Abstract
Objectives
To determine whether the presence of angiographic coronary collaterals is a predictor of long-term clinical outcomes in patients with non-ST elevation myocardial infarction (NSTEMI).
Background
The presence of coronary collaterals on angiography provides prognostic information in patients with STEMI, but it is unknown whether they provide prognostic information in patients with NSTEMI.
Methods
This was a prospective cohort study of 931 consecutive patients undergoing coronary angiography of which 269 (29%) had a NSTEMI. Baseline characteristics, angiographic details, and long-term clinical outcomes including death, recurrent MI, coronary artery bypass graft surgery (CABG), percutaneous coronary intervention (PCI), stroke, and congestive heart failure (CHF) were collected. Each clinical outcome as well as the combined endpoint of death, recurrent MI, CABG, PCI stroke and CHF was compared in subjects with and without collaterals.
Results
At one year, individuals with collaterals had significantly increased rates of the combined endpoint compared to those without (25% vs 16%, p=0.0001). On multivariate analysis, the presence of collaterals was a strong predictor of the combined endpoint of death, recurrent MI, CABG, PCI, stroke and CHF (HR 1.95, CI 95% 1.08–3.52; p=0.027). Similarly, in the subset of 115 patients (43%) in whom the culprit artery was identified, the presence of collaterals was a strong negative predictor (HR 3.71, CI 1.31–10.57, p=0.014), driven by a 13-fold increase in subsequent CABG.
Conclusions
In patients with NSTEMI the presence of angiographic coronary collaterals is a predictor of long-term clinical outcomes primarily driven by increased rates of surgical revascularization.
Indexing words: coronary collaterals, non-ST elevation myocardial infarction
Introduction
Recent trends in the incidence of acute myocardial infarction show that while rates of ST elevation myocardial infarction (MI) have decreased, those of non-ST elevation MI (NSTEMI) have increased by nearly 25% over the past decade.1 While patients with NSTEMI and STEMI share similar cardiac risk factors, their epidemiologic features and clinical presentations are distinct and warrant different management strategies.2 Despite these differences, the long-term clinical outcomes of patients presenting with NSTEMI are quite similar to those presenting with STEMI.3 Thus, identifying clinical and angiographic factors that effect prognosis in patients with NSTEMI is important in this large and growing patient population. In STEMI patients, for example, the presence of angiographic coronary collaterals is associated with smaller infarct size, better left ventricular function 4–8, and decreased incidence of heart failure and need for intra-aortic balloon pump.9 However, investigators studying the significance of collateral flow to the infarct-related artery on long-term clinical outcomes in STEMI have reported no benefit in some 10,11, and worse outcomes in others.12,13 To date, one study evaluating the effect of collaterals on long-term clinical outcome in patients with NSTEMI reported favorable outcomes, but only in those with an occluded culprit artery.14 We performed this study to determine whether the presence of angiographic coronary collaterals is a predictor of long-term outcomes in patients with NSTEMI.
Methods
We prospectively enrolled subjects undergoing coronary angiography at our institution from May 1, 2007 to November 30, 2010, who agreed to participate in the study. We collected baseline clinical, angiographic, and laboratory data and entered it into a computerized registry database. The study was approved by the Institutional Review Board and all subjects provided informed consent. Subjects were contacted via telephone at 6 months and one year to obtain clinical follow-up information including recurrent MI, the need for percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG), the development of congestive heart failure (CHF), stroke and death. Reports of clinical outcomes were verified by chart review. From this registry database we identified patients referred for coronary angiography during their index hospitalization for NSTEMI (defined by chest pain, a positive troponin I level (defined in our clinical laboratory as >0.02 ng/mL), and no evidence of ST segment elevation on 12-lead electrocardiogram).
Selective coronary angiography was performed in multiple orthogonal views using standard techniques. Angiograms for all NSTEMI patients were reviewed independently by two investigators blinded to the clinical data for determination of the culprit artery and for information regarding the presence and extent of angiographic coronary collaterals. The culprit artery was defined as one which had evidence of a complex lesion suggestive of acute plaque rupture including: an intraluminal filling defect, ulcer with overhanging edges, extraluminal contrast, dissection, multiple irregularities within the artery, or acute occlusion as described previously.15 An occlusion was considered acute if it showed an abrupt cut-off with a squared off or convex pattern 15 and was considered chronic if it tapered smoothly to supply a terminal side branch with brisk runoff.16 The presence of collaterals was determined according to the Rentrop score.17 Flow in the culprit artery was visually estimated by the method used in the Thrombolysis in Myocardial Infarction (TIMI) trials and graded on a scale of 0 to 3.18 Discrepancies in angiographic findings were refereed by a third blinded investigator and data reflecting agreement from 2 of the 3 readers was used for analysis. The primary outcome was as a composite endpoint of death, recurrent MI, PCI, CABG, stroke, and CHF. Data regarding left ventricular systolic function assessed by left ventriculography or peri-procedure transthoracic echocardiography, when available, was collected. Normal left ventricular systolic function was defined as an ejection fraction of ≥55%, mild to moderately depressed left ventricular function was defined as an ejection fraction of 40–54%, and severely depressed left ventricular systolic function was defined as an ejection fraction of <40%.
