Summary
The Sestrins constitute a family of evolutionarily-conserved stress-inducible proteins that suppress oxidative stress and regulate adenosine monophosphate-dependent protein kinase (AMPK)-mammalian target of rapamycin (mTOR) signaling. By virtue of these activities, the Sestrins serve as important regulators of metabolic homeostasis. Accordingly, inactivation of Sestrin genes in invertebrates resulted in diverse metabolic pathologies, including oxidative damage, fat accumulation, mitochondrial dysfunction and muscle degeneration that resemble accelerated tissue aging. Likewise, Sestrin deficiencies in mice led to accelerated diabetic progression upon obesity. Further investigation of Sestrin function and regulation should provide new insights into age-associated metabolic diseases, such as diabetes, myopathies and cancer.
Introduction
Sestrins are highly conserved proteins encoded by genes whose expression is upregulated in cells exposed to a variety of environmental stresses including DNA damage, oxidative stress and hypoxia. Sestrins are universally found throughout the animal kingdom, but no Sestrin homologs were identified in plants or fungi. Most vertebrates including mammals express three Sestrins (Sesn1-3), while most invertebrate genomes contain only a single Sestrin (Sesn) gene. Mammalian Sestrins are encoded by three independent genomic loci, Sesn1-3, and the Sesn1 and Sesn3 genes are subject to alternative splicing, thus generating several Sestrin proteins (Peeters et al., 2003). It has been challenging to identify biochemical functions associated with the Sestrins, partially because the proteins do not contain any known structural domains or catalytic motifs. Only a very distant sequence homology to bacterial oxidoreductases was detected, which led to discovery of Sestrins’ antioxidant function (Budanov et al., 2004). Sestrins also bind to Keap1 and p62/SQSTM, and thereby suppress autophagic degradation of Keap1 and lead to enhanced Nrf2-dependent antioxidant gene transcription (Bae et al., 2013). Independently of these redox-regulating activities, Sestrins can suppress mTOR complex 1 (mTORC1) activity through the activation of AMPK (Budanov and Karin, 2008; Chen et al., 2010; Lee et al., 2010). Sestrins are able to bind AMPK, and through direct physical association, as well as through indirect transcriptional regulation, stimulate formation of the AMPK holoenzyme and its phosphorylation and activation by upstream kinases such as LKB1 (Budanov and Karin, 2008; Chen et al., 2010). However, the exact biochemical mechanisms through which the Sestrins function as antioxidants or as AMPK activators are unclear; protein structure determination through X-ray crystallography or nuclear magnetic resonance (NMR) would provide better clues on the physicochemical basis of Sestrins function. Although a lot of work is still needed to reveal the detailed molecular functions of the Sestrins, genetic studies have clearly shown that the Sestrins maintain metabolic homeostasis and protect cells and organisms from age-related physiological abnormalities, mainly through regulation of the AMPK-TORC1 axis. Although most of the data describing the anti-aging functions of Sestrins were initially obtained from dSesn-deficient Drosophila (Lee et al., 2010), recent results from knockout (KO) mouse strains deficient in Sesn2 and Sesn3 support the critical role of Sestrins in regulation of metabolism and suppression of age- and obesity-associated metabolic disorders (Bae et al., 2013; Lee et al., 2012a). Furthermore a recent study using C. elegans shows that cSesn is an important regulator of healthspan and lifespan in worms (Yang et al., 2013), suggesting that Sestrins’ anti-aging function is evolutionarily conserved throughout the animal kingdom. In this review, we will discuss the physiological functions of the Sestrins with an emphasis on the regulation of metabolism and aging.
Regulation of Sestrin Expression
In response to diverse insults, cells adjust their metabolic timbre to support cellular adaptation to stress, for example by facilitating damage repair, ceasing anabolic processes and stimulating catabolic reactions. Through these adjustments, cells can prevent accumulation of damaged macromolecules and save scarce resources for diverse repair processes. Because Sestrins expression is stress-inducible (Budanov et al., 2002; Velasco-Miguel et al., 1999) the Sestrins can be involved in cellular or organism-level adaptation to diverse metabolic challenges. It is therefore critical to understand how Sestrin expression is regulated under diverse physiological and pathological contexts.
Genotoxic stress
Dysregulation of different metabolic pathways can lead to release of genotoxic compounds that damage DNA, such as reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive carbonyl species, lipid peroxidation products and DNA-alkylating agents. Excessive genotoxic damage during metabolic stress can activate DNA damage-sensing signaling pathways, including up-regulation of tumor suppressor p53, which exerts cell cycle-inhibitory and pro-apoptotic activities as well as affecting metabolic regulation. Sesn1 (also known as PA26), the founding member of the Sestrin family, was originally discovered as a p53-inducible gene (Velasco-Miguel et al., 1999). Different challenges, including gamma-irradiation, UV and genotoxic metabolites, stimulate transcription of the Sesn1 and Sesn2 genes through p53 (Budanov et al., 2002; Velasco-Miguel et al., 1999). Activation of p53 by Nutlin-3, a drug that disrupts the inhibitory association between Mdm2 and p53, can lead to induction of Sesn1 and Sesn2 without DNA damage (Budanov and Karin, 2008). p53 is also involved in Sesn2 regulation by the orphan nuclear receptor TR3/Nur77 (Lee et al., 2012b). Correspondingly, p53-responsive cis-elements were identified in the first and second introns of the Sesn1 gene (Velasco-Miguel et al., 1999; Wei et al., 2006) and in the first exon and 9.6kb downstream of the Sesn2 gene (Lee et al., 2012b; Wei et al., 2006). In addition to the genotoxic challenges, paclitaxel-induced mitotic block can also induce Sesn1 and Sesn2 through an unknown mechanism (Rocha et al., 2011).
Hypoxia, energy deficiency and desiccation
Hypoxia, insufficient oxygen availability, is one of the most severe metabolic insults. Sesn2 (also known as Hi95) was originally isolated as a gene activated by hypoxia in human neuroblastoma cells (Budanov et al., 2002). In many human cancer cell lines, hypoxia mimetics upregulate the expression of both Sesn1 and Sesn2. Although Sesn1 is activated strictly in a p53-dependent manner, transcriptional activation of Sesn2 upon hypoxia is p53-independent, (Budanov et al., 2002), but is HIF-1-dependent in mouse epithelial tracheal cells (Olson et al., 2011). However, in many other cell types, Sesn2 is induced upon hypoxia independently of HIF-1 and shows expression kinetics that are distinct from other HIF-1 target genes (Budanov et al., 2002). Presumably, in most cases, Sesn2 transcription is induced not by hypoxia itself but as a consequence of energy deprivation caused by prolonged hypoxia. Many compounds that decrease cellular ATP concentration, such as 2-deoxyglucose (inhibitor of glycolysis) and metformin (inhibitor of mitochondrial respiration), induce Sesn2 expression via a mechanism that is yet-to-be established (Ben-Sahra et al., 2013a). Interestingly, Belgica antarctica, the only insect residing in Antarctica, strongly upregulates Sesn expression in response to desiccation, which is considered to be critical for survival of this species in the freezing Antarctic climate (Teets et al., 2012).
