Abstract
Aims
Mephedrone is a stimulant drug of abuse with close structural and mechanistic similarities to methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA). Although mephedrone does not damage dopamine nerve endings it increases the neurotoxicity of amphetamine, methamphetamine and MDMA. The effects of mephedrone on serotonin (5HT) nerve endings are not fully understood, with some investigators reporting damage while others conclude it does not. Presently, we investigate if mephedrone given alone or with methamphetamine or MDMA damages 5HT nerve endings of the hippocampus.
Main methods
The status of 5HT nerve endings in hippocampus of female C57BL mice was assessed through measures of 5HT by HPLC and by immunoblot analysis of serotonin transporter (SERT) and tryptophan hydroxylase 2 (TPH2), selective markers of 5HT nerve endings. Astrocytosis was assessed through measures of glial fibrillary acidic protein (GFAP) (immunoblotting) and microglial activation was determined by histochemical staining with Isolectin B4.
Key findings
Mephedrone alone did not cause persistent reductions in the levels of 5HT, SERT or TPH2. Methamphetamine and MDMA alone caused mild reductions in 5HT but did not change SERT and TPH2 levels. Combined treatment with mephedrone and methamphetamine or MDMA did not change the status of 5HT nerve endings to an extent that was different from either drug alone.
Significance
Mephedrone does not cause toxicity to 5HT nerve endings of the hippocampus. When co-administered with methamphetamine or MDMA, drugs that are often co-abused with mephedrone by humans, toxicity is not increased as is the case for dopamine nerve endings when these drugs are taken together.
Keywords: mephedrone, methamphetamine, MDMA, serotonin, bath salts, neurotoxicity
Introduction
Abuse of illicit “bath salts” compounds continues to rise and now represents a significant public health crisis. One psychoactive ingredient of “bath salts” is mephedrone (4-methylmethcathinone). From a chemical perspective, mephedrone is a cathinone derivative and structural analog of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA). Other psychoactive ingredients of “bath salts” include methylone, butylone and 3, 4-methylenedioxypyrovalerone (MDPV). As abuse of β-ketoamphetamines continues to rise, the list of their adverse effects has grown to include cardiovascular complications, agitation, insomnia, psychosis and depression (Prosser and Nelson, 2012; Schifano et al., 2011; Wood and Dargan, 2012). Mephedrone is also being used increasingly to supplement more established club drugs such as MDMA and cocaine (Moore et al., 2013).
Mephedrone has many of the same effects on the central nervous system as methamphetamine and MDMA. For example, it interacts with the monoamine plasma membrane transporters for dopamine (DA), serotonin (5HT) and norepinephrine (Baumann et al., 2012; Cozzi et al., 1999; Hadlock et al., 2011; Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012; Nagai et al., 2007; Simmler et al., 2013; Sogawa et al., 2011), blocks uptake of DA, 5HT and norepinephrine (Cozzi et al., 1999; Hadlock et al., 2011; Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012; Simmler et al., 2013) and stimulates release of these neurotransmitters (Baumann et al., 2012; Hadlock et al., 2011; Kehr et al., 2011) via its ability to serve as a transporter substrate (Baumann et al., 2012; Eshleman et al., 2013; Nagai et al., 2007). Mephedrone increases locomotor activity (Angoa-Pérez et al., 2012; Baumann et al., 2012; Marusich et al., 2012; Motbey et al., 2012a; Shortall et al., 2012), causes hyperthermia (Angoa-Pérez et al., 2013; Angoa-Pérez et al., 2012; Baumann et al., 2012; Hadlock et al., 2011) and provokes a Fos expression pattern in brain that closely resembles those of methamphetamine and MDMA (Motbey et al., 2012a). The simultaneous stimulation of DA release and inhibition of its uptake, along with increases in body temperature and locomotor activity mirror the critical elements underlying the neurotoxicity associated with methamphetamine (Cadet et al., 2007; Fleckenstein et al., 2007; Kuhn et al., 2008; Yamamoto and Bankson, 2005).
In view of the close structural and mechanistic overlap of mephedrone with methamphetamine and MDMA, it was predicted that mephedrone would cause toxicity to DA nerve endings. However, a number of studies have established that this drug does not cause persistent reductions in function of the DA neuronal system (Angoa-Pérez et al., 2012; Baumann et al., 2012; den Hollander et al., 2013; Hadlock et al., 2011; Motbey et al., 2012b; Shortall et al., 2012). The issue of whether mephedrone damages 5-HT nerve endings remains unsettled as one study documented possible neurotoxic effects (Hadlock et al., 2011) while others have been negative in this regard (Baumann et al., 2012; den Hollander et al., 2013; Motbey et al., 2012b; Shortall et al., 2012). The present studies were carried out to determine if mephedrone alone or combined with methamphetamine or MDMA would unmask a neurotoxic effect on 5HT nerve endings.