Statistical Methods
The baseline characteristics were analyzed using Wilcoxon rank-sum test for continuous variables and Chi-Square test or Fisher’s Exact test for categorical variables. Kaplan Meier Failure estimates were reported at 365 days for the endpoints. Hazard ratios, p values and confidence intervals were reported using a Cox Proportional hazards regression model for both unadjusted and adjusted models. Multivariable analysis for the endpoints were adjusted using age, gender, history of diabetes, history of CHF, history of MI, extent and severity of coronary artery disease, peak troponin I level, and initial culprit artery Thrombolysis in Myocardial Infarction (TIMI) flow (in those in whom the culprit artery could be determined). The Schoenfeld residuals test was used to test the proportionality of the hazards. None of the models violated the proportional hazards assumption. All analyses were performed using Stata/IC version 10.1 (Statacorp).
Results
Over the study period, a total of 931 patients undergoing coronary angiography agreed to enroll in the research registry. Of these 931 patients, 269 (29%) were diagnosed with NSTEMI and form our patient population. The baseline characteristics and angiographic information for these 269 subjects are shown in Table 1. Patients with collaterals were disproportionately men and had a history of prior CABG and peripheral arterial disease. In addition, those with collaterals had significantly higher LDL cholesterol and were less often on chronic angiotensin converting-enzyme inhibitor therapy (Table 1). Left ventricular systolic function was available in 74% of the patients either by left ventriculography or transthoracic echocardiography performed in the peri-procedure period. Left ventricular systolic function data was not available in 45 of the 173 patients without collaterals (26%), and 25 of the 96 patients with collaterals (26%). There was no significant difference in left ventricular ejection fractions in those with and without collaterals. Subjects with collaterals, however, had more extensive and severe coronary artery disease compared to patients without collaterals (Table 2).
Table 1.
Baseline characteristics of patients according to the presence or absence of angiographic collaterals
| Characteristic | No Collaterals (n=173) | Collaterals (n=96) | P value |
|---|---|---|---|
| Age (years) mean +/− SD | 60 (+/− 12) | 62 (+/− 11) | 0.086 |
| Men | 116 (67%) | 76 (79%) | 0.035 |
| Hypertension * | 138 (80%) | 71 (74%) | 0.273 |
| Hyperlipidemia † | 138 (80%) | 82 (85%) | 0.25 |
| Diabetes mellitus | 63 (36%) | 31 (32%) | 0.497 |
| Current smoker | 57 (33%) | 26 (27%) | 0.318 |
| Former smoker | 49 (28%) | 37 (39%) | 0.085 |
| Prior angina | 76 (44%) | 34 (36%) | 0.195 |
| Prior MI | 54 (31%) | 27 (28%) | 0.597 |
| Prior PCI | 51 (30%) | 25 (26%) | 0.530 |
| Prior CABG | 10 (6%) | 16 (17%) | 0.004 |
| History of CHF | 26 (15%) | 11 (11%) | 0.415 |
| History of arrhythmia | 11 (6%) | 7 (7%) | 0.769 |
| Prior stroke | 12 (7%) | 9 (9%) | 0.475 |
| Family history of CAD | 75 (44%) | 46 (48%) | 0.496 |
| Peripheral arterial disease | 23 (13%) | 23 (24%) | 0.026 |
| Left ventricular ejection fraction ‡ | 0.872 | ||
| Normal | 90 (52%) | 49 (51%) | |
| Mild-moderately depressed | 30 (17%) | 20 (21%) | |
| Severely depressed | 8 (5%) | 2 (2%) | |
| Labs | |||
| Total cholesterol (mg/dL) | 159 [136–196] | 170 [143–235] | 0.073 |
| LDL (mg/dL) | 95 [77–124] | 108 [87–147] | 0.025 |
| HDL (mg/dL) | 35 [27–41] | 34 [28–43] | 0.886 |
| Triglycerides (mg/dL) | 124 [79–199] | 139 [86–211] | 0.429 |
| Troponin I (ng/mL) | 0.98 [0.13–4.88] | 1.0 [0.16–8.38] | 0.515 |
| Medications | |||
| Aspirin | 157 (91%) | 87 (91%) | 0.857 |
| Clopidogrel | 68 (40%) | 39 (41%) | 0.891 |
| Statin | 147 (86%) | 81 (84%) | 0.724 |
| Oral Hypoglycemic | 20 (12%) | 9 (9%) | 0.559 |
| Insulin | 36 (21%) | 25 (26%) | 0.339 |
| Beta Blocker | 128 (75%) | 75 (78%) | 0.602 |
| Calcium Channel Blocker | 17 (10%) | 10 (10%) | 0.902 |
| ACE-Inhibitor | 99 (58%) | 40 (42%) | 0.009 |
Data are expressed as mean ± standard deviation (SD), median [25–75% interquartile range (IQR)], or as number (percentage), ACE= angiotensin converting-enzyme, CABG= coronary artery bypass graft surgery, CAD= coronary artery disease, CHF= congestive heart failure, HDL= high density lipoprotein, LDL = low density lipoprotein, MI= myocardial infarction, PCI= percutaneous coronary intervention.
