Abstract
Nicotine stimulates brain reward circuitries, most prominently the mesocorticolimbic dopamine system, and this action is considered critical in establishing and maintaining the tobacco smoking habit. Compounds that attenuate nicotine reward are considered promising therapeutic candidates for tobacco dependence, but many of these agents have other actions that limit their potential utility. Nicotine is also highly noxious, particularly at higher doses, and aversive reactions to nicotine after initial exposure can decrease the likelihood of developing a tobacco habit in many first time smokers. Nevertheless, relatively little is known about the mechanisms of nicotine aversion. The purpose of this review is to present recent new insights into the neurobiological mechanisms that regulate avoidance of nicotine. First, the role of the mesocorticolimbic system, so often associated with nicotine reward, in regulating nicotine aversion is highlighted. Second, genetic variation that modifies noxious responses to nicotine and thereby influences vulnerability to tobacco dependence, in particular variation in the CHRNA5-CHRNA3-CHRNB4 nicotinic acetylcholine receptor (nAChR) subunit gene cluster, will be discussed. Third, the role of the habenular complex in nicotine aversion, primarily medial habenular projections to the interpeduncular nucleus (IPN) but also lateral habenular projections to rostromedial tegmental nucleus (RMTg) and ventral tegmental area (VTA) are reviewed. Forth, brain circuits that are enriched in nAChRs, but whose role in nicotine avoidance has not yet been assessed, will be proposed. Finally, the feasibility of developing novel therapeutic agents for tobacco dependence that act not by blocking nicotine reward but by enhancing nicotine avoidance will be considered.
Introduction
Nicotine is considered the major reinforcing component of tobacco responsible for addiction in human smokers (Stolerman and Jarvis, 1995), and it has been shown that humans, non-human primates and rodents will volitionally self-administer the drug (Corrigall and Coen, 1989; Goldberg et al., 1981; Harvey et al., 2004; Watkins et al., 1999). Volitionally consumed nicotine is known to stimulate activity in brain reward circuitries (Kenny and Markou, 2006), with this action considered central to the establishment and maintenance of the tobacco habit in human smokers. It is important to note, however, that instead of hedonic reactions, most smokers report their initial smoking experiences as unpleasant. This reflects the fact that in addition to its rewarding effects, nicotine is also highly noxious. Highlighting this dichotomous nature of nicotine, doses of the drug that support maximal rates of responding in squirrel monkeys also induce marked symptoms of aversion, such as vomiting, when the drug-taking habit is being acquired. Moreover, monkeys work to avoid non-contingent delivery of intravenous nicotine infusions even though they will work equally hard to obtain those same nicotine infusions when they are available for contingent delivery (Goldberg and Spealman, 1982, 1983; Goldberg et al., 1981; Goldberg et al., 1983; Spealman and Goldberg, 1982). These aversive reactions to nicotine are important in the context of tobacco dependence, as stronger aversive reactions to nicotine after initial exposure are negatively correlated with the development of habitual tobacco use in first time smokers (Sartor et al., 2010).
Aversive responses to nicotine also appear to play key roles in determining the overall amounts of tobacco smoke consumed and patterns of intake. Indeed, when levels of nicotine contained in tobacco are varied, smokers are far more efficient at titrating their intake downwards when consuming high-nicotine-content tobacco to avoid noxious effects of the drug (Henningfield and Goldberg, 1983a; Henningfield et al., 1986; Russell et al., 1975), than they are at adjusting their intake upward to compensate for reduced nicotine in low-content tobacco (Sutton et al., 1978). Hence, self-regulation of consumption to avoid noxious effects of nicotine is far better regulated that compensation upwards to avoid a reduction in nicotine intake. Also consistent with a key role for noxious nicotine effects in controlling tobacco consumption, a treatment strategy previously employed to facilitate smoking cessation, but no longer typically used (Hajek and Stead, 2004), is to encourage smokers to inhale tobacco smoke more rapidly and deeply than usual. This results in aversive reactions to nicotine, with this increased nicotine exposure from more rapid consumption resulting in persistent suppression of intake (Norton and Barske, 1977). It is likely, therefore, that tolerance to the unpleasant effects of nicotine, and learning to efficiently control tobacco smoking to avoid these effects, must develop in order for habitual tobacco use to be established (Russell, 1979). As such, it is probable that discrete circuitries in the brain respond to the noxious properties of nicotine and that learning to titrate patterns of tobacco consumption in order to avoid activation of these circuitries plays a key role in the acquisition of smoking behavior. Indeed, the nicotinic acetylcholine receptor antagonist mecamylamine has been shown to block both the rewarding and aversive effects of nicotine, delivered by intravenous infusions to human volunteers (Lundahl et al., 2000), consistent with their being at least two discrete populations of nAChRs with each regulating either rewarding or aversive effects of the drug. Diminished sensitivity of nicotine-related aversion systems in the brain is therefore likely to increase vulnerability to develop habitual smoking. As such, it may be possible to target such circuitries in brain to enhance the noxious properties of nicotine with small molecule drugs, offering a novel treatment strategy to facilitate lower levels of tobacco consumption, and perhaps increased ability to cease tobacco smoking altogether. Nevertheless, until recently relatively little was known about which circuits in the brain regulate nicotine aversion, in sharp contrast to our burgeoning knowledge on mechanisms of nicotine reward. Here, we summarize much of the current knowledge on the mechanisms of nicotine aversion.
The mesocorticolimbic system and nicotine aversion
As noted above, the reward-enhancing actions of nicotine are hypothesized to play a key role in the establishment and maintenance of the tobacco habit in human smokers (Kenny and Markou, 2006). The reward-related actions of nicotine are thought to be related to the stimulatory effects of the drug on neuronal nicotinic acetylcholine receptors (nAChRs) containing α4 and β2 subunits (denoted α4β2* nAChRs), particularly those located in the ventral tegmental area (VTA) (Corrigall et al., 1992; Ikemoto et al., 2006a; Maskos et al., 2005; Picciotto et al., 1998; Tapper et al., 2004). Indeed, nicotine-induced activation of α4β2* nAChRs in the VTA increases forebrain dopamine transmission, most prominently in the shell region of the nucleus accumbens (NAc), and this has been shown to contribute to the reinforcing properties of the drug in laboratory animals (Iyaniwura et al., 2001; Nisell et al., 1997; Pontieri et al., 1996). Because of their central role in nicotine reinforcement, α4β2* nAChRs are considered important targets for the development of smoking cessation agents. Varenicline (Chantix) is the only FDA-approved smoking cessation agent that was rationally designed through traditional drug discovery and was developed as an α4β2* nAChR partial agonist (Coe et al., 2005; Dwoskin et al., 2009; Lerman et al., 2007; Reus et al., 2007).
