Abstract
A key element for the successful development of novel therapeutic antibodies is to fully understand their pharmacokinetic and pharmacodynamic behavior before performing clinical trials. While many in vitro modeling approaches exist, these simply cannot substitute for data obtained from appropriate animal models. It was established quite early that the unusual long serum half-life of immunoglobulin G’s (IgGs) and Fc domains are due to their rescue and recycling by the neonatal Fc receptor (FcRn). The diverse roles of FcRn became apparent after isolation and cloning. Interesting are the significant species differences between rodent and human FcRn reactivity, rendering wild type rodents an inadequate model for studying IgG serum half-life. With the advance of genetic engineering mouse models have been established expressing human FcRn, and lacking mouse FcRn protein. These models have become highly relevant tools for serum half-life analysis of Fc-containing compounds.
Keywords: neonatal Fc receptor, humanized mice, monoclonal antibody, serum half-life, pharmacokinetics, efficacy
Introduction
Paul Ehrlich created the term of a “magic bullet” to illustrate how a therapeutic compound would specifically target and eliminate a disease-causing agent. With the breakthroughs of hybridoma technology and monoclonal antibody (mAb) development in 1975 [1], antibodies with their target-specific binding specificities could now be envisioned as treatment for of a wide range of diseases, potentially realizing the idea of a magic bullet. This vision is being increasingly realized by the current success of therapeutic mAbs, with over thirty FDA approved mAbs, spanning a range of disorders, including cancer, autoimmune disorders, infectious diseases, neurodegenerative diseases, macular degeneration, osteoporosis and transplant rejection.
Compared to other serum proteins, mAbs are unique in several features, sharing a common structural framework, while exhibiting a unique epitope specific binding site. Compared to other serum proteins, mAbs are unique in several features, sharing a common structural framework, while exhibiting a unique epitope specific binding site. The Fab (fragment of antigen binding) fragment confers high antigen specificity while the Fragment crystallizable (Fc) region equips immunoglobulin G (IgG) with three key features: (a) immune effector functions, (b) persistence in circulation and (c) transport across cellular barriers. The overall versatility of the IgG framework is immense and open to engineering approaches. With cloning and the ease of genetic engineering property of mAb can now be optimized in line with its anticipated use. The phenomenon of long IgG half-life in circulation was investigated early on. In 1965, Spiegelberg and Weigle found that IgG molecules serum half-life was dependent on the presence of the Fc fragment [2]. The half-life of IgGs is typically 7–22 days, while other antibody classes like IgM, IgA, IgD and IgE half-life is shorter, between 2–6 days [3]. All other serum proteins, with the exemption of serum albumin, have a very short serum half-life in the range of only minutes to hours. This remarkable serum half-life of IgG was hypothesized to be the result of a receptor that engages the Fc fragment rescuing IgG from catabolic elimination. This putative Fc receptor eventually proved to be the neonatal Fc receptor (FcRn) which was isolated from rat in 1989 [4]. However, as the name implies, FcRn was first characterized in the context of its transporter functions. It was known that IgG is readily transported across the materno-fetal barrier transfer providing the newborn with passive immunity before its own immune system develops [5,6]. In rodents, but not humans, FcRn additionally transports IgG from maternal colostrum across the neonatal intestine [7]. It was only later that FcRn was shown to be operative throughout lifespan, being responsible for the extended serum half-life of IgG and also serum albumin, and for the transport across endothelial and epithelial barriers, increasing the overall bioavailability of IgG and serum albumin [8,9,10,11,12]. The cellular trafficking mechanisms by which FcRn rescues, transports and recycles IgG is based on pH-dependent interactions and has been reviewed in detail and are not further described here [9,10,13,14,15,16].
FcRn forms a heterodimer consisting of the alpha-chain and beta-2-microglobulin (B2M) light chain. The alpha chain, also referred to as heavy chain, is a major histocompatibility complex (MHC) class I-like molecule, with the official gene name the “Fc receptor, IgG, alpha chain transporter” (FCGRT). As is common for all MHC class I proteins, FCGRT must complex with B2M light chain to exit from the endoplasmic reticulum, and for efficient pH-dependent binding of IgG [17]. B2M is ubiquitously expressed and associates with the alpha chain of all conventional MHC class I molecules, and MHC class I-like molecules including CD1, Azgp1 (alpha-2-glycoprotein 1, zinc-binding), Procr (protein C receptor, endothelial) and HFE (hemochromatosis) protein. B2M is a highly conserved protein with amino acid identity between human and mouse of 68.1 % (81/119 amino acids). Highest conservation is detected in the functional domains, e.g. transmembrane helices (95% identical) and cytoplasmic loops (93% identical), indicating that B2M may function across species and may be capable to heterodimerize with alpha chain proteins from other species.
