Abstract
Obesity is associated with chronic low-grade inflammation in peripheral tissues caused, in part, by the recruitment of inflammatory monocytes into adipose tissue. Studies in rodent models have also shown increased inflammation in the central nervous system (CNS) during obesity. The goal of this study was to determine whether obesity is associated with recruitment of peripheral immune cells into the CNS. To do this we used a bone marrow chimerism model to track the entry of green-fluorescent protein (GFP) labeled peripheral immune cells into the CNS. Flow cytometry was used to quantify the number of GFP+ immune cells recruited into the CNS of mice fed a high-fat diet compared to standard chow fed controls. High-fat feeding resulted in obesity associated with a 30% increase in the number of GFP+ cells in the CNS compared to control mice. Greater than 80% of the GFP+ cells recruited to the CNS were also CD45+ CD11b+ indicating that the GFP+ cells displayed characteristics of microglia/macrophages. Immunohistochemistry further confirmed the increase in GFP+ cells in the CNS of the high-fat fed group and also indicated that 93% of the recruited cells were found in the parenchyma and had a stellate morphology. These findings indicate that peripheral immune cells can be recruited to the CNS in obesity and may contribute to the inflammatory response.
Keywords: Obesity, Inflammation, Neuroinflammation, Microglia, High-fat diet, Bone marrow chimera
1. Introduction
Obesity is a major health problem in the U.S. affecting greater than 35% of the population and contributing to an estimated $147 billion in annual medical costs Odgen et al., 2012. One characteristic of obesity is chronic low-grade inflammation mediated by the recruitment of macrophages and other immune cells into adipose tissue. This inflammation in peripheral tissues plays a critical role in the progression and development of a wide range of obesity-associated co-morbidities such as type 2 diabetes and cardiovascular disease (Hotamisligil, 2006).
Obesity is also associated with an increased incidence of neurologic disease including a staggering 74% increased risk of dementia (Whitmer et al., 2005). While often regarded as an immune privileged organ, during disease states the central nervous system (CNS) exhibits some of the classical characteristics of inflammation, including increased cytokine production and recruitment of immune cells from the periphery (Muldoon et al., 2013). Recent data suggests that obesity is associated with CNS inflammation which may contribute to the increased susceptibility to neurologic disease, reflecting what has been observed in peripheral tissues. For example, increased production of pro-inflammatory cytokines and reactive oxygen species was shown to occur in the hypothalamus in response to both acute and chronic high-fat feeding in mice (De Souza et al., 2005; Freeman et al., 2013; Thaler et al., 2012). Inflammation in the CNS may also have an impact on the development and perpetuation of obesity; for example, reduction in the NFκB inflammatory signaling cascade through targeted deletion of Iκκβ in the brains of mice led to a reduction in weight gain on high-fat diet and improved glucose tolerance (Zhang et al., 2008). Furthermore, reducing obesity-associated hypothalamic inflammation through infusion of a tumor necrosis factor-α neutralizing antibody has a beneficial effect on insulin sensitivity in the liver (Milanski et al., 2012). This emerging evidence suggests that inflammation in the CNS may be a mediator of disease pathogenesis and pathophysiology in obesity.
In the CNS, glial cells, including microglia and astrocytes, have a critical role in the pathology of inflammatory diseases such as Alzheimer’s (Griffin et al., 1989) and are attractive therapeutic targets. Derived from myeloid progenitors during late embryonic development (Ginhoux et al., 2010), microglia share many functional and molecular characteristics with peripheral tissue macrophages (Ransohoff and Perry, 2009). We and others have demonstrated that high-fat feeding and obesity are associated with the activation of microglia (Thaler et al., 2012; Yi et al., 2012a) and astrocytes (Buckman et al., 2013; Hsuchou et al., 2009; Thaler et al., 2012), which suggests an inflammatory state in the CNS. We hypothesized that peripheral immune cells are recruited to the CNS during obesity in a similar manner to macrophages recruited to white adipose tissue during the disease pathology. Due to their common myeloid origin during embryogenesis, distinguishing between resident microglia and infiltrating monocytes is highly challenging (Chan et al., 2007). One method to overcome this issue is to use a radiation bone marrow chimeric mouse, where bone marrow from donor mice with ubiquitous expression of GFP is used to reconstitute the bone marrow of irradiated hosts (Vallieres and Sawchenko, 2003). This method allows the movement of myeloid cells into tissue to be tracked, and has been used to demonstrate immune cell infiltration in the CNS during neuropathologies such as Alzheimer’s disease (Simard et al., 2006) and viral encephalitis (Getts et al., 2008). In this study, we generated bone marrow chimeras (BM chimeras) to investigate recruitment of immune cells into the CNS during high-fat diet induced obesity.