In addition to α4β2* nAChRs in VTA, accumulating evidence suggests that α7 nAChRs in this site may also play a role in nicotine reinforcement. Nicotine-induced increases in glutamatergic transmission in the VTA is thought to occur through actions at both α4β2* and α7 nAChRs, leading to increases in mesoaccumbens dopamine transmission and regulation of nicotine self-administration behavior (Besson et al., 2012; Gao et al., 2010; Mansvelder and McGehee, 2000; Mao et al., 2011; Melis et al., 2013; Pons et al., 2008; Schilstrom et al., 2000). Supporting a key role for nicotine-induced increases in glutamatergic transmission in the VTA with nicotine reinforcement, blockade of local NMDA receptors profoundly decreases nicotine intake in rats (Kenny et al., 2009b). Considering the well-established role for the VTA and mesocorticolimbic dopamine transmission in regulating the reinforcing properties of nicotine and other drugs of abuse, it is interesting to note that accumulating evidence implicates this same system in nicotine aversion. Lesions of cholinergic innervation of the VTA arising from the pedunculopontine tegmental nucleus (PPTg) blocked the expression of reward-related behaviors in response to nicotine and rendered ‘rewarding’ doses of nicotine aversive, reflected in increased avoidance of the drug (Laviolette et al., 2002). Similarly, blockade of α7 nAChRs or NMDA receptors in the VTA switched the effects of nicotine from rewarding to aversive (Kenny et al., 2009b; Laviolette and van der Kooy, 2003b). Projections from the VTA into the striatum and NAc also appear to be influenced by nicotinic signaling. Presynaptic nAChRs directly regulate the release of dopamine from dopaminergic terminals (Rice and Cragg, 2004; Zhou et al., 2001), in which α4α5β2 nAChR subtype regulates DA release in the dorsal caudate/putamen and the α4α6β2β3 nAChR is involved in the NAc core (Exley et al., 2012). In these regions, several subtypes of nAChRs have been identified, including the α4, α5, α6, α7, β2 and β3 (Exley et al., 2012; Marks et al., 1992; Seguela et al., 1993; Wada et al., 1989). As such, nicotine has the potential to directly and/or indirectly modulate the activity of many neurotransmitter systems in striatum. An intricate network of cholinergic interneurons is present throughout the NAc and striatum. In addition to releasing acetylcholine, these interneurons may also corelease glutamate (Higley et al., 2011). Presynaptic α4β2* nAChRs have been shown to enhance GABA release from inhibitory interneurons, which may subsequently inhibit the cholinergic interneurons (Grilli et al., 2009; Koos and Tepper, 2002; Sullivan et al., 2008). Increased accumbal acetylcholine release is found during expression of a conditioned taste aversion (Mark et al., 1995), and pharmacologically enhancing cholinergic signaling in the NAc is sufficient to produce a conditioned taste aversion (Taylor et al., 2011). It has been proposed that dopamine and acetylcholine act in an opposing manner within the NAc, in which dopamine mediates reward-related processing, whereas acetylcholine signals aversion-related events (Hoebel et al., 2007). Support for this hypothesis derives from the findings that drugs of abuse, such as nicotine, stimulate mesolimbic dopamine release (Mifsud et al., 1989), and in contrast, increased acetylcholine in the NAc attenuates drug self-administration and promotes escape behavior (Glowa et al., 1997; Rada and Hoebel, 2001; Williams and Adinoff, 2008). Furthermore, nicotine withdrawal elicits a decrease in dopaminergic signaling that is paralleled by an increase in acetylcholine (Rada et al., 2001). Although evidence supports this concept of dopamine/acetylcholine opponent processing, more recent findings suggest that acetylcholine can directly enhance dopamine release in this brain region as well (Cachope et al., 2012; Threlfell et al., 2012). Moreover, rather than diminishing its rewarding effects, blockade of dopamine receptors can in some instances enhance the rewarding effects of nicotine, as measured using place conditioning procedures (Grieder et al., 2012; Laviolette and van der Kooy, 2003a). Laviolette and colleagues have shown that blockade of dopamine D2 receptors in the shell region of the nucleus accumbens (NAc), or D1 receptors in the core region of the accumbens, can switch the effects of intra-VTA infusions of higher doses of nicotine from aversive to rewarding during place conditioning (Laviolette et al., 2008). Clarke and co-workers have similarly shown that lesioning dopamine inputs to the NAc shell, achieved using local infusions of the toxin 6-hydroxydopamine, abolished the rewarding effects of intravenously delivered nicotine infusions (Sellings et al., 2008). Conversely, lesions of dopamine inputs into the NAc core region increased the rewarding effects and abolished the aversion-related responses to nicotine, as measured by conditioned taste aversion (Sellings et al., 2008). These findings suggest that dopamine signaling, at least somewhat compartmentalized between the NAc shell and core regions, regulate the rewarding and aversive effects of nicotine, respectively.
One may question how mesoaccumbens dopamine transmission can be critical for both the reinforcing properties of nicotine that support consumption of the drug and also the aversive effects of the drug that support avoidance? Two recent findings appear to shed important light on this issue. First, Malenka and colleagues have shown that distinct populations of VTA dopamine neurons may separately encode reward-related and aversion-related information, and thus guide approach or avoidance behaviors accordingly. Specifically, they found that the cholinergic inputs to the VTA from the laterodorsal tegmental nucleus (LDTg), and inputs to the VTA from the lateral habenula (LHb), regulate reward and aversion behaviors in mice, respectively (Lammel et al., 2012). Moreover, LDTg neurons preferentially synapse onto dopamine neurons that project to the NAc shell, whereas LHb neurons synapse onto dopamine neurons that project to the medial prefrontal cortex or the rostromedial tegmental nucleus (RMTg), both areas of the brain involved in response suppression (Lammel et al., 2012). Hence, these findings are consistent with the concept that discrete populations of dopamine neurons receive information from brain regions that regulate appetitive or aversive responses, which in turn project to discrete areas of the accumbens (and other brain sites) to regulate approach versus avoidance behaviors. Consistent with this possibility, Suto and Wise have shown recently that enhancing dopamine transmission by co-infusion of a D1 and a D2 receptor agonist in the NAc core, but not shell, increased satiety-like responses to intravenously self-administered cocaine infusions (Suto and Wise, 2011); see also (Suto et al., 2009; Suto et al., 2010).
In addition to dopamine and acetylcholine, opioid signaling also appears to be an important mediator of aversive processing within the NAc, most notably through the κ opioid receptor (KOR) (McCutcheon et al., 2012; Resendez et al., 2012). KOR agonists attenuate nicotine-induced hyperlocomotion in rats (Hahn et al., 2000), and chronic nicotine treatment can enhance the effectiveness of KOR agonists to induce a place aversion or anxiogenic-like response in adult rats (Tejeda et al., 2012). Following chronic nicotine exposure, the somatic signs of nicotine withdrawal may also be potentiated by KOR activation, an effect that can be reversed by co-administration of the KOR antagonist, nor-BNI (Tejeda et al., 2012). At the intracellular level, it is an interesting possibility that nicotine signaling in accumbens may exert its aversive effects by stimulating cAMP signaling cascades, resulting in activation of cAMP response element-binding protein (CREB) and consequently the increased transcription of the endogenous KOR ligand, dynorphin (McCarthy et al., 2012). Taken together, the above findings suggest that neurotransmission in the NAc shell regulates the reinforcing effects of nicotine and other drugs of abuse that drive their consumption. Conversely, the NAc core may regulate satiety responses and avoidance behaviors that limit consumption of nicotine and other addictive drugs.
Genetics of tobacco dependence and nicotine aversion
In mice, it has been shown that genetic factors play a key role in regulating sensitivity to the aversive effects of nicotine (Risinger and Brown, 1996). Emerging data from genome-wide association studies (GWAS) are identifying polymorphisms that increase vulnerability to tobacco dependence in humans, and also support the notion that sensitivity to nicotine aversion may be influenced by genetics. A prominent gene in which allelic variation has been associated with risk of developing tobacco dependence is CYP2A6. This gene encodes the cytochrome P450 enzyme responsible for ~80% of nicotine metabolism in the human liver (Raunio and Rahnasto-Rilla, 2012; Ray et al., 2009; Thorgeirsson et al., 2010). CYP2A6 polymorphisms are more commonly found in individuals of Asian descent and less frequently in Caucasians (Johansson and Ingelman-Sundberg, 2011). The CYP2A6 gene is highly polymorphic, with over 60 allelic variants known. Of these, 17 alleles have been shown to exhibit reduced function, whereas two of the variants result in increased nicotine metabolism (Mwenifumbo et al., 2008; Raunio and Rahnasto-Rilla, 2012). Allelic variation that results in slower nicotine metabolism would be expected to allow for more prolonged actions of nicotine. As such, nicotine clearance is expected to be decreased and doses required to experience the rewarding and aversive effects of the drug would be lower (e.g., leftward shift of the dose-response curve). Consistent with this prediction, individuals with a slow metabolizer CYP2A6 genotype are less vulnerable to develop tobacco dependence than those with normal metabolism (Audrain-McGovern et al., 2007; Bloom et al., 2011; Thorgeirsson et al., 2010), potentially due to a greater sensitivity to aversive effects of nicotine at doses that do not trigger aversion in those who metabolize nicotine normally. Further supporting this notion, slow metabolizers consume less nicotine per day and are more successful when attempting to quit smoking than individuals with normal metabolism (Patterson et al., 2008; Rodriguez et al., 2011; Strasser et al., 2007). Thus, a slower metabolism permits smaller quantities of the drug to be consumed that lead to prolonged activity at nAChRs. As such, it may be more difficult for the individual to titrate their intake to obtain rewarding nicotine effects yet to avoid activation of those nAChR subtypes involved in nicotine aversion (see nAChR subtype discussion below). In contrast, faster rates of nicotine metabolism would allow for quicker recovery from the actions of nicotine, resulting in diminished sensitivity to the aversive effects of the drug. Indeed, individuals that rapidly metabolize nicotine exhibit lower cessation rates and more severe withdrawal symptoms (Bloom et al., 2011; Patterson et al., 2008). Given that both withdrawal duration and severity predict likelihood to relapse (Piasecki et al., 2000), the aversive state elicited during nicotine withdrawal likely motivates the individual to seek and continue to consume the drug, thus maintaining the tobacco smoking habit. Interestingly, metabolism regulated by CYP2A6 may be directly modulated by tobacco exposure and contribute to the development of dependence. Smokers exhibit a slower clearance of nicotine compared to non-smokers (Hukkanen et al., 2005), and nicotine appears to reduce CYP2A6 transcript and protein levels in monkeys (Ferguson et al., 2012; Schoedel et al., 2003). Finally, given that the CYP2A6 gene is also expressed in respiratory tissues, it is perhaps not surprising that CYP2A6 allelic variation is associated with smoking-related diseases, such as COPD and cancer (Ariyoshi et al., 2002; Hukkanen et al., 2002; Wassenaar et al., 2011).