While in silico and in vitro modeling is of great value in early stage drug development, alone it is not necessarily predictive of pharmacokinetic (PK) behavior nor is it sufficient to select the best candidates for further clinical development [18]. Especially, in the case of therapeutic mAbs PK analysis is quite complex, requiring careful analysis of a multitude of factors. Such factors comprise of the overall protein structure, glycosylation status, post-translational modifications, isoelectric point (pI), immunogenicity, FcRn binding affinity and interactions with the antigen target; each and all of which are important, potentially confounding factors. Absorption, distribution, metabolism and excretion of administered antibodies are all critical considerations and require in vivo testing. In general, the mouse is a well-established and preferred model for in vivo validation, disease modeling and preclinical pharmacokinetic (PK) analysis. Factors in its favor include ease of handling, cost, availability of numerous disease models, and accessibility of genetically defined animals.
However, conventional inbred or outbred mice, have proven to be inadequate in studying the PK of human Fc-based compounds. In 2001, Ober et al. described significant species differences for FcRn which greatly impact model selection for preclinical testing and development of mAbs [19]. They compared the binding affinities between mouse and human by immobilizing IgG from different species (human, mouse, rat, rabbit, guinea pig, bovine and sheep) to CM5 sensor chips in a Biacore assay, and injected purified human or mouse recombinant FcRn proteins at pH 6.0. In these assays mouse FcRn binds to all IgGs tested, including human and mouse IgG1, while human FcRn shows no effective binding to mouse or rat IgG1. Human FcRn binds besides human IgG1, only rabbit IgG and guinea pig IgG2. Recently, Andersen et al. confirmed these observed differences between rodent and human FcRn using a complementary Biacore assay where FcRn was immobilized and IgG was injected. In addition, Andersen et al. also demonstrated a cross-species difference for albumin binding to the FcRn receptor [20]. Species differences between rodent and primate FcRn receptor are therefore highly relevant in preclinical evaluation of human mAbs and other humanized Fc-containing as well as albumin-based compounds in rodent models.
We have addressed these differences by developing a series of humanized transgenic mouse models designed to study the PK of human Fc-based compounds. These mouse lines are transgenic for human FCGRT and in addition engineered for a deletion in mouse Fcgrt, i.e. they do not express mouse FcRn, but express human FcRn protein. This collection of transgenic lines express human FcRn either under the control of a ubiquitous CAG promoter or human regulatory elements of FcRn [21,22]. The applications and considerations in the use of these humanized mouse models are discussed below.
2. Materials and Methods
2.1 Mice
Mice described here are available from The Jackson Laboratory, Bar Harbor, Maine, USA.
B6.Cg-Fcgrt<tm1Dcr> Tg(CAG-FCGRT)276Dcr/DcrJ (Tg276 mice), stock number 004919, express a human FCGRT cDNA under the control of the ubiquitous CAG promoter,
B6.Cg-Fcgrt<tm1Dcr> Tg(FCGRT)32Dcr/DcrJ (Tg32 mice), stock number 0014565, carry a human FCGRT gene by insertion of a 33 kb cosmid clone containing the complete FCGRT gene of approximately 11 kb, as well as 10 kb of 5′ and 3′ flanking sequences [22,23,24].
B6.Cg-Rag1<tm1Mom> Fcgrt<tm1Dcr> Tg(CAG-FCGRT)276Dcr/DcrJ; (Tg276-Rag1-null mice), stock number 016919, developed by backcrossing Tg276 mice to Rag1-null mice (stock number 002216).
B6.Cg-Fcgrt<tm1Dcr> Prkdc<scid >Tg(CAG-FCGRT)276Dcr/DcrJ (Tg276-SCID mice), stock number 021146, developed by backcrossing Tg276 mice to B6.CB17-Prkdc<scid>/SzJ mice (stock number 001913).
B6.Cg-Fcgrt<tm1Dcr> Prkdc<scid >Tg(FCGRT)32Dcr/DcrJ (Tg32-SCID mice), stock number 018441, developed by backcrossing Tg276 mice to B6.CB17-Prkdc<scid>/SzJ mice (stock number 001913).
B6.Cg-Fcgrt<tm1Dcr> Tg(FCGRT)32Dcr Tg(B2M)55Hpl/Dcr (Tg32/hB2M) mice were created by intercrossing Tg32 mice with hB2M transgenic mice. The hB2M transgenic mice are described in Krimpenfort et al. [25].
As controls wild type C57BL/6J mice (abbreviated as B6), stock number 000664 and B6.129X1-Fcgrt<tm1Dcr>/DcrJ (FcRn-null mice), stock number 003982 were used [21], see also Table 1.
Table 1.