Oxidative stress
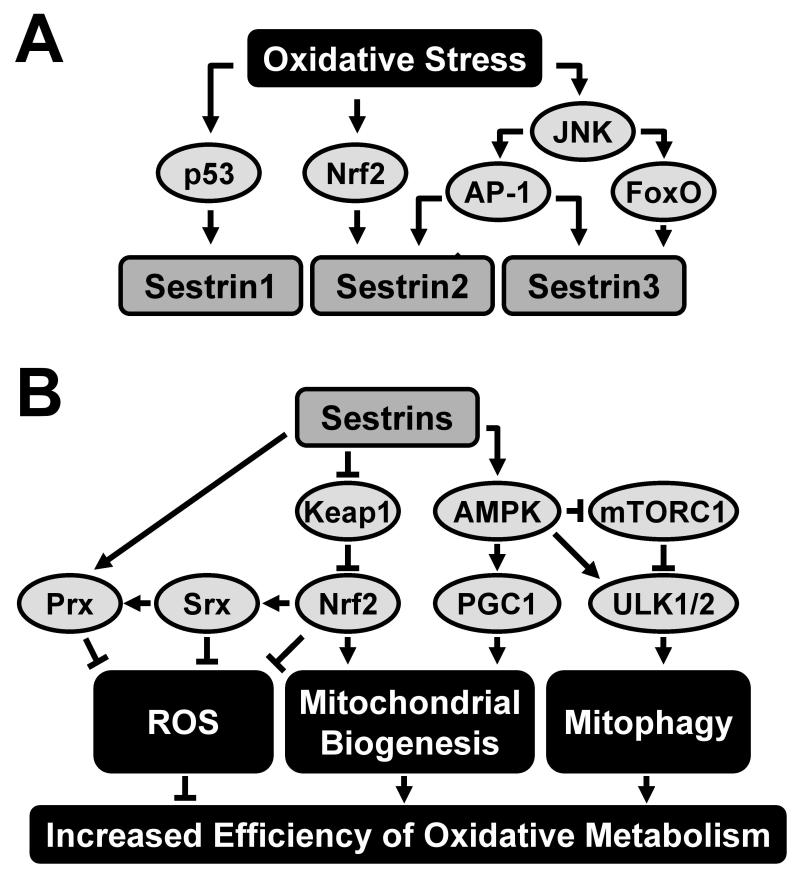
Oxidative stress reflects an imbalance in ROS and RNS metabolism and impairment of the cell’s ability to detoxify ROS, RNS and other reactive metabolic intermediates. All members of Sestrin family are induced by oxidative stress, although they are subject to different induction mechanisms (Figure 1A) (Budanov et al., 2004; Hagenbuchner et al., 2012; Nogueira et al., 2008). Sesn1 is induced by hydrogen peroxide in a p53-dependent manner, whereas induction of Sesn2 by oxidative stress is only partially p53-dependent (Sablina et al., 2005). In neurons, Sesn2 is induced upon NMDA receptor activation, which stimulates the production of ROS, in a c/EBPβ-dependent manner (Papadia et al., 2008). More recently, oxidative stress was found to induce Sesn2 via activation of transcription factor Nrf2 (Shin et al., 2012) and via the JNK-AP-1 signaling axis (Zhang et al., 2013). Binding sites for c/EBPβ, Nrf2 and AP-1 are all present in the Sesn2 promoter region. Sesn3 is stimulated by oxidative damage via activation of FoxO transcription factors (Chen et al., 2010; Hagenbuchner et al., 2012). Similarly, dSesn is regulated by a JNK-dFoxO signaling axis in response to chronic dTORC1-induced oxidative stress in Drosophila (Lee et al., 2010).
Figure 1. Regulation of Oxidative Metabolism by Sestrin-Family Proteins.
(A) Regulation of Sestrin expression by oxidative stress. Although p53 is essential for Sestrin1 expression after oxidative stress, it is dispensable for induction of Sestrin2 and Sestrin3. While Nrf2 and AP-1 are required for Sestrin2 induction, FoxO1 and FoxO3 are required for Sestrin3 induction upon oxidative stress. (B) Signaling pathways through which Sestrins control oxidative stress. Sestrins can recycle peroxiredoxin (Prx) as a part of an oxidoreductase enzyme complex that includes sulfiredoxin (Srx). Alternatively, Sestrins activate an antioxidant transcriptional program by stabilizing Nrf2 through removal of its inhibitor Keap1. Sestrin-induced AMPK activation can lead to activation of PPARγ coactivator 1α (PGC1α) resulting in increased mitochondrial biogenesis, whereas Sestrin-mediated AMPK activation can lead to upregulation of autophagy that removes dysfunctional mitochondria (mitophagy). Through these activities Sestrins decrease ROS accumulation and stimulate anti-oxidant defenses.
Hypernutrition, obesity and chronic mTORC1 activation
Hypernutrition and lack of exercise promote development of obesity and the metabolic syndrome, both of which have become very prevalent in modern societies. We observed that Sesn2 is uniquely induced upon obesity in multiple mouse tissues including liver and skeletal muscle (Lee et al., 2012a). Although the mechanism of this induction response is currently elusive, it is plausible that chronic mTORC1 activation, which is associated with hypernutrition, is involved. It has been previously shown in Drosophila that hyperactive dTORC1 results in dSesn induction through a ROS-JNK-dFoxO signaling pathway (Lee et al., 2010). In several human cancer cell lines, conditions that lead to chronic mTORC1 activation, such as prolonged treatments with insulin, IGF1 or serum, also lead to increased Sesn2 expression (Budanov, unpublished results). However, the mechanism of mammalian Sesn2 induction upon mTORC1 activation can be different from the one responsible for dSesn induction. For instance, unlike dSesn, the only mTORC1-inducible Sesn2 is not regulated by FoxOs in mammalian cells, whereas Sesn1 and Sesn3, which are not induced upon chronic mTORC1 activation or obesity (Lee et al., 2012a), are FoxO targets (Chen et al., 2010; Greer and Brunet, 2005). Therefore, alternative transcription factors and signaling pathways are likely to be involved in obesity- and chronic mTORC1-mediated Sesn2 induction in mammalian cells.
