Abstract
Objective
Many confirmed genetic loci for obesity are expressed in regions of the brain that regulate energy intake and reward-seeking behavior. Whether these loci contribute to the development of specific eating behaviors has not been investigated. We examined the relationship between a genetic susceptibility to obesity and cognitive restraint, uncontrolled and emotional eating.
Design and Methods
Eating behavior and body mass index (BMI) were determined by questionnaires for 1471 men and 2381 women from two U.S cohorts. Genotypes were extracted from genome-wide scans and a genetic-risk score (GRS) derived from 32 obesity-loci was calculated.
Results
The GRS was positively associated with emotional and uncontrolled eating(P<0.002). In exploratory analysis, BMI-increasing variants of MTCH2, TNNI3K and ZC3H4 were positively associated with emotional eating and those of TNNI3K and ZC3H4 were positively associated with uncontrolled eating. The BMI-increasing variant of FTO was positively and those of LRP1B and TFAP2B were inversely associated with cognitive restraint. These associations for single SNPs were independent of BMI but were not significant after multiple-testing correction.
Conclusions
An overall genetic susceptibility to obesity may also extend to eating behaviors. The link between specific loci and obesity may be mediated by eating behavior but larger studies are warranted to confirm these results.
Keywords: obesity, emotional eating, uncontrolled eating, cognitive restraint, genotype, population
INTRODUCTION
Obesity represents a major public health issue contributing to risk of cardiovascular diseases, type 2 diabetes, and various cancers (1). Although environmental factors play an important role in the development of obesity, genetic factors also make a significant contribution to its etiology (2). With the advent of genome-wide association studies (GWAS) we have gained significant knowledge on the common genetic variants underlying the condition (3). However, the mechanisms by which many of these loci affect the development of obesity are not well understood.
A review of the current list of candidate loci points to a key role of the central nervous system (CNS) in body weight regulation. For example, FTO, MC4R, TMEM18, GNPDA2, SH2B1, KCTD15 and BDNF are expressed particularly in the hypothalamus, a crucial center for energy balance and regulation of food intake (4). Furthermore, variants in/near FTO, MC4R, SH2B1, KCTD15, BDNF, TNN13K, MTCH2 and NEGR1 have been linked to energy intake and/or nutrient-specific preferences in humans (5–12). Behavioral factors specific to eating may link these loci to obesity but few epidemiological studies have been conducted on this topic. FTO and MC4R variants have been implicated in feelings of hunger and satiety (9, 13, 14), while common variants in BDNF have been linked to clinical eating disorder-related traits (15).
The Three-Factor Eating Questionnaire (TFEQ) was developed for healthy populations to assess three aspects of eating behavior: uncontrolled eating, emotional eating, and cognitive restraint (16, 17). Uncontrolled eating refers to a tendency to overeat, with the feeling of being out of control. Emotional eating reflects a propensity to overeat in response to negative emotions (i.e. when feeling lonely, anxious or depressed). Cognitive restraint refers to a tendency to consciously restrict one’s food intake instead of using physiological cues (i.e. hunger and satiety) as regulators of intake. Four studies examined genome-wide significant risk variants for obesity and their relationship to eating behaviors, but these were limited to FTO and MC4R (9, 13, 18, 19). We therefore conducted a more comprehensive investigation by examining the effect of 32 previously confirmed GW significant obesity loci on eating behavior in two populations of U.S. men and women.
METHODS AND PROCEDURES
Study sample
The Nurses’ Health Study (NHS) was established in 1976 when 121,700 female registered nurses aged 30–55 years and residing in 11 large U.S. states completed a mailed questionnaire on medical history and lifestyle characteristics (20). Every two years, follow-up questionnaires have been sent to update information on exposures and newly diagnosed diseases and every 2 to 4 years diet is assessed using a validated semi-quantitative FFQ. Blood was collected from 32,826 NHS members between 1989 and 1990. DNA was extracted from white blood cells using the QIAmp™ (QIAGEN Inc., Chatsworth, CA) blood protocol and all samples were processed in the same laboratory. Women contributing to the current analysis were those previously selected as a control for one of 4 independent GWAS in nested case-control studies of the NHS cohort, initially designed for outcomes of type 2 diabetes (T2D), coronary heart disease (CHD), breast cancer (BrCa) and kidney stone disease (KS). Details pertaining to study design have been reported elsewhere (21).