2. Animals and methods
2.1. Mice and diets
Animal studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Vanderbilt University. Male C57BL/6J (Stock #000664) and global GFP transgenic mice on a C57BL/6J background (C57BL/6-Tg (CAG-GFAP) 131Obs/LeySopJ; Stock #006567), 8-weeks old, were purchased from the Jackson Laboratory, ME. For bone marrow transplants, 10-week-old male GFP transgenic mice, bred at Vanderbilt University from a founder purchased at Jackson laboratories, were used as donors with age-matched C57BL/6J wild type (WT) mice as recipients. Global GFP transgenic mice express GFP cDNA under the control of a chicken β-actin promoter and human cytomegalovirus enhancer (Okabe et al., 1997). Animals were housed at 21 ± 2 °C and fed a standard chow diet (Std Chow; 13% kcal from fat; Picolab rodent diet 20, PMI Nutrition International, MO) for approximately 4 weeks before bone marrow transplantation (BMT). Two weeks after bone marrow replacement mice were randomly divided into two groups and placed on a high fat diet (HFD; 60% of total calories from fat; Research Diets Inc, NJ) or maintained on standard laboratory chow (Std Chow) for a total of 15 or 30 weeks (Fig. 1A). Food and water were available ad libitum throughout experimentation periods.
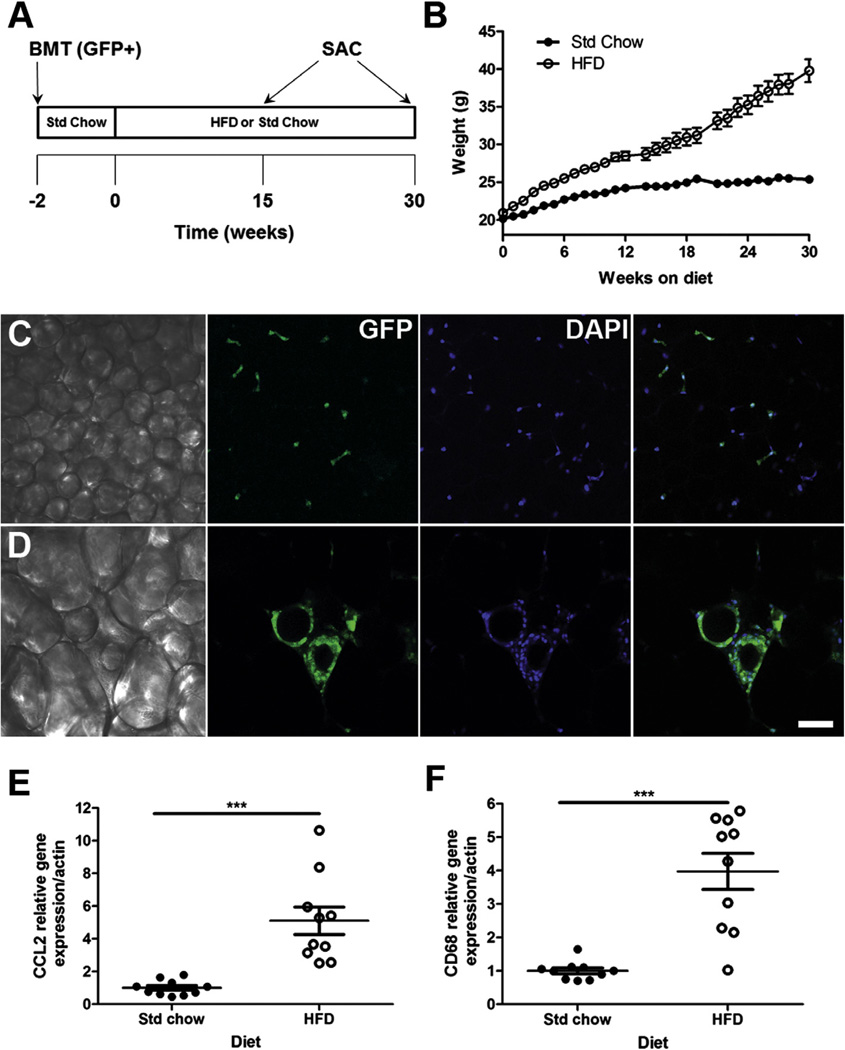
Fig. 1.