Sestrins and Oxidative Metabolism
As described above, all members of Sestrin family are induced by oxidative stress, which implicates their involvement in the metabolism of ROS and other reactive metabolites. Silencing any of Sesn1-3 by shRNA causes accumulation of ROS in various cell lines (Budanov et al., 2004; Nogueira et al., 2008), leading to DNA damage and chromosomal instability (Kopnin et al., 2007; Sablina et al., 2005) or cell death (Budanov et al., 2002; Budanov et al., 2004; Hagenbuchner et al., 2012; Nogueira et al., 2008). Inactivation of dSesn in Drosophila causes ROS accumulation and oxidative cell damage in skeletal muscle (Lee et al., 2010). Importantly, a small conserved region of the Sestrins (100-175 a.a in Sesn1) shows distant but traceable sequence homology to Mycobacterium tuberculosis AhpD protein (Budanov et al., 2004). AhpD is a critical component of the bacterial antioxidant defense system that regenerates overoxidized AhpC, a bacterial peroxiredoxin (Prx), through catalytic reduction. Similar to AhpD, the Sestrins interact with and promote regeneration of overoxidized Prx in mammalian cells (Budanov et al., 2004). Although the Sestrins do not exhibit direct catalytic activity towards Prx that leads to its reduction (Woo et al., 2009), they may promote the activity of other oxidoreductases, such as sulfiredoxin (Srx), that also regenerate Prx. Indeed, one recent study showed that Sestrins can increase Srx expression through activation of Nrf2 (Figure 1B) (Bae et al., 2013). Sestrin-dependent regulation of Prx is important for antioxidant defense in neurons and macrophages (Essler et al., 2009; Papadia et al., 2008).
Independently of their Prx-regulating activity, the Sestrins contribute to redox homeostasis through regulation of the AMPK-mTORC1 signaling pathway. Sestrins prevent mTORC1 hyperactivation that stimulates ROS production through its effects on metabolism and mitochondrial function (Lee et al., 2010). Sestrin-dependent activation of AMPK and suppression of mTORC1 activity are critical for maintaining basal autophagy (Maiuri et al., 2009). Thus, Sestrins can be important for autophagic elimination of dysfunctional mitochondria that leak electrons and produce pathogenic amounts of ROS (Ishihara et al., 2013). Correspondingly, dSesn deficiency in Drosophila results in accumulation of abnormal, ROS-producing, mitochondria in skeletal muscle (Lee et al., 2010). Importantly, ROS accumulation can be suppressed by a mutant dSesn that is unable to regenerate Prx but is able to inhibit mTORC1, indicating this property of the Sestrins is mediated through their effect on mTORC1. Indeed, ROS accumulation in Sestrin-deficient muscle was alleviated by pharmacological mTORC1 inhibitors (Lee et al., 2010). Sestrin-dependent inhibition of mTORC1 can be also important for autophagy-mediated degradation of Keap1, an inhibitor of Nrf2-dependent antioxidant gene expression (Bae et al., 2013). A recent report also demonstrated that, through AMPK activation, Sestrin2 can inhibit NADPH oxidase 4 (NOX4) that generates pathogenic amounts of cytosolic ROS (Eid et al., 2010; Eid et al., 2013). These findings suggest that the AMPK-mTORC1-regulating activity of Sestrins may be more physiologically significant than their Prx-regulating activities in preventing excessive ROS accumulation.
The Sestrins were also shown to mediate the antioxidant activities associated with the p53 and FoxO transcription factors (Hagenbuchner et al., 2012; Nogueira et al., 2008; Sablina et al., 2005). While high levels of oxidative stress can lead to cell death through p53- and FoxO-dependent apoptotic gene transcription, low levels of oxidative stress cause moderate activation of p53 and FoxO that can induce Sestrins to reduce oxidative stress and prevent cell death. Thus, Sestrins are important genetic components of a regulatory circuit that attenuates the detrimental consequences of oxidative stress and ensures cell viability and function. Sestrin2-mediated oxidative stress suppression can also be important for other physiological processes, as one recent study suggests that Sestrin2’s antioxidant function is important for decreasing neuropathic pain; Sesn2−/− mice exhibited highly increased neuropathic pain behavior after peripheral nerve injury, which was suppressible by antioxidant administration (Kallenborn-Gerhardt et al., 2013). Nevertheless, the exact role of Sestrins in oxidative stress adaptation still awaits more studies to be conducted with Sestrin-deficient mouse models.
Sestrins and Nutrient Signaling
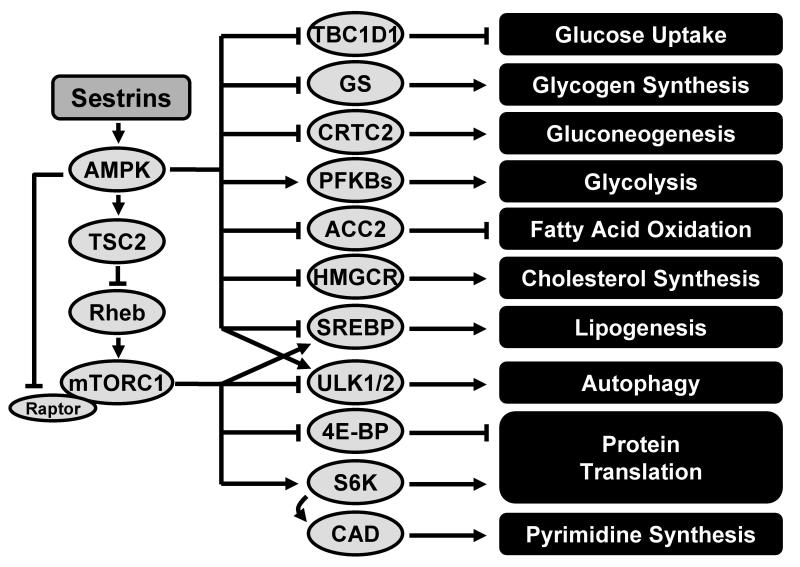
AMPK and mTORC1 are important nutrient-sensing protein kinases that have diametrically antagonistic functions in metabolic homeostasis (Hardie et al., 2012; Zoncu et al., 2011). AMPK phosphorylates diverse anabolic enzymes, such as acetyl-CoA carboxylase (ACC), glycerol phosphate acyl transferase (GPAT), 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) and glycogen synthase (GS), directly inhibiting their enzymatic activities in glycogen and lipids biosynthesis (Hardie et al., 2012). In addition to modulating these traditional target enzymes, AMPK inhibits the transcriptional activity of sterol regulatory element binding protein (SREBP) through direct phosphorylation, thereby decreasing lipogenic gene expression (Li et al., 2011). On the other hand, AMPK can phosphorylate and activate autophagy-initiating protein kinase ULK1/2, stimulating cellular autophagic catabolism (Egan et al., 2011; Kim et al., 2011). Importantly, AMPK can also inhibit mTORC1 activity through phosphorylation-dependent activation of tuberous sclerosis complex 2 (TSC2) and subsequent inhibition of the mTORC1-activating GTPase Rheb (Inoki et al., 2003). Another report shows that AMPK can directly inhibit mTORC1 through phosphorylation-dependent inhibition of its regulatory subunit Raptor (Gwinn et al., 2008). Through these activities, AMPK stimulates cellular catabolism of sugar, protein and lipids, while inhibiting anabolism.