patients treated with antihypertensive medication, and untreated patients with known systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg
patients with total cholesterol level >200mg/dl, or current use of lipid-lowering drugs
as determined by left ventriculography or transthoracic echocardiography, normal left ventricular systolic function was defined as an ejection fraction of ≥55%, mild to moderately depressed left ventricular function was defined as an ejection fraction of 40–54%, and severely depressed left ventricular systolic function was defined as an ejection fraction of <40%.
Table 2.
Angiographic and procedural information according to the presence or absence of collaterals
| Characteristic | No Collaterals (n=173) | Collaterals (n=96) | P value |
|---|---|---|---|
| Extent of CAD | |||
| 1 vessel disease | 56 (32%) | 27 (28%) | <0.0001 |
| 2 vessel disease | 33 (19%) | 34 (35%) | <0.0001 |
| 3 vessel disease | 18 (10%) | 35 (36%) | <0.0001 |
| Severity of CAD | |||
| <70% stenosis | 101 (58%) | 59 (61%) | 0.67 |
| ≥70% stenosis | 107 (62%) | 96 (100%) | <0.0001 |
| ≥70%, <90% stenosis | 141 (81%) | 96 (100%) | 0.01 |
| >90% stenosis | 71 (41%) | 96 (100%) | <0.0001 |
| 100% stenosis | 5 (3%) | 14 (15%) | 0.065 |
| Culprit artery | |||
| Known culprit | 63 (36%) | 52 (54%) | 0.014 |
| Occluded culprit | 5 (8%) | 14 (27%) | 0.068 |
| culprit vessel LAD | 3 (1.7%) | 12 (13%) | 0.118 |
| collateral to culprit | NA | 8 (53%) | |
| culprit vessel RCA | 26 (15%) | 18 (19%) | 0.597 |
| collateral to culprit | NA | 15 (34%) | |
| culprit vessel LCX | 30 (17%) | 13 (14%) | 0.589 |
| collateral to culprit | NA | 5 (12%) | |
| culprit vessel LM | 2 (1.2%) | 0 | |
| collateral to LM | NA | None | |
| culprit vessel SVG | 2 (1.2%) | 9 (9%) | 0.203 |
| collateral to culprit | NA | 1 (9%) | |
| PCI performed at the time of the index angiogram | 128 (74%) | 84 (88%) | 0.974 |
Data presented as number (percentage), CAD= coronary artery disease, LAD= left anterior descending coronary artery, LCX= left circumflex coronary artery, LM= left main coronary artery, PCI percutaneous coronary intervention, RCA= right coronary artery, SVG= saphenous vein graft
The culprit artery responsible for the NSTEMI could be determined in 115 (43%) patients. Baseline characteristics and angiographic information for subjects with NSTEMI in whom a culprit artery could be determined are shown in Table 3. Compared to those with collaterals to the culprit artery, those without collaterals were more likely to have a history of CHF and to be on chronic angiotensin converting-enzyme inhibitor therapy. Left ventricular systolic function was available in 69% of the patients either by left ventriculography or transthoracic echocardiography performed in the peri-procedure period. Left ventricular systolic function data was not available in 20 of the 63 patients without collaterals (32%), and 16 of the 52 patients with collaterals (31%). There was no significant difference in left ventricular ejection fractions in those with and without collaterals. Of the 115 NSTEMI subjects in whom the culprit artery could be identified, 52 (45%) had angiographic evidence of collaterals and of these, 29 (56%) had collateral filling of the culprit artery territory (Table 4). Subjects with collateral filling of the culprit artery had more severe and extensive coronary artery disease compared to those without collateral filling, and higher rates of an occluded culprit artery.
Table 3.