Bone marrow chimeras show obesity and adipose tissue inflammation in response to a high-fat diet. C57BL/6J mice transplanted with GFP+ (donor) bone marrow were randomly divided into two groups and fed either a 60% high-fat (HFD) or standard chow (Std Chow) diet for 15 or 30 weeks (A). Body weight was increased by high-fat diet (B). Confocal imaging demonstrated adipocyte hypertrophy (C, Std chow; D, HFD) and recruitment of GFP+ (green) cells into white adipose. DAPI nuclear stain indicated in blue. White adipose tissue gene expression of inflammatory markers CCL2 (E) and CD68 (F) was significantly higher in mice fed a HF diet after 30 weeks. For real-time RT-PCR data, data are presented as mean ± S.E.M., n = 10 mice per diet. An unpaired Student’s t-test was performed for statistical evaluation of the data. ***P < 0.001. Scale bar = 50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Generation of GFP chimeric mice
All animals used in this study were bone marrow chimeras generated by injecting bone marrow cells obtained from global GFP donor mice into the retro-orbital venous plexus of WT recipient mice 6 h after they received whole body lethal irradiation (without head shielding) with 900 rads from a Cesium gamma source. One week prior to BMT, and for one week following, recipient mice were given antibiotics in their drinking water. All BMT studies were performed with 10 week old donor and recipient male mice.
2.3. Body weight and body composition
Mice were weighed weekly. Prior to euthanasia at 15 or 30 weeks after placement on a Std Chow or HFD diet, body composition was measured using a Bruker Minispec Analyzer (Bruker Optics, TX) in the Vanderbilt Mouse Metabolic Phenotyping Center.
2.4. Real-time RT-PCR
Total RNA was extracted from epididymal white adipose tissue with Trizol reagent (Life Technologies Corp., NY) and complementary DNA (cDNA) synthesized using iScript cDNA synthesis kits (Bio-Rad Laboratories, CA), according to manufacturer’s instructions. Real-time RT-PCR reactions were performed using a CFX96 thermal cycler (Bio-Rad Laboratories, CA) and FAM-conjugated primer/probe sets (TaqMan Gene Expression Assays; all from Life Technologies Corp, NY) normalized to β-actin (catalogue no 4352341E). The assays used were CD68 (Mm00839636_g1) and CCL2 (Mm00441242_m1). Data was analyzed using the ΔΔCt method and presented as relative expression to the Std Chow-fed controls at the representative time point. All samples were run in duplicate.
2.5. Immunohistochemistry
Tissues were collected from mice sacrificed 30 weeks after bone marrow reconstitution (n = 3/diet). Epididymal adipose tissue was harvested from 0.9%-saline-perfused mice and a portion fixed in 1% paraformaldehyde (PFA) for whole mount imaging. For preparation of brain slices, deeply anesthetized mice were transcardially perfused with 0.9% saline followed by 4% PFA. Immunohistochemical labeling was performed on free-floating coronal brain sections, cut at a thickness of 30 µ per slice and stained as previously described (Buckman et al., 2013). For single staining, a primary antibody against GFP (dilution 1:5000; A-11122, Life Technologies, NY) was followed by secondary detection with a horse radish peroxidase conjugated anti-rabbit IgG (dilution 1:500; W4018, Promega, WI) and visualized using Immpact DAB (Vector Laboratories, CA). For confocal microscopy a primary antibody against Iba1 was used as a marker for microglia (dilution 1:1000; 019–19741, Wako, Germany) followed by secondary detection with donkey anti-rabbit Alexa 594 (dilution 1:500; Life Technologies Corp., NY). For fluorescence studies, donor-derived cells were identified in brain and adipose tissue sections by their endogenous expression of GFP. For adipose tissue immunohistochemical analysis, DAPI staining was used to visualize cell nuclei. All fluorescence images were obtained using a Zeiss LSM 710 confocal microscope (Carl Zeiss, DE) in the Vanderbilt Cell Imaging Shared Resource (CISR). Brightfield images were obtained using a wide-field microscope, AxioImager Z1 (Zeiss, NY). Brightness and contrast were adjusted in the digital images to improve quality but this manipulation was performed equally across all groups.