In contrast to AMPK, mTORC1 activates anabolic processes while inhibiting catabolic processes. mTORC1 promotes protein translation by regulating its well-characterized substrates such as p70 S6 kinase (S6K) and eukaryotic translation initiation factor 4E-binding protein (4E-BP) (Zoncu et al., 2011). mTORC1 can also boost cellular lipid synthesis by activating SREBP, through suppression of the SREBP inhibitor Lipin-1 (Peterson et al., 2011). mTORC1 is also an important negative regulator of autophagy that inhibits the autophagy-initiating ULK1/2 complex by direct phosphorylation (Chan, 2009; Kim et al., 2011). Recent studies suggest that mTORC1 can also stimulate synthesis of pyrimidines, which can be used as building blocks for nucleic acids (Ben-Sahra et al., 2013b; Robitaille et al., 2013). Therefore, while subjected to AMPK-mediated suppression, mTORC1 stimulates anabolic processes that are antagonized by AMPK action. However, defects in the regulatory network that modulates the AMPK-mTORC1 balance in cells can lead to aberrant activation of mTORC1, culminating in age- and obesity-associated metabolic pathologies (Howell and Manning, 2011).
When induced in response to stress, Sestrins inhibit mTORC1 through activation of AMPK (Figure 2) (Budanov and Karin, 2008). Consequently, Sestrin-deficient cells and tissues exhibit lower AMPK and higher mTORC1 activities under both normal and stressed conditions (Budanov and Karin, 2008; Lee et al., 2010; Wempe et al., 2010). In mammalian cells, Sestrin2 is found within a high molecular weight protein complex containing TSC1, TSC2 and AMPK (Budanov and Karin, 2008). Although it is not yet clear how the Sestrins activate AMPK, it has been suggested that Sestrin2 can bring an upstream kinase LKB1 and regulatory AMPKβ/γ subunits to the catalytic AMPKα subunit, thereby facilitating LKB1-dependent activatory Thr172 phosphorylation of AMPKα (Sanli et al., 2012). However, Sestrin2-dependent AMPK activation was also observed in LKB1-deficient cells, such as HeLa cells (Wang and Karin, unpublished results), suggesting that Sestrin-induced AMPK activation can also be LKB1 independent.
Figure 2. Regulation of Nutrient-Sensing Signaling Pathways by Sestrins.
Sestrins control metabolism through AMPK and mTORC1. Sestrins potentiate AMPK activation and thereby suppress mTORC1 activity, leading to inhibition of cellular anabolism and augmentation of catabolic processes such as beta-oxidation and autophagy. Abbreviations: TBC1D1, TBC1 domain family member 1; GS, glycogen synthase; CRTC2, CREB regulated transcription coactivator 2; PKFB, fructose-6-phosphate kinase; ACC2, acetyl-coA carboxylase 2; HMGCR, HMG-CoA reductase; CAD, carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase.
Through regulation of the AMPK-mTORC1 signaling axis, the Sestrins promote metabolic adaptation of cells in response to diverse stress insults. Sesn1 and Sesn2, as p53-inducible genes, mediate DNA damage-induced activation of AMPK and suppression of mTORC1 (Budanov and Karin, 2008). Sesn1 and Sesn2 are also responsible for mitotic arrest-induced AMPK activation and mTORC1 inhibition (Rocha et al., 2011). The inhibition of mTORC1 activity leads to hypophosphorylation of S6K and 4E-BP, ultimately resulting in cessation of protein synthesis. Thus, Sesn1 and Sesn2 are essential for p53-mediated suppression of protein translation during genotoxic stress (Braunstein et al., 2009; Loayza-Puch et al., 2013). Induction of Sesn2 upon genotoxic stress can also promote autophagic catabolism, probably through the regulation of AMPK-mTORC1, enabling cells to obtain extra nutrients and energy sources (Maiuri et al., 2009). Both of these effects may be critical for suppressing cell growth during genotoxic stress and for channeling the saved energy to the DNA repair machinery, thereby promoting the survival of stressed cells. Similarly, Sesn3, as a FoxO-inducible protein, was shown to mediate oxidative stress-induced suppression of mTORC1 and be required for the maintenance of cellular energy stores during oxidative challenge (Chen et al., 2010). It was also shown that overexpressed Sesn1 and Sesn2 can protect cells from oxidative stress- and hypoxia-induced cell death (Budanov et al., 2002; Budanov et al., 2004). Therefore, the Sestrins can be viewed as stress-inducible regulators of cellular metabolism that ensure cell survival under stressful conditions.
At the organismal level, Sestrins’ roles in metabolic regulation are not limited to acute stresses. In Drosophila, dSesn acts as a physiological feedback regulator of dTORC1 that suppresses various age-associated metabolic pathologies (Lee et al., 2010). Loss of dSesn causes moderate downregulation of AMPK and upregulation of dTORC1 in the fat body, thus leading to increased expression of mRNAs encoding lipogenic enzymes, ultimately resulting in triglyceride accumulation. This excessive fat accumulation can be suppressed by pharmacological activation of AMPK and inhibition of dTORC1. Similarly in mice, Sesn2, as an obesity-inducible Sestrin, was found to be an important suppressor of liver fat accumulation (hepatosteatosis) upon dietary or genetically-induced obesity (Lee et al., 2012a). Sesn2 also suppresses acute hepatosteatosis induced by short-term feeding with a high-carbohydrate diet after prolonged fasting (Bae et al., 2013). Hepatosteatosis in the Sesn2-deficient mouse liver is associated with decreased beta-oxidation of lipids as well as with reduced mitochondrial mass and diminished hepatic autophagy. Sesn2 was also found to regulate SREBP-1 activity; however, hepatic de novo lipogenesis did not change very much upon loss of Sesn2. Importantly, hepatosteatosis in Sesn2-deficient mice can be corrected by pharmacological or virus-mediated restoration of AMPK activity, supporting a role for AMPK as a major mediator of Sestrin effects on metabolism and implying that Sestrin-dependent AMPK regulation is important for liver lipid homeostasis.
Although Sestrin-dependent regulation of protein synthesis through mTORC1 can be also important for the control of cell growth and proliferation, the Sestrins do not seem to act as bona fide cell growth regulators. Neither dSesn deficiency in Drosophila nor Sesn2/3 deficiency in mice results in increased body size or cell/tissue growth (Bae et al., 2013; Lee et al., 2010; Lee et al., 2012a). Nevertheless, the Sestrins’ role in protein metabolism and cell growth can still be detected under conditions of mTORC1 hyperactivation, when Sestrin expression is induced. For example, in Drosophila, dSesn suppresses hyperactive dTORC1-induced phosphorylation of dS6K and d4E-BP and subsequent tissue overgrowth (Lee et al., 2010). Moreover, Sesn2 suppresses cell growth and proliferation in many cancer cell lines (Budanov et al., 2002; Budanov and Karin, 2008). Therefore, it is plausible that Sestrin-dependent regulation of protein synthesis and cell growth can be important only in the context of hyperactive mTORC1, for instance during tumorigenesis, which will be further discussed below. Collectively, these results show that Sestrins are evolutionarily conserved regulators of protein and lipid metabolism acting through the AMPK-mTORC1 signaling axis.