The Health Professionals Follow-Up Study (HPFS) was initiated in 1986 when 51,529 male health professionals between 40 and 75 years of age years and residing in the U.S. completed an FFQ and a questionnaire on lifestyle and medical history. The participants have been followed with repeated questionnaires on lifestyle and health every 2 years and FFQs every 4 years. Between 1993 and 1996, a blood sample was requested from all active participants in the HPFS and collected from 18,225 men (22). DNA was extracted from white blood cells using the QIAmp™ blood protocol and all samples were processed in the same laboratory. Men contributing to the current analysis were those previously selected as a control for one of 3 independent GWAS in nested case-control studies of the HPFS cohort, initially designed for outcomes of T2D, CHD and KS disease.
Study protocols were approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Measures
Three Factor Eating Questionnaire (TFEQ)-R18
The TFEQ-R18 was developed on the basis of a factor analysis of the original 51-item TFEQ in a large sample of obese subjects (16) and was also shown to be applicable to the general population (17). In 2010, the TFEQ-R18 was included on a supplementary questionnaire mailed to NHS and HPFS subjects previously selected for GWAS to acquire additional information amendable to genetic investigation. Scores for ‘cognitive restraint’, ‘uncontrolled eating’ and ‘emotional eating’ were calculated as previously described by de Lauzon et al (17) and correspond to the ‘cognitive restraint’, ‘hunger’ and ‘disinhibition’ scales of the original 51-item TFEQ (16). Accordingly, each of the 18 items was scored from 1 to 4, and the scores were summed to obtain scale scores. The theoretical ranges for the items were 6–24 for cognitive restraint, 9–36 for uncontrolled eating, and 3–12 for emotional eating. The factor structure of the TFEQ-R18 was explored using Principal Components Analysis with a Varimax rotation. A cut-off point of 0.35 was used for the factor loadings. As shown in Table S1 the original factor structure of the TFEQ-R18 was replicated in both our NHS and HPFS samples. Cronbach’s alpha values for NHS were 0.80 for cognitive restraint, 0.87 for uncontrolled eating, and 0.92 for emotional eating. Corresponding values for HPFS were 0.79, 0.85 and 0.91.
Other Covariates
Additional characteristics of the participants were obtained from questionnaires administrated to the entire NHS and HPFS cohorts preceding the 2010 supplementary questionnaire. Current self-reported anthropometrical data, physical activity and smoking status were available in 2008. Physical activity was expressed as metabolic equivalent task (MET) hours of moderate to vigorous exercise per week. Self-administered questionnaires assessing body weight and physical activity have been validated as described previously (23–25). Total dietary energy intake, proportion of energy from carbohydrate, protein, total fat and saturated fat as well as intakes of alcohol and energy-adjusted cereal fiber were calculated based on the mean of self-administered FFQs in 2002 and 2006.
Genotyping, quality control and imputation
Genotyping was performed using the Affymetrix Genome-Wide Human (Affy) 6.0 array (NHS-T2D, HPFS-T2D, NHS-CHD, HPFS-CHD), Illumina Human-Hap550 array (NHS-BrCa) or Illumina 610Q array (NHS-KS, HPFS-KS). Genotyping, quality control (QC) and imputation for each data set have been described in detail previously (21). Although exact QC protocols varied by sample set, at a minimum DNA samples that did not meet a 90% completion threshold, and SNPs with low call rates (<90%), were dropped. Any self-reported “white” samples that had substantial genetic similarity to non-European reference samples (either the HapMap YRI or CHB+JPT samples) were also excluded.
For each of the 7 GWAS data sets we used MACH (26) to impute up to ~ 2.5 million autosomal SNPs with NCBI build 36 of Phase II HapMap CEU data (release 22) as the reference panel. Genotypes were imputed for SNPs not present on the genome-wide arrays or for those where genotyping had failed to meet the QC criteria.
For the current analysis we selected 32 genotyped or imputed SNPs previously associated with obesity traits in GWAS (3). SNP quality matrices by GWAS set varied and are detailed in Table S2. Approximately 72% of the SNPs were imputed and among those imputed the average SNP quality score (MACH’s derived ‘Rsq’(26)) for imputation was 0.95. Genotype frequencies were tested for Hardy-Weinberg equilibrium (HWE) by using a χ2 test with 1 DF. Only rs3810291 (near ZC3H4) deviated from HWE in men (P=0.005); with fewer homozygote minor allele carriers than expected.
Genetic risk score
The combined effect of the 32 SNPs was assessed by calculating a genetic risk score (GRS) for each participant. We assumed an additive genetic model for each SNP, applying a linear weighting of 0, 1, and 2 to genotypes containing 0, 1, or 2 alleles previously associated with higher BMI (3), respectively. The GRS theoretically ranges from 0 to 64, with higher scores indicating a higher genetic susceptibility to obesity.