Materials and methods
Drugs and Reagents
Mephedrone hydrochloride and 3,4-methylenedioxymethamphetamine (MDMA) hydrochloride were obtained from the NIDA Research Resources Drug Supply Program. (+) Methamphetamine hydrochloride, Isolectin B4 (ILB4), pentobarbital, 5HT, 5-hydroxyindole acetic acid (5HIAA), and all buffers and HPLC reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bicinchoninic acid protein assay kits were obtained from Pierce (Rockford, IL, USA). Polyclonal antibodies against SERT were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and antibodies against GFAP were obtained from Neomarkers (Fremont, CA, USA). Polyclonal antibodies against TPH2 were prepared and used as previously described (Sakowski et al., 2006). HRP-conjugated anti-IgG secondary antibodies were provided by Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Animals
Female C57BL/6 mice (Harlan, Indianapolis, IN, USA) weighing 20–25 g at the time of experimentation were housed 5 per cage in large shoe-box cages in a light- (12 h light/dark) and temperature-controlled room. Female mice were used because they are known to be very sensitive to neuronal damage by the neurotoxic amphetamines and to maintain consistency with our previous studies of methamphetamine neurotoxicity (Thomas et al., 2010; Thomas et al., 2008; 2009). Mice had free access to food and water. The Institutional Care and Use Committee of Wayne State University approved the animal care and experimental procedures. All procedures were also in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Pharmacological procedures
Mice were treated with mephedrone using a binge-like regimen comprised of 4 injections of 20 mg/kg with a 2 h interval between each injection. This binge treatment regimen, when used to inject substituted amphetamines and cathinone derivatives, results in extensive DA nerve ending damage. The doses of mephedrone used presently were previously shown to be non-toxic to DA nerve endings (Angoa-Pérez et al., 2013; Angoa-Pérez et al., 2012). Mice were treated with methamphetamine (4X 5 mg/kg) or MDMA (4X 20 mg/kg) alone or in combination with mephedrone. When treated with two drugs, mice received a mephedrone injection 30 min prior to each of the 4 injections of methamphetamine or MDMA. Controls received injections of physiological saline on the same schedule used for mephedrone alone or in combination with other amphetamines. All injections were given via the i.p. route. Mice were sacrificed 2 days after the last drug treatment when amphetamine-associated neurotoxicity has reached maximum.
Determination of hippocampal 5HT and 5HIAA content
Hippocampus was dissected bilaterally from the brain after treatment and stored at –80°C. Frozen tissues were weighed and sonicated in 10 volumes of 0.16 N perchloric acid at 4°C. Insoluble protein was removed by centrifugation and 5HT and 5HIAA were determined by HPLC with electrochemical detection as previously described (Thomas et al., 2010).
Determination of SERT and TPH2 protein levels by immunoblotting
The effects of drug treatments on hippocampal SERT and TPH2 were determined by immunoblotting as an index of toxicity to 5HT nerve endings as previously reported (Thomas et al., 2010). Mice were sacrificed by decapitation after treatment and hippocampus was dissected bilaterally. Tissue was stored at −80°C. Frozen tissue was disrupted by sonication in 1% SDS at 95°C and insoluble material was sedimented by centrifugation. Protein was determined by the bicinchoninic acid method and equal amounts of protein (70 µg/lane) were resolved by SDS-polyacrylamide gel electrophoresis and then electroblotted to nitrocellulose. Blots were blocked in Tris buffered saline containing Tween 20 (0.1% v/v) and 5% non-fat dry milk for 2 h at room temperature. Primary antibodies against SERT (1:250) or TPH2 (1:200) were added to blots and allowed to incubate for 16 h at 4°C. Blots were washed 3X in Tris-buffered saline to remove unreacted antibodies and then incubated with HRP-conjugated anti-IgG secondary antibody (1:4000) for 1 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence and the relative densities of SERT- and TPH2-reactive bands were determined by imaging with a Kodak Image Station (Carestream Molecular Systems, Rochester, NY, USA) and quantified using ImageJ software (NIH).
Assessment of hippocampal gliosis
The effects of drug treatments on hippocampal astrocytosis were determined by immunoblotting for GFAP (1:2000) as described above for SERT and TPH2 and microglial activation was assessed by histochemical staining with ILB4 as previously reported (Thomas et al., 2004a; Thomas et al., 2004b).
