Abstract
A fast growing number of studies identify pentatricopeptide repeat (PPR) proteins as major players in gene expression processes. Among them, a subset of PPR proteins called PRORP possesses RNase P activity in several eukaryotes, both in nuclei and organelles. RNase P is the endonucleolytic activity that removes 5′ leader sequences from tRNA precursors and is thus essential for translation. Before the characterization of PRORP, RNase P enzymes were thought to occur universally as ribonucleoproteins, although some evidence implied that some eukaryotes or cellular compartments did not use RNA for RNase P activity. The characterization of PRORP reveals a two-domain enzyme, with an N-terminal domain containing multiple PPR motifs and assumed to achieve target specificity and a C-terminal domain holding catalytic activity. The nature of PRORP interactions with tRNAs suggests that ribonucleoprotein and protein-only RNase P enzymes share a similar substrate binding process.
Keywords: RNase P, pentatricopeptide repeat, tRNA maturation, RNA binding protein, evolution
Introduction
Pentatricopeptide repeat (PPR) proteins compose a family of RNA binding proteins specific to eukaryotes and mostly involved in gene expression processes in organelles. PPR proteins are particularly numerous in land plants with up to 450 representatives in Arabidopsis thaliana.1 They are composed of tandem arrays of PPR motifs whose primary sequence is very degenerate,2,3 although their tertiary structure seems to be conserved, with each repeat folding into two antiparallel α helices.4-6 A succession of PPR motifs would thus make a superhelix that could act as a platform to bind RNA.2 The combinatorial nature of PPR proteins allows substrate specificity because individual PPR motifs appear to ensure the selection for individual nucleotides.6,7 Since their discovery over a decade ago, functional studies of PPR proteins have helped to answer many persistent questions regarding organellar gene expression processes.1 For example, studies are beginning to unravel how sequence specificity is achieved for hundreds of C to U RNA editing sites in transcripts from higher plant organelles.8 The characterization of PPR proteins has also helped to settle the long-standing debate over the existence of protein-only RNase P enzymes in eukaryotes.9
RNase P is a key enzyme of tRNA maturation. It was initially described as the endonuclease activity that removes the 5′ leader sequences of tRNA precursors. It is therefore essential for producing functional tRNAs and, hence, indispensable for translation.10,11 RNase P was first characterized on a molecular level in Escherichia coli, where it is composed of an RNA molecule together with a single protein.12 The discovery that RNase P RNA held the actual catalytic activity of the enzyme13 won Sidney Altman the Nobel prize in 1989 and helped to establish the “RNA world” theory proposing that one stage in prebiotic evolution consisted of RNA molecules that were able both to catalyze biochemical reactions and to store genetic information.14 Subsequently, RNase P enzymes were characterized as similar ribonucleoprotein (RNP) enzymes in numerous other organisms and organelles including bacteria, archaea, yeast nuclei and mitochondria and animal cell nuclei.15,16 Isolated RNA subunits from Bacteria, Archaea and Eukarya demonstrate catalytic activity only under extreme ionic conditions, whereas the corresponding RNA•protein holoenzymes are maximally active under physiological conditions.13 Apart from tRNAs, RNP RNase P enzymes are involved in the maturation of a wide array of substrates including rRNAs, protein-coding mRNAs, tmRNA, riboswitches, viral RNA and snoRNA.10,16,17 From a mechanistic point of view, RNP RNases P interact with tRNA mainly in the horizontal stacking domain consisting of the T stem-loop and acceptor stem; they utilize two catalytic metal ions and conserved RNA residues for RNA cleavage.18,19 The structures of RNase P enzymes differ greatly, each containing an RNA molecule (whose structure is considerably reduced in size in some instances20) bound by a variable number of protein subunits ranging from one in bacteria to at least nine in eukaryotes.10,21-23 Still, the central point remained that all RNase Ps contained an RNA moiety responsible for catalytic activity, so that the ribonucleoprotein nature of RNase P became a dogma. RNase P, together with the ribosome, was viewed as one of the ultimate universally conserved vestiges of the RNA world.15
Nevertheless, long before the discovery of the PPR protein family, some experimental evidence contradicted the prevailing dogma and suggested that some eukaryotes could use a different kind of enzyme, devoid of RNA, for RNase P activity. Here we review both the early evidence for the existence of protein-only RNase P and the studies describing the actual identification and characterization on a molecular level of the proteinaceous RNase P enzymes belonging to the PPR family.
Early evidence for the existence of protein-only RNase P
Origins and expectations
The earliest reports of protein-only RNase P came from eukaryotic organelles—chloroplasts and mitochondria—that typically encode some or, in plant chloroplasts and vertebrate mitochondria, all of the tRNAs needed for translation of organellar-encoded proteins. In animals, mitochondrial tRNA genes are interspersed among protein-coding genes, such that production of functional mRNA species requires excision of mature tRNAs by precise 5′- and 3′-terminal endonucleolytic cleavages.24 In chloroplasts, most tRNA genes are transcribed into end-extended precursors bearing 5′- and 3′-terminal extensions that must be removed to yield mature tRNA.25
The earliest expectations for the nature of RNase P from these organelles were based on their established bacterial origins:26 mitochondria descended from the α-proteobacteria27 and chloroplasts arose from within the cyanobacteria.28 Members of both bacterial phyla possess “conventional” (E. coli-like) ribonucleoprotein forms of RNase P. In particular, bacterial-like RNase P RNA has been identified in all sequenced red algae chloroplasts and in many green algae in the Prasinophyte lineage.10 For example, the cyanelle of the alga Cyanophora paradoxa encodes a homolog of cyanobacterial RNase P RNA.29 This RNA alone exhibits weak catalytic activity at high salt concentrations, but can be restored to activity under physiological conditions by assembly with a cyanobacterial protein subunit30 (The equivalent protein subunit in C. paradoxa is presumably nuclear-encoded and imported into the cyanelle.) Likewise, the mitochondrion of the early-branching protozoan Reclinomonas americana encodes a proteobacterial-type RNase P RNA31 that is dependent upon a proteobacterial protein subunit for activity.32
Initial evidence for an RNA component
Early support for a bacterial-like composition of mitochondrial RNase P was provided by genetic and biochemical determinations that in mitochondria of budding yeast (Saccharomyces cerevisiae), RNase P contained an essential, mitochondrial encoded, RNA distantly related to the RNA subunit of bacterial RNase P33 and a nuclear-encoded protein unrelated to the bacterial protein subunit.34
The earliest characterizations of a putative vertebrate mitochondrial RNase P (from rat liver35 or human cells36), did not directly test for the presence of an RNA component. Further efforts by one group, however, led to a claim that human mitochondrial RNase P activity could be attributed entirely to a small amount of nuclear RNase P imported into mitochondria.37 Because these investigations employed a precursor to E. coli tRNATyrsuIII—a substrate for nuclear but not for vertebrate mitochondrial RNase P38—the enzyme described is now thought to be the abundant nuclear RNase P present in the starting cytosolic extracts.35,39
A critical assay: The substrate unmasked
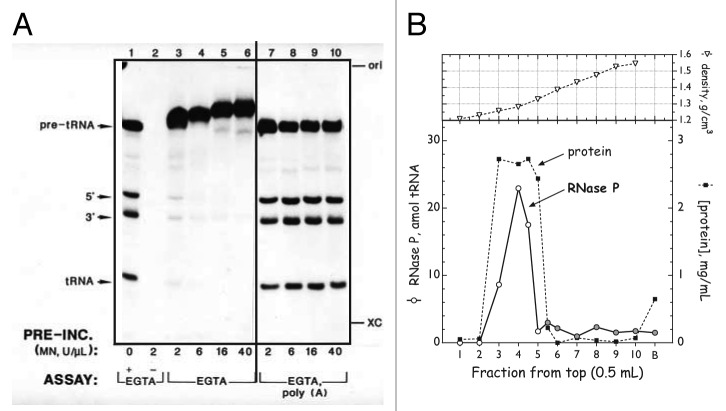
Meanwhile, in the plant kingdom, transcription and processing of chloroplast tRNAs had been demonstrated in 1983 by Gruissem and Hallick.25 Further investigation of chloroplast RNase P was initiated with the expectation that it, too, would resemble the bacterial enzyme. Preliminary evidence accumulated by 1986–87 suggested that crude preparations of RNase P from both spinach and tobacco chloroplasts were sensitive to treatment both with protease and with nuclease, consistent with activity residing in an RNA•protein complex (ref. 40 and Wang et al., poster presentation, 1986 Cold Spring Harbor RNA Processing Meeting). An apparent inhibition of chloroplast RNase P by S. aureus micrococcal nuclease (MN) is shown in Figure 1A, lanes 3–6.