Baseline characteristics of patients with an identifiable culprit artery
| Characteristic | No Collaterals (n=63) | Collaterals (n=52) | P value |
|---|---|---|---|
| Age (years) mean +/− SD | 60 (+/− 12) | 62 (+/− 11) | 0.542 |
| Men | 46 (73%) | 43 (83%) | 0.217 |
| Hypertension * | 52 (83%) | 39 (75%) | 0.322 |
| Hyperlipidemia † | 52 (83%) | 44 (85%) | 0.765 |
| Diabetes mellitus | 21 (33%) | 16 (31%) | 0.770 |
| Current smoker | 26 (41%) | 15 (29%) | 0.166 |
| Former smoker | 18 (29%) | 18 (35%) | 0.487 |
| Prior angina | 32 (51%) | 21 (40%) | 0.265 |
| Prior MI | 18 (29%) | 17 (33%) | 0.633 |
| Prior PCI | 18 (29%) | 16 (31%) | 0.797 |
| Prior CABG | 8 (13%) | 11 (21%) | 0.224 |
| History of CHF | 6 (10%) | 0 | 0.031 |
| History of arrhythmia | 2 (3%) | 5 (10%) | 0.150 |
| Prior stroke | 2 (3%) | 4 (8%) | 0.407 |
| Family history of CAD | 35 (56%) | 24 (46%) | 0.273 |
| Peripheral arterial disease | 7 (11%) | 11 (21%) | 0.140 |
| Left ventricular ejection fraction ‡ | 0.644 | ||
| Normal | 34 (54%) | 23 (44%) | |
| Mild-moderately depressed | 8 (13%) | 13 (25%) | |
| Severely depressed | 1 (2%) | 0 | |
| Labs | |||
| Total cholesterol (mg/dL) | 173 [138–204] | 184 [150–227] | 0.438 |
| LDL (mg/dL) | 110 [80–136] | 117 [90–133] | 0.416 |
| HDL (mg/dL) | 31 [24–41] | 33 [28–43] | 0.390 |
| Triglycerides (mg/dL) | 152 [76–199] | 163 [86–215] | 0.766 |
| Troponin I (ng/mL) | 2.30 [0.38–16.53] | 2.14 [0.39–9.87] | 0.527 |
| Medications | |||
| Aspirin | 59 (94%) | 48 (92%) | 1.000 |
| Clopidogrel | 29 (47%) | 24 (46%) | 0.947 |
| Statin | 55 (89%) | 47 (90%) | 0.772 |
| Oral Hypoglycemic | 4 (6%) | 4 (8%) | 1.000 |
| Insulin | 10 (16%) | 16 (31%) | 0.057 |
| Beta Blocker | 51 (82%) | 41 (79%) | 0.646 |
| Calcium Channel Blocker | 4 (6%) | 4 (8%) | 1.000 |
| ACE-Inhibitor | 37 (60%) | 19 (37%) | 0.014 |
Data are expressed as mean ± standard deviation (SD), median [25–75% interquartile range (IQR)], or as number (percentage), ACE= angiotensin converting-enzyme, CABG= coronary artery bypass graft surgery, CAD= coronary artery disease, CHF= congestive heart failure, HDL= high density lipoprotein, LDL = low density lipoprotein, MI= myocardial infarction, PCI= percutaneous coronary intervention.
patients treated with antihypertensive medication, and untreated patients with known systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg
patients with total cholesterol level >200mg/dl, or current use of lipid-lowering drugs
as determined by left ventriculography or transthoracic echocardiography, normal left ventricular systolic function was defined as an ejection fraction of ≥55%, mild to moderately depressed left ventricular function was defined as an ejection fraction of 40–54%, and severely depressed left ventricular systolic function was defined as an ejection fraction of <40%.
Table 4.