Humanized FcRn Mouse Models
| Stock Number | Mouse Strain Name | Short Name | References |
|---|---|---|---|
| 003982 | B6.129X1-Fcgrt<tm1Dcr>/DcrJ | FcRn-null | [21] |
| 004919 | B6.Cg-Fcgrt<tm1Dcr> Tg(CAG-FCGRT)276Dcr/DcrJ | Tg276 | [22,23,24,27] |
| 014565 | B6.Cg-Fcgrt<tm1Dcr> Tg(FCGRT)32Dcr/DcrJ | Tg32 | [22,23,24] |
| 016919 | B6.Cg-Rag1<tm1Mom> Fcgrt<tm1Dcr> Tg(CAG-FCGRT)276Dcr/DcrJ | Tg276-Rag1-null | [27] |
| 021146 | B6.Cg-Fcgrt<tm1Dcr> Prkdc<scid >Tg(CAG-FCGRT)276Dcr/DcrJ | Tg276-SCID | |
| 018441 | B6.Cg-Fcgrt<tm1Dcr> Prkdc<scid>Tg(FCGRT)32Dcr/DcrJ | Tg32-SCID |
For in vivo studies performed at The Jackson Laboratory, all mice were maintained under specific pathogen-free conditions and the procedures were approved by The Jackson Laboratory Animal Care and Use Committee.
2.2 Development of the humanized FcRn Mouse Models
We have developed a series of humanized transgenic mouse models designed to study the PK of humanized mAbs and other human Fc-based compounds (Table 1). These mouse lines are transgenic for human FCGRT and in addition carry a deletion in mouse Fcgrt, i.e. they do not express mouse FcRn, but only express human FcRn. This collection of transgenic lines express human FcRn either under the control of a ubiquitous CAG promoter or human FCGRT regulatory elements [21,22]. Initially the mouse FcRn gene was inactivated using gene targeting in the mouse embryonic stem (ES) cell line ESV/J-1182 derived from the mouse strain 129X1/SvJ. For this, a gene targeting vector was constructed to replace 1588 nucleotides which encodes the promoter sequence 5′ at the transcriptional start site, exon 1 and most of exon 2 linked to a neomycin resistance gene. Embryonic stem (ES) cells carrying the correct target event were microinjected in C57BL/6J (B6) blastocysts to generate chimeric mice, which were bred and tested for germline transmission. The resulting offspring were genotyped for presence of the targeted FcRn allele. Heterozygous offspring carrying the targeted allele were backcrossed to B6 for 11 generation, and then intercrossed to generate homozygous FcRn-null mice.
To generate the Tg276 mouse transgenic strain, a human FcRn expression construct was generated containing the human cytomegalovirus immediate early promoter/enhancer plus the chicken beta-actin/rabbit beta-globin hybrid promoter (CAG) promoter and the human FCGRT cDNA. This construct was microinjected into B6 fertilized oocytes. Offspring were screened for expression of human FCGRT and positive transgenic lines were backcrossed onto the B6 congenic FcRn-null mice, resulting in several lines expressing human FCGRT while lacking mouse FcRn [22,23,24]. One of these transgenic lines, called Tg276, has been studied more extensively and is described here.
To generate the Tg32 mouse strain, a human 33 kb cosmid clone encoding the complete human FCGRT gene and including a approximately 10 kb of 5′ flanking sequences and 10 kb of 3′ flanking sequences was microinjected into fertilized B6 mouse oocytes. Resulting offspring were screened for expression of the human FCGRT gene, and selected strains were backcrossed to the B6 congenic FcRn-null mice, and homozygosed for the FcRn-null deletion plus the human FCGRT transgene [21,22,23,24]. One of the resulting transgenic line discussed here is Tg32.
2.3 IgG Administration and Dosing
For PK studies in mice, IgGs are commonly administered by intravenous (IV) tail vein injection. However, we also find that intraperitoneal (IP) injections provide comparable results when evaluating the β phase PK. In published studies antibody doses used ranged from 2 mg/kg to 10 mg/kg, with the majority of studies using 2 mg/kg or 5 mg/kg. As the FcRn pathway is saturable substantial dose increases to the range of >50 mg/kg could be counterproductive [18,23,24,26,27].
