Abstract
Pentatricopeptide repeat (PPR) proteins are widespread in eukaryotes and in particular, include several hundred members in land plants. The majority of PPR proteins are localized in mitochondria and plastids, where they play a crucial role in various aspects of RNA metabolism at the post-transcriptional level in gene expression. However, many of their functions remain to be characterized. In contrast to vascular plants, the moss Physcomitrella patens has only 105 PPR genes. This number may represent a minimum set of PPR proteins required for post-transcriptional regulation in plant organelles. Here, we review the overall structure of the P. patens PPR gene family and the current status of the functional characterization of moss PPR proteins.
Keywords: Physcomitrella patens, DYW domain, PPR protein, RNA cleavage, RNA editing, RNA splicing, homologous recombination, moss, targeted gene disruption
Introduction
Pentatricopeptide repeat (PPR) proteins are nucleus-encoded and constitute an extraordinarily large family in land plants, composed of more than 450 members in vascular plants.1,2 Surprisingly, the lycophyte Selaginella moellendorffii has over 800 PPR genes.3 The majority of plant PPR proteins are localized in mitochondria or plastids, and play important roles in a wide range of physiological and developmental functions such as cytoplasmic male sterility, photosynthesis, respiration and embryogenesis.4 Most PPR proteins that have been investigated are required for various post-transcriptional steps associated with RNA in plant organelles (for recent review, see ref. 5).
The PPR proteins are structurally divided into four classes, P, PLS, E/E+ and DYW, based on their PPR motif and characteristic C-terminal domain structures.1 In Arabidopsis and rice, P-class PPR proteins represent half of all PPR proteins and the remaining half are the E/E+ and DYW-class proteins (Table 1). Extensive functional analyses of PPR proteins have been performed using flowering plants: Arabidopsis, rice and maize. However, the function of most PPR proteins is unknown, and their characterization remains one of the major challenges in plant science. In contrast to studies performed in flowering plants, knowledge regarding the PPR proteins required for organelle biogenesis in early land plants is limited. However, studies on the moss P. patens organelles have made rapid progress using recently established technologies that generated a wealth of information on the genomes of the nucleus and organelles.6,7 Here, we describe the current data of the overall structure of the P. patens PPR protein family and their function in plastids and mitochondria, and attempt to highlight the differences and similarities of mosses and angiosperms.
Table 1. Number of PPR genes in Arabidoopsis, rice and moss.
| Plant species | Total | P | PLS | E/E+ | DYW |
|---|---|---|---|---|---|
|
Arabidopsis thaliana |
450 |
250 |
7 |
106 |
87 |
|
Oryza sativa |
477 |
235 |
14 |
138 |
90 |
| Physcomirella patens | 105 | 89 | 6 | 0 | 10 |
P, PLS, E/E+ and DYW classes are defined by Lurin et al. (2004).1
The Physcomitrella PPR protein family
Moss PPR genes were first described in 2004 by Hattori et al., who identified over 30 PPR genes in P. patens.8 Subsequently, the whole genome sequence of this moss was disclosed7 and a total of 103 PPR genes were annotated in the genome.2 The P. patens PPR genes are named PpPPR_#, and are numbered sequentially (Table 2). The genome database was updated (The Physcomitrella patens resource COSMOSS, www.cosmoss.org/) and two additional PPR proteins were identified and designated PpPPR_104 and PpPPR_105. The Physcomitrella PPR gene family is rather small compared with PPR gene families in vascular plants, and contains only 10 DYW-class PPR proteins and no E/E+-class proteins (Table 1).
Table 2. List of Physcomitrella PPR proteins.