For quantification of GFP+ recruited cells from immunohistochemistry, data were collected from five different brain regions from Std Chow and HFD fed mice: cortex, septum and striatum (coordinates: 1.10–0.38 mm from the bregma); thalamus and hypothalamus (coordinates: −0.70–2.54 mm from the bregma). The meninges and choroid plexus were excluded from the quantification. The number of GFP+ cells was counted in five sections per brain region per animal. The morphology of the cells was classified according to previously published work (Vallieres and Sawchenko, 2003). Quantification was performed by an investigator blinded to the diet of the animals at 40× magnification using brightfield microscopy (AxioImager Z1; Zeiss, NY). Guidance on nomenclature and anatomic boundaries was taken from (Paxinos and Franklin, 2001).
2.6. Flow cytometry
Blood was taken at euthanasia by cardiac puncture, followed by brief erythrolysis with water to isolate peripheral blood leukocytes. Whole brain was removed from saline-perfused mice, the cerebellum and hindbrain removed, and the right cerebral hemisphere minced in phosphate buffered saline (PBS) and processed to obtain a single-cell suspension using a papain dissociation system (Worthington Biochemical Corp., NJ) according to the manufacturers’ instructions. Samples were incubated with rat anti-mouse CD16/CD32 in FACS buffer (PBS plus 2% fetal calf serum, filter sterilized) for blockade of Fc receptors and immunostained as appropriate with fluorophore-conjugated antibodies: Rat anti-CD11b (clone M1/70; PE-conjugated), and Rat anti-CD45 (clone 30-F11; APC-conjugated; all from BD Biosciences, CA). Data from 20,000 live, single-cell events was acquired using FACSDiva software (BD Biosciences, CA). Cells were initially gated on forward-scatter and side-scatter to exclude cell doublets from analysis and collection. Dead cells were excluded based on DAPI labeling. Fluorescent compensations were performed using cells isolated from normal non-chimeric wild-type control animals and stained with each fluorochrome separately. The relative percentage of bone marrow-derived cells of donor origin was determined by their intrinsic GFP fluorescence and cell populations (i.e. CD11b+CD45+, microglia) were defined using size, viability, and fluorescence-minus-one controls (Maecker and Trotter, 2006). Cells were processed using a 5 Laser BD LSR Fortessa machine (BD Biosciences, CA) in the Vanderbilt Flow Cytometry Core. All data were analyzed using FlowJo software version 9.3.2 (Tree Star, Ashland, OR).
2.7. Statistical analysis
Differences between groups of mice (Std Chow vs. HFD) were examined using unpaired Student’s t-test (2-tailed); a Welch-correction was applied as needed in the event of a statistically significant difference in variance between groups. Two-way ANOVA was used to determine the relationship between morphology (stellate vs. elongated/amoeboid) and diet in the immunohistochemical analyses. Spearman’s correlation analysis was used to quantify relationships between % GFP+ brain-infiltrating immune cells and variables of interest (i.e. fat mass). All data are shown as mean ± standard error of mean (SEM) and statistical outliers were excluded from each data set if outside the range of the mean by more than two times the standard deviation. GraphPad Prism version 5.4 (GraphPad Software, San Diego, CA) was used for all statistics analyses and a significance level of P < 0.05 was used for statistical inference.