Statistical analysis
All statistical analyses were performed using the SAS statistical package (version 9.1 for UNIX; SAS Institute, Cary, NC) unless indicated otherwise. 2782 men and 4964 women returned the 2010 supplementary questionnaire. We excluded individuals selected as a case in any one of the GWAS sets (1127 men, 2100 women) or with missing data for any of the TFEQ items (109 men, 102 women). Of those remaining, 1,471 men and 2,381 women had available genetic data passing QC and therefore constituted the final sample for analysis. We calculated Pearson correlation coefficients between each of the scores and with other dietary factors. Linear regression was used to analyze the association between the GRS and each of the eating behavior scores, adjusting for current age and GWAS-set. To facilitate interpretation of results and comparison with the magnitude of the GRS-BMI association (see below), we standardized each eating behavior score [i.e. mean of 0 and standard deviation (SD) of 1]. We further examined whether associations were independent of adiposity by adjusting for BMI in multivariable models. We subsequently explored each individual SNP included in the GRS in relation to BMI and eating behaviors by linear regression analysis. Meta-analyses of gender-specific results were conducted using a fixed effects model and inverse-variance weighting as implemented in METAL(27). Between-gender heterogeneity was tested using the Q-statistic. All tests were two-tailed and P values <0.017 (0.05/3 traits) were considered statistically significant for our analysis of the GRS in relation to eating behaviors. For our analysis of individual SNPs, we did not adjust for multiple testing given the exploratory nature of this secondary analysis. For these analyses, we present all associations with P values <0.05. Power estimates for these analyses are also provided in Table S3.
In a sensitivity analysis, we excluded currently obese (BMI ≥ 30 kg/m2) participants because reporting of eating behaviors may be less accurate in this group (28). We also examined the impact on results when adjusting our primary models for dietary energy and cereal fiber intake, alcohol consumption, physical activity and smoking status.
RESULTS
Characteristics of NHS and HPFS participants included in the current study are shown in Table S4. As shown in Table 1, all three eating behavior scores were positively correlated with one another and with BMI and protein intake in both men and women. On absolute score scales, each point of the cognitive restraint score (18-point scale) was associated with a BMI increase of 0.07 kg/m2 and 0.06 kg/m2 for women and men, respectively. Corresponding increases in BMI for the emotional (9-point scale) and uncontrolled (27-point scale) eating scores were 0.73 and 0.34 kg/m2 among women and 0.45 and 0.25 kg/m2 among men. In addition, all eating behavior scores were inversely correlated with age. Cognitive restraint was inversely correlated with total and saturated fat intake. Uncontrolled eating was positively correlated with energy and fat intake and inversely correlated with physical activity and alcohol intake. Emotional eating was positively correlated with fat intake and inversely correlated with alcohol intake.
Table 1.
Pearson correlation coefficients between eating behavior scores and participant characteristics
| Women | Men | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cognitive restraint | Uncontrolled eating | Emotional eating | Cognitive restraint | Uncontrolled eating | Emotional eating | |
| Age, years | −0.14a | −0.18a | −0.19a | −0.12a | −0.14a | −0.12a |
| BMI, kg/m2 | 0.09a | 0.32a | 0.37a | 0.102 | 0.32a | 0.27a |
| Dietary energy, kcal | −0.04 | 0.11a | 0.07b | −0.07d | 0.06d | 0.03 |
| Carbohydrate intake, %energy | 0.01 | −0.13a | −0.09a | 0.05 | −0.05 | −0.02 |
| Protein intake, %energy | 0.16a | 0.11a | 0.09a | 0.13a | 0.13a | 0.10a |
| Total fat intake, %energy | −0.06c | 0.16a | 0.13a | −0.11a | 0.11a | 0.09b |