| Name | Class | Auxiliary | Locationa) | Arabidopsis PPR proteinb) |
|---|---|---|---|---|
| |
|
domain/motif |
|
homologus to PpPPR_# |
| PpPPR_1 |
P |
|
M |
At5g50280 (EMB1006)16 |
| PpPPR_2 |
P |
|
C |
|
| PpPPR_3 |
P |
RRM |
C |
At5g04810 (AtPPR4)19 |
| PpPPR_4 |
P |
|
C |
|
| PpPPR_5 |
P |
|
M |
|
| PpPPR_6 |
P |
|
C |
|
| PpPPR_7 |
P |
LAGLIDADG |
C |
|
| PpPPR_8 |
P |
|
M |
|
| PpPPR_9 |
PLS |
|
M |
|
| PpPPR_10 |
P |
|
C or M |
At4g21190 (EMB1417)17 |
| PpPPR_11 |
P |
|
M |
|
| PpPPR_12 |
P |
|
C |
|
| PpPPR_13 |
P |
|
C |
|
| PpPPR_14 |
P |
|
C |
At3g46610 |
| PpPPR_15 |
P |
|
C* |
|
| PpPPR_16 |
P |
|
M |
|
| PpPPR_17 |
P |
|
C |
At4g39620 (AtPPR5)20 |
| PpPPR_18 |
P |
|
M |
|
| PpPPR_19 |
P |
|
C* |
At3g34830 (MRL1)21 |
| PpPPR_20 |
P |
|
C* |
At1g01970 |
| PpPPR_21 |
P |
|
C* |
At5g02860 |
| PpPPR_22 |
P |
LAGLIDADG |
M |
|
| PpPPR_23 |
P |
|
C |
At3g59040 |
| PpPPR_24 |
P |
|
M |
|
| PpPPR_25 |
PLS |
|
M |
|
| PpPPR_26 |
P |
|
- |
|
| PpPPR_27 |
P |
|
- |
At3g53170 |
| PpPPR_28 |
P |
|
C |
|
| PpPPR_29 |
P |
|
M |
|
| PpPPR_30 |
P |
Smr |
M |
At1g18900 |
| PpPPR_31 |
PLS |
|
M* |
|
| PpPPR_32 |
P |
|
C |
|
| PpPPR_33 |
P |
|
M |
|
| PpPPR_34 |
PLS |
|
C |
|
| PpPPR_35 |
P |
|
M |
At3g53170 |
| PpPPR_36 |
P |
|
M |
|
| PpPPR_37 |
P |
|
- |
|
| PpPPR_38 |
P |
|
C* |
|
| PpPPR_39 |
P |
|
C |
|
| PpPPR_40 |
P |
|
- |
|
| PpPPR_41 |
P |
|
C* |
|
| PpPPR_42 |
P |
Smr |
M |
|
| PpPPR_43 |
DYW |
|
M* |
|
| PpPPR_44 |
P |
|
Mt |
|
| PpPPR_45 |
DYW |
|
C* |
|
| PpPPR_46 |
P |
|
C or M |
At5g39980 |
| PpPPR_47 |
P |
|
M |
|
| PpPPR_48 |
P |
|
C* |
|
| PpPPR_49 |
P |
|
M |
|
| PpPPR_50 |
P |
|
M |
|
| PpPPR_51 |
P |
|
C* |
At4g34830 (MRL1)21 |
| PpPPR_52 |
P |
|
C* |
At3g18110 |
| PpPPR_53 |
P |
|
C |
At5g02860 |
| PpPPR_54 |
P |
|
M |
|
| PpPPR_55 |
P |
|
C |
|
| PpPPR_56 |
DYW |
|
M* |
|
| PpPPR_57 |
P |
|
M |
|
| PpPPR_58 |
P |
|
M |
At4g35850 |
| PpPPR_59 |
P |
Smr |
C* |
At5g02830 |
| PpPPR_60 |
P |
|
M |
|
| PpPPR_61 |
P |
|
M |
At4g35850 |
| PpPPR_62 |
P |
Smr |
M |
|
| PpPPR_63 |
P |
NYN |
Nuc* |
At2g16650 (PRORP2)25 |
| |
|
|
|
At4g21900 (PRORP3)25 |
| PpPPR_64 |
P |
|
C* |
At1g74850 (pTAC2)24 |
| PpPPR_65 |
DYW |
|
M* |
|
| PpPPR_66 |
P |
|
C |
At2g35130 |
| PpPPR_67 |
P |
NYN |
C/M* |
At2g32230 (RPORP1)25 |
| PpPPR_68 |
P |
|
M |
|
| PpPPR_69 |
PLS |
|
- |
|
| PpPPR_70 |
P |
CBS |
C |
At5g10690 (CBSPPR1)22 |
| PpPPR_71 |
DYW |
|
M* |
|
| PpPPR_72 |
P |
|
C |
At2g35130 |
| PpPPR_73 |
P |
|
M |
|
| PpPPR_74 |
P |
|
C |
|
| PpPPR_75 |
P |
Smr |
C |
At2g31400 (GUN1)23 |
| PpPPR_76 |
P |
RRM |
M |
At5g04810 (AtPPR4)19 |
| PpPPR_77 |
DYW |
|
M* |
|
| PpPPR_78 |
DYW |
|
M* |
|
| PpPPR_79 |
DYW |
|
M* |
|
| PpPPR_80 |
P |
|
C |
At4g39620 (AtPPR5)20 |
| PpPPR_81 |
P |
Smr |
M |
|
| PpPPR_82 |
P |
|
C* |
|
| PpPPR_83 |
P |
|
- |
At2g41720 (EMB2654)18 |
| PpPPR_84 |
P |
|
- |
|
| PpPPR_85 |
P |
Smr |
C |
At2g31400 (GUN1)23 |
| PpPPR_86 |
P |
|
ER |
|
| PpPPR_87 |
P |
|
M |
|
| PpPPR_88 |
P |
|
M |
|
| PpPPR_89 |
P |
|
M |
|
| PpPPR_90 |
P |
|
C |
At5g42310 |
| PpPPR_91 |
DYW |
|
M* |
|
| PpPPR_92 |
P |
|
C |
At4g308252 |
| PpPPR_93 |
P |
|
C |
|
| PpPPR_94 |
P |
|
C |
At4g308252 |
| PpPPR_95 |
P |
|
C |
|
| PpPPR_96 |
P |
Smr |
C* |
|
| PpPPR_97 |
P |
|
M |
|
| PpPPR_98 |
DYW |
|
M* |
|
| PpPPR_99 |
P |
|
C |
|
| PpPPR_100 |
P |
|
C |
At2g30100 |
| PpPPR_101 |
P |
|
C |
|
| PpPPR_102 |
P |
|
C* |
|
| PpPPR_103 |
P |
|
- |
|
| PpPPR_104 |
P |
NYN |
C/M* |
At2g32230 (RPORP1)25 |
| PpPPR_105 | PLS | C* | - |
Location indicates chloroplast (C), mitochondria (M), both C and M (C/M), either C or M, endoplasmic reticulum (ER), nucleus (Nuc) or unknown (-). Asterisks indicate location experimentally determined. (b)Arabidopsis PPR proteins listed show more than 35% amino acid identity with PpPPR proteins, excluding PLS and DYW-class proteins.