Since the main site of action of nicotine in the brain is the nAChRs, it may be expected that genetic variation in the genes encoding nAChR receptor subunits would be implicated in tobacco dependence vulnerability. However, what may be surprising is the fact that the subunit genes most implicated in dependence vulnerability are not those that encode the high-affinity nAChRs responsible for regulating the rewarding effects of nicotine (α4β2* nAChRs), but instead lesser studies α5, α3 and β4 nAChRs nAChR subunit genes best known for their role in regulating ganglionic nAChR transmission (Fig. 1). Indeed, recent GWAS studies have shown that variation in the CHRNA3-CHRNA5-CHRNAB4 gene cluster, which encodes the α3, α5 and β4 nAChR receptor subunits, respectively, is prominently associated with predisposition to develop tobacco dependence. Variation in this cluster is also associated with vulnerability to many smoking-associated diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer (Bierut et al., 2008; Hung et al., 2008; Saccone et al., 2010; Saccone et al., 2007; Thorgeirsson et al., 2008). Strikingly, the risk of developing tobacco dependence appears to be more than doubled in individuals carrying two copies of the CHRNA5 risk allele, rs16969968, a polymorphism that results in an aspartic acid to asparagine substitution at amino acid residue 398 (D398N). The D398N risk variant is also associated with early onset of smoking behavior (Weiss et al., 2008), a self-reported “pleasurable buzz” during smoking (Sherva et al., 2008), and heavy smoking (Berrettini et al., 2008; Bierut et al., 2008; Grucza et al., 2008; Stevens et al., 2008). As described in detail below, genetic variation in the CHRNA5-CHRNA3-CHRNB4 subunit cluster, and resultant alterations in the function of mature nAChRs incorporating these altered subunits, appears to regulate tobacco dependence vulnerability not by altering the stimulatory effects of nicotine on brain reward systems, but instead by diminishing the aversive effects of nicotine.
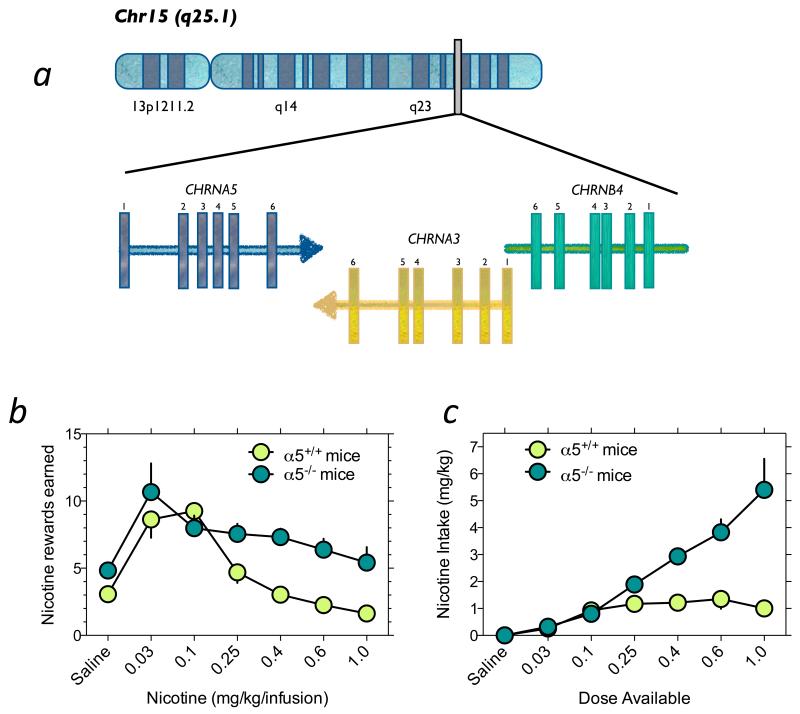
Fig. 1.
Allelic variation in the CHRNA5-CHRNA3-CHRNB4 gene cluster contributes to vulnerability to tobacco dependence. (a) Graphical representation of the genomic organization of the CHRNA5-CHRNA3-CHRNB4 nAChR subunit gene cluster on chromosome 15 (Chr15 q25.1). (b) Mice with null mutation in the α5 subunit gene self-administer more nicotine than wildtype mice. Mice were responding under a fixed-ratio 5 time-out 20 sec schedule of reinforcement. (c) Data from panel B were transformed such that the total amount of nicotine self-administered by wildtype and knockout mice could be examine. Data are modified with permission from (Fowler et al., 2011).
The CHRNA5-CHRNA3-CHRNB3 gene cluster and nicotine aversion
Genetic variation in enzymes responsible for nicotine metabolism can influence tobacco dependence vulnerability as noted above, perhaps by regulating sensitivity to the aversive effects of nicotine. Considering that genetic variation in the CHRNA5-CHRNA3-CHRNB4 gene cluster, particularly genetic variation that diminishes α5* nAChR activity, also influences tobacco dependence vulnerability, our laboratory sought to determine if this effect was because of enhanced rewarding effects of nicotine or instead diminished sensitivity the aversive properties of the drug.
Using a mouse intravenous nicotine self-administration procedure, we assessed nicotine intake in wild-type (WT) and α5 subunit knockout (KO) mice on a C57BL6 background; for full details, see (Fowler et al., 2011). We found that both genotypes responded for intravenous nicotine infusions according to an inverted U-shaped dose-response (D-R) curve (Fig. 1). Levels of intake were similar between WT and KO mice when lower doses of nicotine were available. The ascending portion of the D-R curve is thought to reflect increasing reinforcing properties of nicotine as the unit dose increases (Henningfield and Goldberg, 1983b; Lynch and Carroll, 2001). Therefore, our data suggest that the rewarding effects of nicotine are unaltered by α5 subunit deletion. In contrast, strikingly different patterns of intake were revealed when higher unit doses of nicotine were available, with the KO mice responding far more vigorously than WT mice (Fig. 1). In control experiments, it was shown that this increased nicotine intake in KO mice was not secondary to alterations in operant performance or alterations in sensitivity to drug or non-drug paired conditioned stimuli.
When we examined total amounts of nicotine consumed at each dose available in the WT and α5 KO mice, we found that WT mice consumed ~1.5 mg kg−1 per session independent of the available dose (Fig. 1). In contrast, KO mice consumed progressively greater amounts of the drug as the dose increased. This suggests that WT mice efficiently titrate their intake whereas this behavior is largely absent in α5 subunit KO mice. Similar to human smokers, diminished responding of rodents for nicotine as the unit dose increases is thought to reflect greater restraint over intake to avoid the increasingly aversive effects of the drug (Henningfield and Goldberg, 1983b; Lynch and Carroll, 2001). As such, these findings suggest that diminished aversive effects of nicotine, measured by increased responding on the descending portion of the D-R curve for self-administered nicotine, likely explains the increased nicotine intake in α5 KO mice see (Fowler et al., 2011) and perhaps in humans carrying CHRNA5 risk alleles (Bierut et al., 2008). Consistent with these findings, it was also reported that high nicotine doses continued to support place-conditioning behavior in the knockout mice even though these doses were no longer rewarding in WT mice (Jackson et al., 2010). While it is plausible that rodents may have increased sensitivity to aversive effects of nicotine compared to humans, the similarities between the findings in both species supports the notion that similar aversive states contribute to the regulation of nicotine intake in both species. Indeed, the plasma concentrations achieved by self-administering wildtype mice are comparable to those achieved in humans after 5 hours of smoking their preferred cigarette (Fowler and Kenny, 2011; Fowler et al., 2011; Matta et al., 2007; Russell et al., 1975). Moreover, self-administration of intravenous nicotine in both rodents and humans decreases when higher doses of the drug are provided (Fowler and Kenny, 2011; Harvey et al., 2004), similar to that found with cigarette smoking in humans (Henningfield and Goldberg, 1983a; Henningfield et al., 1986; Russell et al., 1975). These data, combined with the findings that altered expression of the α5* nAChRs in mice, rats and humans results in a similar behavior profile in relation to nicotine consumption, support the contention that aversion induced by nicotine is likely conserved across species. Even so it remains unclear precisely which psychological state(s) manifested in response to high doses of nicotine are responsible for cessation of nicotine intake, this type of assessment may be most readily investigated in humans.
In addition to α5* nAChRs, β4* nAChRs also play an important role in regulating the aversive properties of nicotine (Frahm et al., 2011). Specifically, it was shown that transgenic overexpression of this subunit in mice increased their sensitivity to the aversive properties of the drug and consequently decreased nicotine drinking behavior (Frahm et al., 2011). No studies to date have reported the effects of genetic ablation or modification of α3 nAChR subunit expression on nicotine intake, likely because deletion of this subunit results in post-natal lethality. Taken together, the above findings suggest that the CHRNA5-CHRNA3-CHRNB4 gene cluster plays a key role in nicotine aversion but not nicotine reward, with genetic variation in this cluster increasing vulnerability to tobacco dependence by attenuating the aversive effects of nicotine and thereby diminishing avoidance of the drug.