Data analysis
The effects of drug treatments on hippocampal 5HT and 5HIAA content, expression levels of SERT and TPH2 and gliosis were tested for significance by one-way ANOVA followed by Tukey’s multiple comparison test. Differences were considered statistically significant if p < 0.05. All statistical analyses were carried out using GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
Results
Effects of mephedrone and methamphetamine on 5HT nerve endings
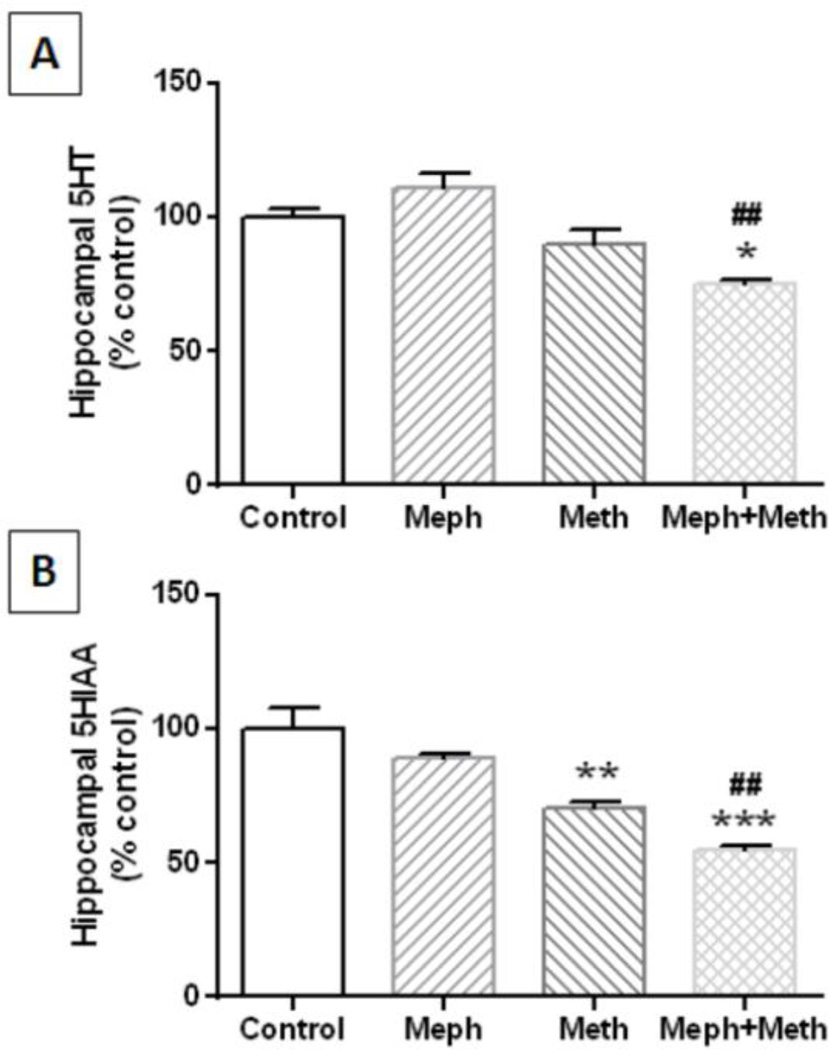
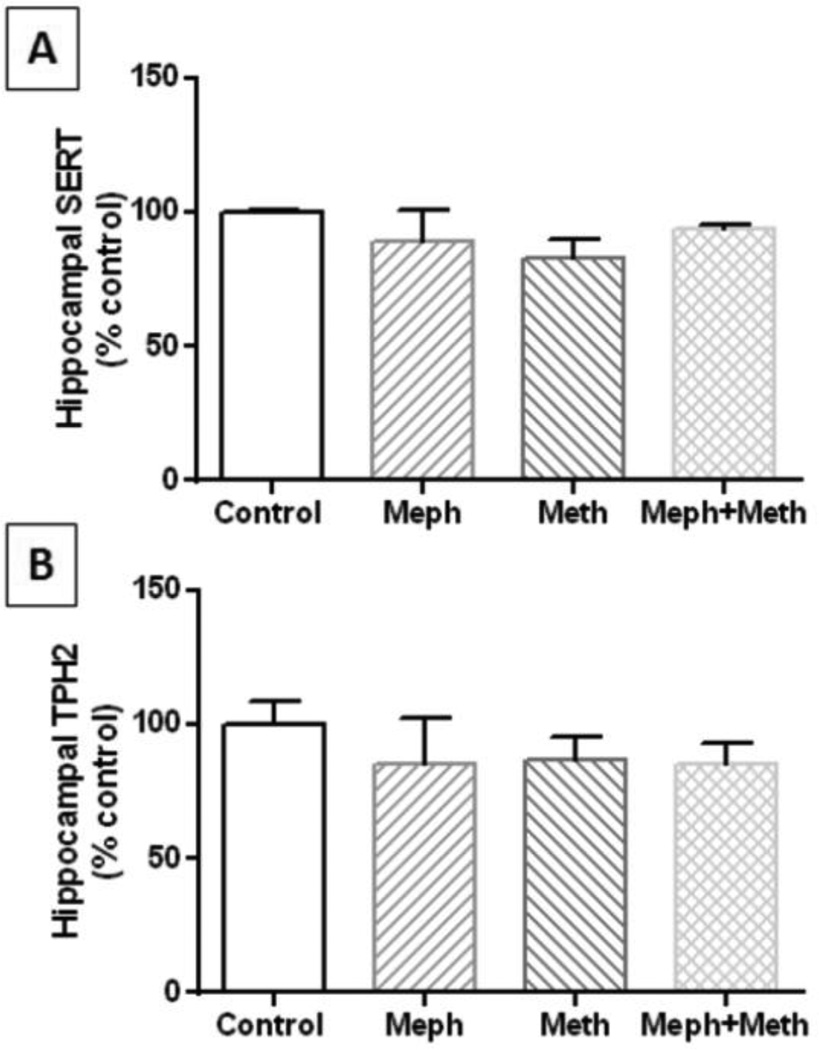
Mephedrone (20 mg/kg) was administered 30 min before each injection of methamphetamine (4X 5 mg/kg) and the effects are presented in Fig. 1A. The main effect of drug treatment on 5HT levels was significant (F3,30 = 5.02, p = 0.006) but neither mephedrone nor methamphetamine alone changed hippocampal 5HT levels. Combined treatment with mephedrone + methamphetamine did lower 5HT levels slightly (~20%) but significantly by comparison with controls (p < 0.05) and mephedrone alone (p < 0.001). The main effect of drug treatment on 5HIAA levels was also significant (F3,14 = 18.56, p < 0.0001) and Fig. 1B shows that methamphetamine (p < 0.001) and the combination of mephedrone + methamphetamine significantly (p < 0.0001) significantly lowered metabolite levels by comparison with controls. This combination treatment was also significantly different from mephedrone alone (p < 0.001). The effects of these drug treatments on hippocampal SERT and TPH2 are presented in Fig. 2A and 2B, respectively, and show that neither mephedrone nor methamphetamine alone or in combination altered the levels of these proteins.