| Saturated fat, %energy | −0.14a | 0.10a | 0.07b | −0.15a | 0.11a | 0.06d |
| Cereal fiber, g/d | 0.08b | −0.01 | 0.004 | 0.02 | −0.01 | −0.01 |
| Alcohol, g/d | 0.002 | −0.07b | −0.09a | −0.02 | −0.12a | −0.11a |
| Physical activity, METs | 0.12a | −0.01 | −0.04 | 0.04 | −0.06d | −0.05d |
| Cognitive restraint score | - | 0.12a | 0.12a | - | 0.10a | 0.11a |
| Uncontrolled eating score | 0.12a | - | 0.68a | 0.10a | - | 0.64a |
| Emotional eating score | 0.12a | 0.68a | - | 0.11a | 0.64a | - |
P<0.0001,
P<0.001,
P<0.01
P<0.05
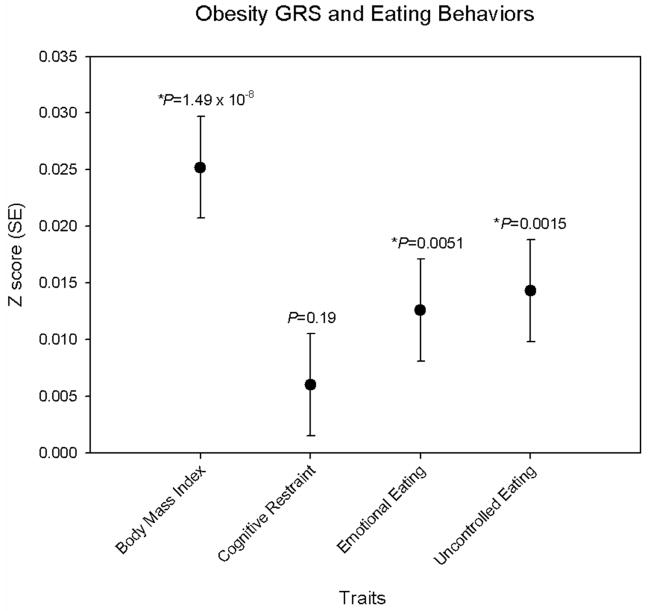
Table S5 presents the main effects of each of the 32 obesity SNPs (herein referenced by their closest gene) on current BMI. In a meta-analysis of men and women combined, FTO, GPRC5B, NEGRI, MTCH2, FAIM2, FANCL, SLC39A8 and BDNF were associated with BMI (P<0.05) and showed directional consistency with those previously reported (3). When combining all 32 variants into a genetic risk score (GRS), the mean (range) score for women and men were 28.6 (17–41) and 28.5 (18–39), respectively. As expected, a significant association was observed between the GRS and BMI (Figure 1).
Figure 1.
Association between the obesity genetic risk score and standardized measures of BMI and eating behaviors. Results from meta-analysis of sex-specific linear regression models adjusted for age and GWAS-set.
The obesity GRS was positively associated with emotional (P=0.005) and uncontrolled (P=0.002) eating in the combined analysis of men and women after correction for multiple testing (Table 2, Figure 1). Each additional BMI increasing allele of the GRS was associated with a 0.016 and 0.013 SD increase in emotional and uncontrolled eating, respectively. By comparison, each additional BMI increasing allele of the GRS was associated with a 0.025 SD increase in BMI.
Table 2.
Associations between confirmed obesity-SNPs and eating behavior scores
| Obesity Loci, SNP | Womenb | Menb | Allc | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| β (SE) | P | β (SE) | P | β (SE) | P | P het | |
| Cognitive restraint
| |||||||
| Obesity GRS | 0.004 (0.006) | 0.47 | 0.009 (0.007) | 0.22 | 0.006 (0.005) | 0.19 | 0.61 |
| FTO, rs1558902 | 0.08 (0.03) | 0.005 | 0.10 (0.04) | 0.01 | 0.09 (0.02) | 0.0002 | 0.76 |
| LRP1B, rs2890652 | −0.09 (0.04) | 0.02 | −0.06 (0.05) | 0.22 | −0.08 (0.03) | 0.01 | 0.71 |
| TFAP2B, rs987237 | −0.03 (0.04) | 0.37 | −0.11 (0.05) | 0.02 | −0.06 (0.03) | 0.03 | 0.19 |
|
| |||||||
| Emotional eating
| |||||||
| Obesity GRS | 0.02 (0.006) | 0.005 | 0.007 (0.007) | 0.35 | 0.01 (0.005) | 0.005 | 0.30 |
| MTCH2, rs3817334 | 0.07 (0.03) | 0.02 | 0.07 (0.04) | 0.05 | 0.07 (0.02) | 0.002 | 0.91 |
| TNNI3K, rs1514175 | 0.08 (0.03) | 0.004 | 0.02 (0.04) | 0.60 | 0.06 (0.02) | 0.01 | 0.17 |
| FTO, rs1558902 | 0.05 (0.03) | 0.07 | 0.06 (0.04) | 0.09 | 0.06 (0.02) | 0.01 | 0.86 |
| ZC3H4, rs3810291 | 0.06 (0.03) | 0.04 | 0.05 (0.04) | 0.17 | 0.06 (0.02) | 0.01 | 0.84 |
| QPCTL, rs2287019 | −0.07 (0.04) | 0.09 | −0.06 (0.05) | 0.22 | −0.06 (0.03) | 0.04 | 0.88 |
|
| |||||||
| Uncontrolled eating
| |||||||
| Obesity GRS | 0.01 (0.006) | 0.03 | 0.02 (0.007) | 0.02 | 0.01 (0.005) | 0.002 | 0.65 |
| TNNI3K, rs1514175 | 0.08 (0.03) | 0.004 | 0.02 (0.04) | 0.50 | 0.06 (0.02) | 0.007 | 0.21 |
| MTCH2, rs3817334 | 0.06 (0.03) | 0.04 | 0.06 (0.04) | 0.08 | 0.06 (0.02) | 0.007 | 0.95 |
| FANCL, rs887912 | 0.08 (0.03) | 0.02 | 0.03 (0.04) | 0.46 | 0.06 (0.02) | 0.02 | 0.33 |
| FTO, rs1558902 | 0.06 (0.03) | 0.05 | 0.04 (0.04) | 0.29 | 0.05 (0.02) | 0.03 | 0.72 |
| FAIM2, rs7138803 | 0.07 (0.03) | 0.01 | 0.01 (0.04) | 0.72 | 0.05 (0.02) | 0.03 | 0.21 |
| ZC3H4, rs3810291 | 0.03 (0.03) | 0.38 | 0.09 (0.04) | 0.02 | 0.05 (0.02) | 0.03 | 0.20 |
P het, P for between-gender heterogeneity, GRS for genetic risk score
Presented are results of associations with P values <0.05 for the combined analysis of men and women. β (SE) correspond to changes in eating behavior Z-score for each additional BMI increasing allele (GRS) or allele (SNPs).