Subcellular localization of the Physcomitrella PPR proteins
In silico and in vivo analyses have shown that most PPR proteins are localized in either mitochondria or chloroplasts.1 Similarly, we checked the subcellular localization of 105 PpPPR proteins using in silico analysis and in vivo analyses using transient assay or transgenic moss plants expressing PpPPR-green fluorescent protein (GFP) fusion proteins. The subcellular localization of 29 PpPPR proteins was determined experimentally and that of 68 proteins was derived from prediction (Table 2). At least 95 out of 105 PPR proteins are presumably localized in chloroplasts or mitochondria, or both. The number of chloroplast-targeted PPR proteins is nearly the same as mitochondrial PPR proteins. PpPPR_63 is localized in the nucleus and its paralogs (PpPPR_67 and 104) are located in both chloroplasts and mitochondria. PpPPR_86 is predictably targeted to the endoplasmic reticulum (ER). Subcellular localization of eight PpPPR proteins was not predicted. This could be why PPR ORF models lack the correct initiation codon and, thus, are missing a potential targeting peptide.
Physcomitrella PPR proteins diverge from Arabidopsis and rice proteins
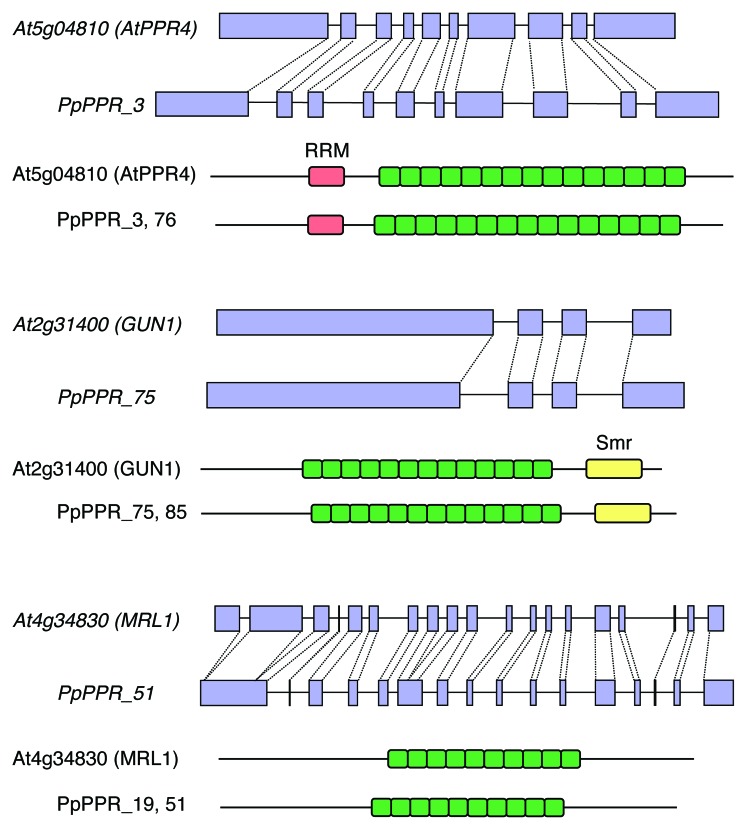
The number of PPR genes in Arabidopsis and rice are strikingly similar (Table 1). More than 80% of Arabidopsis and rice PPR proteins are orthologous pairs.2 In contrast, the Physcomitrella PPR proteins are somewhat diverged from the Arabidopsis and rice PPR proteins. Intron-containing PPR genes represent three-fourths in Physcomitrella but only one-fourth in Arabidopsis and rice.2 Physcomitrella PPR genes are generally intron-rich and alternative splicing variants are often found in PpPPR genes, including PpPPR_389 and PpPPR_43.10 The gene structure and encoded amino acid sequence of many PPR proteins are well conserved in Physcomitrella and Arabidopsis plants (Fig. 1). This conservation suggests that such homologous PPR proteins have the same or similar function in moss and flowering plants. Presumably, intron-rich PPR genes may represent “ancient” PPR genes that pre-dated the occurrence of retrotransposition-mediated expansion of the PPR gene family in land plants.2
Figure 1. Examples of intron conservation in the homologous PPR genes. In each figure, the first panel shows the gene structure and the second panel shows the motif structure of the predicted PPR proteins.