3. Results
3.1. Bone marrow chimeric animals show significant weight gain and adipose tissue inflammation in response to high-fat feeding
To confirm that the chimeric mice showed the “normal” physiologic response to diet-induced obesity we measured body weight, fat mass and peripheral inflammation in epididymal white adipose tissue. There was a statistically significant increase in weight gain (Fig. 1B; 15 weeks, t(14) = 5.15, P = 0.0001; 30 weeks, t(9) = 6.64, P < 0.0001) within groups fed HFD due primarily to increased fat mass (Table 1). Assessment of adipose tissue whole mount preparations revealed adipocyte hypertrophy, and the accumulation of donor-derived GFP+ cells which often formed crown-like structures in obese adipose tissues (Fig. 1D). In contrast, adipocyte hypertrophy and crown-like structures were not present in lean Std Chow control animals (Fig. 1C). Additionally, gene expression of the monocyte chemotactic protein CCL2 (Fig. 1E; t(9) = 4.81, P = 0.001) and the macrophage-specific antigen CD68 (Fig. 1F; t(9) = 5.43, P = 0.0004) were significantly increased, 88% and 75% respectively, in adipose tissue from obese HFD animals as compared to lean Std Chow controls.
Table 1.
The body composition of the radiation bone marrow chimeric mice after 15 or 30 weeks on high-fat (HFD) or standard laboratory chow (Std Chow).
| Group | Weight (g) | Fat mass (g) | Fat mass (%) | Muscle mass (g) |
|---|---|---|---|---|
| 15 weeks (n = 11/diet) | ||||
| Std Chow | 23.64 ± 0.50 | 1.81 ± 0.14 | 7.64 ± 0.53 | 16.26 ± 0.16 |
| HFD | 29.27 ± 0.97 | 5.37 ± 0.75 | 18.62 ± 2.78 | 17.28 ± 0.52 |
| t(14) = 5.15, P = 0.0001 | t(10) = 4.68, P = 0.0009 | t(10) = 3.89, P = 0.003 | t(11) = 1.87, P = 0.09 | |
| 30 weeks (n = 10/diet) | ||||
| Std Chow | 25.05 ± 0.37 | 2.41 ± 0.17 | 9.56 ± 0.60 | 18.15 ± 0.32 |
| HFD | 38.52 ± 1.99 | 13.33 ± 1.51 | 33.61 ± 2.33 | 21.14 ± 0.54 |
| t(9) = 6.64, P < 0.0001 | t(9) = 7.20, P < 0.0001 | t(10) = 10.01, P < 0.0001 | t(18) = 4.78, P = 0.0001 |
Data are expressed as mean ± SEM.
3.2. High-fat diet-induced obesity is associated with increased recruitment of peripheral leukocytes into the CNS
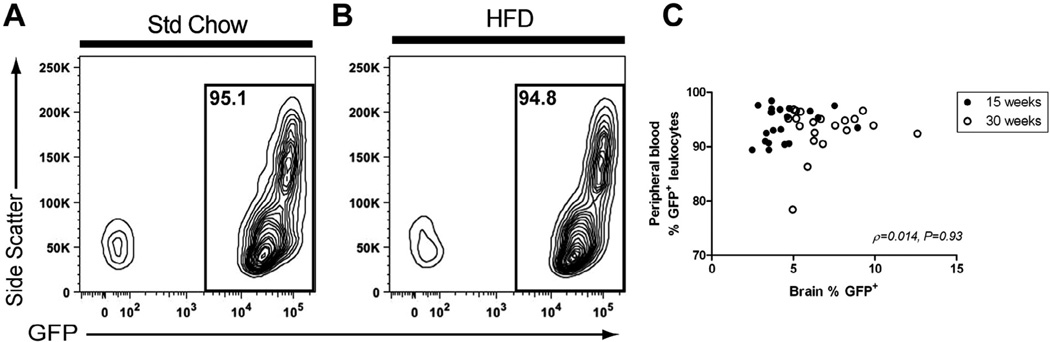
The percentage of GFP+ leukocytes in the blood after bone marrow transplant was examined in order to confirm that the cells that repopulated the peripheral immune system in the chimeric mice were of donor (GFP+) origin (Fig. 2). The mean percentage of GFP+ peripheral leukocytes was greater than 90% across all animals (range 78–98%). There was no significant difference in the % GFP+ leukocytes between the Std chow and HFD fed animals at 30 weeks (Std chow = 91.86 ± 1.75, HFD = 94.36 ± 0.65; P = 0.21, t(11) = 1.13). While there was a small, but statistically significant, difference in the % GFP+ leukocytes between the Std chow and HFD fed animals at 15 weeks (Std chow = 92.29 ± 8.27, HFD = 95.9 ± 0.68; P = 0.003, t(19) = 3.40), there was no statistically significant correlation observed between the percentage of GFP+ peripheral leukocytes and the number of GFP+ single cell events in the CNS (Fig. 2; ρ = 0.014, 95% CI = −0.30–0.33, P = 0.93).