Angiographic and procedural information in patients with an identifiable culprit artery
| Characteristic | No Collaterals (n=63) | Collaterals (n=52) | P value |
|---|---|---|---|
| Extent of CAD | |||
| 1 vessel disease | 37 (59%) | 21 (40%) | 0.021 |
| 2 vessel disease | 20 (32%) | 16 (31%) | 0.021 |
| 3 vessel disease | 6 (10%) | 15 (29%) | 0.021 |
| Severity of CAD | |||
| <70% stenosis | 36 (57%) | 34 (65%) | 0.367 |
| ≥70% stenosis | 63 (100%) | 52 (100%) | --- |
| ≥70%, <90% stenosis | 17 (27%) | 0 | <0.001 |
| >90% stenosis | 45 (71%) | 52 (100%) | <0.001 |
| 100% stenosis | 5 (8%) | 14 (27%) | 0.006 |
| Culprit artery | |||
| Occluded culprit | 5 (8%) | 14 (27%) | 0.006 |
| culprit vessel LAD | 17 (27%) | 12 (23%) | 0.631 |
| collateral to culprit | NA | 8 (67%) | |
| culprit vessel RCA | 26 (41%) | 18 (35%) | 0.465 |
| collateral to culprit | NA | 15 (83%) | |
| culprit vessel LCX | 16 (25%) | 13 (25%) | 0.961 |
| collateral to culprit | NA | 5 (38%) | |
| culprit vessel LM | 2 (3%) | 0 | 0.500 |
| collateral to culprit | NA | 0 | |
| culprit vessel SVG | 2 (3%) | 9 (17%) | 0.022 |
| collateral to culprit | NA | 1 (11%) | |
| PCI performed at the time of the index angiogram | 44 (70%) | 36 (69%) | 0.944 |
Data presented as number (percentage), CAD= coronary artery disease, LAD= left anterior descending coronary artery, LCX= left circumflex coronary artery, LM= left main coronary artery, PCI= percutaneous coronary intervention, RCA= right coronary artery, SVG= saphenous vein graft
At one-year follow-up, patients with evidence of angiographic collaterals had statistically significant higher unadjusted rates of the combined endpoint of death, recurrent MI, PCI, CABG, stroke, and CHF compared to those without collaterals (p=0.0001) (Figure 1). This increased risk began at the time of the index admission and continued to diverge over the one-year follow-up period. In the subset of subjects in whom the culprit artery could be determined, however, there was no significant difference in outcomes at one year (p=0.16) (Figure 2). On multivariate analysis the presence of collaterals was a strong predictor of the combined endpoint of death, recurrent MI, PCI, CABG, stroke, and CHF at one year in both the entire cohort (HR 1.95, CI 1.08–3.52, p=0.027), as well as in the subset in whom a culprit artery could be determined (HR 3.71, CI 95% 1.31–10.57; p=0.014 (Table 5).
Figure 1.
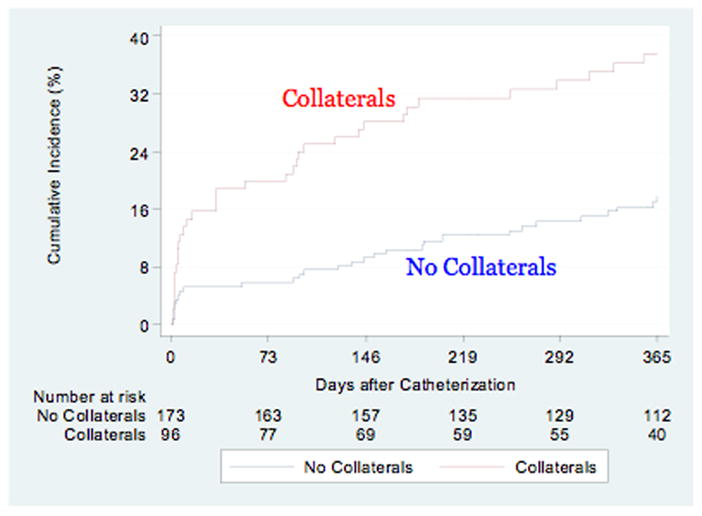
Cumulative event curves for the composite endpoint of death, myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, stroke and congestive heart failure by the presence or absence of collaterals. Red line (top) indicates the presence of collaterals, blue line (bottom) represents patients with no collaterals, log rank p=0.0001.
Figure 2.
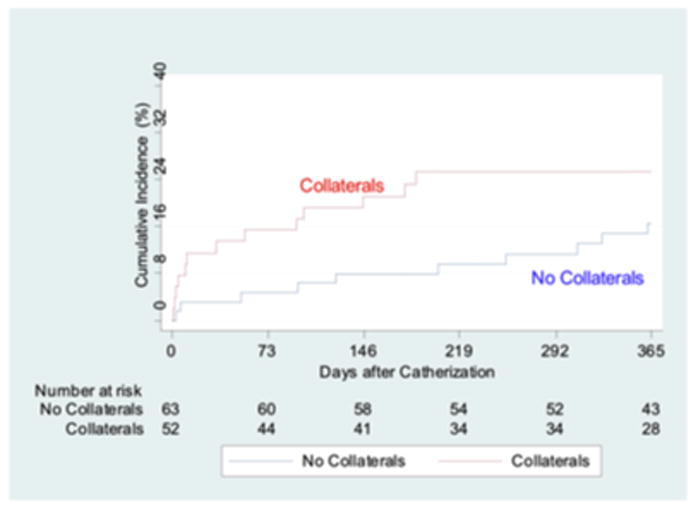
Cumulative event curves for the composite endpoint of death, myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, stroke and congestive heart failure by the presence or absence of collaterals in patients in whom a culprit artery could be identified. Red line (top) indicates the presence of collaterals, blue line (bottom) represents patients with no collaterals to the culprit vessel, log rank p=0.16.
Table 5.