Figure 1. Plant RNase Ps do not contain an RNA component. (A) Resistance of spinach chloroplast RNase P to digestion with micrococcal nuclease (MN). Crude enzyme fraction was incubated with the indicated amounts of MN plus 5 mM CaCl2 (30 min at 37°C) after which excess EGTA was added, followed by substrate and reaction buffer. Lane 1, positive control for RNase P (pre-incubated without MN); lane 2, positive control for MN (as lane 1 but MN not inactivated prior to addition of substrate); lanes 3–6, pre-incubated with 2‒40 U MN/μl and treated with EGTA prior to assay; lanes 7–10, as lanes 3–6 with addition of 1 μg poly(A)/μl prior to assay. Modified from reference 44. (Essentially identical results were obtained with wheat nuclear RNase P.48) (B) Buoyant density of spinach chloroplast RNase P.44 Fraction II chloroplast enzyme (~5 mg) was pretreated with MN (1 U/μl, 20 min; terminated with EGTA), brought up to 1.0 ml with gradient buffer, and layered over 4.0 ml of CsCl solution (1.40 g/ml). After centrifugation to equilibrium, fractions were collected from the top and density was determined by refractometry. CsCl was removed by dialysis and fractions were assayed for RNase P. Lower panel, distribution across the gradient of total protein (filled squares) and RNase P activity (open circles: amol mature tRNA formed; shaded circles: non-tRNA-sized material). Upper panel, observed buoyant density of each fraction.
At the time, three primary criteria were used to confirm the presence of an essential RNA component in an RNA processing activity: (1) sensitivity to pre-treatment with nucleases having little or no specificity for RNA sequence or structure, (2) buoyant density in Cs salts and (3) presence of co-fractionating RNA species of appropriate size (150–400 nucleotide length). In the most common nuclease sensitivity protocol, an enzyme fraction is incubated with micrococcal nuclease (MN) in presence of its catalytic cofactor Ca2+. The nuclease is then inactivated by addition of EGTA, which chelates most divalent cations much more strongly than it does Mg2+, a required cofactor for all RNase Ps. Remaining RNase P activity is then assayed by addition of substrate directly to the treated enzyme fraction. All three assays are, however, susceptible to artifacts or misinterpretation. In particular, nuclease treatment is complicated by the fact that most suitable nucleases are difficult to inhibit cleanly, but residual activity will destroy the reaction substrate. EGTA-inactivated MN often displayed some inhibition of RNA processing.40,41 Moreover, inhibition by active MN of non-RNA-containing enzymes had been observed (e.g., refs. 40 and 42). This was interpreted as resulting from degradation of bulk RNA, present in a crude extract, which was thought to stabilize the RNA processing complex under investigation.42,43
In order to conclusively show whether MN treatment was specifically inactivating chloroplast RNase P, Wang et al. asked whether MN-inhibited RNase P activity could be recovered by addition of non-specific RNA. The dramatic result, as shown in Figure 1A, lanes 7–10, was that addition of yeast RNA or of synthetic polynucleotides completely reversed the apparent inhibition by MN.44 Further work41 showed, not surprisingly, that Ca2+-depleted MN retains substrate binding ability, reversibly binding pre-tRNA with an apparent Kd of 1.35 μM. Polyanions such as heparin or synthetic polynucleotides compete with pre-tRNA for binding MN. The final picture is that binding of catalytically inactive MN to RNA substrate sterically blocks access to the cleavage site. Addition of excess non-specific RNA sequesters the inactive nuclease and frees the pre-tRNA substrate for productive cleavage by the processing enzyme. This phenomenon is referred to as “substrate occlusion” or “substrate masking.”41
Chloroplasts
With a reliable assay in hand, progress was rapid, and Wang et al. determined that chloroplast RNase P is completely insensitive to digestion with concentrations of micrococcal nuclease 20- to 50-fold greater than those required to inactivate E. coli RNase P.44 Furthermore, as shown in Figure 1B, the chloroplast activity has a buoyant density in CsCl (1.28 g/cm3) that is precisely centered within the density distribution of bulk protein.44 In this context, it is essential to note that because observed buoyant densities are a function of the density medium, and for values determined by refractometry, are also influenced by solvent composition, they cannot be directly compared between experiments. (In CsCl gradients, buoyant densities for pure protein, E. coli RNase P, and pure RNA are 1.28, 1.7 and ~2.0 g/cm3; in Cs2SO4 these are 1.23, 1.55 and 1.65 g/cm3.43) The most stringent test for presence or absence of an RNA is the extent to which enzyme activity co-fractionates with bulk protein or with a known protein-only enzyme. The coincidence of protein and enzyme densities for plant chloroplast and human mitochondrial RNase P indicates that neither enzyme could possess more than one copy of a 10- to 20-nt long RNA.44,45
Mechanistic differences between the chloroplast enzyme and the ribozyme-type RNase P affirmed that the chloroplast enzyme could not have an RNA subunit like that of bacterial or yeast nuclear RNase P46 (discussed in detail in the section on structural mimicry). Further studies of the 1,000‒2,000-fold purified chloroplast activity indicated that it does not co-purify with any RNAs that can be 3′-end labeled,47 and that its hydrodynamic size, determined by gel filtration corresponds to a ~70 kDa globular protein.47
Plant nuclei
Knowing that most soluble plastid proteins are encoded in the nucleus, translated in the cytoplasm, and imported into the organelle, Wang et al.44 suggested that chloroplast RNase P or related polypeptides could have been recruited to process pre-tRNAs encoded in the nucleus and mitochondrion. To investigate this possibility, Oommen48 used the techniques successful for chloroplasts to demonstrate that wheat embryo extracts contained an authentic RNase P activity with properties essentially identical to those of chloroplast RNase P. (The reaction requirements and substrate specificity of this activity [ref. 48 and unpublished observations] suggested that it was localized to the nucleus). This activity is resistant to amounts of micrococcal nuclease at least 5-fold greater than required to fully inactivate E. coli RNase P. In CsCl gradients, the distribution of wheat RNase P activity is absolutely coincident with the distribution of bulk protein (1.28–1.29 g/cm3).48 Active fractions across the final ion-exchange column contained no RNA molecules whose abundance was correlated with RNase P activity; trace RNAs larger than tRNA present in the active fractions could be removed without reducing RNase P activity. Finally, gel filtration chromatography in the absence of urea indicated a hydrodynamic size corresponding to a ~120 kDa globular protein or protein complex.
Somewhat later, another group presented essentially identical results: a buoyant density identical with bulk protein and complete resistance to MN treatment.49 On the basis of its reaction requirements, this activity could be identified with nuclear rather than mitochondrial RNase P. At the time, these data were interpreted as consistent with wheat nuclear RNase P containing an RNA subunit associated with a large number of proteins that conferred a protein-like buoyant density and protected the RNA from nuclease attack.49 Other researchers separated two RNase P activities, possibly nuclear and mitochondrial, from carrot cell suspension culture.50 Presence or absence of RNA components was not established: buoyant densities were not determined and results of MN treatment were inconclusive because controls for substrate masking were not included and reaction products were not characterized. Of the two activities, one was inhibited only partially by a 10-fold excess of MN; the second was completely inhibited by either active or inactive MN at all concentrations tested, indicative of unresolved substrate masking.
Plant mitochondria
In 1990, two groups reported processing in vitro of plant mitochondrial pre-tRNAs with homologous mitochondrial extracts. Marchfelder et al. showed that RNase P-like activity in Oenothera mitochondrial lysates was completely inhibited by either inactive or active MN when assayed in the absence of poly(A),51 consistent with substrate masking. Hanic-Joyce and Gray, on the other hand, stated that the activity in wheat mitochondria was insensitive to MN digestion when assayed in the presence of poly(A).52 In the absence of further physical characterization, these observations, though intriguing, were not seen as compelling.
Human mitochondria
The first purification of an authentic mitochondrial RNase P from vertebrates was reported by Rossmanith and colleagues38 in 1995. Using a fully homologous system with a mitochondrial-specific substrate, they achieved a clean separation of human mitochondrial RNase P from the nuclear enzyme, which was by then known to be an RNA•protein complex.53 Using an approach similar to that of Wang et al.,44 Rossmanith then made a rigorous finding that the mitochondrial enzyme consisted entirely of protein.45 First, activity was fully resistant to digestion with a 10-fold excess of MN. Second, in Cs2SO4 gradients, the buoyant density of RNase P activity (1.23 g/cm3) was well within the distribution of bulk protein and was identical with the density of pre-tRNA 3′endonuclease, a known protein enzyme. The mitochondrial activity was cleanly separated from E. coli RNase P, which pelleted at the bottom of the gradient (density > 1.45 g/cm3).45 Third, the most highly-purified enzyme contained only RNAs of tRNA size and smaller, which could be degraded by MN treatment without affecting enzyme activity. Fourth, the mass of human mitochondrial RNase P, determined by rate zonal sedimentation, was about 170 kDa, substantially smaller than the smallest known RNA-containing RNase P. Additionally, since mitochondrial-specific RNase P could be isolated from mitochondrial mutants completely lacking mtDNA,45 the mitochondrial enzyme was definitely encoded in the nucleus and imported into the organelle.