Fig. 2.
The mean percentage of GFP+ peripheral blood leukocytes was greater than 90% across all animals. Donor-derived GFP+ cells in the blood of recipient animals were assessed by flow cytometry and ranged from 78% to 98% of total peripheral blood leukocytes. Representative contour plots are shown gated for GFP+ cells (A and B). No correlation was observed between the percentage of GFP+ cells in the brains and peripheral blood of BM chimeras (C). Spearman correlation was performed for statistical evaluation of the data. ρ and P values for association indicated on graph (n = 10–11/diet/timepoint).
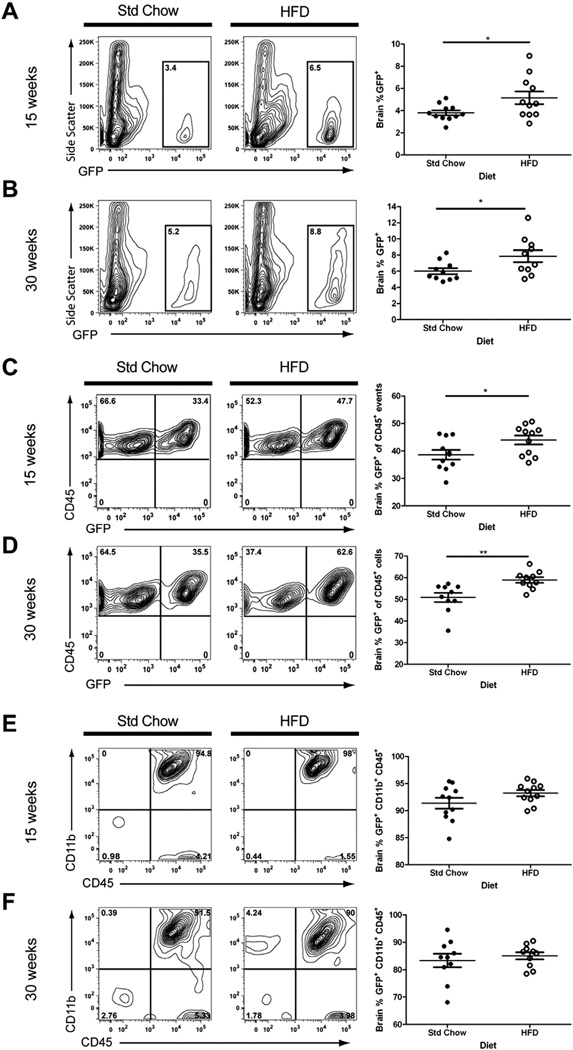
The presence of peripheral immune cells in the brains of Std Chow and HFD mice was quantified using flow cytometry of isolated cells that were gated for their intrinsic GFP expression. Quantitative analysis revealed that the percentage of donor-derived GFP+ peripheral cell infiltrates in the CNS was increased significantly in obese HFD mice at both 15 (Fig. 3A; t(12) = 2.21, P = 0.05) and 30 weeks (Fig. 3B; t(18) = 2.22, P = 0.04) as compared to their lean Std Chow controls when expressed as a percentage of single cell events (Fig. 3A and B) or as a percentage of cells that were also positive for the hematopoietic lineage marker CD45 (Fig. 3C – 15 weeks, t(20) = 2.24, P = 0.04; Fig. 3D – 30 weeks, t(18) = 3.29, P = 0.004). We further analyzed the GFP+ cells in the brain for expression of microglia/monocyte/macrophage marker CD11b. The mean percentage of GFP+ cells that were positive for both CD11b+ and CD45+ was greater than 80% in both Std Chow and HFD fed groups (Fig. 3E and F).
Fig. 3.