3. Results and Discussion
3.1 PK Studies with Fc-engineered Antibodies
The first PK study using Tg276 and Tg32 hFcRn transgenic mice was published by Petkova et al. [23]. Here trastuzumab (Hu4D5) and three genetically engineered variants of Hu4D5 (N434A; T307A/E380A/N434A; I253A) were compared and examined regarding their serum half-lives in Tg276 mice (hemizygous and homozygous), Tg32 mice (hemizygous), FcRn-null mice and wild type B6 mice. These antibodies were selected such to include those engineered to eliminated binding to FcRn (I253A), and those to increase binding to FcRn (N434A; T307A/E380A/N434A) compared to Hu4D5, as determined in vitro. The data showed that the I253A antibody has a very short half-life in all mouse models tested (t1/2 = 24 to 37 h). When these antibodies were assayed in the FcRn-null model, their half-lives were similarly short for all (t1/2 = 24 to 29 h). This is as expected, as the recycling by FcRn is impaired due the genetic deletion of FcRn expression. In wild type B6 mice the longest half-life values were found as would be predicted due to the higher affinity of human antibodies to mouse FcRn. The wild type Hu4D5 antibody performed best in B6 mice, and slightly lower serum half-life values, which were not statistically significant, were measured for the two engineered IgG variants N434A; and T307A/E380A/N434A. These results would have lead to the conclusion that the engineered antibodies do not perform in a superior manner in vivo, and would therefore potentially not have been further investigated. However, when these variants were analyzed in the humanized mouse models, increases in mAb half-live were readily detected for both enhanced IgG variants. The greatest percentage improvement was detected in hFcRn hemizygous Tg276 mice, showing a 2.2 and 2.5 fold extended serum half-life for the variants N434A; and T307A/E380A/N434A, respectively. For this study hemizygous Tg32 mice were pretreated with 2 mg purified human IgG (hIgG) in order to compensate for the low IgG levels observed in this humanized FcRn model [28]. This pretreatment was considered unnecessary for the Tg276 mice, as these show shorter serum half-lives for hIgG when compared to the Tg32 mouse strain. In current studies hIgG pre-treatment is typically not applied, as this complicates the study design and introduces additional variables, which the require the use of by larger cohorts of mice. However, as a general rule we have not found a need to include the pre-treatment option to obtain reproducible and meaningful PK data,. This has been also supported in a recent publication by Tam et al. [26].
3.2 Correlation of PK Studies between hFcRn Tg276 Mice and Monkeys
The first PK data that investigated the correlations between the humanized hemizygous Tg276 FcRn model and non-human primates were published by Zalevesky et al. [27]. In this study the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab, the anti-epidermal growth factor receptor (EGFR) antibody cetuximab and their correlating FcRn-engineered variants were compared regarding their half-life in humanized mice and cynomolgus monkeys (Macaca fascicularis). For the anti-VEGF Mabs, the IgG1 wild type and four Fc engineered variants were studied: YTE (M252Y/S254T/T256E), IF (V259I/V308F), LS (M428L/N434S; Xtend) and IFL (V259I/V308F/M428L). In in vitro affinity FcRn binding studies using a Biacore assay, the ranking was IgG1 > IF > YTE > LS > IFL, with IgG1 having the highest KD value and IFL the lowest. In Tg276 hemizygous mice half-life ranking was IgG1 ≤ IF < YTE < IFL LS, with IgG1 having the shortest half-life. The ranking in cynomolgus monkeys was IgG1 < IF < YTE ≤ IFL < LS. For the cynomolgus monkey the group size was 3, while for the mice two studies were preformed with n=6 each. For the anti-EGFR the LS variant was compared to wild type IgG1, showing in Tg276 hemizygous mice a 4.8 fold increase in half-life, and in monkeys a 3.1 fold increase for the LS variant compared to the IgG1 wild type. These studies were the first to document significant correlations between the PK behaviors of humanized FcRn mice and primates.
3.3 Correlation of PK Studies between hFcRn Tg32 Mice, Monkeys and Humans
Tam et al. have extended the analysis of hFcRn moue models by evaluating a series of antibodies in the hemizygous Tg32 mouse model, compared to non-human primate and human clinical trial data [26]. mAbs were administered IV, at a dose of 2 mg/kg, and a group size of n=4 for mice. Seven mAbs were compared using a Pearson correlation test revealing that the relationship between monkey clearance and mouse clearance values is significant (p = 0.008). Also for seven antibodies with human PK data the correlation test showed that the relationship between human clearance and mouse clearance is significant, (p < 0.001). Five of the seven mAbs were in common between the correlation of monkeys and human data. In summary, these studies indicate that the humanized FcRn mouse model are a convenient, cost-effective alternative to primates for in vivo prediction of hIgG antibody half-life and clearance.
3.4 Additional PK Studies
Other publications have also provided PK analyses data using these humanized mouse models, i.e. e. Wang et al., Gehlsen et al., and Andersen et al. [18,24,29,30]. Here, we compiled data from four different, independent studies, regarding their published PK values determined in the humanized FcRn mouse strains. Table 2 shows that serum half-life values from the different laboratories and studies are in the same range, revealing good reproducibility among different laboratories and experimental set ups.
Table 2.