Kinetoplastid mitochondria
Mitochondria of the kinetoplastid parasite Trypanosoma brucei encode no tRNAs. Instead, all tRNAs are encoded in the nucleus and imported into the mitochondrion. Although it is uncertain whether any tRNAs are imported as 5′-extended precursors, it is known that kinetoplastid mitochondria do possess an active RNase P. In 2001, Salavati used the “masking-free” MN assay44 to demonstrate that highly-purified T. brucei RNase P was unaffected by digestion with a 10-fold excess of MN.54 Some RNAs larger than tRNA were present in active fractions but could be degraded without effect on RNase P activity. Notably, the hydrodynamic size estimated by gel filtration chromatography was about 70 kDa, the same size as chloroplast RNase P. In the absence of buoyant density or mechanistic data, however, these results were not considered definitive.
Hindsight
In retrospect, the ability to recognize the existence of protein-only RNase Ps was hindered by (1) justifiable expectations that organelles would have bacterial-type RNase P, most likely containing an organelle-encoded RNA subunit and an imported, nuclear-encoded polypeptide; (2) knowledge that yeast mitochondrial RNase P conformed to this model; (3) indications that RNase P in C. paradoxa cyanelles and R. americana mitochondria would follow the bacterial paradigm; and (4) evidence that yeast and human nuclear RNase Ps contained an RNA subunit related to the bacterial prototype. On the other hand, there was no obvious reason to doubt the validity of experimental work supporting a protein-only composition for RNase P in animal mitochondria, plant chloroplasts, or plant nuclei, nor was there convincing experimental evidence supporting other interpretations. Nevertheless, these conclusions remained controversial until isolated polypeptides, overexpressed from cloned cDNAs corresponding to defined genetic loci, were shown to possess RNase P activity.
Identification at the molecular level of protein-only RNase P
Characterization of the RNase P enzyme in human mitochondria
The concept of protein-only RNase P was definitely accepted only when the core components responsible for RNase P activity in human mitochondria were identified at the molecular level.55 In that study, Rossmanith and coworkers confirmed that this RNase P activity did not require any RNA component. Using an elegant approach combining proteomic identification of human mitochondrial RNase P (mtRNase P) complexes, in vitro mtRNase P activity assay and reverse genetics, the authors’ work led to the conclusion that only three individual polypeptide subunits were strictly required for the reconstitution of mtRNase P activity and that their mode of action was concerted. These three polypeptides composing the mtRNase P holoenzyme are nuclear-encoded and were named respectively MRPP1, 2 and 3 (for Mitochondrial RNase P Proteins). MRPP1 (or TRMT10C) encodes a putative tRNA:m1G9-methyltransferase whereas MRPP2 (or SDR5C1) encodes a 3-hydroxyacyl-CoA dehydrogenase and MRPP3 encodes a protein containing a metallonuclease domain as well as a PPR domain.9,55
MRPP1 catalyzes the methylation of specific bases (G9 or A9) in mitochondrial tRNAs and interacts with tRNAs in vitro,56 although its methyltransferase activity is not required for tRNA cleavage by the mtRNase P holoenzyme.56 Little is known about the involvement of MRPP2 in mtRNase P activity. Binding to MRPP2 is critical for MRPP1 to perform mitochondrial tRNA methylation, although MRPP2’s dehydrogenase activity seems to be dispensable.56 Reciprocally, although MRPP1 and MRPP2 are essential components of the mtRNase P holoenzyme, neither the methyltransferase nor the dehydrogenase activity, respectively, is required for tRNA processing.56
MRPP3 was the only identified subunit of mtRNase P harboring a predicted nuclease domain. Hence, it was hypothesized from the start that the involvement of MRPP3 in mtRNase P activity would be to perform the actual phosphodiester bond hydrolysis.55 MRPP3 also features PPR motifs. These elements are helical-repeat motifs considered to bind with specificity to single-stranded RNA stretches; they are found in eukaryotic proteins, predominantly those involved in organellar RNA metabolism.1,2,57 Even though the precise role of MRPP3′s PPR motifs in mtRNase P is still unexplored, a tempting proposal is that these repeats contribute to tRNA binding and/or confer base-specific recognition of tRNAs.
Apart from the protein-only RNase P, it was also proposed that RNase P RNA could be imported into human mitochondria, thus leading to the potential cohabitation of both RNP and protein-only RNase P in this organelle.58 The occurrence of RNase P RNA in human mitochondria remains controversial and has been discussed in detail by Rossmanith in 2012.59
The catalytic subunit of protein-only RNase P is the PPR protein
While the three polypeptides that compose the human mitochondrial RNase P enzyme have some RNA-binding potential, only MRPP3 possesses the features of a metallonuclease that could account for the catalytic activity of RNase P. MRPP3 orthologs could be identified in many eukaryotic organisms and define a new protein family that was named PRORP (for PROteinaceous RNase P). Hence, MRPP3 is now also called human PRORP. These proteins are characterized by the presence of a number of PPR and/or PPR-like motifs, a CXXC Zn finger-like motif and a metallonuclease domain belonging to the NYN family.60 The function of putative PRORPs identified by sequence similarities has been explored in depth in Arabidopsis61,62 and in the protist T. brucei.63 Data are also available for Ostreococcus tauri, a primitive unicellular green alga.64 Arabidopsis expresses three PRORP proteins: At-PRORP1 is a 62 kDa protein with a pI of 9 and is localized to both plastids and mitochondria, whereas At-PRORP2 and At-PRORP3 are 59 kDa proteins, with pI of 6 and are localized in the nucleus.61,62 RNase P catalytic activity was first assigned unequivocally to the single protein At-PRORP1 in Arabidopsis organelles61 and later to each of the nuclear proteins on its own.62 In vitro RNase P activity tests using homologous pre-tRNA substrates were performed with purified recombinant forms of the three Arabidopsis RNase P protein candidates, each carefully verified for the absence of contamination by E. coli RNase P.61,62 Precise mapping of the cleavage site was achieved by high-resolution urea-PAGE or circular RT-PCR, and characterization of the 5′ nucleotide of the mature tRNA products showed that each PRORP is a tRNA-specific endonuclease removing 5′ extensions from pre-tRNAs and leaving a phosphate group at the 5′ end of mature tRNAs. Abolition of the RNase P activity of recombinant PRORPs mutated in two conserved aspartates (predicted to be part of the catalytic site) confirmed that each of the three Arabidopsis PRORPs possessed RNase P activity as a single polypeptide.61,62
Two PRORP genes were identified in the fully sequenced trypanosomatid genomes. In Trypanosoma brucei, PRORP1 is localized to the nucleus and PRORP2 to the mitochondrion.63 Using in vitro cleavage assays with purified recombinant proteins, each T. brucei PRORP protein appeared to perform the canonical 5′ tRNA maturation on its own, similar to Arabidopsis PRORPs.63 Although studied to a lesser extent, a recombinant PRORP from the green algae O. tauri is capable of pre-tRNA 5′ processing in vitro.64 The RNase P activity of these eukaryotic PRORP proteins from distant organisms is most likely shared by other members of this family. The association of a nuclease domain with a PPR domain to create RNase P enzymes represents yet another example of the potential and diversity of functions (i.e., RNA editing, splicing or translation1) acquired by the family of PPR proteins.
Beyond the capacity to perform RNase P activity in vitro, an important testimonial to the generality of PRORP tRNA processing capability came with the observation that Arabidopsis organellar PRORP1 and Trypanosoma nuclear PRORP1 could replace, in vivo, the E. coli and yeast nuclear RNase P respectively.61,63 Wild-type At-PRORP1, but not a protein mutated in the two conserved catalytic aspartates, rescues the lethal knockdown of RNase P RNA in E. coli. Similarly, T. brucei nuclear PRORP1 can rescue a deletion of the RNA component of yeast nuclear RNase P. These heterologous complementations led to the remarkable result that a single polypeptide can substitute in vivo for a complex ribonucleoprotein structure. Still, PRORP might not be the exact functional equivalent of RNP RNase P as fitness differences were observed between yeast strains non complemented and complemented by PRORP.63 Similarly, kinetic studies reveal that specificity constants of PRORP are not equivalent, i.e., they are lower than that of RNP RNase P.62,65
Further experiments explored the in vivo roles of the three Arabidopsis PRORPs. The lethality of a single-gene knockout of At-PRORP1 and of the double knockout of At-PRORP2 and At-PRORP3 indicate that both the organellar and the nuclear PRORP enzymes fulfill essential functions in vivo, as expected for the authentic RNase P in cellular compartments encoding tRNA.61,62 The role of At-PRORP in tRNA 5′ maturation in both organelles and the nucleus in planta was further explored by downregulation using virus-induced gene silencing.62 A decrease in PRORP1 specifically affects internal structures of chloroplast and mitochondria and reduces the level of mature organellar tRNAs, while nuclear-encoded tRNA levels are unchanged. Conversely, downregulation of PRORP2 in a prorp3 knockout background has no effect on organellar tRNAs, while the level of nuclear-encoded processed tRNA is reduced compared with control plants. Since downregulation of each PRORP protein causes a reduction of RNase P activity in the cellular compartment where that protein is found, it can be concluded that each PRORP protein is required for processing the tRNA pool in its respective compartment. On the other hand, downregulation of POP1 and POP4, two essential protein components of RNase MRP (a ribonucleoprotein related to the nuclear RNP RNase Ps and involved in cytosolic rRNA maturation) affected rRNA maturation but did not reduce nuclear tRNA levels.62 Altogether, these results are consistent with PRORP proteins being the sole source of RNase P activity in both organelles and the nucleus of plants.