Results from regression analysis adjusted for age and GWAS-set
Results from meta-analysis of men and women
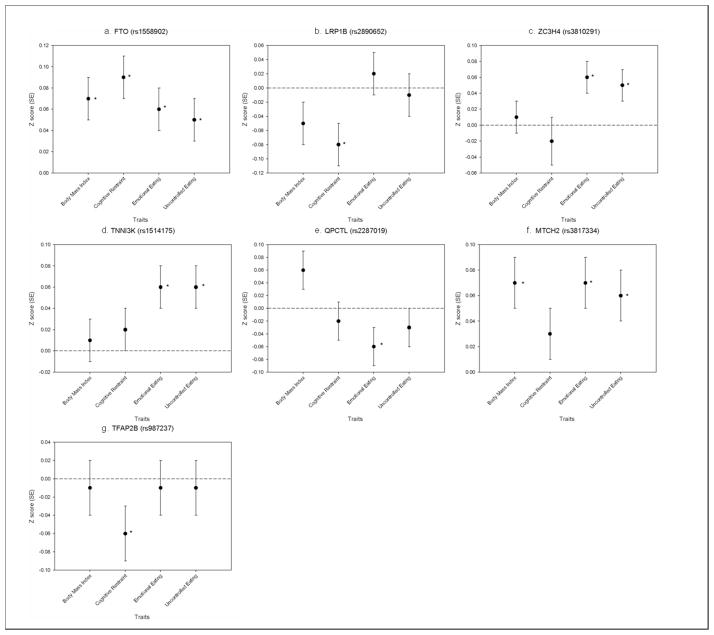
We next explored the individual SNPs that were included in the obesity GRS to gain additional insight to the association between the obesity-related genes and self-reported eating behaviors. Table 2 and Figure 2 presents obesity SNPs that were associated (uncorrected P value <0.05) with eating behavior scores in the combined analysis of men and women (complete results are available from authors upon request). The MTCH2 (P=0.002), TNNI3K (P=0.01), FTO (P=0.01), and ZC3H4 (P=0.01) alleles previously associated with higher BMI were also positively associated with emotional eating, while the BMI-increasing allele of QPCTL (P=0.04) was inversely associated with emotional eating. Each effect allele of MTCH2, TNNI3K, FTO, ZC3H4, and QPCTL was associated with a 0.12 to 0.15 point difference in emotional eating score (corresponding to a 0.06 to 0.07 SD difference).
Figure 2.
Associations between variants in/near a) FTO b) LRP1B c) ZC3H4 d) TNNI3K e) QPCTL f) MTCH2 and g) TFAP2B and standardized measures of BMI and eating behaviors. Results from meta-analysis of sex-specific linear regression models adjusted for age and GWAS-set. * P<0.05 (not corrected for multiple testing)
BMI-increasing alleles of TNNI3K (P=0.007), MTCH2 (P=0.007), FANCL (P=0.02), FTO (P=0.03), FAIM2 (P=0.03) and ZC3H4 (P=0.03) were positively associated with uncontrolled eating; each effect allele being associated with a 0.21 to 0.27 point higher score (corresponding to a 0.05 to 0.06 SD difference).