Although a large mutant collection of targeted gene knockout lines was produced for P. patens,11 no PPR gene-targeted lines are identified. To characterize the function of each PpPPR protein, we constructed gene-targeted knockout or knockdown mutant lines via homologous recombination. To date, in our laboratory, one-third of the PpPPR genes were tagged by an antibiotic-resistant gene cassette and characterization of their mutants are in progress. This reverse-genetics approach has revealed the function of several PpPPR genes as described below.
P-class PPR proteins in Physcomitrella
The P-class PPR proteins are characterized by 35 canonical amino-acid PPR (P) motif. P-class PPR proteins are usually involved in RNA cleavage, RNA splicing, RNA stability, or translation.5 More than half (55%) of the Arabidopsis PPR proteins are grouped into the P-class. Some contain an additional conserved motif or domain, such as an RNA recognition motif (RRM),12 small MutS-related (Smr) domain,13 cystathione β synthase (CBS) domain14 or NYN metallonuclease domain.15 In Physcomitrella, most (85%) of the PpPPR proteins are P-class proteins, and 40% of the P-class PPR proteins show high amino acid identities with Arabidopsis PPR protein sequences, including EMBRYO-DEFECTIVE (EMB) genes,16-18 AtPPR4,19 AtPPR5,20 MRL1,21 AtCBS1,22 GUN1,23 pTAC224 and PRORP1, 2 and 325 (Table 2).
In Physcomitrella, the first functional analysis was achieved for P-class PpPPR_38.9 PpPPR_38 is involved in splicing and cleavage of the clpP pre-mRNA9 and binds specifically to the intergenic spacer of chloroplast clpP–5′-rps12 dicistronic mRNA.26 Although the gene organization of clpP–5′-rps12 is conserved in Physcomitrella and Arabidopsis, PpPPR_38 orthologs are not identified in Arabidopsis. This suggests that an Arabidopsis protein involved in clpP maturation is highly diverged from PpPPR_38. Several P-class PPR proteins are known to be splicing factors for plastid or mitochondrial pre-mRNA in Arabidopsis and maize.27 Among these, at least AtPPR4 and AtPPR5 homologs are found in Physcomitrella.
The nucleus-localized PpPPR_63 possesses a NYN-metallonuclease domain and is likely orthologous to Arabidopsis PRORP2 that possesses RNase P activity.25 Disruption of PpPPR_63 gene resulted in abnormal formation of the branched filaments of protonemata (Komura and Sugita, unpublished), suggesting the involvement of PpPPR_63 in the growth and development of protonemal filaments. PpPPR_59 contains an Smr domain and is plastid-localized. Disruption of the PpPPR_59 gene did not result in a different phenotype than wild-type moss plants (Ide and Sugita, unpublished). Arabidopsis GUN1, pTAC2 and SUPPRESSOR OF VARIEGATION7 (SVR7) are a P-class PPR protein with an Smr domain. GUN1 is known to be involved in plastid-to-nucleus retrograde signaling23 and pTAC2 is a component of the transcriptionally active plastid chromosome and might be involved in plastid gene expression.24 SVR7 could be required for FtsH-medaited chloroplast biogenesis.28 PPR motifs likely act as sequence-specific RNA-binding proteins, and non-PPR domains may take part in some RNA processing steps. At least 11 paralogous pairs are found in Physcomitrella P-class proteins, including PpPPR_3 and 76, PpPPR_75 and 85 and PpPPR_19 and 51 (Fig. 1). Their paralogous pairs may have redundant function.
PLS-class PPR proteins in Physcomitrella
This class of PPR proteins is characterized by canonical PPR (P), PPR long (L) and PPR short (S) motifs. Six PLS-class PPR proteins are present in Physcomitrella (Table 1) but their functions have not been identified. Three proteins are predicted to be localized in the mitochondria and two are predicted to be localized in the plastid. Disruption of the PpPPR_31 gene encoding a mitochondrial protein resulted in severe protonemal growth retardation (Tasaki and Sugita, unpublished). Physcomitrella PLS-class proteins are structurally unrelated to the Arabidopsis proteins.