The medial habenula-interpeduncular systems and nicotine aversion
The above findings show that disruption of α5* nAChR signaling increases, whereas transgenic overexpression of β4 nAChR subunits decreases, nicotine intake. These findings may appear counterintuitive when the role for nAChRs in nicotine addiction has traditionally been to consider their involvement in nicotine reward. Indeed, disruption of nAChR signaling, particularly the high-affinity nAChRs (α4β2*), usually results in diminished reinforcing effects of nicotine and consequently reduced consumption of the drug. Pharmacological blockade of α4β2* nAChRs, or genetic disruption of β2 nAChR subunits, almost invariably decreases nicotine intake in rats and mice (Corrigall et al., 1994; Corrigall et al., 1999; Corrigall et al., 2000; David et al., 2006; Ikemoto et al., 2006b; Kenny et al., 2009a; Maskos et al., 2005; Molles et al., 2006). Disruption of nAChR function in midbrain dopamine systems, particularly the VTA, is generally thought to be responsible for the decreased reinforcing effects of nicotine reported in these studies. In light of these previous studies, it is somewhat paradoxical that genetic ablation of α5* nAChR signaling increases nicotine intake in rats and mice. Hence, these findings suggest that α5* nAChR receptors, and perhaps also α3* and β4* nAChRs, regulate nicotine intake in a manner distinctly different from nAChRs in midbrain dopamine systems.
The α5 nAChR subunit mRNA is expressed in the VTA and substantia nigra pars compacta (SNc), as well as in deep layers of the cortex. However, the α5 nAChR subunit mRNA is most densely expressed in the medial habenula-interpeduncular nucleus (IPN) system (Marks et al., 1992). The α3 and β4 subunits are also predominately found in the MHb-IPN system. The MHb projects almost exclusively to the IPN via the fasciculus retroflexus (Fr) (Herkenham and Nauta, 1979). Functional α5* nAChRs are expressed on MHb afferents to IPN (Grady et al., 2009; Sheffield et al., 2000). Intriguingly, the MHb-IPN tract is known to limit consumption of noxious substances (Donovick et al., 1970; Thornton et al., 1994) and regulate avoidance of aversive stimuli (Donovick et al., 1970; Hammer and Klingberg, 1990; Meszaros et al., 1985; Thompson, 1960; Thornton et al., 1994; Wirtshafter, 1981). This system is also involved in the expression of somatic aspects of the nicotine withdrawal syndrome (Salas et al., 2009). We therefore hypothesized that α5* nAChRs, and perhaps α3* and β4* nAChRs, in the MHb-IPN regulate aversive effects of nicotine. Consistent with this hypothesis, we found that virus-mediated re-expression of the otherwise absent α5 subunit in the MHb-IPN system of the KO mice completely rescued their behavioral phenotype; these “rescued” α5 KO mice reduced their level of nicotine intake at higher doses consistent with the behavioral profile exhibited by WT mice. Conversely, virus-mediated knockdown of this subunit in the MHb-IPN system of rats resulted in greater intake, particularly when high unit doses of the drug were available for consumption (Fowler et al., 2011). These findings suggest that the CHRNA5-CHRNA3-CHRNB4 gene cluster in the MHb-IPN system, and nAChRs in these sites that incorporate the subunits encoded by these genes, regulate the aversive effects of higher nicotine doses that serve to suppress intake. To more directly test this hypothesis, we have examined the effects of nicotine on intracranial self-stimulation (ICSS) thresholds in α5 KO mice and in rats after knockdown of α5 subunits in the MHb-IPN system. Nicotine-mediated lowering of ICSS thresholds are thought to reflect the stimulatory actions of the drug on the brain reward systems responsible for establishing and maintaining the nicotine-taking habit in rodents and perhaps human tobacco smokers (Kenny, 2007). Conversely, elevations of ICSS thresholds induced by higher nicotine doses are thought to reflect an inhibitory action on brain reward systems that drives avoidance of the drug (Fowler et al., 2011). We found that global deletion of α5 nAChR subunits in the knockout mice (Fowler et al., 2013), or restricted knockdown of the subunits in the MHb-IPN system of rats, abolished the ICSS threshold-elevating effects of higher doses of nicotine but did not impact the threshold-lowering effects of lower nicotine doses. The specific role for α5* nAChRs in nicotine aversion but not nicotine reward may explain the shape of the D-R curve for nicotine self-administration in humans, primates and rodents, and why it is altered by α5* nAChR deficiency. Specifically, we propose that nicotine reward and aversion are dissociable effects, with the mesoaccumbens dopamine neurons that project to the accumbens shell region likely playing an important role in the positive reinforcing effects of nicotine, and the habenula-interpeduncular systems regulating nicotine aversion. The rewarding actions of nicotine may occur through the α4α6β2β3* nAChR subtype (Grady et al., 2007), which has the highest sensitivity to nicotine of any native nAChR so far identified (Grady et al., 2007) and would explain the “ascending” portion of the D-R curve. As α6* nAChR subunits are not expressed in the MHB-IPN aversion pathway, the high-affinity α4α6β2β3 nAChR subtype is not expressed locally. Instead, nicotine activates this pathway through lower affinity α5* nAChRs, accounting for the descending portion of the D-R curve at higher nicotine doses. The combinatorial effects of nicotine at the high-affinity α4α6β2β3* nAChRs in the mesoaccumbens systems, and lower affinity α5* nAChRs in the MHb-IPN therefore likely explains the “window” of nicotine doses that are reinforcing, and the emergence of aversion at higher doses resulting in a U-shaped dose-response curve. Taking all the above data together, it seems that nicotine stimulates the MHb-IPN pathway through α5* nAChRs and thereby enhances glutamatergic transmission in, and consequent activation of, the IPN. Most available smoking cessation agents are thought to attenuate smoking behavior by targeting nAChRs in midbrain dopamine systems, perhaps by targeting the α4α6β2β3* nAChRs. As discussed below, it may be possible to rationally design new smoking cessation agents that act independent of nicotine reward and instead act by targeting α5* nAChRs in habenular aversion systems.
Finally, almost exclusively, regions of the posterior septum are responsible for providing afferent input to the MHb. Specifically, the triangular septal and septofimbrial nuclei and the bed nucleus of the anterior commissure (BAC) densely innervate the MHb (Yamaguchi et al., 2013). Interestingly, lesions to the septum or the interpeduncular nucleus can abolish aversive responses to, and avoidance of, noxious bitter tastants such as quinine (Donovick et al., 1970). This suggests that major functions of the septo-habenulo-interpeduncular pathway include regulation of food consumption by controlling avoidance of noxious substances. Interestingly, individuals with deficits in the ability to detect bitter tastants are much more likely to be regular smokers (Saper et al., 2002). Hence, it is an intriguing possibility that constitutive deficits in septo-habenulo-interpeduncular function, reflected in diminished ability to detect bitter tastes, may also result in diminished sensitivity to the aversive effects of nicotine and account for the increased vulnerability to developing tobacco dependence in such individuals. More recently, it was shown that septal inputs to MHb may also regulate anxiety and fear-related behaviors in rodents (Yamaguchi et al., 2013). It is therefore interesting to speculate that mood-regulated effects mediated by the septo-habenulo-interpeduncular system may also influence vulnerability to tobacco dependence.
The lateral habenula-rostromedial tegmental area pathway and nicotine aversion
In addition to the MHb projection to the IPN, recent evidence suggests that the lateral habenula (LHb) may also play a role in nicotine aversion. Unlike the MHb, which projects almost exclusively to IPN, the LHb projects only sparely to IPN and instead sends prominent projections to the rostromedial tegmental nucleus (RMTg) (Jhou et al., 2009), and less prominent projects to the VTA. Through these projections, the LHb inhibits the firing of midbrain dopamine neurons directly (via VTA projections) or indirectly (via RMTg projections) (Bromberg-Martin and Hikosaka, 2011; Hikosaka, 2010; Jhou et al., 2009; Lecourtier and Kelly, 2007; Matsumoto and Hikosaka, 2009). LHb neurons are activated by aversive stimuli or omission of anticipated rewards (Bromberg-Martin and Hikosaka, 2011; Hikosaka, 2010; Lecourtier and Kelly, 2007; Matsumoto and Hikosaka, 2009). This suggests that LHb transmission, and its inputs to RMTg, could encode aspects of nicotine aversion and influence responses to the drug. Consistent with this possibility, Pistis and colleagues have recently shown that nicotine potently and robustly excites neurons in the RMTg (Lecca et al., 2012). This effect was likely related to a stimulatory action of nicotine on α7 nAChRs located presynaptically on excitatory glutamatergic inputs from the LHb (Lecca et al., 2012). The functional consequent of this stimulatory effect of nicotine on RMTg transmission in regulating nicotine aversion and nicotine consumption has not been directly investigated.