A report by Krehan, et al. has shown that RNase P activity as well as RNase MRP RNA are present in a wheat embryo extract immune-precipitated with POP1 antibodies.66 This result has been interpreted as a clue for the presence of an RNP RNase P enzyme in plant nuclei.67 Since the downregulation of POP1 in planta resulted in decreased RNase MRP activity and did not affect RNase P activity,62 we believe that the results instead reflect the presence of both PRORP and RNase MRP in the immunoprecipitated fraction, i.e., that the two enzymes might be present in a single complex in planta as also proposed by Krehan, et al.66
In Trypanosoma, PRORP activity was analyzed after immunodepletion, with anti-PRORP antibodies, of RNase P activity in a whole-cell extract. Depletion of both nuclear PRORP1 and mitochondrial PRORP2 abolishes all activity, suggesting that T. brucei contains no other RNase P.63 More studies are required, however, to understand the function of T. brucei PRORPs in vivo. Since a complete set of tRNAs is imported from the cytosol into mitochondria in Trypanosoma, it will be particularly interesting to identify the substrates of the mitochondrial PRORP2 in vivo.
Collectively, experimental data obtained in distantly-related eukaryotes has clearly established that RNase P activity can reside in a single polypeptide. Moreover, in plants and Trypanosomes, PRORP proteins provide RNase P activity in vivo in both organelles and in the nucleus.
The substrate spectrum of PRORP, like that of RNP RNase Ps, goes beyond tRNAs
RNase P was first defined as the activity performing the 5′ maturation of tRNA precursors. Still, extensive analyses of ribonucleoprotein RNase P functions have revealed that RNase P can be involved in the maturation of a much wider variety of substrates in both prokaryotes and eukaryotes.10 After finding that PRORP proteins could perform the 5′ maturation of tRNA precursors in Arabidopsis, Trypanosoma and Ostreococcus,61,63,64 it was logical to investigate whether PRORP proteins are entirely tRNA-specific or whether they, like RNP RNase Ps, are involved in the maturation of other substrates.
The assumption that PRORP enzymes might be involved in the maturation of other RNAs is supported by the fact that numerous tRNA-derived sequences or structures are present in plant genomes. For instance, tRNA-like sequences called “t-elements” are present in transcripts of plant mitochondrial DNA, where they separate individual mRNAs.68 Similarly, in the nucleus, SINE RNAs are derived from tRNAs, although their canonical cloverleaf structure has apparently been lost.69 Another argument comes from the observation that Arabidopsis PRORP1 can replace E. coli RNP RNase P in vivo.61 Bacterial RNP RNase P is responsible for the maturation of many non-tRNA substrates, including the precursor to the 4.5S RNA.70 Two substrates that contain tRNA-like recognition elements are the precursor to C4 antisense RNA of bacteriophage P1 and P7, which possesses a tRNA-like structure with short D- and T-loops;71 and the precursor to tmRNA, part of whose structure resembles the horizontal stacking domain (acceptor stem plus T-stem and loop) of tRNAAla,72 a known minimal substrate for E. coli RNase P.73 E. coli RNase P is also involved in processing polycistronic mRNAs such as the histidine operon transcript,74,75 and in cleavage of some riboswitches, including those for the coenzyme B12.17 It can thus be speculated that Arabidopsis PRORP1 could catalyze the maturation of all these E. coli non-tRNA substrates. Alternatively, it is possible that some of these non-tRNA maturation steps are not essential or that they can be rescued by other enzymatic systems in the absence of ribonucleoprotein RNase P.
Preliminary results, both in vitro and in vivo, have confirmed that Arabidopsis PRORPs are indeed involved in the maturation of other RNA substrates. In particular, PRORP1 is able to perform in vitro the endonucleolytic cleavage of tRNA-like t-elements present in the mitochondrial transcripts of Arabidopsis nad6 and Brassica napus orf138,61 and PRORP1 activity is required in vivo to accumulate nad6 mRNA.62
Similarly, Arabidopsis PRORP2 and 3 are indirectly involved in the maturation of snoRNA.62 In Arabidopsis, a dicistronic precursor to tRNAGly and the snoRNA snoR43.1 is processed by both RNase P and the pre-tRNA 3′-processing endonuclease RNase Z, with RNase P cleavage of the pre-tRNAGly portion being a prerequisite for the cleavage by RNase Z that separates mature tRNAGly from mature snoR43.1.76 In PRORP downregulation mutants, snoR43 failed to accumulate to normal levels whereas tRNA-snoRNA precursor levels increased, showing that nuclear PRORP activity is required for the accumulation of this snoRNA.62
An initial investigation of the PRORP/tRNA complex has revealed that minimal tRNA structural features are required for recognition by PRORP alone. For example, and like the bacterial RNP RNase P, the tRNA acceptor stem is essential whereas the anticodon domain is not. Unlike the bacterial enzyme, PRORP cleavage is impaired by the absence of the D domain from tRNAs or t-elements.61,77 Thus, maturation of mitochondrial mRNAs by cleavage of some t-elements (such as the one from ccmC mRNA that lacks both D and anticodon domains68) might require additional proteins acting as PRORP partners to recognize these structures. Similarly, in humans, the requirement for MRPP1 and 2 might reflect an inability of Hs-PRORP alone to bind the non-canonical tRNA structures characteristic of vertebrate mitochondria.55 This would also explain why the plant PRORP1 can function in both chloroplasts and mitochondria, since tRNAs from plant chloroplasts and mitochondria closely resemble bacterial tRNAs.
The diversity of substrates identified so far for PRORP remains limited. Other potential RNA substrates will have to be investigated at the transcriptome-wide level, for example, through comparative transcriptome analyses of PRORP downregulation mutants or by global sequencing of RNA partners immune-precipitated in complex with PRORP proteins.
Emergence and distribution of PRORP enzymes in eukaryotes
RNase P is a ubiquitous enzyme, found in all organisms with the exceptions of symbiotic Archaea, such as Nanoarchea equitans, several species of Pyrobaculum and Aquifex aeolicus in which transcription of tRNAs starts at position +1.78 The RNP form of RNase P is widespread as it is present in Bacteria, in Archaea and in Eukarya with characterized activities in both the nucleus and mitochondria (as, for example, in yeast).10 On the contrary, identified PRORP RNase Ps are limited to eukaryotes (Fig. 2), having been identified in human mitochondria,55 Arabidopsis thaliana mitochondria, chloroplasts and nuclei61,62 and in Trypanosoma brucei mitochondria and nuclei.63 In the green alga Ostreococcus tauri, a PRORP protein was found to have RNase P activity but its localization was not determined.64 However, bacterial-type RNase P ribozymes can be found encoded in both mitochondrial and plastidial genomes along with an RNP RNase P protein in the nucleus.29,64 Nonetheless, although all characterized PRORP proteins are eukaryotic, they are not restricted to endosymbiotic organelles as was previously assumed.11
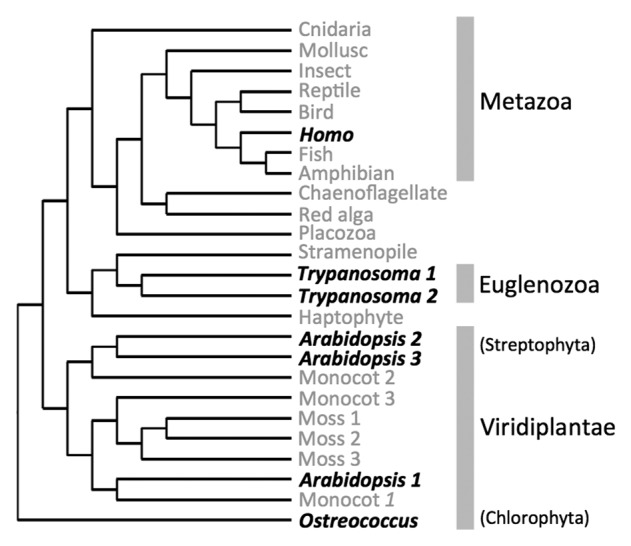
Figure 2. The occurrence of PRORP in eukaryote lineages is represented in an unrooted neighbor-joining phylogenic tree derived from Gobert et al.61 Representative PRORP protein sequences described by Gobert et al. from evolutionarily distant eukaryotes were used for the phylogenetic analysis. Grey names show the incidence of putative PRORP sequences in the respective subgroups whereas black names indicate species where PRORP proteins were experimentally shown to hold RNase P activity. The demonstration that RNase P activity could be held by PRORP proteins in distantly related eukaryote groups such as Metazoa, Euglenozoa and Viridiplantae strongly suggest that PRORP evolved early in eukaryote history.