DYW-class PPR proteins are involved in RNA editing and RNA splicing in Physcomitrella
P. patens has 10 DYW-class PPR proteins. The DYW domains are 95 amino acids and are named after its characteristic C-terminal tripeptide, Asp-Tyr/Phe-Trp. This domain has not been found in any other proteins or in organisms apart from land plants, except for a heterolobosean protist Naegleria gruberi.29 The protist DYW-class PPR proteins are hypothesized to derive from horizontal gene transfer from plants in very early land plant evolution.29Funaria hygrometrica, a closely related species of P. patens, has nine DYW-class PPR proteins homologous to the P. patens proteins but lacks the PpPPR_56 ortholog.30 In contrast, marchantiid liverworts do not possess DYW-class proteins.31
In seed plants, more than 400 C-to-U RNA editing sites have been identified in the mitochondria. To date, more than 30 E/E+ and DYW-class PPR proteins have been identified as editing site-specific factors in flowering plants.5 In contrast, RNA editing occurs at only 11 sites in P. patens mitochondrial mRNAs.32,33 To date, eight out of 10 Physcomitrella DYW-class proteins have been identified as RNA editing factors. PpPPR_56 is involved in editing at the nad3 and nad4 sites,34 PpPPR_77 at the cox2 and cox3 sites34 and PpPPR_91 at the nad5-2 site.34 PpPPR_78 and PpPPR_79 are required for editing at the rps14 and cox1 sites30,35 and the nad5-1 site,35 respectively. PpPPR_71 is a sequence-specific recognition factor for editing at the ccmF-2 site of ccmFc mRNA.33,36 In fact, this was demonstrated using electrophoresis mobility shift assays for detection of RNA binding of PpPPR_71 protein to the target RNA. PpPPR_65 targets the ccmF-1 editing site (Ichinose and Sugita, unpublished; Rüdinger and Knoop, unpublished) and PpPPR_98 is responsible for atp9 editing (Ichinose and Sugita, unpublished). Thus, eight DYW-class PPR proteins function in editing all 11 sites in P. patens mitochondrial transcripts. Among these 11 editing sites, editing at the ccmF-1, ccmF-2 and nad5-1 sites also occurs in Arabidopsis mitochondria. Interestingly, the moss F. hygrometrica lacks both the PpPPR_56 ortholog and its target nad3 and nad4 editing sites.30 This suggests that PPR genes and their cognate editing sites are mutually constrained in evolution.30,37
E/E+-class PPR proteins are required for RNA editing in plastids and mitochondria in flowering plants.38,39 However, no E/E+-class PPR proteins exist in P. patens. In addition, DYW140 and multiple organellar editing factor (MORF)41 proteins have recently been identified as editing factors in Arabidopsis but are not present in Physcomitrella. This suggests that DYW-class PPR proteins are a sole key player required for RNA editing in Physcomitrella. However, we cannot exclude the possibility that non-DYW class PPR or non-PPR proteins (e.g., RRM type RNA-binding proteins42) are necessary for recognition of the RNA editing site or the efficiency of RNA editing events together with DYW-class proteins in Physcomitrella.
In Physcomitrella plastids, RNA editing occurs at only one site in the translated region of rps14 mRNA.43,44 Plastid-localized PpPPR_45 is predicted to be a plastid rps14 RNA editing factor. In contrast, PpPPR_43 protein is not involved in RNA editing, but is required for group II intron splicing of the mitochondrial cox1 transcript.10 The DYW domain of PpPPR_43 is distinct from the other nine DYW domains of Physcomitrella PPR proteins.
Function of the DYW domain
DYW-class proteins are involved in RNA editing,38 RNA splicing10 and RNA cleavage.45 This suggests that the DYW domain itself may have certain catalytic activity for target RNA species. There is a correlation between the presence of nuclear DYW genes and the occurrence of organelle RNA editing among land plants.31,46 Therefore, a hypothesis was provided in which the DYW domains are responsible for RNA editing in plant organelles and catalyze RNA editing.46 In fact, the DYW domain contains a conserved region, which includes invariant residues that match the active site of cytidine deaminases (C/HxE….PCxxC) from various organisms. However, cytidine deaminase activity was not detected by an in vitro assay using the recombinant DYW domain of Arabidopsis protein (At2g02980).47 Alternatively, recombinant DYW domains are found to possess endoribonuclease activity.47 Arabidopsis CRR2, a DYW-class PPR protein, is required for intergenic RNA cleavage of plastid rps7-ndhB dicistronic pre-mRNA.45 The DYW domain of CRR2 has been shown to be indispensable for cleavage of the target RNA in vivo.48 The DYW domain contains the cytochrome c family heme-binding site signature (CxxCH),49 which overlaps with the active site of cytidine deaminase. Mutation of this signature to GxxGH resulted in a significant reduction of RNA cleavage activity.47 This indicates that the CxxCH motif is required for endoribonuclease activity of the DYW domain.