Other brain circuitries that may play a role in nicotine aversion
As described above, the MHb-IPN system densely expresses nAChRs containing α5, α3 and/or β4 subunits. Indeed, it was based on the dense expression of these subunits in the MHb-IPN system that the role of α5* nAChRs in these sites in nicotine aversion was first investigated in mice (Fowler et al., 2011). Interestingly, the nucleus tractus solitarius (NTS) is a hindbrain site that also displays very dense expression of these subunits. The NTS contains at least three types of neurons: catecholaminergic neurons that produce the neurotransmitter norepinephrine (and to a lesser extent epinephrine); glucagon-producing neurons that synthesize the neuropeptide glucagon-like peptide-1 (GLP-1); and neurons that synthesize the feeding-related neuropeptide proopiomelanocortin (POMC). The NTS is perhaps best known for its role in regulating taste reactivity, as it receives dense innervation from the buccal cavity (Appleyard et al., 2005; Grill and Hayes, 2009). NTS neurons also receive vagal inputs from the viscera and NTS activation in response to vagal stimulation can induce cessation of food intake. Specifically, catecholaminergic neurons relay signals to higher feeding centers in the brain from the gastrointestinal (GI) tract related to meal ingestion or gastric distension, and respond also to circulating satiety signals including cholecystokinin (CCK) (Appleyard et al., 2007; Monnikes et al., 1997; Rinaman et al., 1998; Willing and Berthoud, 1997). Catecholaminergic neurons in the NTS have been implicated in the expression of aversive aspects of drug withdrawal (Delfs et al., 2000; Taylor et al., 1998). It has been shown that nicotine can activate NTS catecholaminergic neurons, likely though a mechanism involving increased local glutamatergic transmission (Feng et al., 2012; Hong et al., 2012; Kalappa et al., 2011; Shiraki et al., 1997; Zhao et al., 2007). Moreover, the α and β adrenergic receptor antagonist carvedilol can reduce self-reported aversive responses to nicotine, delivered as a lozenge to human volunteers (Sofuoglu et al., 2006). These findings suggest that nicotine may activate NTS catecholaminergic neurons, increasing adrenergic transmission in forebrain regions, with this effect contributing to aversive aspects of the drug, a hypothesis that has yet to be tested. NTS catecholaminergic neurons have instead been implicated in drug reward rather than aversion. Specifically, it was shown that the rewarding effects of morphine are greatly diminished in dopamine β-hydroxylase knockout (DBH-KO) mice that are unable to synthesize norepinephrine (Olson et al., 2006), and virus-mediated re-expression of DBH in the NTS of the KO mice restores their sensitivity to morphine reward (Olson et al., 2006). As noted above, neurons that produce the neuropeptide GLP-1 are also a major population of neurons in the NTS. Activation of GLP-1 occurs in response to gastric distention, nausea, stress and illness and results in suppression of food intake (Barrera et al., 2011; Hayes et al., 2009; Turton et al., 1996). Hence, the NTS, and in particular GLP-1 neurons, seem well placed to regulate aversion-related actions of nicotine. Nevertheless, the role of the NTS in nicotine avoidance behavior has not yet been investigated.
Projections from the IPN to other brain regions are also of interest as potential mediators of aversive processing. Little is currently known about how aversion-related information from the MHb-IPN circuit is integrated and processed in parallel with reward-related information rom the mesocorticolimbic pathway. The IPN has broad ascending and descending projections to various brain regions. The most prominent of these projections are the medial septum/diagonal band of Broca, hippocampus, dorsal tegmental nucleus, raphe and periaqueductal gray (Groenewegen et al., 1986; Klemm, 2004; Montone et al., 1988; Shibata and Suzuki, 1984). Many of these regions, including the diagonal band of Broca, dorsal tegmental nucleus, and raphe, send projections to the VTA (Groenewegen et al., 1986; Oades and Halliday, 1987; Phillipson, 1979; Wirtshafter, 1981). Thus, MHb-IPN signaling may be integrated within mesocorticolimbic system, perhaps even in the core region of the NAc (see above) via intermediate brain regions that project to specific neuronal populations within the VTA, analogous to the LHb-RMTg-VTA circuit. Thus, understanding how information from the MHb-IPN system is processed at the wider circuit level will be important to delineate in future investigations.
Novel smoking cessation agents that modulate nicotine avoidance
Data described above demonstrate that deficient α5* nAChR signaling, particularly in the MHb-IPN system, increases nicotine intake in rats and mice. Hence, an intriguing approach to facilitate smoking cessation may be the development of small molecule compounds that amplify α5* nAChR signaling. Before such selective compounds can be developed, it is critical to know which subtype of α5* nAChRs regulates nicotine aversion. In heterologous expression systems, α5 subunits can co-assemble into α4β2, α3β2, and α3β4 nAChR subtypes (Fucile et al., 1997; Gerzanich et al., 1998; Tapia et al., 2007). However, in the mammalian brain it appears that α5 subunits predominantly assembles into α4β2* nAChR subtypes (Gotti et al., 2007; Kuryatov et al., 2008; Mao et al., 2008; Perry et al., 2007). Indeed, using immunoprecipitation, it was reported that α5 subunits are almost exclusively in complex with α4β2 subunits in the hippocampus, striatum, cortex and thalamus, with almost undetectable levels of α3* nAChRs containing the α5 subunit in these regions (Mao et al., 2008). In the MHb-IPN pathway ~11% of β2* nAChRs express α5 subunits (Grady et al., 2009), whereas only ~5% of β4* nAChRs express the subunit (Grady et al., 2009). Importantly, in the MHb-IPN pathway α3β4* nAChRs are thought to exclusively regulate acetylcholine release (Grady et al., 2009), whereas α4β2α5* nAChRs regulate glutamate release (Girod et al., 2000). We previously found that disruption of glutamatergic transmission in the IPN increases nicotine intake in rats in a manner similar to genetic disruption of α5* nAChR signaling in rats or mice (Fowler et al., 2011). Hence, 5* nAChRs are likely to be a functional subtype in the MHb-IPN that negatively regulates nicotine intake. Boosting the activity of this particular nAChR subtype in response to nicotine consumption may therefore be a novel strategy to decrease nicotine intake and facilitate smoking cessation efforts.
Nicotinic receptors are pentameric complexes in which acetylcholine (and nicotine) binds at the interface between α and β subunits (orthosteric sites). It is hypothesized that agonist binding at orthosteric sites stabilizes the receptor channel complex in the “open” conformation, thereby increasing receptor activity. Partial agonists less efficiently stabilize the receptor “open” state, and conversely, competitive antagonists stabilize the receptor channel in the “closed” state. Agonists may also stabilize a receptor transition from “active” to an inactive “desensitized” state. There are multiple allosteric sites elsewhere on the multimeric nAChR complex, which by themselves do not stimulate opening or closing of the receptor channel, but instead modify the activity of the receptor once activated by orthosteric ligands (Changeux, 1990; Changeux et al., 1984; Changeux et al., 1992; Chemouilli et al., 1985; Lena and Changeux, 1993). Positive allosteric modulators (PAMs) are ligands that bind to allosteric sites to facilitate agonist-induced stabilization of the “open” conformation or to reduce agonist-facilitated receptor desensitization, with PAMs unable to influence receptor function in the absence of orthosteric agonists. Hence, PAMs can potentiate the stimulatory effects of low agonist concentrations on nAChR function in much the same manner that benzodiazepines potentiate the actions of GABA at the GABAA receptor. A number of features of PAM acting at α4β2α5* suggest that they may be particularly attractive candidates as novel smoking cessation agents. First, because the orthosteric binding site is so well conserved between various nAChR subtypes, it is difficult to engineer agonists with receptor selectivity (Albrecht et al., 2008; Armishaw et al., 2009). Moreover, α5 nAChR subunits co-expressed with β2 or β4 subunits do not co-assemble into functional heteropentameric nAChRs without the presence of another α subunit (Boulter et al., 1987; Couturier et al., 1990). Instead, α5 subunits act as accessory subunits that modulate receptor activation/desensitization kinetics (Ramirez-Latorre et al., 1996). Moreover, α5 subunits play a key role in generating novel allosteric modulatory sites on α5* nAChRs (Taly et al., 2009). Thus, it is likely to be far easier to develop PAMs that are highly selective for α4β2α5* nAChRs compared to the development of orthosteric agonists. Second, it is expected that α4β2α5* PAMs have low intrinsic activity at these nAChRs in the MHb-IPN tract or other brain areas (depending on cholinergic tone). Instead PAMs should potentiate α4β2α5* nAChRs most efficiently only when activated by nicotine in tobacco smoke. This is an important point when considering that MHb-IPN activation typically occurs in response to aversive stimuli (Donovick et al., 1970; Hammer and Klingberg, 1990; Meszaros et al., 1985; Thompson, 1960; Thornton et al., 1994; Wirtshafter, 1981), suggesting that full α4β2α5* nAChR agonists may possess intrinsic aversive properties that would limit their clinical utility. Furthermore, unlike orthosteric agonists, PAMs are unlikely to desensitize and thereby inhibit α4β2α5* nAChRs. Desensitization of nAChRs by full agonists could paradoxically decrease MHb-IPN sensitivity to nicotine, resulting in an increase in the motivational properties of the drug and an undesired increase in tobacco consumption. Finally, by potentiating the deficient function of α4β2α5* nAChRs in individuals carrying CHRNA5 risk alleles, PAMs may be able to attenuate genetic vulnerability to tobacco dependence.