Sestrins Prevent Insulin Resistance and Diabetes
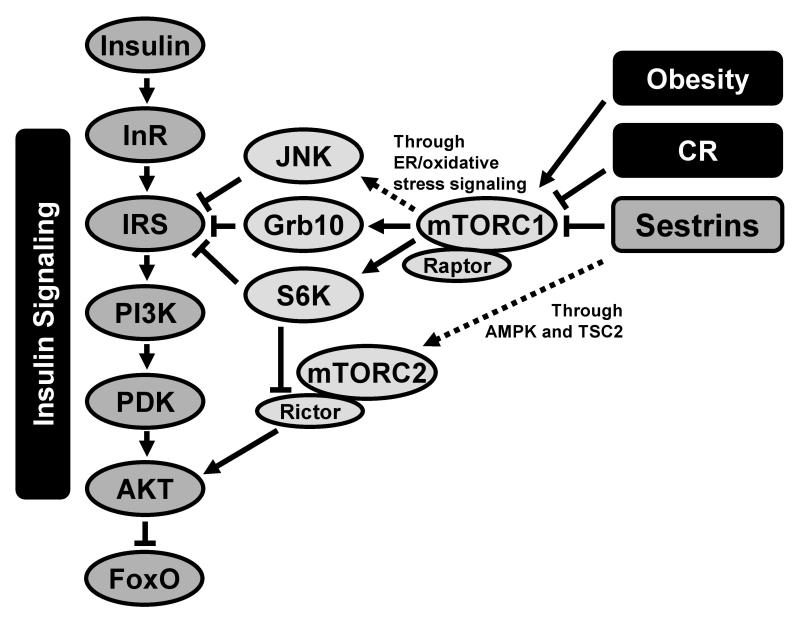
One of the most serious metabolic pathologies associated with chronic mTORC1 activation is insulin resistance and type II diabetes (Howell and Manning, 2011; Zoncu et al., 2011). Through its substrate S6K, mTORC1 activation results in inhibitory phosphorylation of insulin receptor substrates (IRS), thereby contributing to attenuation of insulin-induced phosphoinositide 3 kinase (PI3K)/AKT signal transduction and subsequent development of insulin resistance (Figure 3) (Um et al., 2004). Recently, it was found that mTORC1 also phosphorylates and activates growth factor receptor-bound protein 10 (Grb10), which acts as an inhibitor of the insulin-activated PI3K-AKT signaling pathway (Hsu et al., 2011; Yu et al., 2011). mTORC1-activated S6K also phosphorylates and inhibits Rictor, a component of the mTORC2 complex, that is critical for AKT activation (Julien et al., 2010; Treins et al., 2010). Chronic inhibition of autophagy in liver, which can be caused by prolonged mTORC1 activation, attenuates insulin signaling and induces insulin resistance (Yang et al., 2010). Prolonged mTORC1 activation in hepatocytes can also induce chronic endoplasmic reticulum (ER) stress (Ozcan et al., 2008), which can additionally contribute to development of insulin resistance (Ozcan et al., 2004). Thus, persistent activation of mTORC1 in response to prolonged hypernutrition can give rise to insulin resistance, elevate blood glucose and eventually drive the pathogenesis of type II diabetes.
Figure 3. Regulation of Insulin Signaling by Sestrins.
Sestrin-enhanced activation of AMPK liberates insulin signaling from mTORC1-mediated inhibitory effects. Abbreviations: CR, caloric restriction; InR, insulin receptor; IRS, insulin receptor substrate, PDK, phosphoinositide-dependent kinase; Grb10, Growth factor receptor-bound protein 10.
Importantly, during obesity and also under normal conditions, Sesn2-3 in mice and dSesn in Drosophila were all shown to be critical for maintenance of blood sugar homeostasis (Lee et al., 2012a). Especially, Sesn2, whose expression in liver is induced upon hypernutrition, is important for inhibition of chronic mTORC1 and suppression of insulin resistance in hepatocytes. Sesn2 is also important for the maintenance of insulin responsiveness in adipose tissue. Consequently, Sesn2-deficient mice exhibit impaired glucose homeostasis upon either dietary or genetic obesity, which is mostly due to defects in insulin-mediated suppression of hepatic glucose production. Although Sesn2-deficient mice show no apparent defects in glucose homeostasis on a normal chow diet, concomitant deletion of Sesn2 and Sesn3 renders mice susceptible to spontaneous development of hepatic insulin resistance associated with elevated hepatic mTORC1 activity, even without nutritional overload or obesity. Similarly, dSesn-null Drosophila exhibit an elevated hemolymph trehalose level on a normal diet (Lee et al., 2012). These results indicate that Sestrin-dependent regulation of blood sugar homeostasis is physiologically important and evolutionarily conserved.
It has been also shown in diverse cultured cell lines that Sestrins, including Sesn1-3 and dSesn, can potentiate PI3K-AKT signal transduction even in the absence of insulin (Lee et al., 2012a). Sestrin-induced activation of AKT is dependent on AMPK, TSC2 and Rictor, suggesting that Sestrins upregulate AKT signaling through AMPK and mTORC2. This further suggests that Sestrins can modulate the balance of signaling activity between mTORC1 and mTORC2 through AMPK. Indeed, Sestrins potently inhibit the mTORC1-p70S6K pathway while slightly upregulating the mTORC2-AKT pathway. This very property differentiates the Sestrins from common pharmacological mTOR inhibitors, such as rapamycin, Torin and PP242, which inhibit both mTORC1 and mTORC2 when administered for a long term. Notably pharmacological mTOR inhibitors were found to be inappropriate for treatment of obesity-associated diabetes and other metabolic pathologies because they promote insulin resistance through mTORC2 inhibition (Lamming et al., 2012). Therefore, modulation of Sestrins activity may provide an alternative approach to prevention of obesity, insulin resistance and diabetes through attenuation of mTORC1 and potentiation of mTORC2. These findings also suggest that endogenous Sestrins are physiologically important in keeping mTORC1 activity low and mTORC2-AKT activity high, which is critical for avoiding insulin resistance and other metabolic derangements.