Fig. 1.
Effects of mephedrone and methamphetamine on hippocampal levels of 5HT and 5HIAA. Mice were treated with mephedrone (Meph; 20 mg/kg) 30 min prior to each of the 4 injections of methamphetamine (Meth; 5 mg/kg) and sacrificed 2d later for determination of hippocampal levels of 5HT (A) and 5HIAA (B) by HPLC. Data are mean ± SEM for 6–11 mice per group. *p < 0.05, **p < 0.001, or ***p < 0.0001 vs controls and ##p < 0.001 vs mephedrone alone (Tukey’s multiple comparison test).
Fig. 2.
Effects of mephedrone and methamphetamine on hippocampal levels of SERT and TPH2. Mice were treated with mephedrone (Meph; 20 mg/kg) 30 min prior to each of the 4 injections of methamphetamine (Meth; 5 mg/kg) and sacrificed 2d later for determination of striatal levels of SERT (A) or TPH2 (B) by immunoblotting. Blots were quantified using ImageJ and data are mean ± SEM for 5–7 mice per group.
Effects of mephedrone and MDMA on 5HT nerve endings
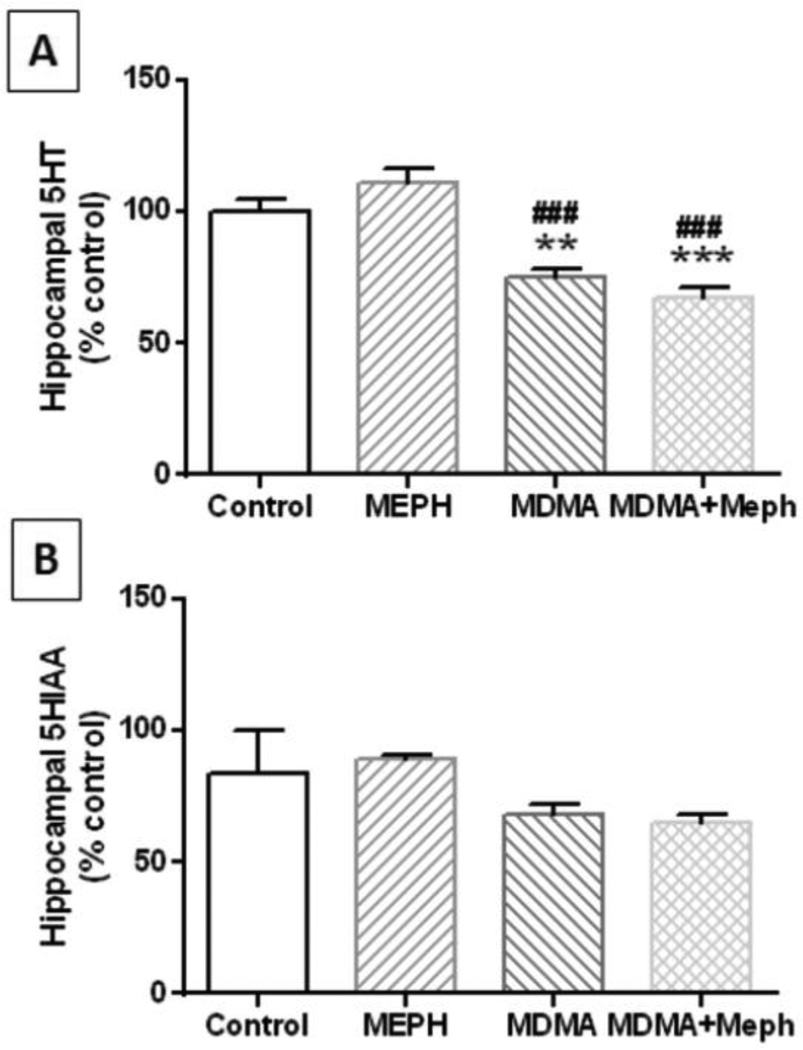
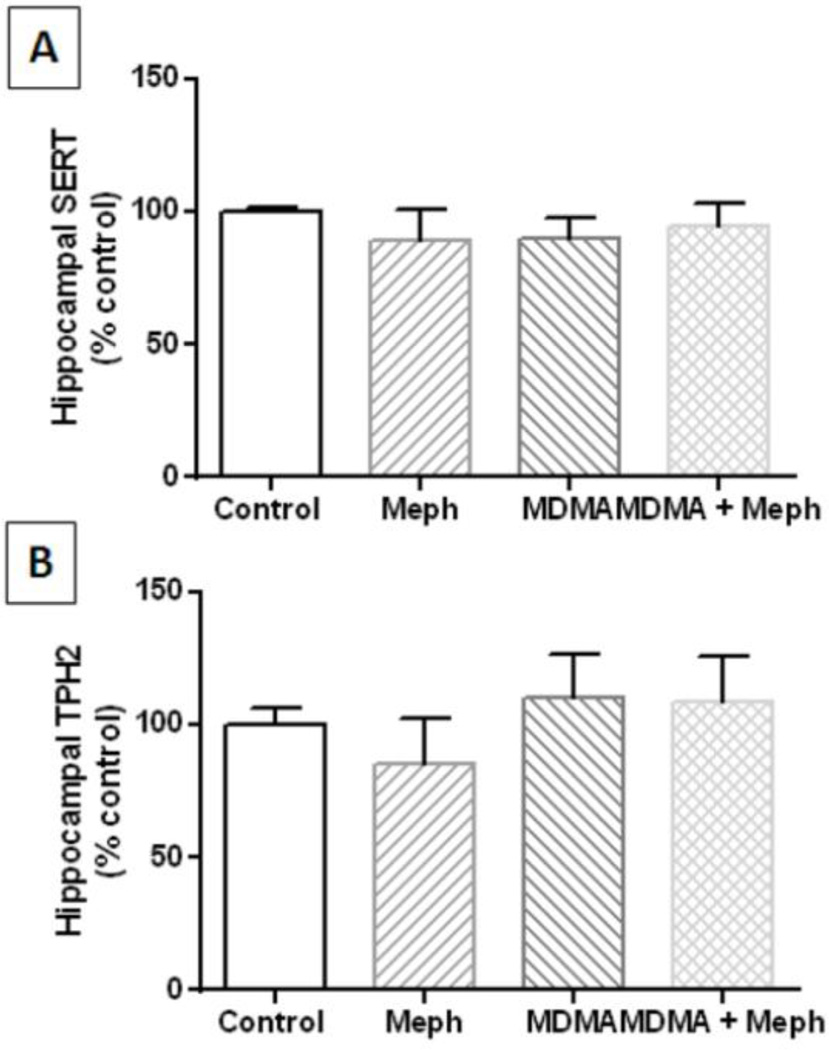
Mephedrone (20 mg/kg) was administered 30 min before each injection of MDMA (4X 20 mg/kg) and the effects are presented in Fig. 3A. The main effect of drug treatment on 5HT levels was significant (F3,17 = 22.52, p < 0.0001). It can be seen that mephedrone did not alter hippocampal 5HT while MDMA significantly reduced 