Database analyses confirm that PRORP proteins constitute a eukaryote-specific family of enzymes. Putative PRORP sequences can be found in nearly all major eukaryotic groups (i.e., in Metazoa, Streptophyta, Chlorophyta, Kinetoplastida, Stramenopiles and Oomycetes) with the notable exceptions of fungi and amoebozoa.61 The appearance of PRORP can essentially be defined by the event that led to the fusion of a PPR domain with an NYN nuclease domain. The precise timing of this event and the evolutionary history of PRORP remain to be established. Still, its occurrence as experimentally shown for Metazoa, Euglenozoa and for both Streptophyta and Chlorophyta in Viridiplantae (Fig. 2), already suggests that PRORP appeared very early in the evolution of eukaryotes.61
The emergence of PRORP has been proposed to be related to the acquisition of organelles.5 Similarly, Howard et al. suggested that the evolutionary drive for RNP replacement by PRORP might have resided in different substrate specificities between nuclear and organelle RNase P enzymes, in the difficulty of importing a large RNA such as that for RNase P into mitochondria, or in the “vulnerability” of organelle RNA toward RNP RNase P enzymes.67 All these propositions assume that PRORP initially arose as an organelle-targeted enzyme, which is not established and not necessarily true. Indeed, PRORP clearly emerged as a nuclear gene by fusion of genes encoding a PPR RNA-binding protein and an NYN metallonuclease domain (discussed in the following section). Because the nuclear RNase P activity of PRORP is found in distantly related eukaryotes, PRORP nuclear activity is most likely ancient. It is thus possible that PRORP might have first functioned as a nuclear enzyme. If so, the evolutionary impetus to replace a RNP complex containing one RNA and up to ten proteins by a protein-only enzyme might have resided in the fact that the simpler enzyme assembles faster, is easier to regulate and requires fewer cellular resources for its biogenesis.
PRORP enzymes are two-domain proteins
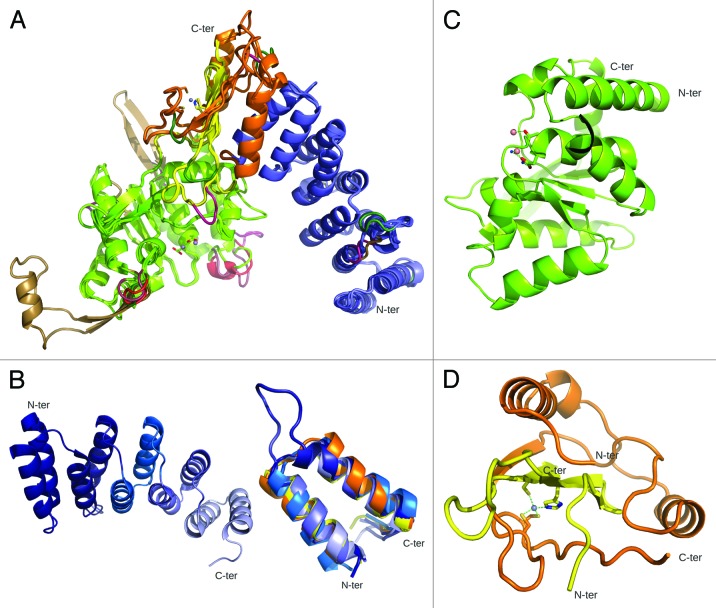
Initial structural predictions of PRORP based on sequence analyses indicated the presence of PPR modules in the N terminus and of a NYN-like catalytic domain in the C terminus. This organization into two α-helix-rich domains was supported by biophysical characterization (circular dichroism and small angle X-ray scattering) of At-PRORPs in solution and is consistent with the X-ray crystal structure of At-PRORP1.77,79 Taking together 1-, 2- and 3-D data available for this enzyme family, comparative models of representative PRORP members are presented in Figure 3A. These models pinpoint the general conservation of the PRORP fold from unicellular algae to humans. Small variations are observed, mainly in peripheral loops. Long insertions are present in plasmodial enzymes, as is often observed in proteins from this parasite family.80
Figure 3. PRORP are two-domain PPR proteins. (A) 3D models were built using SwissModel93 for all characterized members of the PRORP family based on At-PRORP1 crystal structure (PDB ID 4G24). This global view shows superimposed structure and models with PPR domains in blue, N-terminal and C-terminal connecting regions in orange and yellow, respectively, and catalytic NYN domains in green. Insertions/deletions to the reference structure of At-PRORP1 are colored as following: A. thaliana PRORP2, PRORP3, O. tauri PRORP, Trypanosoma PRORP2 and human mitochondrial MRPP3 indels are in violet, red, dark green, light brown and pink, respectively. Little structural variations are observed. (B) At-PRORP1 PPR domain. (Left) This view of the whole domain highlights individual PPR motifs in light to dark blue from N to C terminus. (Right) Superposition of the five PPR motifs from A. thaliana PRORP1 (represented with the same color code as on the left) and the two PPR motifs (in orange and yellow) of human mitochondrial RNA polymerase (PDB ID 3SPA) illustrating the conservation of the PPR fold. (C) At-PRORP1 catalytic domain. Manganese ions shown as pink spheres and two water molecules bridging one Mn2+ ion to conserved Asp474 in the catalytic site. (D) At-PRORP1 connecting region. This region is composed of a N-terminal half (orange) following the PPR domain and a C-terminal half (yellow) following the catalytic domain. It binds a zinc ion (gray) coordinated by C344, C345 (orange) and H548, C565 (yellow).
The N-terminal PPR domain forms a superhelical structure very similar to those described in TPR (TetratricoPeptide Repeat) domains, an evolutionary-related domain involved in protein•protein interactions.2,81 As illustrated in Figure 3B, it contains five PPR and PPR-like motifs: two canonical ones and three displaying remote sequence similarities. Despite their divergent sequences, these PPR modules are structurally similar and superimposable on those found in the only other PPR protein of known three-dimensional structure, i.e., human mitochondrial RNA polymerase.4 This confirms, as was originally proposed,2 that the defining feature of PPR family members is a conserved structural fold of PPR motifs rather than of conserved sequence elements.
The catalytic domain of PRORP adopts an α/β/α sandwich fold (Fig. 3C) belonging to the PIN-like nuclease family.82,83 A similar architecture is found in the nuclease domain of T4 RNase H,84 and of human SMG6 and SMG5, two essential factors in nonsense-mediated mRNA decay,82 as well as of a recently characterized MCPIP1 RNase (MCP-1 induced protein 1) that participates in the regulation of immune response by degrading the mRNA of inflammatory cytokines.83 Among the four aspartate residues involved in the binding of metal ions,79 two are strictly conserved in PRORPs (D474 and D475 in At-PRORP1) and in other nucleases of the PIN/NYN family and are essential for pre-tRNA cleavage.77
These two functional domains are connected by a split zinc-binding module derived from the central and the C-terminal regions of PRORP (Fig. 3D) and which forms the tip of the overall “V shape” of PRORP, the PPR and catalytic domains being the two arms of the V. The concave surface of the PPR superhelix in one arm thus faces the catalytic groove in the other arm, thereby exposing conserved aspartate residues and metal ions, making the overall architecture look like tweezers.
Is PRORP a structural mimic of ribonucleoprotein RNase P?