Sestrins Attenuate Aging
Deregulated nutrient signaling, loss of protein homeostasis and accumulation of oxidative stress are hallmarks of aging (Lopez-Otin et al., 2013). Activation of AMPK, suppression of mTORC1 and stimulation of autophagic signaling were all shown to be beneficial for extending both lifespan and healthspan (Gelino and Hansen, 2012; Harrison et al., 2009; Mair et al., 2011; Miller et al., 2011; Wilkinson et al., 2012). Thus, it seems plausible that the antioxidant, AMPK-activating, mTORC1-suppressing and autophagy-inducing abilities of the Sestrins also contribute to the attenuation of aging and suppression of age-associated diseases. Indeed, Drosophila and mouse models of Sesn deficiencies demonstrate that endogenous Sestrin activity is required to prevent diverse age- and obesity-associated pathologies. Inactivation of dSesn in Drosophila leads to chronic suppression of AMPK and activation of mTORC1, resulting in fat accumulation, blood sugar elevation, and skeletal/cardiac muscle degeneration (Lee et al., 2010). As summarized in the preceding sections, defective lipid and blood sugar homeostasis, which are hallmarks of age-associated metabolic derangements, are observed in both dSesn-null Drosophila and Sesn2/3-knockout mice (Lee et al., 2010; Lee et al., 2012a).
Notably, the cardiac and skeletal muscle phenotypes of the dSesn-null flies closely resemble the degenerative features of age-associated myopathies and cardiomyopathies (Lee et al., 2010). dSesn-null flies show irregularity of heart beat (arrhythmia), cardiac dilation, and decreased heart rate, ultimately leading to decreased cardiac function. The thoracic skeletal muscles of dSesn-null flies exhibit disorganized sarcomeric structure, dysfunctional mitochondria, accumulation of protein aggregates and oxidative stress. Interestingly, similar deterioration of cardiac/skeletal muscle is observed in flies with inactivation of ATG1, a critical regulator of autophagy, implying that regulation of autophagy is at least one mechanism that contributes to dSesn’s myoprotective function (Lee et al., 2010). These phenotypes observed in young dSesn or Atg1 mutant flies (~20 days old) are also similar to the ones typically seen in very old WT flies (~90 days old). Many of the detrimental consequences of dSesn inactivation are prevented by treatment with pharmacological AMPK activators or mTORC1 inhibitors, suggesting that dSesn attenuates tissue aging through modulation of the AMPK-mTORC1 axis (Lee et al., 2010). The degenerative muscle phenotypes were also exhibited by mutant C. elegans deficient in cSesn, suggesting that the role of Sestrins in muscle is evolutionarily conserved (Table 1) (Yang et al., 2013). It is yet to be determined if the mammalian Sestrins are similarly involved in cardiac and skeletal muscle homeostasis during aging and other pathological conditions.
Table 1. Phenotypes of Sestrin-Modified Genetic Model Organisms.
Genetic studies done in C. elegans (Yang et al., 2013), Drosophila (Edwards et al., 2009; Lee et al., 2010) and mice (Bae et al., 2013; Kallenborn-Gerhardt et al., 2013; Lee et al., 2012a; Wempe et al., 2010) suggest that Sestrin-family proteins are critical for suppressing age- and obesity-associated metabolic pathologies in diverse organ systems. Abbreviations: COPD, chronic obstructive pulmonary disease; HFD, high fat diet.
| Organism | Gene(s) mutated |
Nature of mutation(s) |
Context | Phenotype | Reference |
|---|---|---|---|---|---|
|
| |||||
| C. elegans |
cSesn (Sesn-1 or Y74C9A.5) |
Loss of function | Normal aging | Reduced lifespan | Yang et al., 2013 |
| Reduced locomotor activity | |||||
| Increased muscle ROS | |||||
| Muscle actin disorganization | |||||
| Bacterial infection | Normal resistance | ||||
| Oxidative stress (H2O2) | Reduced resistance | ||||
| Heat stress (35°C) | Reduced resistance | ||||
| Heavy metal stress (CuSO4) | Reduced resistance | ||||
| Gain of function | Normal aging | Increased lifespan | Yang et al., 2013 | ||
| Increased locomotor activity | |||||
| Decreased muscle ROS | |||||
| Oxidative stress (H2O2) | Increased resistance | ||||
| Heat stress (35°C) | Increased resistance | ||||
| Heavy metal stress (CuSO4) | Increased resistance | ||||
|
| |||||
| Drosophila |
dSesn
(Sesn or CG11299) |
Loss of function | Normal aging | Less aggressivity | Edwards et al., 2009 |
| Increased fat accumulation | Lee et al., 2010 | ||||
| Cardiac dilation | |||||
| Decreased/arrhythmic heartbeat | |||||
| Muscle degeneration | |||||
| Increased muscle ROS | |||||
| Protein aggregate formation | |||||
| Increased mTORC1 signaling | |||||
| Gain of function | Developing imaginal disc | Growth suppression | Lee et al., 2010 | ||
| Decreased mTORC1 signaling | |||||
|
| |||||
| Mouse |
Sesn2
(Hi-95) |
Loss of function | Normal aging | Normal body weight | Lee et al., 2012 |
| Normal glucose tolerance | |||||
| Normal insulin sensitivity | |||||
| Normal liver fat accumulation | |||||
| Normal mTORC1 signaling | |||||
| Nerve injury | Increased ROS accumulation | Kallenborn-Gerhardt et al., 2013 | |||
| Increased neuropathic pain | |||||
| Mouse model of COPD | Reduced emphysema | Wempe et al., 2010 | |||
| Increased ROS accumulation | |||||
| Increased mTORC1 signaling | |||||
| Reduced TGFβ signaling | |||||
| Fasting | Reduced autophagy | Bae et al., 2013 | |||
| Fasting/Refeeding | Increased hepatosteatosis | ||||
| Increased ROS accumulation | |||||
| Increased liver damage | |||||
| Increased mTORC1 signaling | |||||
| HFD-induced obesity | Body weight same as WT (con) | Lee et al., 2012 | |||
| Increased hepatosteatosis | |||||
| Increased glucose intolerance | |||||
| Increased insulin resistance | |||||
| Increased mTORC1 signaling | |||||
| Lepob-induced obesity | Body weight same as WT (con) | ||||
| Increased hepatosteatosis | |||||
| Increased glucose intolerance | |||||
| Increased insulin resistance | |||||
| Increased mTORC1 signaling | |||||
| Sesn2 & Sesn3 | Loss of function | Normal aging | Unaltered body weight | Lee et al., 2012 | |
| Increased glucose intolerance | |||||
| Increased insulin resistance | |||||
| Increased mTORC1 signaling | |||||
It should be noted that the DNA damage-sensing pathway composed of ATM and p53 is important for expression of endogenous Sestrins and regulation of metabolic homeostasis during aging. The p53Ser15Ala knock-in mouse, which shows dramatic downregulation of Sesn1-3 in liver, exhibits early onset hepatic insulin resistance and hyperglycemia (Armata et al., 2010). On the other hand, p53-overexpressing mice, which have elevated Sesn1 and Sesn2 expression in liver, show significantly increased longevity and a delayed onset of age-associated metabolic pathologies (Matheu et al., 2007). Although both studies attribute the metabolic phenotypes to the modulation of Sestrins’ redox-regulating activities, it should be examined whether Sestrin-dependent regulation of the AMPK-mTOR axis provides a better explanation to the anti-diabetic and anti-aging activities of the ATM-p53 pathway. Regardless of the molecular mechanisms operating downstream of the Sestrins, it is highly likely that the Sestrins mediate the metabolic output of the DNA damage-sensing pathway, which is abrogated during tumorigenesis, aging and metabolic pathologies.