5HT levels by comparison to control (p < 0.001) and mephedrone alone (p < 0.0001). Combined treatment with mephedrone + MDMA also significantly reduced 5HT levels by comparison with controls (p < 0.0001) and mephedrone alone (p < 0.0001), but MDMA + mephedrone did not differ significantly from MDMAM alone. None of these treatments changed hippocampal 5HIAA levels (Fig. 3B). Likewise, none of these drug treatments altered the levels of SERT (Fig. 4A) or TPH2 (Fig. 4B) by comparison with controls.
Fig. 3.
Effects of mephedrone and MDMA on hippocampal levels of 5HT and 5HIAA. Mice were treated with mephedrone (Meph; 20 mg/kg) 30 min prior to each of the 4 injections of methamphetamine (MDMA; 20 mg/kg) and sacrificed 2d later for determination of hippocampal levels of 5HT (A) or 5HIAA (B) by HPLC. Data are mean ± SEM for 5–7 mice per group. **p < 0.001 or ***p < 0.0001 vs control and ###p < 0.0001 vs mephedrone alone (Tukey’s multiple comparison test).
Fig. 4.
Effects of mephedrone and MDMA on hippocampal levels of SERT and TPH2. Mice were treated with mephedrone (Meph; 20 mg/kg) 30 min prior to each of the 4 injections of MDMA (20 mg/kg) and sacrificed 2d later for determination of hippocampal levels of SERT (A) or TPH2 (B) by immunoblotting. Blots were quantified using ImageJ and data are mean ± SEM for 5–7 mice per group.