The bacterial RNP RNase P docks onto the acceptor stem of its pre-tRNA substrate, with an interaction extending from the tRNA corner (T and D loops), which is recognized by the specificity domain (S-domain) to the cleavage point between nucleotides -1 and +1, which is apposed to the catalytic domain (C-domain).18,85,86 In E. coli tRNAs, the 3′ terminal CCA interacts specifically with a complementary sequence in a loop of the RNase P RNA,87 whereas the pre-tRNA leader interacts with the protein subunit of the holoenzyme.88
The bipartite organization of PRORPs (Fig. 3) is reminiscent of that of RNP RNase P, with the PPR domain playing the role of the S-domain to ensure recognition of the pre-tRNA and its orientation in the catalytic domain. In support of this role, removal of the four N-terminal PPR motifs of At-PRORP1 leads to a 34-fold drop of affinity for the substrate and a > 2,000-fold loss of enzymatic activity.79 Similarly, the deletion of the S-domain in the RNP RNase P resulted in 30- to 13,000-fold loss in catalytic performance, depending upon the substrate used. However, the S-domain deletion, surprisingly, led to more accurate cleavage site selection.89
On the substrate side, deletions altering the pre-tRNA structure show that for PRORP, just as for the RNP RNase P, the anticodon stem-loop is dispensable, whereas the D and T loops are required. Footprint experiments confirmed that the corner of the tRNA L-fold interacts with At-PRORP1 to give strong protection of residues U16, G18-19 and C56.77
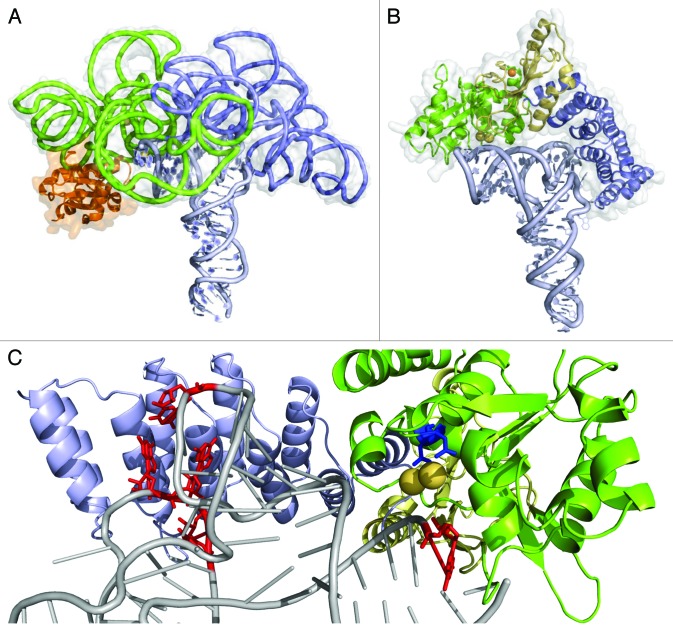
The PRORP/pre-tRNA complex was modeled based on the At-PRORP1 crystal structure using as geometrical restraints the binding of the T/D loops by the PPR domain and the positioning of the cleavage point in the vicinity of conserved aspartate groups constituting the metal-binding site. Figure 4 illustrates the potential similarity between PRORP and RNP RNase P in the way they bind their pre-tRNA substrates.77 Another model of PRORP/pre-tRNA complex has been proposed, it shows PRORP interacting on the side rather as on the top of tRNAs.67 However, the latter does not take in account footprinting and tRNA deletion results that suggested contacts between PRORP and tRNA residues U16, G18, G19 as well as C56, while the anticodon stem is dispensable for recognition.77
Figure 4. The current model of the PRORP/tRNA complex suggests a common mode of RNA binding in RNP and PRORP RNases P. (A) Structure of Thermotoga maritima ribozyme (PDBid 3Q1R18) with the catalytic domain in green, the specificity domain in blue, the RNase P protein subunit in orange, the tRNA product in light blue and the molecular surface of the RNP in gray. (B) The two-domain architecture of At-PRORP1 structure offers a concave surface that can be docked on the tRNA acceptor arm. The protein shown in the same orientation and same color code as the RNP with the catalytic domain in green, with metal ions bound (yellow spheres) close to the RNA cleavage site and the RNA-binding PPR domain in blue interacting with the region of the D-TψC loops. The central region (yellow) stabilized by a zinc ion (orange sphere) connects the two main PRORP domains. (C) A close-up of the PRORP1-tRNA complex model shows conserved catalytic aspartates D474 and D475 (blue) adjacent to tRNA cleavage site at position G+1 (red) as well as U16, G18, G19 and C56 (the nucleotides protected in footprint experiments77 in red) in contact with PPR motifs. Current functional data indicate that PRORP proteins have evolved an RNA recognition process very similar to that of RNP RNase P.
Despite the overall similarity of their substrate-binding modes, however, the two types of RNase P—employing a protein or an RNA catalytic component—are mechanistically distinct.79 Both cleave a phosphodiester bond by nucleophilic attack of hydroxide ion apical to O3ʹ of the upstream ribose, generating products with 3′-hydroxyl and 5′-phosphoryl termini. The presence of metal-binding sites in the structure of At-PRORP1 suggests that the proteinaceous enzymes use a two-metal-ion mechanism90 to deprotonate water and to stabilize the transition state. However, the tolerance of PRORPs to an Rp-phosphorothioate modification of the scissile bond in the presence of Mg2+ as cofactor is a striking difference from the RNP enzyme,65,91 indicating that the metal in PRORP does not directly coordinate the pro-Rp-oxygen of the target phosphodiester. Rather, it appears that, whereas the RNase P RNA subunit employs one hydrated divalent cation to provide the attacking hydroxide and a second metal hydrate to protonate the leaving group,18,79 the proteinaceous RNase P utilizes a more conventional mechanism akin to that of known protein metallonucleases, in which the metal ions serve primarily to stabilize the charge and structure of the trigonal bipyramidal transition state, and general acid-base chemistry is accomplished by the carboxylate groups of aspartate (and possibly the imidazole nitrogen of histidine). The binding affinities of PRORPs for their pre-tRNA substrate are in the micromolar range.65,79 These values are one or two orders of magnitude lower than for RNP RNases P and may indicate more transient interaction with substrates. Nevertheless, these proteinaceous enzymes are efficient enough to complement E. coli RNP RNase P.61 So the precise functional advantages of the PRORP and RNP RNase P mechanistic dissimilarities remain to be identified.
Concluding remarks
Within the PPR family, the characterization of PRORP proteins has finally settled the long-lasting debate over the existence of an alternative system devoid of RNA for RNase P activity in eukaryotes. The discovery of protein-only RNase P indicates that the distribution and evolutionary history of RNase P are more complex than previously thought. The functional and mechanistic comparison of PRORP with RNP RNase P will have important implications for our understanding of the evolution of living systems. Indeed, it will illustrate how convergent evolution has found two independent routes to catalyze the 5′ maturation of tRNAs: either with an RNA-based enzyme or a protein-only enzyme. This mechanistic comparison leads to important questions. For instance, the mechanism by which PPR motifs confer PRORP substrate specificity remains to be elucidated. Future work, in particular determination of the crystal structure of PRORP in complex with tRNA, will establish whether PPR motifs indeed bind conserved residues in the single-stranded D and T loops of tRNAs as was previously suggested,77 and thus whether the PRORP mode of RNA recognition is in conformity with the overall mode of RNA recognition recently proposed for PPR proteins.7,92
Acknowledgments
This work was supported by the French “Centre National de la Recherche Scientifique” and by the University of Strasbourg. A.G. and O.F. were supported by an ANR Blanc research grant “PRO-RNase P, ANR 11 BSV8 008 01” to P.G. and C.S. and by the LabEx consortium “MitoCross.” F.P. and B.G. were supported by PhD grants from the University of Strasbourg.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/25273
References
- 1.Schmitz-Linneweber C, Small I. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 2008;13:663–70. doi: 10.1016/j.tplants.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Small ID, Peeters N. The PPR motif - a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci. 2000;25:46–7. doi: 10.1016/S0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 3.Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringel R, Sologub M, Morozov YI, Litonin D, Cramer P, Temiakov D. Structure of human mitochondrial RNA polymerase. Nature. 2011;478:269–73. doi: 10.1038/nature10435. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb KC, Borah S, Cech TR. RNase P branches out from RNP to protein: organelle-triggered diversification? Genes Dev. 2012;26:1005–9. doi: 10.1101/gad.193581.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipovska A, Rackham O. Modular recognition of nucleic acids by PUF, TALE and PPR proteins. Mol Biosyst. 2012;8:699–708. doi: 10.1039/c2mb05392f. [DOI] [PubMed] [Google Scholar]
- 7.Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, et al. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 2012;8:e1002910. doi: 10.1371/journal.pgen.1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 2011;191:37–47. doi: 10.1111/j.1469-8137.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossmanith W, Holzmann J. Processing mitochondrial (t)RNAs: new enzyme, old job. Cell Cycle. 2009;8:1650–3. doi: 10.4161/cc.8.11.8502. [DOI] [PubMed] [Google Scholar]