The FTO allele linked to higher BMI was also positively associated with cognitive restraint (P=0.0002), whereas BMI-increasing alleles of LRP1B (P=0.01) and TFAP2B (P=0.03) were inversely associated with cognitive restraint. Each BMI-increasing allele of FTO, LRP1B and TFAP2B was associated with a 0.24 to 0.33 point difference in the 18-point cognitive restraint scale (corresponding to a 0.06 to 0.09 SD difference).
Figure 2 shows genetic loci reported in Table 2 in relation to both eating behavior scores and current BMI. All these loci, with the exception of MTCH2, were more strongly associated with one or more eating behaviors than with current BMI.
Adjusting for BMI abolished the association between the GRS and eating behaviors (Table S6). However, the associations between MTCH2, TNNI3K, and ZC3H4 variants and emotional eating remained after adjusting for BMI (all P≤0.01). Associations between TNNI3K and ZC3H4 variants and uncontrolled eating and between FTO, LRP1B and TFAP2B and cognitive restrained also persisted with BMI-adjustment (all P≤0.04).
A post-hoc analysis of the GRS-BMI association (β =0.025), revealed modest attenuation in this association upon adjustment for the three eating behaviors (β=0.020). Similar results were observed after excluding obese participants (Table S6) with the exception of the associations for FTO with cognitive restraint that became substantially stronger both before (P=0.00005) and after (P=0.0001) adjusting for BMI. Results were also similar when further adjusting for dietary factors, physical activity and smoking status (data not shown).
DISCUSSION
The mechanisms by which genetic loci for obesity lead to increased adiposity are not well understood. Many of the genes mapping to or near these loci are expressed in regions of the brain regulating energy balance, appetite, and reward-seeking behavior (4). In the current study, we examined the effect of thirty-two previously confirmed genome-wide significant obesity loci on self-reported eating behaviors including cognitive restraint, emotional eating, and uncontrolled eating in two populations of U.S. men and women. A genetic risk score for obesity based on all thirty-two variants was significantly associated with emotional and uncontrolled eating. In further exploratory analyses, variants in/near FTO, LRP1B, TFAP2B, MTCH2, TNNI3K, ZC3H4 and QPCTL were associated with one or more eating behaviors; independent of current BMI, suggesting their link to obesity may, in part, be mediated by eating behaviors. We discuss these findings below but acknowledge the need for careful interpretation of results as none of the individual SNP-eating behavior associations remain statistically significant after correcting for multiple testing.
Our reported associations of eating behaviors with BMI are consistent with previous literature obtained from populations of varying characteristics; thus supporting the appropriateness of the NHS and HPFS cohorts for the current study. Uncontrolled and emotional eating and cognitive restraint are positively correlated with each other and positively associated with current BMI and weight change (29–32). In both NHS and HPFS these patterns of associations were also observed: all three eating behaviors were directly correlated with BMI and with one another. With respect to the main effects of obesity loci on BMI in our population, we did not replicate all previous reported associations but similar directional effects were observed for 27 of the 32 examined loci. Accounting for the number of SNPs tested, the current study had more than 80% power to detect an effect estimate as small as that reported for the FTO variant in stage 2 of the BMI GWAS reported by Speliotes et al (3) but less than 27% for the remaining 31 loci (Supplementary Table). These findings are in accordance to other reports of replication studies on obesity loci identified by large scale efforts (5, 33).
The association between the GRS and BMI is only modestly attenuated by adjustment for the studied eating behaviors. The reason for this may be that only part of the SNPs included in the GRS affect BMI though the studied eating behaviors, whereas other included SNPs affect BMI through independent biological pathways such as energy expenditure. Our analysis of individual SNPs supports this notion; some variants were associated with eating behaviors with a similar or greater strength of association as for BMI, whereas other SNPs were not associated with eating behaviors.
FTO has been the strongest and most consistent signal in GWAS and follow-up studies of obesity traits to date. In our study the BMI-increasing variant of FTO was positively associated with all three studied eating behaviors. Interestingly, only the association with cognitive restraint remained after adjusting for current BMI and was stronger than the association with BMI in our population. Although all three eating behaviors are characterized by lack of control of eating by physiological cues, it is possible that FTO’s effects on emotional and uncontrolled eating are strongly correlated with its effects on BMI, whereas the effects FTO has on cognitive restraint may be more distinct.
SNPs recently associated with BMI in MTCH2, TNNI3K and ZC3H4 (3) were associated with higher emotional and uncontrolled eating scores in the current study, with minimal attenuation after BMI adjustment. These findings suggest that the effects of these genetic variants on the development of obesity may be mediated through emotional and uncontrolled eating possibly reflecting lack of homeostatic control or greater sensitivity to food reward feedback. The potential impact of the genetic variations on these pathways merits further study as the mechanisms through which these loci affect the development of obesity are currently unknown.