Long-term clinical outcomes according to the presence or absence of angiographic collaterals
| Entire cohort N=269 | Collaterals (N=96) | No Collaterals (N=173) | Unadjusted HR (95% CI) | Unadjusted P value | Adjusted HR* (95% CI) | Adjusted P value |
|---|---|---|---|---|---|---|
| Death, n (%) | 9 (9.9) | 4 (2.4) | 4.17 (1.28–13.53) | 0.018 | 3.52 (0.88–14.00) | 0.074 |
| Recurrent MI, n (%) | 4 (4.6) | 2 (1.4) | 3.72 (0.68–20.34) | 0.129 | 2.60 (0.30–22.47) | 0.386 |
| Recurrent angina, n (%) | 19 (21.0) | 22 (13.6) | 1.67 (0.90–3.08) | 0.102 | 1.31 (0.64–2.69) | 0.466 |
| Stroke, n (%) | 0 | 2 (1.3) | --- | --- | --- | --- |
| Arrhythmia, n (%) | 4 (4.4) | 6 (3.2) | 1.26 (0.36–4.48) | 0.717 | 3.39 (0.48–24.05) | 0.223 |
| CHF, n (%) | 6 (6.7) | 7 (4.4) | 1.59 (0.53–4.73) | 0.405 | 8.05 (1.36–47.58) | 0.021 |
| PCI, n (%) | 6 (6.7) | 5 (3.2) | 2.22 (0.68–7.26) | 0.189 | 1.62 (0.41–6.42) | 0.495 |
| CABG, n (%) | 18 (19.0) | 9 (5.3) | 3.87 (1.74–8.61) | 0.001 | 1.43 (0.62–3.31) | 0.404 |
| Combined endpoint,† n (%) | 35 (37.4) | 29 (17.7) | 2.56 (1.56–4.19) | <0.001 | 1.95 (1.08–3.52) | 0.027 |
| Known culprit artery N=115 | Collaterals (N=52) | No Collaterals (N=63) | Unadjusted HR (95% CI) | Unadjusted P value | Adjusted HR** (95% CI) | Adjusted P value |
|---|---|---|---|---|---|---|
| Death, n (%) | 3 (6.0) | 2 (3.3) | 1.90 (0.32–11.35) | 0.484 | 10.59 (0.61–185.05) | 0.106 |
| Recurrent MI, n (%) | 1 (1.9) | 1 (1.8) | 1.28 (0.08–20.52) | 0.860 | --- | --- |
| Recurrent angina, n (%) | 8 (15.8) | 8 (13.6) | 1.31 (0.49–3.50) | 0.586 | 2.50 (0.68–9.13) | 0.165 |
| Stroke, n (%) | 0 | 0 | --- | --- | --- | --- |
| Arrhythmia, n (%) | 2 (3.9) | 1 (1.8) | 2.55 (0.23–28.20) | 0.444 | --- | --- |
| CHF, n (%) | 2 (4.3) | 2 (3.4) | 1.29 (0.18–9.18) | 0.798 | 1.66 (0.11–24.05) | 0.712 |
| PCI, n (%) | 3 (5.8) | 3 (5.1) | 1.27 (0.26–6.28) | 0.772 | 3.19 (0.24–41.77) | 0.377 |
| CABG, n (%) | 6 (11.5) | 3 (4.9) | 2.59 (0.65–10.36) | 0.179 | 13.46 (1.23–147.62) | 0.033 |
| Combined endpoint,† n (%) | 13 (25.3) | 10 (16.5) | 1.79 (0.78–4.08) | 0.167 | 3.71 (1.31–10.57) | 0.014 |
CABG=coronary artery bypass surgery; CHF=congestive heart failure; CI=confidence interval; HR=hazard ratio; MI=myocardial infarction; PCI=percutaneous coronary intervention.
Adjusted for age (>=65 vs <65), gender, history of diabetes, history of CHF, history of MI, extent of coronary artery disease, and peak troponin;
Adjusted for age (>=65 vs <65), gender, history of diabetes, history of CHF, history of MI, extent of coronary artery disease, peak troponin, and initial TIMI flow in culprit artery, and stent.
combined endpoint of death, recurrent MI, PCI, CABG stroke, and PCI.
Discussion
The impact of coronary collaterals on clinical outcomes in patients with NSTEMI is incompletely understood and remains an important area of research in light of the growing population of NSTEMI patients. While it seems intuitive that collaterals would exert a protective, beneficial effect in patients with MI, the available data is conflicting. In STEMI patients for example, angiographic studies following thrombolytic therapy showed preserved left ventricular function in patients who failed to reperfuse but had evidence of collateral filling.5,22 Moreover, in patients with persistent occlusion of the infarct-related artery and in those with late presentations, collateral filling of the infarct-related artery has been associated with better myocardial viability and improved clinical outcomes.5,23,24 Studies of early reperfusion, however, show mixed results with some showing improved clinical outcomes in patients with angiographic evidence of collaterals 6–9,25–26 and others showing either no difference 10–11,27 or worse outcomes.12–13 One explanation may be differences in clinical endpoints across studies including assessment of infarct size.