Summary of Serum Half-Life Data in humanized FcRn mice
| Antibody | Route | Dose | Half-Life (h) | Mouse Strain | Reference |
|---|---|---|---|---|---|
| Adalimumab | IV | 10 mg/kg | 86 | Tg276 hemi | [18] |
| Basiliximab | IV | 10 mg/kg | 120 | Tg276 hemi | [18] |
| Bevacizumab | IV | 10 mg/kg | 76 | Tg276 hemi | [18] |
| Bevacizumab | IV | 2 mg/kg | 67–70 | Tg276 hemi | [27] |
| Cetuximab | IV | 10 mg/kg | 66 | Tg276 hemi | [18] |
| Cetuximab | IV | 2 mg/kg | 70 | Tg276 hemi | [27] |
| Trastuzumab | IV | 10 mg/kg | 81 | Tg276 hemi | [18] |
| Trastuzumab | IV | 10 mg/kg | 56 | Tg276 hemi | [24] |
| Trastuzumab | IP | 5 mg/kg | 41 | Tg276 hemi | [23] |
| Trastuzumab | IP | 5 mg/kg | 139 | Tg276 homo | [23] |
| Trastuzumab | IP | 5 mg/kg | 155 | Tg32 hemi | [23] |
| Trastuzumab | IP | 5 mg/kg | 242 | C57BL6/J | [23] |
3.5 hFcRn Mouse Models Tg276 and Tg32
Both humanized FcRn models, Tg276 and Tg32 are well suited to screen human/ized IgGs regarding their serum half-life, assist in the prioritization of candidates and develop meaningful PK data. The major difference between Tg276 and Tg32 is that half-life values are the shortest in hemizygous Tg276 mice, and the longest in Tg32 homozygous mice. As a general rule, hemizygous Tg276 mice allow to better detect subtle differences in serum half-life between administered mAbs compared to Tg32. For hIgG testing, both hemizygous Tg276 and hemizygous Tg32 are currently the preferred models.
3.5 Additional Applications
The humanized FcRn mouse models are of general of use for testing Fc-containing compounds, not only IgGs, but also any Fc-fusion molecules, as for example recombinant factor VIII-Fc fusion [31], single-chain variable fragment antibody-Fc proteins [30], and CH2 domain proteins [29]. Other applications may include in vivo quality control of IgGs and Fc fusion proteins. In one example these mice were used for mAb stability testing as it has been shown that oxidation negatively affects FcRn binding and serum half-life [24]. Both humanized FcRn models are also available on immunodeficient backgrounds, allowing efficacy testing in a human FcRn context. As a well established model human cancer cell lines can be transplanted to develop a mouse tumor model. Alternatively, primary human tumor biopsies or human cells can be engrafted. When the human mAb is administered, its circulation has the appropriate half-life instead of an abnormally extended half-life in the case of wild type mouse expressing mouse FcRn, and therefore resulting in more accurate efficacy data. Zalevesky et al. used the Tg276-Rag-1 null mice for xenograft studies with two human cell lines, human ovarian carcinoma SKOV-3 and human epidermoid carcinoma A431 cells. They were able to demonstrate anti-tumor activity of mAbs targeting VEGF or EGFR, revealing the superiority of the Xtend engineered mAbs compared to the corresponding wild type mAbs [27].
Currently, three immunodeficient variants have been developed: Tg276-Rag1-null mice, Tg276-SCID and Tg32-SCID mice (Table 1). These immunodeficient hFcRn mouse models can be used directly in xenograft studies and for the testing of anti-tumor activities of the mAbs in vivo. Further, they allow PK analysis of immunogenic mAbs. Other areas where the current hFcRn mouse models can be easily adapted for efficacy testing are induced disease models, such as diet-induced, pathogen or chemical induced, for example for infectious diseases, inflammatory bowel diseases and lung disorders [32,33,34,35,36,37,38,39,40,41]. However, in such models, it needs be ensured that the target in mouse will be recognized by the human mAb.
3.6 Humanized FcRn mice expressing human B2M
A potential caveat in the use of the current hFcRn Tg models is they do not express human B2M and therefore have a human-mouse heterodimer FcRn receptor. There has been one case reported in Madin-Darby Canine Kidney (MDCK) cells that cross-species heterodimers may not yield full functionality, for example, when only human FcRn is expressed. Only after co-expressing human B2M in the MDCK cells full human FcRn activity could be detected [42]. To address whether the human-mouse heterodimer FcRn receptor is a confounding factor, we intercrossed the Tg32 mice to mouse strain expressing human B2M [25]. The resulting mice lack mouse FcRn expression (FcRn-null), while expressing human FcRn, human B2M and mouse B2M. The resulting strain is called here Tg32/hB2M (B6.Cg-Fcgrt<tm1Dcr>Tg(FCGRT)32Dcr Tg(B2M)55Hpl/Dcr). For a negative control, we intercrossed the FcRn-null mice with mice expressing human B2M, generating a strain lacking FcRn expression, while expressing human B2M and mouse B2M (FcRn-null/hB2M).