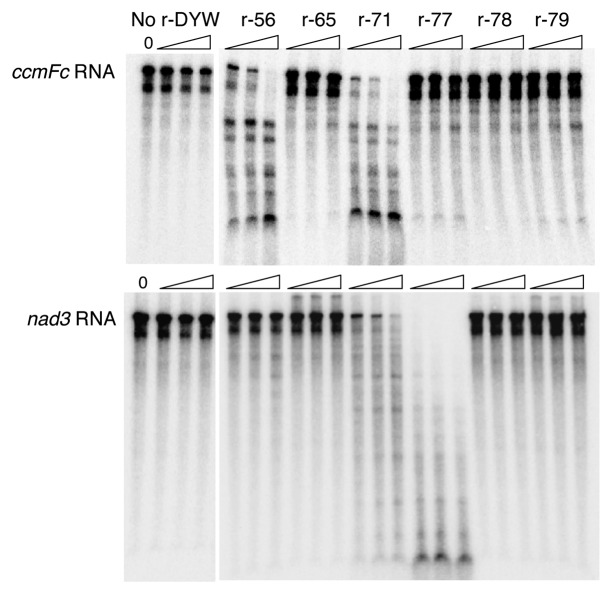
Physcomitrella DYW domains are well conserved (60–80% amino acid identities among DYW-class proteins, excluding PpPPR_43) and contain HSE….CxDCH residues. This suggests that Physcomitrella DYW domains may have potential endoribonuclease activity and/or cytidine deaminase activity. This possibility was tested and at least three DYW domains of PpPPR_56, 71 and 77 showed endoribonuclease-like activity (Fig. 2). Interestingly, its activity tightly depends on the substrate RNA used for the assay. For instance, the DYW of PpPPR_56 (r-56) digested ccmFc RNA but not nad3 RNA, whereas that of PpPPR_77 (r-77) rapidly cleaved the nad3 RNA but not ccmFc RNA. In contrast, r-71 efficiently degraded both RNAs. This implies that some DYW domains have potential RNA degradation activity. In contrast, no cytidine deaminase activity was detected. As described above, Physcomitrella DYW-class proteins are involved in RNA editing but also may function in certain RNA processing events in organelles. This possibility will be further investigated.
Figure 2. Detection of RNase activity of the recombinant moss DYW proteins. (α-32P-UTP)-labeled mitochondrial ccmFc RNA (402 nt) or nad3 RNA (433 nt) was incubated at 28°C for 15, 30 or 60 min (indicated as wedge-shape) with the indicated r-DYW proteins (r-56, r-65, r-71, r-77, r-78 or r-79, 50 ng each) or without protein (No r-DYW) in the presence of 6 mM MgCl2 and 25 mM EDTA as previously described.47 (32P)-labeled RNA was analyzed on 6% polyacrylamide gels containing 6 M urea, and detected by autoradiography.
Perspectives
Plastid genomes of land plants are relatively uniform in size and their gene content and organization are well conserved.50 However, mitochondrial genome structures largely differ between Physcomitrella and flowering plants.51 The extraordinarily large number of E/E+ and DYW-class PPR proteins in vascular plants can be correlated with large number of RNA editing sites in mitochondria.39 The number of P-class PPR proteins in Physcomitrella is less than half of those of flowering plants. This may reflect certain differences of regulatory processes in organellar gene expression between early land plants and flowering plants. Identification of all target RNA molecules recognized by Physcomitrella PPR proteins and characterization of their functions will provide clues for understanding the basal molecular mechanism of post-transcriptional regulation that evolved in land plant organelles.
Acknowledgments
We thank Prof Volker Knoop and Dr Mereike Rüdinger for communicating their unpublished results. This work was supported by JSPS KAKENHI Grant Number 23657003, 25291059 and by a Research Grant from DAIKO FOUNDATION (Nagoya).
Submitted
02/21/13
Revised
04/10/13
Accepted
04/22/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/24772
References
- 1.Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Toole N, Hattori M, Andres C, Iida K, Lurin C, Schmitz-Linneweber C, et al. On the expansion of the pentatricopeptide repeat gene family in plants. Mol Biol Evol. 2008;25:1120–8. doi: 10.1093/molbev/msn057. [DOI] [PubMed] [Google Scholar]
- 3.Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, dePamphilis C, et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–3. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz-Linneweber C, Small I. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 2008;13:663–70. doi: 10.1016/j.tplants.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Gutmann B, Gobert A, Giegé P. Mitochondrial genome evolution and the emergence of PPR proteins. Adv Bot Res. 2012;63:253–313. doi: 10.1016/B978-0-12-394279-1.00010-7. [DOI] [Google Scholar]
- 6.Cove D, Bezanilla M, Harries P, Quatrano R. Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol. 2006;57:497–520. doi: 10.1146/annurev.arplant.57.032905.105338. [DOI] [PubMed] [Google Scholar]
- 7.Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–9. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 8.Hattori M, Hasebe M, Sugita M. Identification and characterization of cDNAs encoding pentatricopeptide repeat proteins in the basal land plant, the moss Physcomitrella patens. Gene. 2004;343:305–11. doi: 10.1016/j.gene.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Hattori M, Miyake H, Sugita M. A Pentatricopeptide repeat protein is required for RNA processing of clpP Pre-mRNA in moss chloroplasts. J Biol Chem. 2007;282:10773–82. doi: 10.1074/jbc.M608034200. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose M, Tasaki E, Sugita C, Sugita M. A PPR-DYW protein is required for splicing of a group II intron of cox1 pre-mRNA in Physcomitrella patens. Plant J. 2012;70:271–8. doi: 10.1111/j.1365-313X.2011.04869.x. [DOI] [PubMed] [Google Scholar]
