumbens (NAc) (Panel B, top and bottom). In the dStr (Panel C), TH protein expression was elevated significantly in G2 DEF adolescent rats relative to the control protein, tubulin. Conversely, in the dStr of G2 DEF adult rats, protein levels of VMAT2 were elevated significantly, while TH protein expression was reduced significantly compared to ADQ-fed counterparts. Representative images are shown correspondingly below each bar graph for both the protein of interest and tubulin. Upper histology panels represent coronal rat brain atlas diagrams (66) indicating regions of interest dissected for analyses. Data are shown as mean optical density of protein of interest relative to tubulin ± SEM, n = 6–8/group, * p < 0.05 compared to age-matched respective G2 ADQ group.
DISCUSSION
Dietary n-3 PUFA deficiency across consecutive generations of rats produces behavioral deficiencies and alterations in brain markers of dopamine-related neurotransmission that are distinct in adolescents compared to adults. While n-3 PUFA deficient animals appear to be in general good health, we observed diet-induced changes in behavioral measures in adolescents that reflected hyperactivity, increased anxiety, increased goal-irrelevant and decreased goal-directed activity, and reduced behavioral flexibility. In most cases, these behavioral differences were only significant, or more pronounced, in the second generation of deficient adolescents. Given that dietary trends toward lower n-3 PUFA consumption began in the 1960’s and 1970’s when most parents of current adolescents and young adults were born, the consecutive generational model may be relevant to the current state of n-3 PUFA deficiency in humans. This model therefore makes a strong case for the nutritional contribution to dopamine-related cognitive and affective functioning and vulnerability to psychiatric illness in adolescents. Below we discuss two main findings of the present work, which indicate that the detrimental effects of n-3 PUFA dietary deficiency 1) worsen after consecutive generations and 2) are different in adolescents and adults.
Progressive worsening of behavioral disruptions in consecutive generations of adolescents
In tasks where we compared G1 and G2 DEF adolescents, we consistently observed more substantial behavioral disruptions in the G2 DEF group. In the open field, significantly less center exploration but overall hyperlocomotion was observed in G2 but not G1 DEF rats. This suggests an elevated state of anxiety and hyperactivity in G2 DEF adolescents. Data from the object recognition task during the acquisition phase also suggests that G2 DEF adolescents are hyperactive. Furthermore, the observation that hyperlocomotion occurs in the absence of increased exploratory behavior suggests enhanced goal-irrelevant behavior in G2 DEF animals. Of note, both generations of n-3 PUFA DEF rats explored the familiar object for durations of time comparable with ADQ groups, indicating that reduced novel object exploration in G2 DEF rats is not because diminished visual abilities (54). During the retention phase, G2 DEF animals spent less time exploring the novel object, suggesting impairment in spatial recognition memory. This task is analogous to the visual paired comparison task, which measures declarative memory in humans and monkeys (55). Thus, these data further suggest that consecutive generational n-3 PUFA deficiency produces deficits in declarative memory.
In the instrumental learning task, G2 DEF adolescents displayed slower learning rates throughout the training sessions, higher latencies for pellet retrieval and a trend for higher task-irrelevant pokes, which further support the notion that these animals engage in goal-irrelevant behavior and are less motivated to stay on task. Although G1 DEF animals also displayed reduced learning rates, this effect was less pronounced than in G2 DEF rats. The G1 DEF animals performed similarly to ADQ groups in other measures, further complementing the results of open field and object recognition tasks indicating that n-3 PUFA deficiency over consecutive generations worsens behavioral outcomes.
Dietary n-3 PUFA deficiency impacts adolescents differently than adults
Our study focused on adolescent behaviors because this is a period of psychiatric vulnerability and the typical time of symptomatic onset of several psychopathologies, including schizophrenia [reviewed in (56)]. Preventive measures with n-3 PUFA supplementation also appear to be most effective during this period (19). Thus, while most previous animal and human research with n-3 PUFA deficiency and supplementation has focused on adults, (e.g. (57) the adolescent-early adulthood range may be more relevant to understanding biological processes that contribute to psychiatric illness progression and prevention. Furthermore, adolescence may be an especially vulnerable developmental period because dopamine and endocannabinoid systems, which have been reported to be affected in adult n-3 PUFA deficient animals (23, 51, 52, 58, 59), undergo significant remodeling during this time (60, 61). These neurotransmitter systems are also implicated in various aspects of cognitive functioning including behavioral flexibility and goal-directed behaviors (27–29, 48, 49). In addition, dopamine availability in the DS is implicated in vulnerabilities to schizophrenia in young at-risk individuals (30, 31).