In the context of developing α4β2α5* nAChR PAMs for smoking cessation, it is interesting to note that that the acetylcholinesterase (AChE) inhibitors galantamine and physostigmine are PAMs of α4β2* nAChRs (Maelicke et al., 2001; Pereira et al., 1994; Pereira et al., 1993; Samochocki et al., 2003; Samochocki et al., 2000; Storch et al., 1995), and codeine may also be an α4β2* nAChR PAM (Storch et al., 1995). This action is not thought to be related to AChE inhibitor activity, as other AChE inhibitors including tacrine, metrifonate, rivastigmine and donepezil do not share this action (Samochocki et al., 2000). Importantly, the FK1 monoclonal antibody, which binds selectively to α nAChR subunits (Schroder et al., 1994), completely abolishes the PAM effects of galantamine, physostigmine and codeine on α4β2* nAChR function (Pereira et al., 1994; Storch et al., 1995), thereby verifying a direct allosteric action on the α subunit, and not an orthosteric action at the interfac subunits. Intriguingly, a recent study reported that galantamine is a PAM only at α4β2α5* nAChRs, and is practically inactive α4β2* nAChRs that do not contain α5 subunits (Kuryatov et al., 2008). Galantamine has been shown to reduce the number of cigarettes smoked in a recent clinical trial (Diehl et al., 2006) and also reduced intravenous nicotine self-administration behavior in rats (Hopkins et al., 2012; Liu and Stewart, 2009). These actions of galantamine may be related in part to its PAM action at α4β2α5* nAChRs. Nevertheless, galantamine is likely to have very limited clinical utility for smoking cessation. Its PAM action occurs only at low concentrations, and at higher concentrations it inhibits α4β2α5* nAChRs. Also, AChE inhibition by galantamine and other known PAMs is likely to be a major confound. Therefore, it will be important to develop and test novel α5* nAChR PAMs for smoking cessation that are efficacious across a broad dose-range and are devoid of “off-target” effects.
Aversion or satiety?
At first glance, the data reviewed above appear to support the concept of enhancing nicotine aversion as a potential therapeutic strategy for tobacco dependence. This may bring to mind other types of approaches used in the past, not always successfully, to treat substance abuse disorders, most notably alcohol dependence. Disulfiram (Antabuse) is an “aversive therapy” use to facilitate abstinence from alcohol. Disulfiram acts by irreversibly inhibiting an enzyme involved in alcohol metabolism, acetaldehyde dehydrogenase (Center for Substance Abuse Treatment, 2009). When alcohol consumption by those treated with disulfiram there is accumulation of acetaldehyde, resulting in moderate to severe physical reactions to alcohol that include nausea, vomiting, hypotension and facial flushing. When alcohol is not consumed, side effects are minimal and may include headache and fatigue (Fuller and Gordis, 2004). The clinical effectiveness of disulfiram has been variable (Brewer et al., 2000; Fuller and Gordis, 2004), most notably due to issues with patient compliance (Suh et al., 2006). Given these considerations, a therapeutic that enhances the aversive effects of nicotine may not be as straightforward as one would hope in the clinical setting due to the potential confound of patient adherence to the dosing schedule.
This issue necessitates a more refined consideration of the concepts of aversion and avoidance, and how these processes could potentially be leveraged to develop novel smoking cessation agents. In this regard, it is important to note that the role for α5* nAChRs in the habenula-interpeduncular system in regulating nicotine intake may be more subtle that just regulating aversive effects of nicotine and thereby promoting avoidance. Instead, we propose that these receptors may serve to transmit a “satiety” signal for nicotine that decreases the motivation to further consume the drug, with this satiety signal occurring at does of nicotine that are not overtly aversive. In essence, such a satiety signal would serve to indicate that consumption of nicotine beyond the point at which these receptors have been engaged will reverse the stimulatory actions of nicotine on brain reward systems that motivate consumption of the drug, and yet further consumption will lead to frank aversion. From a conceptual perspective, nicotine intake could be envisioned as being regulated by opposing brain systems: those that promote consumption to obtain reward-enhancing properties of the drug, analogous to hunger systems in the brain that drive food consumption in states of negative energy balance. Conversely, putative satiety systems may promote cessation of consumption to avoid excessive activation of nAChR transmission in brain, analogous to satiety systems in the brain that promote cessation of food consumption in states of positive energy balance. If this is indeed the case, then α5* nAChRs in the habenula-interpeduncular system may represent a novel “satiety” system for nicotine, boosting the activity of which would decrease nicotine intake in a manner fundamentally different from previous “aversion therapy” approaches. In essence, boosting α5* nAChR signaling would lower the amount of nicotine required to trigger a satiety signal in reward systems, and cessation of intake, at concentrations of nicotine that are not yet sufficient to induce states of aversion. Such an action would different fundamentally from how Disulfiram and other aversion therapies work. The proposed existence of satiety systems for nicotine, and potentially other drugs of abuse, may offer new approaches to understanding how compulsive drug use may emerge, and how long-term abstinence from drug use can be achieved.
Summary
The findings reviewed above demonstrate that, in addition to the rewarding effects of nicotine, noxious effects of the drug also likely influence the development and persistence of the tobacco smoking habit in humans. Specifically, avoidance of the aversive properties of nicotine play a key role in determining the amounts of nicotine consumed, patterns of consumption, and hence the magnitude by which nicotine induces neuroplasticity in addiction-relevant brain reinforcement circuits. Habenulo-interpedunular glutamatergic transmission and aspects of mesoaccumbens dopamine, acetylcholine and opioid transmission appear to regulate the aversive effects of nicotine, and thereby control avoidance of the drug. Moreover, allelic variation in genes highly expressed in the aversion-related circuitries, in particular the CHRNA5 gene that encodes the α5 nAChR subunit, can influence vulnerability to tobacco dependence in humans, highlighting the importance of nicotine aversion in controlling vulnerability to addiction. Much work still remains to precisely understand how the aversive effects of nicotine are encoded in the brain, and how aversion-related circuits may interact with reward circuits to control nicotine intake. Nevertheless, the available data support the interesting possibility that amplifying the noxious properties of nicotine via small molecule drugs may serve as a novel strategy to develop efficacious smoking cessation agents. Finally, it is interesting to speculate that in addition to brain reward systems that drive drug-seeking behaviors, brain satiety systems may exist that serve to suppress drug-seeking behaviors, with α5* nAChR signaling in the habenula-interpeduncular systems representing on such satiety system.
Highlights.
Role for nicotine aversive in controlling tobacco consumption highlighted.
Genetics to tobacco addiction reviewed.
Mesoaccumbens dopamine systems in nicotine aversion reviewed.
Habenulo-interpeduncular system in nicotine aversion is reviewed.
Novel smoking cessation therapeutics that increase nicotine aversion considered.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. This work was supported by a grant from the National Institute on Drug Abuse (DA020686 to P.J.K. and DA032543 to C.D.F.). This is manuscript number 23035 from The Scripps Research Institute.