- 10.Lai LB, Vioque A, Kirsebom LA, Gopalan V. Unexpected diversity of RNase P, an ancient tRNA processing enzyme: challenges and prospects. FEBS Lett. 2010;584:287–96. doi: 10.1016/j.febslet.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esakova O, Krasilnikov AS. Of proteins and RNA: the RNase P/MRP family. RNA. 2010;16:1725–47. doi: 10.1261/rna.2214510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark BC, Kole R, Bowman EJ, Altman S. Ribonuclease P: an enzyme with an essential RNA component. Proc Natl Acad Sci USA. 1978;75:3717–21. doi: 10.1073/pnas.75.8.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–57. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 14.Cech TR. The efficiency and versatility of catalytic RNA: implications for an RNA world. Gene. 1993;135:33–6. doi: 10.1016/0378-1119(93)90046-6. [DOI] [PubMed] [Google Scholar]
- 15.Altman S. A view of RNase P. Mol Biosyst. 2007;3:604–7. doi: 10.1039/b707850c. [DOI] [PubMed] [Google Scholar]
- 16.Evans D, Marquez SM, Pace NR. RNase P: interface of the RNA and protein worlds. Trends Biochem Sci. 2006;31:333–41. doi: 10.1016/j.tibs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Altman S, Wesolowski D, Guerrier-Takada C, Li Y. RNase P cleaves transient structures in some riboswitches. Proc Natl Acad Sci USA. 2005;102:11284–9. doi: 10.1073/pnas.0505271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiter NJ, Osterman A, Torres-Larios A, Swinger KK, Pan T, Mondragón A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature. 2010;468:784–9. doi: 10.1038/nature09516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter NJ, Osterman AK, Mondragón A. The bacterial ribonuclease P holoenzyme requires specific, conserved residues for efficient catalysis and substrate positioning. Nucleic Acids Res. 2012;40:10384–93. doi: 10.1093/nar/gks744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seif ER, Forget L, Martin NC, Lang BF. Mitochondrial RNase P RNAs in ascomycete fungi: lineage-specific variations in RNA secondary structure. RNA. 2003;9:1073–83. doi: 10.1261/rna.5880403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann E, Hartmann RK. The enigma of ribonuclease P evolution. Trends Genet. 2003;19:561–9. doi: 10.1016/j.tig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Marvin MC, Engelke DR. RNase P: increased versatility through protein complexity? RNA Biol. 2009;6:40–2. doi: 10.4161/rna.6.1.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrous N, Gopalan V. Archaeal/eukaryal RNase P: subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–94. doi: 10.1093/nar/gkq701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–4. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 25.Gruissem W, Greenberg BM, Zurawski G, Prescott DM, Hallick RB. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983;35:815–28. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- 26.Gray MW, Cedergren R. The new age of RNA. FASEB J. 1993;7:4–6. doi: 10.1096/fasebj.7.1.7678565. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Oyaizu Y, Oyaizu H, Olsen GJ, Woese CR. Mitochondrial origins. Proc Natl Acad Sci USA. 1985;82:4443–7. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannoni SJ, Turner S, Olsen GJ, Barns S, Lane DJ, Pace NR. Evolutionary relationships among cyanobacteria and green chloroplasts. J Bacteriol. 1988;170:3584–92. doi: 10.1128/jb.170.8.3584-3592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum M, Cordier A, Schön A. RNase P from a photosynthetic organelle contains an RNA homologous to the cyanobacterial counterpart. J Mol Biol. 1996;257:43–52. doi: 10.1006/jmbi.1996.0145. [DOI] [PubMed] [Google Scholar]
- 30.Pascual A, Vioque A. Functional reconstitution of RNase P activity from a plastid RNA subunit and a cyanobacterial protein subunit. FEBS Lett. 1999;442:7–10. doi: 10.1016/S0014-5793(98)01621-4. [DOI] [PubMed] [Google Scholar]
- 31.Lang BF, Burger G, O’Kelly CJ, Cedergren R, Golding GB, Lemieux C, et al. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–7. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 32.Seif E, Cadieux A, Lang BF. Hybrid E. coli--Mitochondrial ribonuclease P RNAs are catalytically active. RNA. 2006;12:1661–70. doi: 10.1261/rna.52106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingsworth MJ, Martin NC. RNase P activity in the mitochondria of Saccharomyces cerevisiae depends on both mitochondrion and nucleus-encoded components. Mol Cell Biol. 1986;6:1058–64. doi: 10.1128/mcb.6.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales MJ, Dang YL, Lou YC, Sulo P, Martin NC. A 105-kDa protein is required for yeast mitochondrial RNase P activity. Proc Natl Acad Sci USA. 1992;89:9875–9. doi: 10.1073/pnas.89.20.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manam S, Van Tuyle GC. Separation and characterization of 5′- and 3′-tRNA processing nucleases from rat liver mitochondria. J Biol Chem. 1987;262:10272–9. [PubMed] [Google Scholar]
- 36.Doersen CJ, Guerrier-Takada C, Altman S, Attardi G. Characterization of an RNase P activity from HeLa cell mitochondria. Comparison with the cytosol RNase P activity. J Biol Chem. 1985;260:5942–9. [PubMed] [Google Scholar]
- 37.Puranam RS, Attardi G. The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol Cell Biol. 2001;21:548–61. doi: 10.1128/MCB.21.2.548-561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossmanith W, Tullo A, Potuschak T, Karwan R, Sbisà E. Human mitochondrial tRNA processing. J Biol Chem. 1995;270:12885–91. doi: 10.1074/jbc.270.21.12885. [DOI] [PubMed] [Google Scholar]
- 39.Doersen CJ, Guerrier-Takada C, Altman S, Attardi G. Characterization of an RNaseP activity from HeLa cell mitochondria. J Biol Chem. 1985;260:5942–9. [PubMed] [Google Scholar]
- 40.Yamaguchi-Shinozaki K, Shinozaki K, Sugiura M. Processing of precursor tRNAs in a chloroplast lysate. Processing of the 5′ end involves endonucleolytic cleavage by an RNase P-like enzyme and precedes 3′ end maturation. FEBS Lett. 1987;215:132–6. doi: 10.1016/0014-5793(87)80127-8. [DOI] [Google Scholar]
- 41.Wang MJ, Gegenheimer P. Substrate masking: binding of RNA by EGTA-inactivated micrococcal nuclease results in artifactual inhibition of RNA processing reactions. Nucleic Acids Res. 1990;18:6625–31. doi: 10.1093/nar/18.22.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryner LC, Manley JL. Requirements for accurate and efficient mRNA 3′ end cleavage and polyadenylation of a simian virus 40 early pre-RNA in vitro. Mol Cell Biol. 1987;7:495–503. doi: 10.1128/mcb.7.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall TA, Brown JW. The ribonuclease P family. Methods Enzymol. 2001;341:56–77. doi: 10.1016/S0076-6879(01)41145-1. [DOI] [PubMed] [Google Scholar]
- 44.Wang MJ, Davis NW, Gegenheimer P. Novel mechanisms for maturation of chloroplast transfer RNA precursors. EMBO J. 1988;7:1567–74. doi: 10.1002/j.1460-2075.1988.tb02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossmanith W, Karwan RM. Characterization of human mitochondrial RNase P: novel aspects in tRNA processing. Biochem Biophys Res Commun. 1998;247:234–41. doi: 10.1006/bbrc.1998.8766. [DOI] [PubMed] [Google Scholar]
- 46.Thomas BC, Chamberlain J, Engelke DR, Gegenheimer P. Evidence for an RNA-based catalytic mechanism in eukaryotic nuclear ribonuclease P. RNA. 2000;6:554–62. doi: 10.1017/S1355838200991477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas BC, Gao L, Stomp D, Li X, Gegenheimer PA. Spinach chloroplast RNase P: a putative protein enzyme. Nucleic Acids Symp Ser. 1995;33:95–8. [PubMed] [Google Scholar]
- 48.Oommen A. Characterization of transfer RNA processing enzymes from wheat embryo. PhD Dissertation. Lawrence: University of Kansas, 1991. [Google Scholar]
- 49.Arends S, Schon A. Partial purification and characterization of nuclear ribonuclease P from wheat. Eur J Biochem. 1997;244:635–45. doi: 10.1111/j.1432-1033.1997.t01-1-00635.x. [DOI] [PubMed] [Google Scholar]
- 50.Franklin SE, Zwick MG, Johnson JD. Characterization and partial purification of two pre-tRNA 5′-processing activities from Daucus carrota (carrot) suspension cells. Plant J. 1995;7:553–63. doi: 10.1046/j.1365-313X.1995.7040553.x. [DOI] [PubMed] [Google Scholar]
- 51.Marchfelder A, Schuster W, Brennicke A. In vitro processing of mitochondrial and plastid derived tRNA precursors in a plant mitochondrial extract. Nucleic Acids Res. 1990;18:1401–6. doi: 10.1093/nar/18.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanic-Joyce PJ, Gray MW. Processing of transfer RNA precursors in a wheat mitochondrial extract. J Biol Chem. 1990;265:13782–91. [PubMed] [Google Scholar]