Our data demonstrate that, while dietary n-3 PUFA deficiency influences behavior and dopamine neurotransmission in both adults and adolescents, the nature of this influence differs between the age groups. We compared the G2 adult and adolescent behaviors in open field and two operationally distinct learning paradigms, a T-maze set-shifting task and operant instrumental learning task. The adult G2 DEF rats displayed slower rate of acquisition in both tasks, suggesting that this age group may suffer from a general learning deficit. Adolescents, in addition to slower learning rate in the instrumental task, displayed goal-irrelevant behavior and reduced behavioral flexibility. Both of these tasks depend on the functional integrity of dopamine projections to cortical and striatal regions. The instrumental learning task is a measure of reward-driven goal-directed behavior. Elevated task-irrelevant responses and reward retrieval latency by G2 DEF adolescents in this task suggest increased goal-irrelevant behavior and reduced motivation. The T-maze set-shifting task is analogous to the Wisconsin Card Sorting Test (41, 62) and assesses rule learning and extra-dimensional rule shifting. The G2 DEF adolescents acquired the rule but exhibited impaired ability to shift rule contingencies. This group also displayed reduced performance accuracy from RA starts, suggesting increased distractibility and a potentially more general, non-perseverative cognitive impairment (41, 63). In contrast, G2 DEF adults displayed rule acquisition, but not set-shifting deficits. These data indicate that, while initial rule learning in adolescents was not affected, their cognitive flexibility was compromised. Thus, the dietary manipulation induces a modality-selective and task-dependent impairment in cognitive and motivated behavior distinct from the deficits observed in adults.
In both age groups, n-3 PUFA deficiency altered the expression of proteins critical for regulating dopamine neurotransmission selectively in the dorsal striatum. The direction of this change, however, was opposite in adolescents compared to adults. Adolescents displayed a significant increase in TH expression in DS, indicating enhanced dopamine availability, which is consistent with the observed hyperactivity and enhanced goal irrelevant activity in this age group (44, 56). In contrast, adults displayed a small reduction in TH and a large increase in VMAT2 expression in DS, which may indicate a homeostatic response in which VMAT2 compensates for the impact of reduced TH by increasing vesicular packaging of newly synthesized dopamine. Nonetheless, these data indicate that the dopamine system is differentially disrupted in these age groups by n-3 PUFA deficiency, and that this may in part account for the observed behavioral differences.
Clinical Implications
These findings bear relevance to public health given that the second generation of deficient adolescents may mimic the current state of n-3 PUFA deficiency in some human adolescents. In addition to compromising optimal behavioral health, this common dietary deficiency may be a critical environmental factor that contributes to illness progression in individuals at risk for developing major psychiatric disorders including mood disorders or schizophrenia (13–16). We found that, while animals were generally healthy with intact motor and learning skills, there were subtle cognitive deficits, increased anxiety and other behavioral effects relevant to some of the deficits observed during the prodromal phase of schizophrenia (64). In addition, we found evidence of elevated dopamine availability specific to the dorsal regions of the striatum of adolescents, which is especially important in the context of schizophrenia, as it corresponds closely to reports of increased capacity for dopamine synthesis specific to the DS in individuals at high risk for developing schizophrenia (30, 31). Thus, selective enhancement of dopamine neurotransmission in DS may be a key neuronal mechanism that contributes to the potential detrimental effects of n-3 PUFA deficiency for mental health. Of note, the brain fatty acid concentration differences in the DEF compared with ADQ diet rats in this study were substantial, showing that DHA was largely replaced by DPAn-6. While the clinical relevance of these changes remains to be validated, DPAn-6s are precursors to endocanninoids (65), the function of which are affected by n-3 PUFA deficiency (23, 59) and implicated in the etiology of schizophrenia (13, 14). Thus this animal model may allow for further dissection of neuronal mechanisms through which dietary deficiencies and supplementation contribute to the behavioral health of adolescents and, in combination with other risk factors, to psychiatric illnesses.