Obesity is associated with increased immune cell entry into the CNS. Brain cells were isolated from recipient mice after 15 or 30 weeks on a HFD or Std Chow diet and analyzed by flow cytometry. High-fat feeding led to increased CNS infiltration of GFP+ immune cells at both the 15 and 30 week time points, shown as a percentage of single cell events (A and B) or as a percentage of cells that were positive for the hematopoietic lineage marker CD45 (C and D). Greater than 80% of the GFP+ cells expressed microglial markers CD11b and CD45 (E and F, upper right quadrants). Representative contour plots are shown for each diet/time point. Data are presented as mean ± S.E.M. (n = 10–11). An unpaired Student’s t-test was performed for statistical evaluation of the data. *P < 0.05, **P < 0.001.
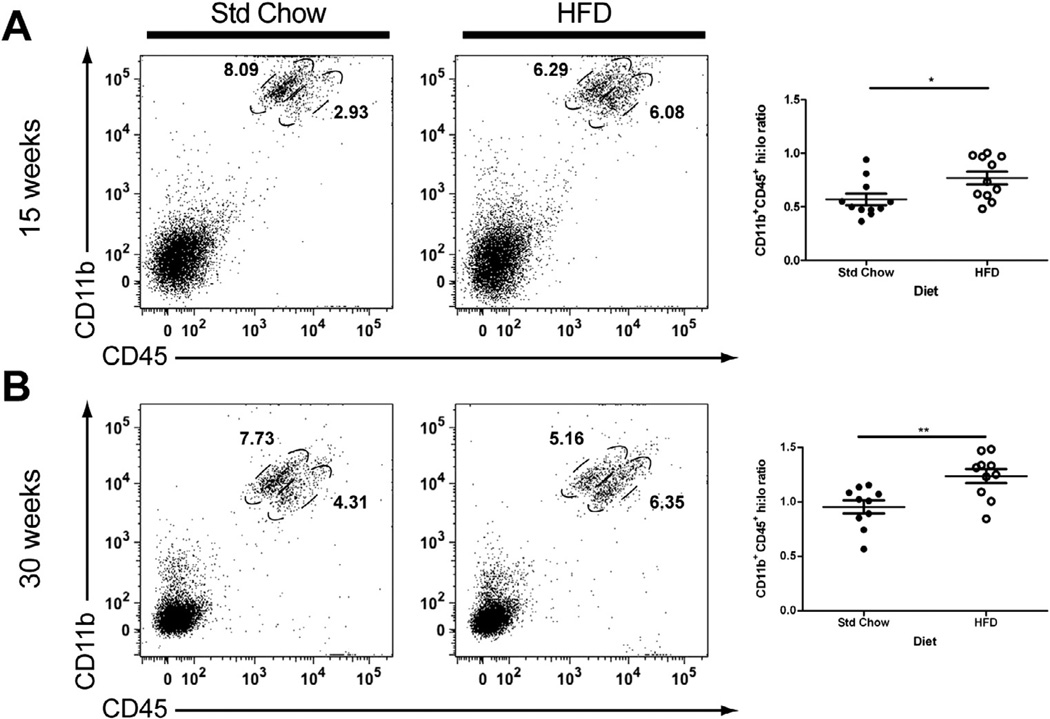
3.3. High-fat feeding is associated with an increased number of CD45hi expressing microglia/macrophages in the brain
CD45 is expressed on all cells of the hematopoietic lineage; however, with respect to microglia, regulation of CD45 expression occurs in an activation-dependent manner. For example, ramified/resting microglia have a CD45 low phenotype (CD45lo) and increase their cell surface expression to a high level (CD45hi) upon activation (Sedgwick et al., 1991; Zhang et al., 2002). In contrast, macrophages in peripheral tissues constitutively express high levels of CD45; consequently CNS-infiltrating peripheral monocytes are also characterized by CD45hi expression levels. In this study we observed two distinct CD45 expressing populations: CD11b+/CD45lo cells and CD11b+/CD45hi cells. There was a significant increase in the ratio of CD11b+CD45hi cells to CD11b+CD45lo cells in the brains of obese compared to lean mice at both 15 (Fig. 4A; t(20) = 2.53, P = 0.02) and 30 (Fig. 4B; t(18) = 3.24, P = 0.005) weeks post-HFD. Fewer than 10% of the cells found in the CD45hi gates were GFP−, suggesting that the majority of the CD45hi cells were GFP+ CNS-infiltrating peripheral monocytes.
Fig. 4.