CITED LITERATURE
- Albrecht BK, Berry V, Boezio AA, Cao L, Clarkin K, Guo W, Harmange JC, Hierl M, Huang L, Janosky B, Knop J, Malmberg A, McDermott JS, Nguyen HQ, Springer SK, Waldon D, Woodin K, McDonough SI. Discovery and optimization of substituted piperidines as potent, selective, CNS-penetrant alpha4beta2 nicotinic acetylcholine receptor potentiators. Bioorg Med Chem Lett. 2008;18:5209–5212. doi: 10.1016/j.bmcl.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi N, Miyamoto M, Umetsu Y, Kunitoh H, Dosaka-Akita H, Sawamura Y, Yokota J, Nemoto N, Sato K, Kamataki T. Genetic polymorphism of CYP2A6 gene and tobacco-induced lung cancer risk in male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:890–894. [PubMed] [Google Scholar]
- Armishaw C, Jensen AA, Balle T, Clark RJ, Harpsoe K, Skonberg C, Liljefors T, Stromgaard K. Rational design of alpha-conotoxin analogues targeting alpha7 nicotinic acetylcholine receptors: improved antagonistic activity by incorporation of proline derivatives. J Biol Chem. 2009;284:9498–9512. doi: 10.1074/jbc.M806136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31:3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Baudonnat M, Cazala P, Guilloux JP, Reperant C, Cloez-Tayarani I, Changeux JP, Gardier AM, Granon S. Alpha7-nicotinic receptors modulate nicotine-induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice. Psychopharmacology (Berl) 2012;220:1–14. doi: 10.1007/s00213-011-2422-1. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr., Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J, Hinrichs AL, Wang JC, von Weymarn LB, Kharasch ED, Bierut LJ, Goate A, Murphy SE. The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics. 2011;21:403–416. doi: 10.1097/FPC.0b013e328346e8c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C, Meyers RJ, Johnsen J. Does disulfiram help to prevent relapse in alcohol abuse? Cns Drugs. 2000;14:329–341. [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nature neuroscience. 2011;14:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment, C. Incorporating Alcohol Pharmacotherapies Into Medical Practice: A Review of the Literature*. In: (US) S. A. a. M. H. S. A., editor. Treatment Improvement Protocol (TIP) Series. Rockville, MD: 2009. [PubMed] [Google Scholar]
- Changeux JP. The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol Sci. 1990;11:485–492. doi: 10.1016/0165-6147(90)90049-e. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Galzi JL, Revah F. The acetylcholine receptor: a model of an allosteric membrane protein mediating intercellular communication. Ciba Found Symp. 1992;164:66–89. doi: 10.1002/9780470514207.ch6. discussion 87-97. [DOI] [PubMed] [Google Scholar]
- Chemouilli P, Heidmann T, Changeux JP, Bachy A, Morre M. Allosteric effects of diprobutine on acetylcholine receptors. Eur J Pharmacol. 1985;117:205–214. doi: 10.1016/0014-2999(85)90605-3. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL. Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology (Berl) 1999;145:412–417. doi: 10.1007/s002130051075. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology. 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Couturier S, Erkman L, Valera S, Rungger D, Bertrand S, Boulter J, Ballivet M, Bertrand D. Alpha 5, alpha 3, and non-alpha 3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. J Biol Chem. 1990;265:17560–17567. [PubMed] [Google Scholar]
- David V, Besson M, Changeux JP, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, Croissant B, Smolka MN, Batra A, Mann K. Galantamine reduces smoking in alcohol-dependent patients: a randomized, placebo-controlled trial. Int J Clin Pharmacol Ther. 2006;44:614–622. doi: 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- Donovick PJ, Burright RG, Zuromski E. Localization of quinine aversion within the septum, habenula, and interpeduncular nucleus of the rat. J Comp Physiol Psychol. 1970;71:376–383. doi: 10.1037/h0029114. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks PA, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem Pharmacol. 2009;78:732–743. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal alpha5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci. 2012;32:2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Sametsky EA, Gusev AG, Uteshev VV. Responsiveness to nicotine of neurons of the caudal nucleus of the solitary tract correlates with the neuronal projection target. J Neurophysiol. 2012;108:1884–1894. doi: 10.1152/jn.00296.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CS, Miksys S, Palmour RM, Tyndale RF. Differential effects of nicotine treatment and ethanol self-administration on CYP2A6, CYP2B6 and nicotine pharmacokinetics in African green monkeys. J Pharmacol Exp Ther. 2012;343:628–637. doi: 10.1124/jpet.112.198564. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Tuesta L, Kenny PJ. Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3235-1. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to Nicotine Is Regulated by the Balanced Activity of beta4 and alpha5 Nicotinic Receptor Subunits in the Medial Habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alema S, Ballivet M, Eusebi F. Alpha 5 subunit forms functional alpha 3 beta 4 alpha 5 nAChRs in transfected human cells. Neuroreport. 1997;8:2433–2436. doi: 10.1097/00001756-199707280-00005. [DOI] [PubMed] [Google Scholar]
- Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction. 2004;99:21–24. doi: 10.1111/j.1360-0443.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–13825. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Rice KC, Matecka D, Rothman RB. Phentermine/fenfluramine decreases cocaine self-administration in rhesus monkeys. Neuroreport. 1997;8:1347–1351. doi: 10.1097/00001756-199704140-00006. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Maintenance and suppression of behavior by intravenous nicotine injections in squirrel monkeys. Fed Proc. 1982;41:216–220. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224:334–340. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav. 1983;19:1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder TE, George O, Tan H, George SR, Le Foll B, Laviolette SR, van der Kooy D. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc Natl Acad Sci U S A. 2012;109:3101–3106. doi: 10.1073/pnas.1114422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. International journal of obesity. 2009;33(Suppl 1):S11–15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- Grilli M, Zappettini S, Raiteri L, Marchi M. Nicotinic and muscarinic cholinergic receptors coexist on GABAergic nerve endings in the mouse striatum and interact in modulating GABA release. Neuropharmacology. 2009;56:610–614. doi: 10.1016/j.neuropharm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Jr., Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ. A Risk Allele for Nicotine Dependence in CHRNA5 Is a Protective Allele for Cocaine Dependence. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP, Shoaib M. Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology. 2000;39:2848–2855. doi: 10.1016/s0028-3908(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database Syst Rev. 2004:CD000546. doi: 10.1002/14651858.CD000546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer KH, Klingberg F. Active avoidance is permanently abolished after lesions of the nucleus interpeduncularis in rat. Biomed Biochim Acta. 1990;49:489–497. [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav. 1983a;19:1021–1026. doi: 10.1016/0091-3057(83)90409-4. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983b;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR, Herning RI, Jasinski DR, Lukas SE, Miyasato K, Nemeth-Coslett R, Pickworth WB, Rose JE, Sampson A, et al. Human studies of the behavioral pharmacological determinants of nicotine dependence. NIDA Res Monogr. 1986;67:54–65. [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nature reviews. Neuroscience. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LZ, Cheng PW, Cheng WH, Chen SR, Wang LL, Tseng CJ. Involvement of NMDA receptors in nicotine-mediated central control of hypotensive effects. Chin J Physiol. 2012;55:337–345. doi: 10.4077/CJP.2012.BAA059. [DOI] [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology. 2012;37:2310–2321. doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Pelkonen O, Hakkola J, Raunio H. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Crit Rev Toxicol. 2002;32:391–411. doi: 10.1080/20024091064273. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006a;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006b;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyaniwura TT, Wright AE, Balfour DJ. Evidence that mesoaccumbens dopamine and locomotor responses to nicotine in the rat are influenced by pretreatment dose and strain. Psychopharmacology (Berl) 2001;158:73–79. doi: 10.1007/s002130100852. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Ingelman-Sundberg M. Genetic polymorphism and toxicology - with emphasis on cytochrome P450. Toxicological Sciences. 2011;120:1–13. doi: 10.1093/toxsci/kfq374. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Feng L, Kem WR, Gusev AG, Uteshev VV. Mechanisms of facilitation of synaptic glutamate release by nicotinic agonists in the nucleus of the solitary tract. Am J Physiol Cell Physiol. 2011;301:C347–361. doi: 10.1152/ajpcell.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009a;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009b;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–273. [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci. 2002;22:8653–8660. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lauzon NM, Bishop SF, Sun N, Tan H. Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci. 2008;28:8025–8033. doi: 10.1523/JNEUROSCI.1371-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003a;8:50–59. doi: 10.1038/sj.mp.4001197. 59. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology (Berl) 2003b;166:306–313. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Allosteric modulations of the nicotinic acetylcholine receptor. Trends Neurosci. 1993;16:181–186. doi: 10.1016/0166-2236(93)90150-k. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Liu X, Stewart C. Suppression of nicotine self-administration by acetylcholinesterase inhibition in rats; 2009 Neuroscience Meeting Planner; Society for Neuroscience; Chicago, IL. 2009. Online 648.5/W17. [Google Scholar]