Acknowledgements
This research was supported by grants from the US National Institute of Health grants R21 (MH097219) and the National Institute on Aging Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 3.Oh G, Petronis A. Environmental studies of schizophrenia through the prism of epigenetics. Schizophr Bull. 2008;34:1122–1129. doi: 10.1093/schbul/sbn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "Just the Facts": what we know in 2008 part 1: overview. Schizophr Res. 2008;100:4–19. doi: 10.1016/j.schres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- 6.Bourre JM. Fatty acids, cognition, behavior, brain development, and mood diseases. In: Chow CK, editor. "Fatty acids in foods and their health implications". Third Ed. USA: CRC Press Taylor & Francis Group; 2007. pp. 935–954. [Google Scholar]
- 7.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 9.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Evolutionary aspects of the dietary omega-6:omega-3 fatty acid ratio: medical implications. World Rev Nutr Diet. 2009;100:1–21. doi: 10.1159/000235706. [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Omega-6/omega-3 essential fatty acids: biological effects. World Rev Nutr Diet. 2009;99:1–16. doi: 10.1159/000192755. [DOI] [PubMed] [Google Scholar]
- 13.Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biological psychiatry. 1991;30:795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- 14.Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biological psychiatry. 2001;49:510–522. doi: 10.1016/s0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- 15.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biological psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 16.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 18.Vancassel S, Durand G, Barthelemy C, Lejeune B, Martineau J, Guilloteau D, et al. Plasma fatty acid levels in autistic children. Prostaglandins Leukot Essent Fatty Acids. 2001;65:1–7. doi: 10.1054/plef.2001.0281. [DOI] [PubMed] [Google Scholar]
- 19.Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 20.Carrie I, Clement M, de Javel D, Frances H, Bourre JM. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J Lipid Res. 2000;41:473–480. [PubMed] [Google Scholar]
- 21.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 22.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Larrieu T, Madore C, Joffre C, Laye S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J Physiol Biochem. 2012 doi: 10.1007/s13105-012-0179-6. [DOI] [PubMed] [Google Scholar]
- 24.Volkmar FR. Childhood and adolescent psychosis: a review of the past 10 years. Journal of the American Academy of Child and Adolescent psychiatry. 1996;35:843–851. doi: 10.1097/00004583-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Pine DS. Brain development and the onset of mood disorders. Seminars in clinical neuropsychiatry. 2002;7:223–233. doi: 10.1053/scnp.2002.35218. [DOI] [PubMed] [Google Scholar]
- 26.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman-Rakic P. The cortical dopamine system: Role in memory and cognition. Advances in Pharmacology. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 28.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 29.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual review of neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 31.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 33.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, et al. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 34.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 35.Igarashi M, Kim HW, Gao F, Chang L, Ma K, Rapoport SI. Fifteen weeks of dietary n-3 polyunsaturated fatty acid deprivation increase turnover of n-6 docosapentaenoic acid in rat-brain phospholipids. Biochim Biophys Acta. 2012;1821:1235–1243. doi: 10.1016/j.bbalip.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N., Jr An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav Neurosci. 2009;123:196–205. doi: 10.1037/a0013801. [DOI] [PubMed] [Google Scholar]
- 37.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 38.Igarashi M, Kim HW, Chang L, Ma K, Rapoport SI. Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain. J Neurochem. 2012;120:985–997. doi: 10.1111/j.1471-4159.2011.07597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biological psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- 42.Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, et al. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews M, Bondi CO, Torres GE, Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neurospsychopharmacology. 2013 doi: 10.1038/npp.2013.32. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harlan Laboratories. 2008 Sprague Dawley® Outbred Rat Growth Curve At: http://www.harlan.com/products_and_services/research_models_and_services/research_models/sprague_dawley_outbred_rat.hl.
- 46.Kim HW, Rao JS, Rapoport SI, Igarashi M. Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation. Prostaglandins Leukot Essent Fatty Acids. 2011;85:361–368. doi: 10.1016/j.plefa.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Research Revues. 1983;6:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 48.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 49.Wise RA. Dopamine, learning and motivation. Nature reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 50.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem. 2008;106:662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- 51.Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- 52.Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 53.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209–1219. [PubMed] [Google Scholar]
- 54.Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biological psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 58.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 59.Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 60.Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 64.Larson MK, Walker EF, Compton MT. Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert review of neurotherapeutics. 2010;10:1347–1359. doi: 10.1586/ern.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caramia G. [Essential fatty acids and lipid mediators. Endocannabinoids] La Pediatria medica e chirurgica : Medical and surgical pediatrics. 2012;34:65–72. doi: 10.4081/pmc.2012.2. [DOI] [PubMed] [Google Scholar]
- 66.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]