- 53.Altman S, Baer MF, Bartkiewicz M, Gold H, Guerrier-Takada C, Kirsebom LA, et al. Catalysis by the RNA subunit of RNase P--a minireview. Gene. 1989;82:63–4. doi: 10.1016/0378-1119(89)90030-9. [DOI] [PubMed] [Google Scholar]
- 54.Salavati R, Panigrahi AK, Stuart KD. Mitochondrial ribonuclease P activity of Trypanosoma brucei. Mol Biochem Parasitol. 2001;115:109–17. doi: 10.1016/S0166-6851(01)00273-0. [DOI] [PubMed] [Google Scholar]
- 55.Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462–74. doi: 10.1016/j.cell.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase--extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–93. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams-Carrier R, Kroeger T, Barkan A. Sequence-specific binding of a chloroplast pentatricopeptide repeat protein to its native group II intron ligand. RNA. 2008;14:1930–41. doi: 10.1261/rna.1077708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, Chen HW, Oktay Y, Zhang J, Allen EL, Smith GM, et al. PNPASE regulates RNA import into mitochondria. Cell. 2010;142:456–67. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossmanith W. Of P and Z: mitochondrial tRNA processing enzymes. Biochim Biophys Acta. 2012;1819:1017–26. doi: 10.1016/j.bbagrm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anantharaman V, Aravind L. The NYN domains: novel predicted RNAses with a PIN domain-like fold. RNA Biol. 2006;3:18–27. doi: 10.4161/rna.3.1.2548. [DOI] [PubMed] [Google Scholar]
- 61.Gobert A, Gutmann B, Taschner A, Gössringer M, Holzmann J, Hartmann RK, et al. A single Arabidopsis organellar protein has RNase P activity. Nat Struct Mol Biol. 2010;17:740–4. doi: 10.1038/nsmb.1812. [DOI] [PubMed] [Google Scholar]
- 62.Gutmann B, Gobert A, Giegé P. PRORP proteins support RNase P activity in both organelles and the nucleus in Arabidopsis. Genes Dev. 2012;26:1022–7. doi: 10.1101/gad.189514.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taschner A, Weber C, Buzet A, Hartmann RK, Hartig A, Rossmanith W. Nuclear RNase P of Trypanosoma brucei: a single protein in place of the multicomponent RNA-protein complex. Cell Rep. 2012;2:19–25. doi: 10.1016/j.celrep.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai LB, Bernal-Bayard P, Mohannath G, Lai SM, Gopalan V, Vioque A. A functional RNase P protein subunit of bacterial origin in some eukaryotes. Mol Genet Genomics. 2011;286:359–69. doi: 10.1007/s00438-011-0651-y. [DOI] [PubMed] [Google Scholar]
- 65.Pavlova LV, Gössringer M, Weber C, Buzet A, Rossmanith W, Hartmann RK. tRNA processing by protein-only versus RNA-based RNase P: kinetic analysis reveals mechanistic differences. Chembiochem. 2012;13:2270–6. doi: 10.1002/cbic.201200434. [DOI] [PubMed] [Google Scholar]
- 66.Krehan M, Heubeck C, Menzel N, Seibel P, Schön A. RNase MRP RNA and RNase P activity in plants are associated with a Pop1p containing complex. Nucleic Acids Res. 2012;40:7956–66. doi: 10.1093/nar/gks476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howard MJ, Liu X, Lim WH, Klemm BP, Koutmos M, Engelke DR, et al. RNase P enzymes: Divergent scaffolds for a conserved biological reaction. RNA Biol. 2013;10 doi: 10.4161/rna.24513. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forner J, Weber B, Thuss S, Wildum S, Binder S. Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Res. 2007;35:3676–92. doi: 10.1093/nar/gkm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramerov DA, Vassetzky NS. Short retroposons in eukaryotic genomes. Int Rev Cytol. 2005;247:165–221. doi: 10.1016/S0074-7696(05)47004-7. [DOI] [PubMed] [Google Scholar]
- 70.Peck-Miller KA, Altman S. Kinetics of the processing of the precursor to 4.5 S RNA, a naturally occurring substrate for RNase P from Escherichia coli. J Mol Biol. 1991;221:1–5. doi: 10.1016/0022-2836(91)80194-Y. [DOI] [PubMed] [Google Scholar]
- 71.Hartmann RK, Heinrich J, Schlegl J, Schuster H. Precursor of C4 antisense RNA of bacteriophages P1 and P7 is a substrate for RNase P of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:5822–6. doi: 10.1073/pnas.92.13.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9223–7. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClain WH, Guerrier-Takada C, Altman S. Model substrates for an RNA enzyme. Science. 1987;238:527–30. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- 74.Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 1994;8:3021–31. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Altman S. A specific endoribonuclease, RNase P, affects gene expression of polycistronic operon mRNAs. Proc Natl Acad Sci USA. 2003;100:13213–8. doi: 10.1073/pnas.2235589100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kruszka K, Barneche F, Guyot R, Ailhas J, Meneau I, Schiffer S, et al. Plant dicistronic tRNA-snoRNA genes: a new mode of expression of the small nucleolar RNAs processed by RNase Z. EMBO J. 2003;22:621–32. doi: 10.1093/emboj/cdg040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gobert A, Pinker F, Fuchsbauer O, Gutmann B, Boutin R, Roblin P, et al. Structural insights into protein-only RNase P complexed with tRNA. Nat Commun. 2013;4:1353. doi: 10.1038/ncomms2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Randau L, Schröder I, Söll D. Life without RNase P. Nature. 2008;453:120–3. doi: 10.1038/nature06833. [DOI] [PubMed] [Google Scholar]
- 79.Howard MJ, Lim WH, Fierke CA, Koutmos M. Mitochondrial ribonuclease P structure provides insight into the evolution of catalytic strategies for precursor-tRNA 5′ processing. Proc Natl Acad Sci USA. 2012;109:16149–54. doi: 10.1073/pnas.1209062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frugier M, Bour T, Ayach M, Santos MA, Rudinger-Thirion J, Théobald-Dietrich A, et al. Low Complexity Regions behave as tRNA sponges to help co-translational folding of plasmodial proteins. FEBS Lett. 2010;584:448–54. doi: 10.1016/j.febslet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Zeytuni N, Zarivach R. Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure. 2012;20:397–405. doi: 10.1016/j.str.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Glavan F, Behm-Ansmant I, Izaurralde E, Conti E. Structures of the PIN domains of SMG6 and SMG5 reveal a nuclease within the mRNA surveillance complex. EMBO J. 2006;25:5117–25. doi: 10.1038/sj.emboj.7601377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu J, Peng W, Sun Y, Wang X, Xu Y, Li X, et al. Structural study of MCPIP1 N-terminal conserved domain reveals a PIN-like RNase. Nucleic Acids Res. 2012;40:6957–65. doi: 10.1093/nar/gks359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhagwat M, Meara D, Nossal NG. Identification of residues of T4 RNase H required for catalysis and DNA binding. J Biol Chem. 1997;272:28531–8. doi: 10.1074/jbc.272.45.28531. [DOI] [PubMed] [Google Scholar]
- 85.LaGrandeur TE, Darr SC, Haas ES, Pace NR. Characterization of the RNase P RNA of Sulfolobus acidocaldarius. J Bacteriol. 1993;175:5043–8. doi: 10.1128/jb.175.16.5043-5048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan T, Loria A, Zhong K. Probing of tertiary interactions in RNA: 2′-hydroxyl-base contacts between the RNase P RNA and pre-tRNA. Proc Natl Acad Sci USA. 1995;92:12510–4. doi: 10.1073/pnas.92.26.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirsebom LA, Svärd SG. Base pairing between Escherichia coli RNase P RNA and its substrate. EMBO J. 1994;13:4870–6. doi: 10.1002/j.1460-2075.1994.tb06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurz JC, Niranjanakumari S, Fierke CA. Protein component of Bacillus subtilis RNase P specifically enhances the affinity for precursor-tRNAAsp. Biochemistry. 1998;37:2393–400. doi: 10.1021/bi972530m. [DOI] [PubMed] [Google Scholar]
- 89.Wu S, Kikovska E, Lindell M, Kirsebom LA. Cleavage mediated by the catalytic domain of bacterial RNase P RNA. J Mol Biol. 2012;422:204–14. doi: 10.1016/j.jmb.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 90.Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci USA. 1993;90:6498–502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas BC, Li X, Gegenheimer P. Chloroplast ribonuclease P does not utilize the ribozyme-type pre-tRNA cleavage mechanism. RNA. 2000;6:545–53. doi: 10.1017/S1355838200991465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yagi Y, Hayashi S, Kobayashi K, Hirayama T, Nakamura T. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS One. 2013;8:e57286. doi: 10.1371/journal.pone.0057286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]