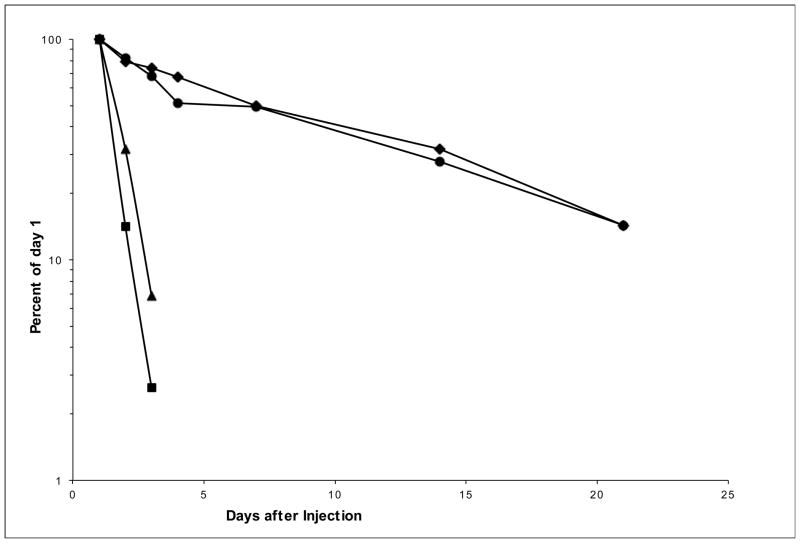
For PK analysis, 100 μg of purified IgG (GammaGard; Baxter Laboratories) was IP administered IP toTg32 mice, Tg32/hB2M mice and as control to mice lacking FcRn expression, FcRn-null and FcRn-null/hB2M mice. Serial blood samples were collected 1, 2, 3, 4, 7, 14, and 21 days after injection. Serum concentrations of hIgG were determined by ELISA analysis as previously described [22]. Fig. 1 shows no detectable differences in half-lives between Tg32 and Tg32/hB2M mice, indicating that at least in this system adding human B2M does not impact the serum half-life of hIgG. It is therefore suggested that the human B2m/hFcRn heterodimer is fully functional in vivo, at least in the Tg32 mouse model.
Fig. 1.
Addition of a human B2m transgene does not affect the serum half-life of IgG in Tg32 mice
hB2M transgenic mice [25] were crossed onto FcRn-null and Tg32 mice. For FcRn-null (filled square), and FcRn-null expressing hB2M (filled triangle), and, Tg32/hB2M mice (filled diamond) 4 mice per group, and for Tg32 mice (filled circle) 3 mice per group were injected with 100 μg purified hIgG (GammaGard; Baxter Laboratories). Serial blood samples were collected at 1, 2, 3, 4, 7, 14, and 21 days after injection. Serum concentrations of hIgG were determined by ELISA analysis as previously described [22]. Results are presented as the percent of hIgG concentrations measured 24 h after IP injections. Addition of a human B2M transgene does not alter human FcRn dependent pharmacokinetics of hIgG.
3.7 Considerations
The humanized FcRn mouse models discussed here present a strong and viable alternative to primates for the study of serum half-life with human Fc-based compounds. This now makes it feasible to screen a whole range of genetically engineered mAb candidates, in a relevant in vivo model and select the best molecules for further development. As it has been shown previously, in silico models and in vitro assays alone are not optimal or predictive for ranking mAbs regarding their performance in vivo, making it difficult to limit which of many mAb candidate should be tested in non-human primates [18,43]. The humanized FcRn mouse models presented here provide a reliable tool for studying serum half-life of human/ized mAbs and other Fc-containing proteins. In most cases the studies are not complicated by target-mediated antibody degradation, due to the fact that in most cases human target antigens are sufficiently different from mouse, i.e. the mAb will not recognize its target in mouse. In target-mediated antibody elimination receptor and soluble targets are viewed separately. In the case of cell surface receptors, receptor meditated endocytosis of IgG is the general route of IgG elimination. For soluble targets the typical degradation pathways is catabolism. The formation of large complexes has also been observed, which are typically removed by phagocytosis. Most marketed antibodies show a dose-dependent elimination which corresponds with target-mediated elimination [18].