Notably, current data from Drosophila and C. elegans indicate that the association between Sestrin and lifespan is not as strong as the antagonistic relationship between Sestrin and age-associated pathologies. We and others observed that the lifespan of dSesn-null flies under normal conditions is not significantly different from that of WT flies (Lee and Karin, unpublished results; P. Kapahi, personal communication). The lifespan of cSesn-null worms was only slightly shorter than that of WT worms (Yang et al., 2013). This weak association between Sestrins and lifespan is in stark contrast to the robust linkage between Sestrins and age-associated metabolic pathologies. However, according to recent studies, most (if not all) of the age-associated deaths in flies and worms are caused by, or associated with, intestinal disintegration (McGee et al., 2011; Rera et al., 2012). As Sestrins may not play major roles in regulating intestinal barrier function, it is not very surprising that Sestrin-deficient invertebrates show almost normal lifespan. However, because Sestrin mutants show strong age-associated pathologies in other metabolic organs such as fat body, liver and skeletal/cardiac muscle in virtually all of the model organisms examined, including C. elegans, Drosophila and mice, Sestrins still can be considered as an important determinants of healthspan, if not lifespan. It should be also noted that, in mammals, the cause of age-related death is different from invertebrates: cardiac dysfunction and malignant neoplasms are the most significant causes of age-related death in humans. Cardiac dysfunction in Drosophila is not immediately associated with organismal death (Ocorr et al., 2007) and C. elegans even does not have a distinct cardiac structure. Death caused by malignancy also does not normally occur in flies or in worms. Because Sestrins are suppressors of age-associated cardiac pathologies and tumorigenesis, it is still plausible that Sestrins regulate lifespan as well as healthspan in mammals.
Sestrins and Cancer
Advanced age is the major risk factor for cancer. As a result, cancer is one of the most significant causes of age-related human death. Cancer is a disease tightly linked to metabolic dysregulation, oxidative stress and genomic instability. Given that Sestrins are important for suppressing oxidative damage, inactivation of Sestrins and accumulation of ROS and RNS might contribute to carcinogenesis. Accordingly, silencing of Sestrins in cancer cells stimulates mutagenesis, genomic instability and growth of tumor xenografts; features that are largely eliminated by antioxidant treatment (Sablina et al., 2005). In addition, as suppressors of mTORC1 activity, Sestrins can inhibit cancer cell growth and therefore may be preferentially lost during tumorigenesis. Indeed, Sesn1 and Sesn2 are generally downregulated in cancer, probably due to the inactivation of their master regulator-p53 (Loayza-Puch et al., 2013). Constitutively active Ras oncogene also downregulates Sesn1 and Sesn3 (Kopnin et al., 2007). Although the mechanism of Ras-induced suppression has not been determined, it has been recently shown that the transcription factor HSF1 mediates Sesn3 regulation by Ras (Zamkova et al., 2013). Loss-of-heterozygocity in Sesn1 (6q21) and Sesn2 (1p35) is also often observed in diverse human cancers (Ragnarsson et al., 1999; Velasco-Miguel et al., 1999). A mutation in the Sesn2 gene (Pro87Ser) was recently identified as a cancer-driving mutation in myeloproliferative neoplasms through genome sequencing (Hou et al., 2012), although how this mutation specifically affects Sestrin2 function is not yet known. Another mechanism that can lead to Sestrin depletion has been described in endometrial cancers where Sesn3 is heavily methylated in 20% of tumors (Zighelboim et al., 2007).
Despite their involvement in tumor suppression and genome protection, Sestrins are still expressed in many cancers (Budanov et al., 2002) and might actually be required for maintaining the viability of cancer cells under certain conditions. Very high levels of oxidative stress, associated with chronic inflammation, prolonged growth factor signaling and dysfunctional mitochondria, can be detrimental for the survival and propagation of cancer cells. For this reason, many cancer cells upregulate the expression of antioxidant proteins, and Sestrins are part of the antioxidant defense system. Another potential benefit to keep Sestrin function intact is activation of autophagy (Ishihara et al., 2013; Maiuri et al., 2009), which can support tumor growth and metabolism during conditions of limited nutrient and oxygen supply. Finally, Sestrin-dependent activation of mTORC2-AKT (Lee et al., 2012a), a well recognized oncogenic pathway, might contribute to carcinogenesis through promotion of cancer cell proliferation and survival.
Being stress-responsive genes, Sestrins can also contribute to the effects of cytotoxic anticancer therapies. Sestrins were shown to promote DNA damage-induced cell death upon irradiation and genotoxic drug treatments through a poorly understood mechanism that may be related to the inhibition of mTORC1 (Sanli et al., 2012). On the other hand, Sestrins may hamper oxidative damage-associated chemotherapy by promoting cancer cell survival (Budanov et al., 2002; Budanov et al., 2004; Hagenbuchner et al., 2012). Furthermore, it is also possible that hypoxia-induced Sesn2 may contribute to the well-known resistance of hypoxic cancer cells to radiotherapy and chemotherapy. Thus the Sestrins might be important modulators of the outcome of cancer therapy, although whether they are advantageous or disadvantageous would have to be validated in various cancer models. In any case, it is probable that Sestrins may serve as predictive markers for the efficiency of cancer therapy and help choose the best strategy for treatment. Modulation of Sestrin expression or activity by small molecules might improve the efficiency of radiotherapy or chemotherapy.
Unanswered Questions and Future Directions
Apparently, there are many remaining and unanswered questions regarding Sestrin biology and biochemistry. First and foremost, the chemical basis of Sestrins’ antioxidant and AMPK-activating/mTORC1-inhibiting activities should be clarified. Elucidation of Sestrin structure and determination of critical amino acid residues should be one of the first steps towards a better understanding of the biochemical properties of Sestrin proteins. These studies would also facilitate future development of Sestrin-mimicking small molecules. Second, we need to understand how Sestrin expression is regulated in response to metabolic stressors, such as hypernutrition, energy depletion and ER stress. Third, the detailed molecular mechanisms through which Sestrin induction normalizes metabolic derangements during obesity and normal aging should be determined. Fourth, the role of mammalian Sestrin1 needs to be genetically determined. Because Sestrin1 is highly enriched in skeletal muscle (Peeters et al., 2003), it is plausible that it may be analogous to cSestrin and dSestrin in control of muscle homeostasis (Lee et al., 2010; Yang et al., 2013). However, to test this hypothesis, muscle-specific Sesn1-knockout mice are needed. It would be also interesting to see if Sestrin1 and other mammalian Sestrins are required for preventing cardiac pathologies, as in Drosophila (Lee et al., 2010). Finally, it is important to determine and evaluate the impact of mammalian Sestrins on the regulation of aging, carcinogenesis, the metabolic syndrome and other diseases. The recently generated Sestrin-deficient mouse strains would provide important tools for determining the role of each Sestrin in diverse physiological contexts. Through these future studies, we will be able to understand how Sestrins regulate aging and metabolism in mammals and whether their antioxidant function, effects on AMPK-mTORC1 signaling, or some yet-to-be-defined activities are most important.