Several limitations need to be considered when interpreting the results. Associations between the GRS and emotional and uncontrolled eating were no longer significant after adjusting for BMI. If part of the effect of the GRS on BMI is a consequence of an effect of the GRS on emotional and uncontrolled eating then adjustment for BMI represents over-adjustment. Alternatively, effects of the GRS on eating behaviors may be mediated by BMI with changes in BMI resulting in changes in eating behavior. Although we excluded participants with obesity from our study, we cannot exclude the possibility that the effect of the GRS on eating behaviors was secondary to an effect of the GRS on BMI. The TFEQ may overestimate restraint in obese subjects, thus potentially augmenting the effect of obesity-associated genetic variants on cognitive restraint scores (28). Recognizing potential TFEQ response differences in currently obese and non-obese participants, we assessed the robustness of our findings when excluding obese subjects (in addition to adjusting for BMI in our statistical models). In most cases, associations between genetic variants and eating behavior scores were strengthened. Within-person variation in eating behaviors (for example due to dieting) may have weakened the observed associations between genetic variants and eating behaviors. We did not consider a history of over-weight/obesity or weight-cycling or other factors that could impact development of eating behavior, thus we cannot exclude the possibility that the effects of genetic loci on eating behavior are not independent of weight history. We also cannot discount the possibility that eating behavior was associated with misreporting of body weight, as previously described (34). How these issues may have impacted our findings is unclear. Our sample consisted predominately of healthy older health professionals of European ancestry, which may limit the generalizability of our findings to younger individuals of other social or racial demographics. One advantage in studying older adults is potential for capturing more stabilized eating behaviors (29, 35).
There is growing evidence suggesting that obesity is related to an imbalance of homeostatic as well as hedonic systems (36). Our study shows that previously confirmed obesity variants combined in a risk score substantially affect habitual eating behaviors. For a subset of these genetic variants, the studied eating behaviors may reflect mechanisms underlying their association with BMI. Indeed, several previous studies have suggested substantial heritability for dietary restraint (heritability 0.23–0.59), disinhibition (heritability 0–0.60) and perceived hunger (heritability 0.23–0.45) (37–39). Moving forward, a focus on genetic underpinnings of dietary behaviors may be one approach to identify factors contributing to obesity. Our findings thus suggest that eating behaviors may contribute importantly to the link between genetic variation and the development of obesity and motivate future investigations on the role these loci play in eating behavior.
Supplementary Material
What is already known about this subject?
Many of the confirmed obesity loci are expressed in regions of the brain that regulate energy intake and reward-seeking behaviors
Variants in/near FTO, MC4R, SH2B1, KCTD15, BDNF, TNN13K, MTCH2 and NEGR1 have been linked to energy intake and/or nutrient-specific preferences in humans
FTO and MC4R variants have been implicated in feelings of hunger and satiety, while common variants in BDNF have been linked to clinical eating disorder-related traits
What does this study add?
The authors conducted a more comprehensive investigation by examining the combined and individual effects of 32 previously confirmed genome-wide significant obesity loci on eating behaviors in men and women.
A genetic-risk score for obesity was positively associated with emotional eating and uncontrolled eating. Further, several loci were associated with cognitive restraint, emotional eating and uncontrolled eating independent of current BMI. A subset of these loci were more strongly associated with one or more eating behaviors than with current BMI
Findings suggest that an overall genetic propensity for obesity may also extend to eating behaviors and that the link between some obesity loci and the development of obesity may be partly mediated by eating behaviors
Acknowledgments
MCC and RMD designed the study. MCC completed the statistical analysis and prepared the first draft of the manuscript. RMD provided supervision. RMD, FBH, GCC, EBR, PK, and DJH obtained funding. All authors contributed to the data interpretation and critically revised the manuscript. We thank the participants in the NHS and HPFS for their dedication and commitment. This work was supported by grants from the National Institutes of Health [NCI (CA40356, CA087969, CA055075, CA98233), NIDDK (DK058845, DK070756), NHGRI (HG004399), NHLBI (HL35464)] with additional support from Merck/Rosetta Research Laboratories, North Wales, PA. Dr. Cornelis is a recipient of a NARSAD Young Investigator Award.