As with all models there are caveats which must be recognized and circumvented. First, and as predicted the endogenous mouse IgG levels are very low in these models, being only 7–16 % of wild type IgG levels [28]. This is due to mouse IgGs having a very low affinity for human FcRn and are hence not actively recycled. Therefore, administered human/ized mAbs or human Fc do not face competition by endogenous IgG for binding to FcRn in these models. Another caveat is that B2M has not been humanized in these mouse models, therefore producing a human-mouse (B2M) chimeric FcRn receptor. The possible effect of this affecting the overall analysis is still open to speculation, but PK studies with hFcRn mice implicate that the human-mouse heterodimer is functional, as data from such correlate remarkably well to non-human primate and human [26,27]. Another possible interfering aspect is the presence of mouse serum albumin (MSA), and lack of human serum albumin (HSA) in these mice, especially when studying albumin-based molecules. Andersen et al. have analyzed binding affinities of MSA and HSA to human FcRn in vitro using a surface plasmon resonance assay. This revealed that MSA has a higher affinity of 0.8 μM compared to 1–4 μM for HSA [20]. However, since binding sites for HSA and Fc are not competitive, this would not be expected to impact PK analysis of Fc-based compounds. Conversely, it almost certainly would have a complicating impact in the analysis of albumin-base therapeutics. Further development of these models to humanize the albumin locus will help to address this issue.
Besides measuring half-life of Fc-based compounds, these mouse models are available on immunodeficient backgrounds Rag1-null and SCID. These immunocompromised backgrounds can be directly used in xenograft studies, and for the testing of anti-tumor activities of the mAbs in vivo. Other applicable efficacy models include chemically induced disease models, as established for examples for inflammatory and autoimmune diseases [32,33,35], or diet-induced [40,41] or pathogen-induced models [36,37,38,39]. However, in the case of induced models it needs be ensured that the target in mouse will recognized by the human mAb.
In spite of minor limitations, half-life data obtained from these humanized FcRn mouse models provide a strong correlation to non-human primates and human. To date, these humanized FcRn mouse models are the only rodent models available to perform predictive serum half-life studies for Fc-based compounds, allowing the screening for optimal half-life of many compounds in an in vivo model.
Conclusion
The humanized FcRn mouse models described here have become valuable tools for assessing the in vivo PK of human/ized mAbs and Fc-based therapeutics, especially when assessing Fc-engineered proteins. In addition to simple PK studies, these mice can be readily translated to a number of efficacy screens, especially in the area of xenograft and cancer studies. To date, these humanized FcRn mouse models are the only rodent models available to perform predictive PK studies for Fc-based compounds, allowing the screening for optimal half-life of compounds in an in vivo model. In spite of minor limitations, data obtained from these humanized FcRn mouse models correlate surprisingly well with that of non-human primates and humans. These models are also being sequentially engineered to make them more amenable for albumin-based therapeutics, by replacing the mouse albumin gene with the human, and such developing a solid platform useful for drug development. With the continuous advancement of antibody design and therapeutic development, further refinement of these models is needed and will provide more appropriate preclinical models, also tailored to specific disease areas providing better tools for preclinical testing.
Highlights.
We have developed several humanized FcRn mouse models.
hFcRn mouse models are suitable for PK assays of human/humanized mAbs and Fc-based therapeutics.
The hFcRn mouse models correlate well with non-human primates and human clinical trial data.
These models are also applicable for efficacy validation, especially for xenograft studies.
Acknowledgments
The authors thank Tom Sproule and Greg Christianson for technical assistance and discussions, and Dr. Michael V. Wiles for critical reading and discussions.
Abbreviations
- B2M
beta-2-microglobulin
- EGFR
epidermal growth factor receptor
- ES
embryonic stem
- Fab
fragment of antigen binding
- Fc
fragment crystallizable
- FCGRT
Fc receptor IgG alpha chain transporter
- FcRn
neonatal Fc receptor
- HSA
human serum albumin
- Ig
immunoglobulin
- IP
intraperitoneal
- IV
intravenous
- mAb
monoclonal antibody
- MDCK
Madin-Darby Canine Kidney
- MHC
major histocompatibility complex
- MSA
mouse serum albumin
- p
probability value
- PK
pharmacokinetics
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest
Derry C. Roopenian is an inventor on patent applications containing some of the subject matter described in this report; the patent applications are assigned to The Jackson Laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gabriele Proetzel, Email: gproetzel@earthlink.net.
Derry C. Roopenian, Email: Derry.Roopenian@jax.org.