Compared to the STEMI population, there are few published studies focused on the impact of collaterals on clinical outcomes in the NSTEMI population. In one study relevant to ours, the investigators compared the clinical characteristics and clinical outcomes in NSTEMI patients with and without an occluded culprit artery.14 They found that 29% of the NSTEMI patients had an occluded culprit artery and that those with an occluded culprit artery had larger infarcts and worse long-term outcomes compared to those with a patent culprit artery. They also found that the patients with an occluded culprit artery who had evidence of collateral filling had better clinical outcomes compared to those who did not have collateral filling. One limitation of their study was that the culprit lesion was determined by the cardiologist performing the coronary angiogram without off-line independent review. This may have led to confounding bias especially among those with multi-vessel disease. Furthermore, the investigators did not state what definition was used to determine the culprit artery angiographically. In contrast, we used an off-line, widely-accepted and previously published angiographic definition of the culprit artery 15 by investigators who were not involved in the care of the patient and who were blinded to the clinical information. Our observations are similar to studies where a single culprit lesion was identified in <50% of NSTEMI patients undergoing coronary angiography 15,21. It remains uncertain as to why many NSTEMI patients do not have an identifiable culprit lesion. One possible explanation is the overall low rate of thrombus (regarded as a common feature of the culprit artery) detected on coronary angiography. For example, in the TIMI IIIA trial, investigators noted that only 35% of NSTEMI patients had angiographic evidence of thrombus.16 Another explanation may be higher rates of spontaneous reperfusion in patients with NSTEMI given the increased time delay to coronary angiography when compared to STEMI patients.
In our study, subjects with collaterals had more occluded vessels and the collateral filling may have helped provide information about viable targets for revascularization. This may at least partially explain the 13-fold increase in the rate of subsequent CABG in our patient cohort. In a previous study of patients undergoing coronary angiography collaterals were associated with improved survival in most, but did not influence survival in those treated with subsequent CABG.19 In another study, investigators found no difference in 5-year survival in NSTEMI patients with well-developed coronary collaterals who underwent CABG compared to those who were treated with medical therapy alone.20
Study limitations
Our study has several limitations. First, we do not have assessment of left ventricular systolic function for all subjects and therefore were not able to calculate a clinical SYNTAX score or adjust for this in our multivariate model. However, we did adjust for a history of congestive heart failure. Second, due to the small cohort in whom the culprit artery was identifiable, statistical analysis according to the extent (grade) of collateral filling (as measured by the Rentrop score) could not be performed. Third, we may have underestimated the presence of collaterals by not administering vasodilators routinely prior to angiography and by measuring only spontaneously visible coronary collaterals. Fourth, it is possible that there were differences in factors we did not collect that may play a role in the recruitment of coronary collaterals. Fifth, all patients were referred for coronary angiography therefore our results may not be generalizable to NSTEMI patients who are not referred for angiography. Sixth, we did not have one year clinical follow-up on all patients, however, a statistically significant difference in the clinical outcomes between the two groups occurred early on when only 11% of the subjects were lost to follow-up and this difference remained significant throughout the whole follow-up period. Finally, our results reflect an association but do not establish a causal relationship between the presence of angiographic coronary collaterals and clinical outcomes in patients with NSTEMI.
Conclusions
In patients with NSTEMI the presence of angiographic coronary collaterals is a predictor of long-term clinical outcomes primarily driven by increased rates of surgical revascularization.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [HL97074 to E.C.K.]