- 11.Schween G, Egener T, Fritzowsky D, Granado J, Guitton MC, Hartmann N, et al. Large-scale analysis of 73 329 Physcomitrella plants transformed with different gene disruption libraries: production parameters and mutant phenotypes. Plant Biol (Stuttg) 2005;7:228–37. doi: 10.1055/s-2005-837692. [DOI] [PubMed] [Google Scholar]
- 12.Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–21. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 13.Moreira D, Philippe H. Smr: a bacterial and eukaryotic homologue of the C-terminal region of the MutS2 family. Trends Biochem Sci. 1999;24:298–300. doi: 10.1016/S0968-0004(99)01419-X. [DOI] [PubMed] [Google Scholar]
- 14.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–3. doi: 10.1016/S0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 15.Anantharaman V, Aravind L. The NYN domains: novel predicted RNAses with a PIN domain-like fold. RNA Biol. 2006;3:18–27. doi: 10.4161/rna.3.1.2548. [DOI] [PubMed] [Google Scholar]
- 16.Bryant N, Lloyd J, Sweeney C, Myouga F, Meinke D. Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis. Plant Physiol. 2011;155:1678–89. doi: 10.1104/pp.110.168120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majeran W, Friso G, Asakura Y, Qu X, Huang M, Ponnala L, et al. Nucleoid-enriched proteomes in developing plastids and chloroplasts from maize leaves: a new conceptual framework for nucleoid functions. Plant Physiol. 2012;158:156–89. doi: 10.1104/pp.111.188474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian JO, Braginets R, Schulze WX, Walther D. Characterization and prediction of protein phosphorylation hotspots in Arabidopsis thaliana. Front Plant Sci. 2012;3:207. doi: 10.3389/fpls.2012.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A. A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell. 2006;18:2650–63. doi: 10.1105/tpc.106.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beick S, Schmitz-Linneweber C, Williams-Carrier R, Jensen B, Barkan A. The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol Cell Biol. 2008;28:5337–47. doi: 10.1128/MCB.00563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson X, Wostrikoff K, Finazzi G, Kuras R, Schwarz C, Bujaldon S, et al. MRL1, a conserved Pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in Chlamydomonas and Arabidopsis. Plant Cell. 2010;22:234–48. doi: 10.1105/tpc.109.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushwaha HR, Singh AK, Sopory SK, Singla-Pareek SL, Pareek A. Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics. 2009;10:200. doi: 10.1186/1471-2164-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–9. doi: 10.1126/science.1140516. [DOI] [PubMed] [Google Scholar]
- 24.Pfalz J, Liere K, Kandlbinder A, Dietz KJ, Oelmüller R. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell. 2006;18:176–97. doi: 10.1105/tpc.105.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobert A, Gutmann B, Taschner A, Gössringer M, Holzmann J, Hartmann RK, et al. A single Arabidopsis organellar protein has RNase P activity. Nat Struct Mol Biol. 2010;17:740–4. doi: 10.1038/nsmb.1812. [DOI] [PubMed] [Google Scholar]
- 26.Hattori M, Sugita M. A moss pentatricopeptide repeat protein binds to the 3′ end of plastid clpP pre-mRNA and assists with mRNA maturation. FEBS J. 2009;276:5860–9. doi: 10.1111/j.1742-4658.2009.07267.x. [DOI] [PubMed] [Google Scholar]
- 27.de Longevialle AF, Small ID, Lurin C. Nuclearly encoded splicing factors implicated in RNA splicing in higher plant organelles. Mol Plant. 2010;3:691–705. doi: 10.1093/mp/ssq025. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Yu F, Rodermel S. An Arabidopsis pentatricopeptide repeat protein, SUPPRESSOR OF VARIEGATION7, is required for FtsH-mediated chloroplast biogenesis. Plant Physiol. 2010;154:1588–601. doi: 10.1104/pp.110.164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knoop V, Rüdinger M. DYW-type PPR proteins in a heterolobosean protist: plant RNA editing factors involved in an ancient horizontal gene transfer? FEBS Lett. 2010;584:4287–91. doi: 10.1016/j.febslet.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Rüdinger M, Szövényi P, Rensing SA, Knoop V. Assigning DYW-type PPR proteins to RNA editing sites in the funariid mosses Physcomitrella patens and Funaria hygrometrica. Plant J. 2011;67:370–80. doi: 10.1111/j.1365-313X.2011.04600.x. [DOI] [PubMed] [Google Scholar]
- 31.Rüdinger M, Polsakiewicz M, Knoop V. Organellar RNA editing and plant-specific extensions of pentatricopeptide repeat proteins in jungermanniid but not in marchantiid liverworts. Mol Biol Evol. 2008;25:1405–14. doi: 10.1093/molbev/msn084. [DOI] [PubMed] [Google Scholar]