References
- 1.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelberg HL, Weigle WO. The catabolism of homologous and heterologous 7S γ globulin fragments. J Exp Med. 1965;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy K, Travers P, Walport M. Janeway’s Immunobiology. 7. New York: Garland Science; 2008. [Google Scholar]
- 4.Simister NE, Mostov KE. Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):571–580. doi: 10.1101/sqb.1989.054.01.068. [DOI] [PubMed] [Google Scholar]
- 5.Stern CM. The materno-foetal transfer of carrier protein sensitivity in the mouse. Immunology. 1976;30:443–448. [PMC free article] [PubMed] [Google Scholar]
- 6.Brambell FW, Hemmings WA, Morris IG. A THEORETICAL MODEL OF GAMMA-GLOBULIN CATABOLISM. Nature. 1964;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- 7.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–2927. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 9.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghetie V, Ward ES. FcRn: the MHC class I-related receptor that is more than an IgG transporter. Immunol Today. 1997;18:592–598. doi: 10.1016/s0167-5699(97)01172-9. [DOI] [PubMed] [Google Scholar]
- 14.Roopenian DC, Sun VZ. Clinical ramifications of the MHC family Fc receptor FcRn. J Clin Immunol. 2010;30:790–797. doi: 10.1007/s10875-010-9458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. mABs. 2011;3:422–430. doi: 10.4161/mabs.3.5.16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30:777–789. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin WL, West AP, Jr, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Lu P, Fang Y, Hamuro L, Pittman T, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos. 2011;39:1469–1477. doi: 10.1124/dmd.111.039453. [DOI] [PubMed] [Google Scholar]
- 19.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: Implications for therapeutic antibodies. International Immunology. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem. 2010;285:4826–4836. doi: 10.1074/jbc.M109.081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 22.Roopenian DC, Christianson GJ, Sproule TJ. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol. 2010;602:93–104. doi: 10.1007/978-1-60761-058-8_6. [DOI] [PubMed] [Google Scholar]
- 23.Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, et al. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol. 2011;48:860–866. doi: 10.1016/j.molimm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Krimpenfort P, Rudenko G, Hochstenbach F, Guessow D, Berns A, et al. Crosses of two independently derived transgenic mice demonstrate functional complementation of the genes encoding heavy (HLA-B27) and light (beta 2-microglobulin) chains of HLA class I antigens. EMBO J. 1987;6:1673–1676. doi: 10.1002/j.1460-2075.1987.tb02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam SH, McCarthy SG, Brosnan K, Goldberg KM, Scallon BJ. Correlations between pharmacokinetics of IgG antibodies in primates vs. FcRn-transgenic mice reveal a rodent model with predictive capabilities. mABs. 2013;5:1–9. doi: 10.4161/mabs.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein C, Kling L, Proetzel G, Roopenian DC, de Angelis MH, et al. Clinical chemistry of human FcRn transgenic mice. Mamm Genome. 2012;23:259–269. doi: 10.1007/s00335-011-9379-6. [DOI] [PubMed] [Google Scholar]
- 29.Gehlsen K, Gong R, Bramhill D, Wiersma D, Kirkpatrick S, et al. Pharmacokinetics of engineered human monomeric and dimeric CH2 domains. mABs. 2012;4:466–474. doi: 10.4161/mabs.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen JT, Foss S, Kenanova VE, Olafsen T, Leikfoss IS, et al. Anti-carcinoembryonic antigen single-chain variable fragment antibody variants bind mouse and human neonatal Fc receptor with different affinities that reveal distinct cross-species differences in serum half-life. J Biol Chem. 2012;287:22927–22937. doi: 10.1074/jbc.M112.355131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumont JA, Liu T, Low SC, Zhang X, Kamphaus G, et al. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119:3024–3030. doi: 10.1182/blood-2011-08-367813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldner MJ, Neurath MF. Chemically induced mouse models of colitis. Curr Protoc Pharmacol. 2009;Chapter 5(Unit 5):55. doi: 10.1002/0471141755.ph0555s46. [DOI] [PubMed] [Google Scholar]
- 33.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 34.Paveglio S, Puddington L, Rafti E, Matson AP. FcRn-mediated intestinal absorption of IgG anti-IgE/IgE immune complexes in mice. Clin Exp Allergy. 2012;42:1791–1800. doi: 10.1111/j.1365-2222.2012.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996;14:425–438. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- 36.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina E. Murine model of cutaneous infection with Streptococcus pyogenes. Methods Mol Biol. 2010;602:395–403. doi: 10.1007/978-1-60761-058-8_21. [DOI] [PubMed] [Google Scholar]
- 38.Medina E. Murine model of polymicrobial septic peritonitis using cecal ligation and puncture (CLP) Methods Mol Biol. 2010;602:411–415. doi: 10.1007/978-1-60761-058-8_23. [DOI] [PubMed] [Google Scholar]
- 39.Medina E. Murine model of pneumococcal pneumonia. Methods Mol Biol. 2010;602:405–410. doi: 10.1007/978-1-60761-058-8_22. [DOI] [PubMed] [Google Scholar]
- 40.Kalish BT, Le HD, Gura KM, Bistrian BR, Puder M. A metabolomic analysis of two intravenous lipid emulsions in a murine model. PLoS One. 2013;8:e59653. doi: 10.1371/journal.pone.0059653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- 42.Claypool SM, Dickinson BL, Yoshida M, Lencer WI, Blumberg RS. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human beta 2-microglobulin. J Biol Chem. 2002;277:28038–28050. doi: 10.1074/jbc.M202367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, et al. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos. 2012;40:1545–1555. doi: 10.1124/dmd.112.045864. [DOI] [PubMed] [Google Scholar]