Footnotes
No conflicts of interest
References
- 1.Giugliano RP, Braunwald E. The year in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 58:2342–2354. doi: 10.1016/j.jacc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 3.Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study) Eur Heart J. 2010;31:2755–2764. doi: 10.1093/eurheartj/ehq326. [DOI] [PubMed] [Google Scholar]
- 4.Charney R, Cohen M. The role of the coronary collateral circulation in limiting myocardial ischemia and infarct size. Am Heart J. 1993;126:937–945. doi: 10.1016/0002-8703(93)90710-q. [DOI] [PubMed] [Google Scholar]
- 5.Habib GB, Heibig J, Forman SA, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation. 1991;83:739–746. doi: 10.1161/01.cir.83.3.739. [DOI] [PubMed] [Google Scholar]
- 6.Rogers WJ, Hood WP, Jr, Mantle JA, et al. Return of left ventricular function after reperfusion in patients with myocardial infarction: importance of subtotal stenoses or intact collaterals. Circulation. 1984;69:338–349. doi: 10.1161/01.cir.69.2.338. [DOI] [PubMed] [Google Scholar]
- 7.Clements IP, Christian TF, Higano ST, et al. Residual flow to the infarct zone as a determinant of infarct size after direct angioplasty. Circulation. 1993;88:1527–1533. doi: 10.1161/01.cir.88.4.1527. [DOI] [PubMed] [Google Scholar]
- 8.Hatada K, Sugiura T, Kamihata H, et al. Clinical significance of coronary flow to the infarct zone before successful primary percutaneous transluminal coronary angioplasty in acute myocardial infarction. Chest. 2001;120:1959–1963. doi: 10.1378/chest.120.6.1959. [DOI] [PubMed] [Google Scholar]
- 9.Elsman P, van ‘t Hof AW, de Boer MJ, et al. Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J. 2004;25:854–858. doi: 10.1016/j.ehj.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Antoniucci D, Valenti R, Moschi G, et al. Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am J Cardiol. 2002;89:121–125. doi: 10.1016/s0002-9149(01)02186-5. [DOI] [PubMed] [Google Scholar]
- 11.Sorajja P, Gersh BJ, Mehran R, et al. Impact of collateral flow on myocardial reperfusion and infarct size in patients undergoing primary angioplasty for acute myocardial infarction. Am Heart J. 2007;154:379–384. doi: 10.1016/j.ahj.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Gohlke H, Heim E, Roskamm H. Prognostic importance of collateral flow and residual coronary stenosis of the myocardial infarct artery after anterior wall Q-wave acute myocardial infarction. Am J Cardiol. 1991;67:1165–1169. doi: 10.1016/0002-9149(91)90920-g. [DOI] [PubMed] [Google Scholar]
- 13.Nicolau JC, Nogueira PR, Pinto MA, et al. Early infarct artery collateral flow does not improve long-term survival following thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 1999;1:83:21–26. doi: 10.1016/s0002-9149(98)00776-0. [DOI] [PubMed] [Google Scholar]
- 14.Bahrmann P, Rach J, Desch S, et al. Incidence and distribution of occluded culprit arteries and impact of coronary collaterals on outcome in patients with non-ST-segment elevation myocardial infarction and early invasive treatment strategy. Clin Res Cardiol. 2010;100:457–467. doi: 10.1007/s00392-010-0269-9. [DOI] [PubMed] [Google Scholar]
- 15.Kerensky RA, Wade M, Deedwania P, et al. Revisiting the culprit lesion in non-Q-wave myocardial infarction. Results from the VANQWISH trial angiographic core laboratory. J Am Coll Cardiol. 2002;39:1456–1463. doi: 10.1016/s0735-1097(02)01770-9. [DOI] [PubMed] [Google Scholar]
- 16.Early effects of tissue-type plasminogen activator added to conventional therapy on the culprit coronary lesion in patients presenting with ischemic cardiac pain at rest. Results of the Thrombolysis in Myocardial Ischemia (TIMI IIIA) Trial. Circulation. 1993;87:38–52. doi: 10.1161/01.cir.87.1.38. [DOI] [PubMed] [Google Scholar]
- 17.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 18.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 19.McMurtry MS, Lewin AM, Knudtson ML, et al. The clinical profile and outcomes associated with coronary collaterals in patients with coronary artery disease. Can J Cardiol. 2011;27:581–588. doi: 10.1016/j.cjca.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Tatli E, Aktoz M, Cakar MA, et al. Survival of patients with well-developed collaterals undergoing CABG or medical treatment: an observational case-controlled study. Anadolu Kardiyol Derg. 2012;12:97–101. doi: 10.5152/akd.2012.033. [DOI] [PubMed] [Google Scholar]
- 21.Wang TY, Zhang M, Fu Y, et al. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am Heart J. 2009;157:716–723. doi: 10.1016/j.ahj.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Nitzberg WD, Nath HP, Rogers WJ, et al. Collateral flow in patients with acute myocardial infarction. Am J Cardiol. 1985;56:729–736. doi: 10.1016/0002-9149(85)91124-5. [DOI] [PubMed] [Google Scholar]
- 23.Sabia PF, Powers ER, Ragosta M, et al. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–1831. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 24.Kurotobi T, Sato H, Kinjo K, et al. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol. 2004;44:28–34. doi: 10.1016/j.jacc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Castellano N, Garcia EJ, Abeytua M, et al. Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am Coll Cardiol. 1998;31:512–518. doi: 10.1016/s0735-1097(97)00521-4. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro P, Antunes A, Goncalves LM, Providencia LA. Long-term clinical impact of coronary collateral vessels after acute myocardial infarction. Rev Port Cardiol. 2003;22:1051–1061. [PubMed] [Google Scholar]
- 27.Boehrer JD, Lange RA, Willard JE, Hillis LD. Influence of collateral filling of the occluded infarct-related coronary artery on prognosis after acute myocardial infarction. Am J Cardiol. 1992;69:10–12. doi: 10.1016/0002-9149(92)90668-o. [DOI] [PubMed] [Google Scholar]