- Lundahl LH, Henningfield JE, Lukas SE. Mecamylamine blockade of both positive and negative effects of IV nicotine in human volunteers. Pharmacol Biochem Behav. 2000;66:637–643. doi: 10.1016/s0091-3057(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, Zerlin M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry. 2001;49:279–288. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Weinberg JB, Rada PV, Hoebel BG. Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res. 1995;688:184–188. doi: 10.1016/0006-8993(95)00401-b. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nature neuroscience. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Duchemin AM, Neff NH, Hadjiconstantinou M. CREB involvement in the regulation of striatal prodynorphin by nicotine. Psychopharmacology (Berl) 2012;221:143–153. doi: 10.1007/s00213-011-2559-y. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, Cadeddu F, Frau R, Fattore L, Fadda P, Ennas MG, Castelli MP, Fratta W, Schilstrom B, Banni S, De Montis MG, Pistis M. PPARalpha Regulates Cholinergic-Driven Activity of Midbrain Dopamine Neurons via a Novel Mechanism Involving alpha7 Nicotinic Acetylcholine Receptors. J Neurosci. 2013;33:6203–6211. doi: 10.1523/JNEUROSCI.4647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros J, Gajewska S, Tarchalska-Krynska B. Habenulo-interpeduncular lesions: the effects on pain sensitivity, morphine analgesia and open-field behavior in rats. Pol J Pharmacol Pharm. 1985;37:469–477. [PubMed] [Google Scholar]
- Mifsud JC, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- Molles BE, Maskos U, Pons S, Besson M, Guiard P, Guilloux JP, Evrard A, Cormier A, Mameli-Engvall M, Cloez-Tayarani I, Nakatani H, Dufour N, Bemelmans AP, Mallet J, Cazala P, Gardier AM, David V, Faure P, Granon S, Changeux JP. Targeted in vivo expression of nicotinic acetylcholine receptors in mouse brain using lentiviral expression vectors. Journal of molecular neuroscience: MN. 2006;30:105–106. doi: 10.1385/jmn:30:1:105. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770:277–288. doi: 10.1016/s0006-8993(97)00865-2. [DOI] [PubMed] [Google Scholar]
- Montone KT, Fass B, Hamill GS. Serotonergic and nonserotonergic projections from the rat interpeduncular nucleus to the septum, hippocampal formation and raphe: a combined immunocytochemical and fluorescent retrograde labelling study of neurons in the apical subnucleus. Brain Res Bull. 1988;20:233–240. doi: 10.1016/0361-9230(88)90183-9. [DOI] [PubMed] [Google Scholar]
- Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, Krasnow RE, Swan GE, Benowitz NL, Tyndale RF. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin Pharmacol Ther. 2008;83:115–121. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm. 1997;104:1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- Norton GR, Barske B. The role of aversion in the rapid-smoking treatment procedure. Addict Behav. 1977;2:21–25. doi: 10.1016/0306-4603(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, Hawk LW, Tyndale RF, Benowitz N, Lerman C. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J, Whiting P, Albuquerque EX. Physostigmine and galanthamine: probes for a novel binding site on the alpha 4 beta 2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J Pharmacol Exp Ther. 1994;270:768–778. [PubMed] [Google Scholar]
- Pereira EF, Reinhardt-Maelicke S, Schrattenholz A, Maelicke A, Albuquerque EX. Identification and functional characterization of a new agonist site on nicotinic acetylcholine receptors of cultured hippocampal neurons. J Pharmacol Exp Ther. 1993;265:1474–1491. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rada PV, Hoebel BG. Aversive hypothalamic stimulation releases acetylcholine in the nucleus accumbens, and stimulation-escape decreases it. Brain Res. 2001;888:60–65. doi: 10.1016/s0006-8993(00)02865-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Raunio H, Rahnasto-Rilla M. CYP2A6: genetics, structure, regulation, and function. Drug Metabol Drug Interact. 2012;27:73–88. doi: 10.1515/dmdi-2012-0001. [DOI] [PubMed] [Google Scholar]
- Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. kappa-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus VI, Obach RS, Coe JW, Faessel H, Rollema H, Watsky E, Reeves K. Varenicline: new treatment with efficacy in smoking cessation. Drugs Today (Barc) 2007;43:65–75. doi: 10.1358/dot.2007.43.2.1069956. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. The American journal of physiology. 1998;275:R262–268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM. Genetic differences in nicotine-induced conditioned taste aversion. Life Sci. 1996;58:223–229. doi: 10.1016/0024-3205(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Cook DG, Gaunt TR, Nightingale CM, Whincup PH, Day IN. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J Psychopharmacol. 2011;25:915–923. doi: 10.1177/0269881111405352. [DOI] [PubMed] [Google Scholar]
- Russell MA. Tobacco dependence: is nicotine rewarding or aversive? NIDA Res Monogr. 1979:100–122. [PubMed] [Google Scholar]
- Russell MA, Wilson C, Patel UA, Feyerabend C, Cole PV. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. Br Med J. 1975;2:414–416. doi: 10.1136/bmj.2.5968.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliovaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nothen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lubbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Zerlin M, Jostock R, Groot Kormelink PJ, Luyten WH, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol Scand Suppl. 2000;176:68–73. doi: 10.1034/j.1600-0404.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, Grant JD, Jacob T, Xian H. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav. 2010;35:771–778. doi: 10.1016/j.addbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, Svensson TH. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Sellers EM, Palmour R, Tyndale RF. Down-regulation of hepatic nicotine metabolism and a CYP2A6-like enzyme in African green monkeys after long-term nicotine administration. Mol Pharmacol. 2003;63:96–104. doi: 10.1124/mol.63.1.96. [DOI] [PubMed] [Google Scholar]
- Schroder B, Reinhardt-Maelicke S, Schrattenholz A, McLane KE, Kretschmer A, Conti-Tronconi BM, Maelicke A. Monoclonal antibodies FK1 and WF6 define two neighboring ligand binding sites on Torpedo acetylcholine receptor alpha-polypeptide. J Biol Chem. 1994;269:10407–10416. [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Baharnouri G, McQuade LE, Clarke PB. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci. 2008;28:342–352. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Suzuki T. Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: a retrograde horseradish peroxidase study. Brain Res. 1984;296:345–349. doi: 10.1016/0006-8993(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Shiraki T, Toyoda A, Sugino H, Hori A, Kobayashi S. Possible nicotinic receptor-mediated modulation of synaptic transmission in nucleus of the solitary tract. The American journal of physiology. 1997;272:R869–873. doi: 10.1152/ajpregu.1997.272.3.R869. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Yoo S, Kosten T. Adrenergic blocker carvedilol attenuates the cardiovascular and aversive effects of nicotine in abstinent smokers. Behav Pharmacol. 2006;17:731–735. doi: 10.1097/FBP.0b013e32801155d4. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14-20. [DOI] [PubMed] [Google Scholar]
- Storch A, Schrattenholz A, Cooper JC, Abdel Ghani EM, Gutbrod O, Weber KH, Reinhardt S, Lobron C, Hermsen B, Soskic V, et al. Physostigmine, galanthamine and codeine act as ‘noncompetitive nicotinic receptor agonists’ on clonal rat pheochromocytoma cells. Eur J Pharmacol. 1995;290:207–219. doi: 10.1016/0922-4106(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9:511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, Wise RA. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology (Berl) 2009;205:431–439. doi: 10.1007/s00213-009-1553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Wise RA. Satiating effects of cocaine are controlled by dopamine actions in the nucleus accumbens core. J Neurosci. 2011;31:17917–17922. doi: 10.1523/JNEUROSCI.1903-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SR, Feyerabend C, Cole PV, Russell MA. Adjustment of smokers to dilution of tobacco smoke by ventilated cigarette holders. Clin Pharmacol Ther. 1978;24:395–405. doi: 10.1002/cpt1978244395. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Punch LJ, Elsworth JD. A comparison of the effects of clonidine and CNQX infusion into the locus coeruleus and the amygdala on naloxone-precipitated opiate withdrawal in the rat. Psychopharmacology (Berl) 1998;138:133–142. doi: 10.1007/s002130050655. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Mark GP, Hoebel BG. Conditioned taste aversion from neostigmine or methyl-naloxonium in the nucleus accumbens. Physiol Behav. 2011;104:82–86. doi: 10.1016/j.physbeh.2011.04.050. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 2012;224:289–301. doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. Interpeduncular nucleus and avoidance conditioning in the rat. Science. 1960;132:1551–1553. doi: 10.1126/science.132.3439.1551. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, Consortium E, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton EW, Murray M, Connors-Eckenrode T, Haun F. Dissociation of behavioral changes in rats resulting from lesions of the habenula versus fasciculus retroflexus and their possible anatomical substrates. Behav Neurosci. 1994;108:1150–1162. doi: 10.1037//0735-7044.108.6.1150. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, Piper ME, Fiore MC, Scholand MB, Connett JE, Kanner RE, Gahring LC, Rogers SW, Hoidal JR, Leppert MF. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. The American journal of physiology. 1997;272:R59–67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D. The role of interpeduncular connections with the tegmentum in avoidance learning. Physiol Behav. 1981;26:985–989. doi: 10.1016/0031-9384(81)90197-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct Roles of Segregated Transmission of the Septo-Habenular Pathway in Anxiety and Fear. Neuron. 2013 doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Chen H, Sharp BM. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdala is mediated by N-methyl-D-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J Pharmacol Exp Ther. 2007;320:837–844. doi: 10.1124/jpet.106.112474. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]