Conclusion
Chronic stress, metabolic derangements and organ dysfunction are hallmarks of aging, obesity and their co-morbidities. Formerly discovered as stress-responsive and p53-regulated proteins, the Sestrins are now recognized as important regulators of metabolism that ensure physiological homeostasis, suppression of age- and obesity-associated diseases, muscle degeneration, cardiac malfunction and cancer. Consequently, the Sestrins may be imminent therapeutic targets for attenuation of aging and prevention of cancer and obesity-related metabolic derangements.
Acknowledgements
Work was supported by grants and fellowships from the NIH (CA118165, CA155120, and P42-ES010337 to M.K., DK082080 and CA172660 to A.B., and AG045432, P30-AG013283, P30-AG024824 and P30-DK089503 to J.H.L.), Ellison Medical Foundation (AG-SS-2440-10 to M.K. and AG-NS-0932-12 to J.H.L.), American Diabetes Association (7-08-MN-29 to M.K. and 1-13-BS-106 to J.H.L.) and American Association for the Study of Liver Diseases/American Liver Foundation (to J.H.L). M.K. is an American Cancer Society Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases. We thank Myungjin Kim and Haeli Park for help in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armata HL, Golebiowski D, Jung DY, Ko HJ, Kim JK, Sluss HK. Requirement of the ATM/p53 Tumor Suppressor Pathway for Glucose Homeostasis. Mol Cell Biol. 2010;30:5787–5794. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, Tanti JF, Bost F. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 2013a;20:611–619. doi: 10.1038/cdd.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013b;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29:5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal. 2009;2:e51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Zwarts L, Yamamoto A, Callaerts P, Mackay TF. Mutations in many genes affect aggressive behavior in Drosophila melanogaster. BMC Biol. 2009;7:29. doi: 10.1186/1741-7007-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent eNOS uncoupling and matrix protein expression. Mol Cell Biol. 2013 doi: 10.1128/MCB.00217-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler S, Dehne N, Brune B. Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett. 2009;583:3531–3535. doi: 10.1016/j.febslet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Gelino S, Hansen M. Autophagy - An Emerging Anti-Aging Mechanism. J Clin Exp Pathol. 2012;(Suppl 4) doi: 10.4172/2161-0681.s4-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuchner J, Kuznetsov A, Hermann M, Hausott B, Obexer P, Ausserlechner MJ. FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci. 2012;125:1191–1203. doi: 10.1242/jcs.092098. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Song L, Zhu P, Zhang B, Tao Y, Xu X, Li F, Wu K, Liang J, Shao D, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell. 2012;148:873–885. doi: 10.1016/j.cell.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, et al. Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013 doi: 10.1152/ajprenal.00642.2012. In press. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenborn-Gerhardt W, Lu R, Syhr KM, Heidler J, von Melchner H, Geisslinger G, Bangsow T, Schmidtko A. Antioxidant Activity of Sestrin 2 Controls Neuropathic Pain After Peripheral Nerve Injury. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.4958. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Talukdar S, Park EJ, Park H, Park H-W, Bandyopadhyay G, Li N, Aghajan M, Jang I, et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012a;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012b;31:3265–3276. doi: 10.1038/onc.2011.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013;14:R32. doi: 10.1186/gb-2013-14-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, Hall DH, Melov S. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell. 2011;10:699–710. doi: 10.1111/j.1474-9726.2011.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr KA, Crawley T, Gibson G, Bodmer R. Genetic variation for cardiac dysfunction in Drosophila. PLoS One. 2007;2:e601. doi: 10.1371/journal.pone.0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N, Hristova M, Heintz NH, Lounsbury KM, van der Vliet A. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2011;301:L993–L1002. doi: 10.1152/ajplung.00250.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters H, Debeer P, Bairoch A, Wilquet V, Huysmans C, Parthoens E, Fryns JP, Gewillig M, Nakamura Y, Niikawa N, et al. PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Hum Genet. 2003;112:573–580. doi: 10.1007/s00439-003-0917-5. [DOI] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnarsson G, Eiriksdottir G, Johannsdottir JT, Jonasson JG, Egilsson V, Ingvarsson S. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br J Cancer. 1999;79:1468–1474. doi: 10.1038/sj.bjc.6690234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- Rocha GZ, Dias MM, Ropelle ER, Osorio-Costa F, Rossato FA, Vercesi AE, Saad MJ, Carvalheira JB. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17:3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS One. 2012;7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BY, Jin SH, Cho IJ, Ki SH. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med. 2012;53:834–841. doi: 10.1016/j.freeradbiomed.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Teets NM, Peyton JT, Colinet H, Renault D, Kelley JL, Kawarasaki Y, Lee RE, Jr., Denlinger DL. Gene expression changes governing extreme dehydration tolerance in an Antarctic insect. Proc Natl Acad Sci U S A. 2012;109:20744–20749. doi: 10.1073/pnas.1218661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–1016. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Wempe F, De-Zolt S, Koli K, Bangsow T, Parajuli N, Dumitrascu R, Sterner-Kock A, Weissmann N, Keski-Oja J, von Melchner H. Inactivation of sestrin 2 induces TGF-beta signaling and partially rescues pulmonary emphysema in a mouse model of COPD. Dis Model Mech. 2010;3:246–253. doi: 10.1242/dmm.004234. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HA, Bae SH, Park S, Rhee SG. Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins. Antioxid Redox Signal. 2009;11:739–745. doi: 10.1089/ars.2008.2360. [DOI] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Loh KS, Liou BY, Chu IH, Kuo CJ, Chen HD, Chen CS. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp Gerontol. 2013;48:371–379. doi: 10.1016/j.exger.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkova M, Khromova N, Kopnin BP, Kopnin P. Ras-induced ROS upregulation affecting cell proliferation is connected with cell type-specific alterations of HSF1/SESN3/p21 (Cip1/WAF1) pathways. Cell Cycle. 2013;12:826–836. doi: 10.4161/cc.23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Wu XQ, Deng R, Sun T, Feng GK, Zhu XF. Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal. 2013;25:150–158. doi: 10.1016/j.cellsig.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Zighelboim I, Goodfellow PJ, Schmidt AP, Walls KC, Mallon MA, Mutch DG, Yan PS, Huang TH, Powell MA. Differential methylation hybridization array of endometrial cancers reveals two novel cancer-specific methylation markers. Clin Cancer Res. 2007;13:2882–2889. doi: 10.1158/1078-0432.CCR-06-2367. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]