- 32.Rüdinger M, Funk HT, Rensing SA, Maier UG, Knoop V. RNA editing: only eleven sites are present in the Physcomitrella patens mitochondrial transcriptome and a universal nomenclature proposal. Mol Genet Genomics. 2009;281:473–81. doi: 10.1007/s00438-009-0424-z. [DOI] [PubMed] [Google Scholar]
- 33.Tasaki E, Hattori M, Sugita M. The moss pentatricopeptide repeat protein with a DYW domain is responsible for RNA editing of mitochondrial ccmFc transcript. Plant J. 2010;62:560–70. doi: 10.1111/j.1365-313X.2010.04175.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani S, Ichinose M, Tasaki E, Aoki Y, Komura Y, Sugita M. Targeted gene disruption identifies three PPR-DYW proteins involved in RNA editing for five editing sites of the moss mitochondrial transcripts. Plant Cell Physiol. 2010;51:1942–9. doi: 10.1093/pcp/pcq142. [DOI] [PubMed] [Google Scholar]
- 35.Uchida M, Ohtani S, Ichinose M, Sugita C, Sugita M. The PPR-DYW proteins are required for RNA editing of rps14, cox1 and nad5 transcripts in Physcomitrella patens mitochondria. FEBS Lett. 2011;585:2367–71. doi: 10.1016/j.febslet.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Tasaki E, Sugita M. The moss Physcomitrella patens, a model plant for the study of RNA editing in plant organelles. Plant Signal Behav. 2010;5:727–9. doi: 10.4161/psb.5.6.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes ML, Giang K, Mulligan RM. Molecular evolution of pentatricopeptide repeat genes reveals truncation in species lacking an editing target and structural domains under distinct selective pressures. BMC Evol Biol. 2012;12:66. doi: 10.1186/1471-2148-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammani K, Okuda K, Tanz SK, Chateigner-Boutin A-L, Shikanai T, Small I. A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell. 2009;21:3686–99. doi: 10.1105/tpc.109.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 2011;191:37–47. doi: 10.1111/j.1469-8137.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 40.Boussardon C, Salone V, Avon A, Berthomé R, Hammani K, Okuda K, et al. Two interacting proteins are necessary for the editing of the NdhD-1 site in Arabidopsis plastids. Plant Cell. 2012;24:3684–94. doi: 10.1105/tpc.112.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takenaka M, Zehrmann A, Verbitskiy D, Kugelmann M, Härtel B, Brennicke A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc Natl Acad Sci USA. 2012;109:5104–9. doi: 10.1073/pnas.1202452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun T, Germain A, Giloteaux L, Hammani K, Barkan A, Hanson MR, et al. An RNA recognition motif-containing protein is required for plastid RNA editing in Arabidopsis and maize. Proc Natl Acad Sci USA. 2013;110:E1169–78. doi: 10.1073/pnas.1220162110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyata Y, Sugiura C, Kobayashi Y, Hagiwara M, Sugita M. Chloroplast ribosomal S14 protein transcript is edited to create a translation initiation codon in the moss Physcomitrella patens. Biochim Biophys Acta. 2002;1576:346–9. doi: 10.1016/S0167-4781(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 44.Miyata Y, Sugita M. Tissue- and stage-specific RNA editing of rps 14 transcripts in moss (Physcomitrella patens) chloroplasts. J Plant Physiol. 2004;161:113–5. doi: 10.1078/0176-1617-01220. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 2003;36:541–9. doi: 10.1046/j.1365-313X.2003.01900.x. [DOI] [PubMed] [Google Scholar]
- 46.Salone V, Rüdinger M, Polsakiewicz M, Hoffmann B, Groth-Malonek M, Szurek B, et al. A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett. 2007;581:4132–8. doi: 10.1016/j.febslet.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Sugita M. A conserved DYW domain of the pentatricopeptide repeat protein possesses a novel endoribonuclease activity. FEBS Lett. 2008;582:4163–8. doi: 10.1016/j.febslet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Okuda K, Chateigner-Boutin AL, Nakamura T, Delannoy E, Sugita M, Myouga F, et al. Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell. 2009;21:146–56. doi: 10.1105/tpc.108.064667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathews FS. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45:1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 50.Sugiura C, Kobayashi Y, Aoki S, Sugita C, Sugita M. Complete chloroplast DNA sequence of the moss Physcomitrella patens: evidence for the loss and relocation of rpoA from the chloroplast to the nucleus. Nucleic Acids Res. 2003;31:5324–31. doi: 10.1093/nar/gkg726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terasawa K, Odahara M, Kabeya Y, Kikugawa T, Sekine Y, Fujiwara M, et al. The mitochondrial genome of the moss Physcomitrella patens sheds new light on mitochondrial evolution in land plants. Mol Biol Evol. 2007;24:699–709. doi: 10.1093/molbev/msl198. [DOI] [PubMed] [Google Scholar]