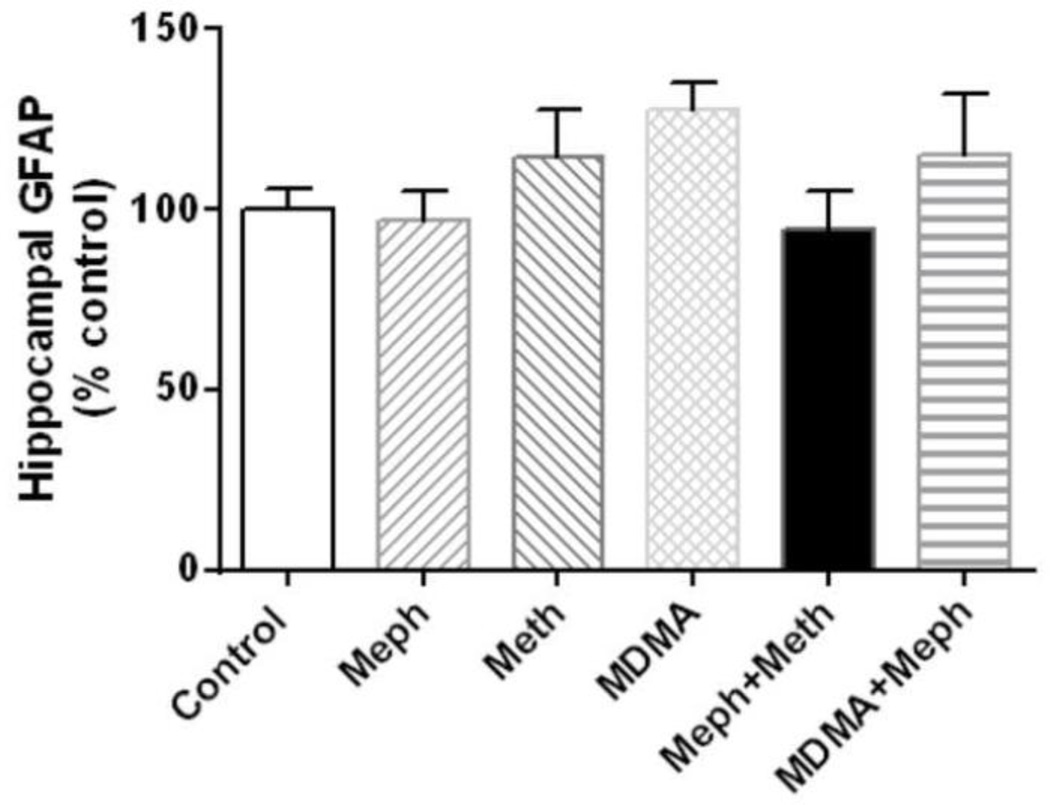
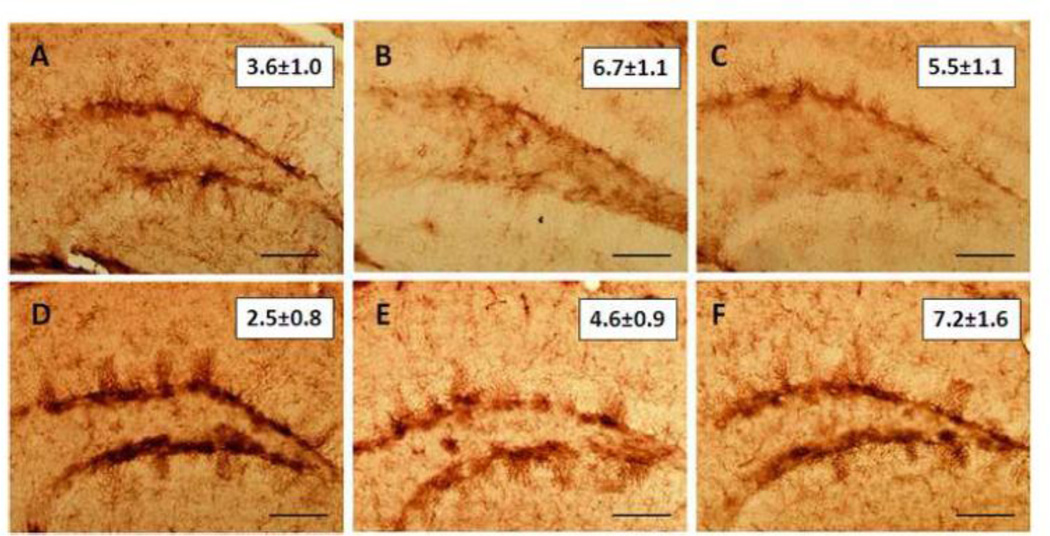
Effects of mephedrone in combination with methamphetamine or MDMA on hippocampal gliosis
We have shown previously that both methamphetamine and MDMA cause microglial activation in striatum (Thomas et al., 2004a). Therefore, hippocampus was examined for evidence of astrogliosis via measures of GFAP and for microglial activation via histochemical staining with ILB4. Results in Fig. 5 show that mephedrone, methamphetamine or MDMA alone did not change GFAP levels in hippocampus. Similarly, combined treatment with mephedrone + methamphetamine or mephedrone + MDMA did not alter hippocampal levels of GFAP. The effects of these same drug treatments on microglial status were assessed and results in Fig. 6 show that none of the drugs given alone or in combination caused microglial activation in hippocampus.
Fig. 5.
Effects of mephedrone, methamphetamine and MDMA on hippocampal GFAP. Mice were treated with mephedrone (Meph; 20 mg/kg), methamphetamine (Meth; 5 mg/kg) or MDMA (20 mg/kg), or the combinations of mephedrone + methamphetamine or MDMA. When given in combination, mephedrone was given 30 min prior to each of the 4 injections of methamphetamine or MDMA. Mice were sacrificed 2d after treatment for determination of hippocampal GFAP by immunoblotting. Blots were quantified using ImageJ. Data are mean ± SEM for 5–7 mice in each group.
Fig. 6.