Footnotes
Competing interests: the authors have no competing interests
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Dubois L, Ohm Kyvik K, Girard M, Tatone-Tokuda F, Perusse D, Hjelmborg J, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an international study of over 12,000 twin pairs. PLoS One. 2012;7:e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual review of psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 5.Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90:951–9. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- 6.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 7.Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–8. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961–5. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 9.Stutzmann F, Cauchi S, Durand E, Calvacanti-Proenca C, Pigeyre M, Hartikainen AL, et al. Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes (Lond) 2009;33:373–8. doi: 10.1038/ijo.2008.279. [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet. 2008;17:3502–8. doi: 10.1093/hmg/ddn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009;90:1483–8. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaffery JM, Papandonatos GD, Peter I, Huggins GS, Raynor HA, Delahanty LM, et al. Obesity susceptibility loci and dietary intake in the Look AHEAD Trial. Am J Clin Nutr. 2012;95:1477–86. doi: 10.3945/ajcn.111.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 14.den Hoed M, Westerterp-Plantenga MS, Bouwman FG, Mariman EC, Westerterp KR. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr. 2009;90:1426–32. doi: 10.3945/ajcn.2009.28053. [DOI] [PubMed] [Google Scholar]
- 15.Mercader JM, Ribases M, Gratacos M, Gonzalez JR, Bayes M, de Cid R, et al. Altered brain-derived neurotrophic factor blood levels and gene variability are associated with anorexia and bulimia. Genes, brain, and behavior. 2007;6:706–16. doi: 10.1111/j.1601-183X.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24:1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 17.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–80. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 18.Rutters F, Nieuwenhuizen AG, Bouwman F, Mariman E, Westerterp-Plantenga MS. Associations between a single nucleotide polymorphism of the FTO Gene (rs9939609) and obesity-related characteristics over time during puberty in a Dutch children cohort. J Clin Endocrinol Metab. 2011;96:E939–42. doi: 10.1210/jc.2010-2413. [DOI] [PubMed] [Google Scholar]
- 19.Valladares M, Dominguez-Vasquez P, Obregon AM, Weisstaub G, Burrows R, Maiz A, et al. Melanocortin-4 receptor gene variants in Chilean families: association with childhood obesity and eating behavior. Nutritional neuroscience. 2010;13:71–8. doi: 10.1179/147683010X12611460763643. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu NF, Spiegelman D, Yu J, Rifai N, Hotamisligil GS, Rimm EB. Plasma leptin concentrations and four-year weight gain among US men. Int J Obes Relat Metab Disord. 2001;25:346–53. doi: 10.1038/sj.ijo.0801549. [DOI] [PubMed] [Google Scholar]
- 23.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–8. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inference. Am J Hum Genet. 2006;S79:2290. [Google Scholar]
- 27.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Strien T, Herman CP, Engels RC, Larsen JK, van Leeuwe JF. Construct validation of the Restraint Scale in normal-weight and overweight females. Appetite. 2007;49:109–21. doi: 10.1016/j.appet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Drapeau V, Provencher V, Lemieux S, Despres JP, Bouchard C, Tremblay A. Do 6-y changes in eating behaviors predict changes in body weight? Results from the Quebec Family Study. Int J Obes Relat Metab Disord. 2003;27:808–14. doi: 10.1038/sj.ijo.0802303. [DOI] [PubMed] [Google Scholar]
- 30.Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. Am J Clin Nutr. 2002;75:476–83. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- 31.Stice E, Ng J, Shaw H. Risk factors and prodromal eating pathology. Journal of child psychology and psychiatry, and allied disciplines. 2010;51:518–25. doi: 10.1111/j.1469-7610.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- 32.Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychological bulletin. 1993;114:100–21. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- 33.Holzapfel C, Grallert H, Huth C, Wahl S, Fischer B, Doring A, et al. Genes and lifestyle factors in obesity: results from 12,462 subjects from MONICA/KORA. Int J Obes (Lond) 2010;34:1538–45. doi: 10.1038/ijo.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vartanian LR, Germeroth LJ. Accuracy in estimating the body weight of self and others: Impact of dietary restraint and BMI. Body image. 2011;8:415–8. doi: 10.1016/j.bodyim.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Hays NP, Bathalon GP, Roubenoff R, McCrory MA, Roberts SB. Eating behavior and weight change in healthy postmenopausal women: results of a 4-year longitudinal study. J Gerontol A Biol Sci Med Sci. 2006;61:608–15. doi: 10.1093/gerona/61.6.608. [DOI] [PubMed] [Google Scholar]
- 36.Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–19. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 37.de Castro JM, Lilenfeld LR. Influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutrition. 2005;21:446–55. doi: 10.1016/j.nut.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Steinle NI, Hsueh WC, Snitker S, Pollin TI, Sakul H, St Jean PL, et al. Eating behavior in the Old Order Amish: heritability analysis and a genome-wide linkage analysis. Am J Clin Nutr. 2002;75:1098–106. doi: 10.1093/ajcn/75.6.1098. [DOI] [PubMed] [Google Scholar]
- 39.Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. Am J Clin Nutr. 2005;81:564–9. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.