Effects of mephedrone, methamphetamine or MDMA on hippocampal microglial activation. Mice were treated with mephedrone (Meph; 20 mg/kg), methamphetamine (Meth; 5 mg/kg) or MDMA (20 mg/kg), or the combinations of mephedrone + methamphetamine or MDMA. When given in combination, mephedrone was given 30 min prior to each of the 4 injections of methamphetamine or MDMA. Mice were sacrificed 2d after treatment for determination of hippocampal microglial activation using histochemical staining with ILB4 and representative results are shown for (A) control, (B) methamphetamine, (C) MDMA, (D) mephedrone, (E) mephedrone + methamphetamine and (F) mephedrone + MDMA. Numbers in the insets of each panel represent microglial counts and are the mean ± SEM for 5–7 mice per group. The calibration bar is 40 µm.
Discussion
Mephedrone has become one of the most common drug of abuse following cannabis, MDMA and cocaine (Morris, 2010; Winstock et al., 2011b) and its use will likely surpass that of MDMA as the purity of this latter drug continues to fall (Brunt et al., 2011; Tanner-Smith, 2006; Teng et al., 2006). Mephedrone induces stronger feelings of craving in humans by comparison to MDMA (Brunt et al., 2011) and users who snort mephedrone rate it as more addictive than cocaine (Winstock et al., 2011b). The strong abuse potential of mephedrone has also been confirmed by emerging preclinical studies showing its ability to cause locomotor sensitization (Lisek et al., 2012; Shortall et al., 2012), establish a conditioned place preference (Lisek et al., 2012) and sustain intravenous self-administration (Hadlock et al., 2011).
Considering the close structural and mechanistic overlaps between mephedrone and the neurotoxic amphetamines, it was surprising to find that mephedrone does not cause damage to DA nerve endings of the striatum in mice (Angoa-Pérez et al., 2012; Baumann et al., 2012; den Hollander et al., 2013; Hadlock et al., 2011; Motbey et al., 2012b; Shortall et al., 2012). On the other hand, given the manner in which it interacts with DA and 5HT transporters, it has been predicted that mephedrone would protect against methamphetamine- or MDMA-induced neurotoxicity. This latter possibility has been ruled out by studies showing that mephedrone enhances damage to DA nerve endings caused by methamphetamine, MDMA and amphetamine (Angoa-Pérez et al., 2013). Based on common patterns of abuse of mephedrone and other “bath salts” ingredients (i.e., consumed in a binge-like fashion and often taken with other drugs such as cannabis and the amphetamine psychostimulants (Fass et al., 2012; Kelly, 2011; Moore et al., 2013; Schifano et al., 2011; Torrance and Cooper, 2010; Winstock et al., 2011a)) it becomes important to determine if mephedrone damages 5HT nerve endings and if it can interact with methamphetamine and MDMA to enhance their toxicity.
In the present study, we found that mephedrone alone did not cause damage to hippocampal 5HT nerve endings. The levels of 5HT (and its main metabolite 5HIAA), SERT and TPH2 were unchanged by a binge-treatment regimen using relatively high doses of mephedrone. This result is in good agreement with other recent studies showing that mephedrone did not cause persistent depletion of 5HT (Baumann et al., 2012; den Hollander et al., 2013; Motbey et al., 2012b; Shortall et al., 2012). The one study reporting that mephedrone caused 5HT deficits (Hadlock et al., 2011) used similar experimental subjects and treatment conditions as employed by the studies referenced above, so the reason for this discrepancy is not clear. By extending these studies to a poly-drug use paradigm, it was also observed that mephedrone did not enhance the effects of either methamphetamine or MDMA on hippocampal 5HT neuronal markers. Although methamphetamine did cause a mild reduction in 5HIAA levels, this effect was not modified by mephedrone. MDMA caused a significant reduction in hippocampal 5HT levels and this effect was likewise unmodified by mephedrone. Mephedrone alone or in combination with methamphetamine or MDMA did not alter the levels of SERT or TPH2, two highly specific protein markers for 5HT nerve endings.
Increased expression of GFAP is an index of astrogliosis and has been used extensively as a marker of amphetamine-induced CNS damage (O'Callaghan and Miller, 1993; 1994). It has also been shown that microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines (Bowyer et al., 1994; LaVoie et al., 2004; Thomas et al., 2004a). Gliosis was assessed in hippocampus after mephedrone injections and it was observed that the levels of GFAP were not changed from control, and microglial activation was not seen. Neither methamphetamine nor MDMA caused gliosis in hippocampus when given alone and combined treatment with mephedrone + methamphetamine or mephedrone + MDMA likewise did not change the levels of GFAP or cause microglial activation. Both methamphetamine and MDMA cause extensive astrogliosis (O'Callaghan and Miller, 1993; 1994) and microglial activation in striatum (Bowyer et al., 1994; LaVoie et al., 2004; Thomas et al., 2004a; Thomas et al., 2009; Thomas and Kuhn, 2005; Thomas et al., 2004b) in a manner that is associated with amphetamine-induced neurotoxicity to DA nerve endings in this brain region. Although our previous research on the effects of mephedrone on the DA neuronal system did not report data on striatal gliosis (Angoa-Pérez et al., 2013; Angoa-Pérez et al., 2012), we have not yet observed any evidence of gliosis in striatum (unpublished observations).
Taken together, the results of the present experiments suggest that mephedrone does not cause damage to 5HT nerve endings of the hippocampus. Methamphetamine and MDMA alone lead to minor disruptions in 5HT neurochemistry (i.e., reduced turnover) but these do not appear to rise to the level of neurotoxicity because stable protein markers such as SERT and TPH2 were not altered and gliosis was not observed. We purposefully used high doses of methamphetamine and MDMA because their effects on 5HT nerve endings outside of the striatum are somewhat mild and possibly non-toxic (Baumann et al., 2007). Nevertheless, when mephedrone was administered with either methamphetamine or MDMA, an enhancement of any drug effect on 5HT, SERT or TPH2 levels was not observed. Likewise, these combined drug treatments did not result in hippocampal gliosis. The question of whether MDMA damages the 5HT neuronal system remains a contested issue (Baumann et al., 2007; McLane et al., 2011; Wang et al., 2005; Wang et al., 2004; Xie et al., 2006). Mephedrone, on the other hand, does not damage DA or 5HT nerve endings despite being a chemical congener of the neurotoxins methamphetamine and MDMA. Our conclusion that mephedrone has low neurotoxic potential within the 5HT neuronal system should be tempered somewhat in view of the fact that mice are not as sensitive to the 5HT-damaging effects of MDMA as rats and other species (Baumann et al., 2007; Colado et al., 2004). This could lead to an underestimation of mephedrone-induced toxicity to 5HT nerve endings. Furthermore, brain regions in addition to the hippocampus should be studied for their response to mephedrone to determine if this drug exerts toxic effects on 5HT nerve endings that are brain-region specific. We have shown previously that mephedrone does not prevent the hyperthermia caused by methamphetamine or MDMA (Angoa-Pérez et al., 2012, 2013), ruling out the possibility that its low toxicity is related to prevention of amphetamine-induced increases in core temperature. Future research on methamphetamine- and MDMA-induced neurotoxicity should focus on the minor structural differences with mephedrone that renders the latter drug non-neurotoxic.
Conclusion
Mephedrone is a powerful psychostimulant with high abuse potential. Mephedrone shares many chemical and mechanistic properties with the neurotoxic amphetamines but does not cause neurotoxic effects on the DA neuronal system. The present work confirms other reports that mephedrone does not appear to cause persistent deficits in 5HT nerve ending function. The ability of mephedrone to accentuate the toxic effects of methamphetamine and MDMA on DA nerve endings does not extend to 5HT nerve endings of the hippocampus. It will be interesting to determine if other psychoactive ingredients of “bath salts” such as methylone, butylone and MDPV are also devoid of neurotoxic effects.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All the authors declared no competing interests.
References
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, et al. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem. 2013;125:102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, et al. Mephedrone, an abused psychoactive component of 'bath salts' and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, et al. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol (Oxf) 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, 3rd, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of "bath salt" designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.04.004. http://dx.org/10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann Pharmacother. 2012;46:436–441. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann N Y Acad Sci. 2008;1139:118–126. doi: 10.1196/annals.1432.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, et al. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in "bath salts" on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLane MW, McCann U, Ricaurte G. Identifying the serotonin transporter signal in Western blot studies of the neurotoxic potential of MDMA and related drugs. Synapse. 2011;65:1368–1372. doi: 10.1002/syn.20958. [DOI] [PubMed] [Google Scholar]
- Moore K, Dargan PI, Wood DM, Measham F. Do novel psychoactive substances displace established club drugs, supplement them or act as drugs of initiation? The relationship between mephedrone, ecstasy and cocaine. Eur Addict Res. 2013;19:276–282. doi: 10.1159/000346678. [DOI] [PubMed] [Google Scholar]
- Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375:1333–1334. doi: 10.1016/s0140-6736(10)60559-4. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addiction Biol. 2012a;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Karanges E, Li KM, Wilkinson S, Winstock AR, Ramsay J, et al. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS One. 2012b;7:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Res Monogr. 1993;136:188–212. [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RT, Jayson R, Korsah C, Pillidge KE, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;169:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa C, Sogawa N, Ohyama K, Kikura-Hanajiri R, Goda Y, Sora I, et al. Methylone and monoamine transporters: correlation with toxicity. Curr Neuropharmacol. 2011;9:58–62. doi: 10.2174/157015911795017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner-Smith EE. Pharmacological content of tablets sold as "ecstasy": results from an online testing service. Drug Alcohol Depend. 2006;83:247–54. doi: 10.1016/j.drugalcdep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Teng SF, Wu SC, Liu C, Li JH, Chien CS. Characteristics and trends of 3,4-methylenedioxymethamphetamine (MDMA) tablets found in Taiwan from 2002 to February 2005. Forensic Sci Int. 2006;161:202–208. doi: 10.1016/j.forsciint.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Angoa-Pérez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004a;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem. 2009;109:1745–1755. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202:e62–e63. doi: 10.1016/j.forsciint.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Morales M, Rothman RB. (+/−)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011a;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011b;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Dargan PI. Mephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:227–233. doi: 10.1016/j.pnpbp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, McLane MW, Hatzidimitriou G, Yuan J, McCann U, et al. Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology. 2006;31:2639–2651. doi: 10.1038/sj.npp.1301031. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]