Obesity is associated with increased CD45hi-expressing microglia/macrophages in the CNS. Flow cytometric analysis revealed that the percentage of CD11b+ cells that expressed high levels of CD45 was increased in HFD-induced obese mice at 15 (A) and 30 (B) week time points as compared to lean Std Chow-fed controls. Representative dot plots are shown gated for CD45hi and CD45lo cells. Data are presented as mean ± S.E.M. (n = 10–11). An unpaired Student’s t-test was performed for statistical evaluation of the data. *P < 0.05; **P < 0.01.
3.4. Donor-derived GFP+ cells in the brain express the microglial marker Iba-1
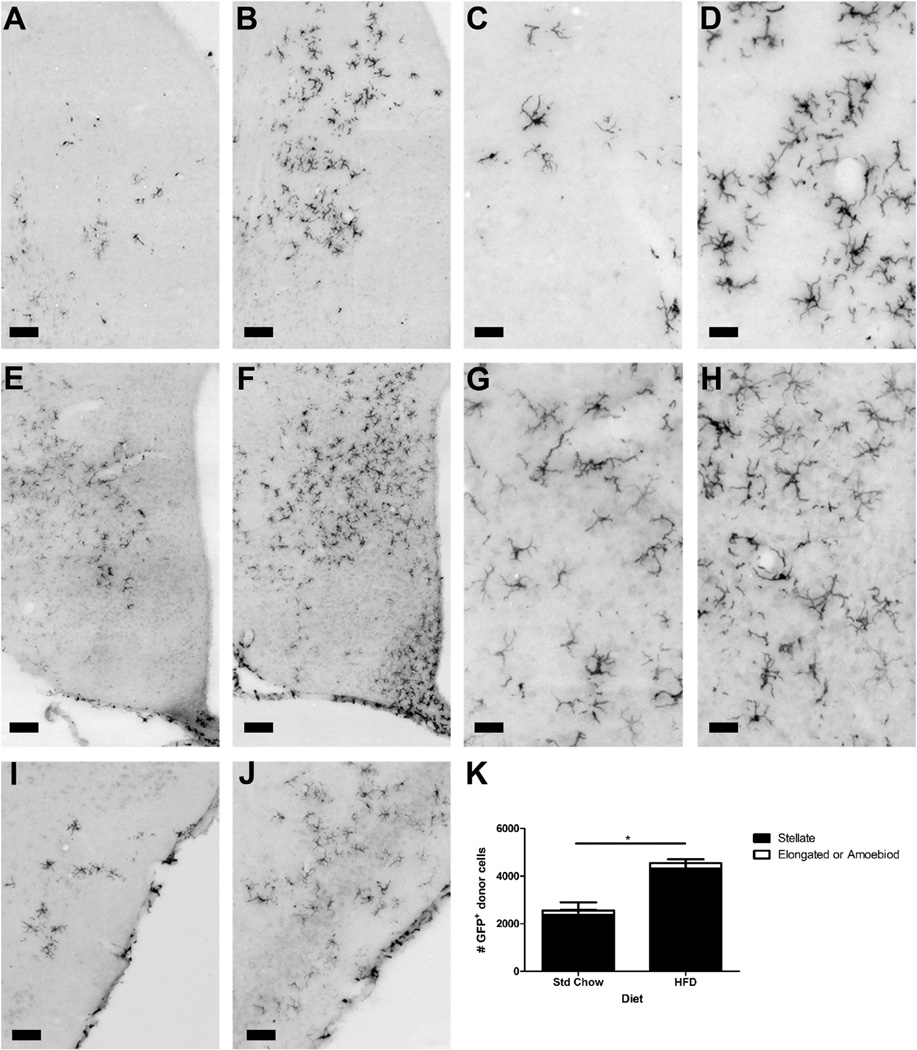
Using immunohistochemistry we determined that the regional distribution of GFP+ cells in the CNS was comparable with what has previously been reported using the radiation bone marrow chimera model (Simard and Rivest, 2004). Greater than 92% of the GFP+ cells across both standard chow and HFD groups were found in the parenchyma and had a stellate morphology. In agreement with the flow cytometry data there was a statistically significant increase in GFP+ cells in the brains of the HFD animals compared with Std Chow fed controls and this was due to an increase in the number of stellate cells as opposed to cells with an elongated or amoeboid morphology (Fig. 5: Fdiet(1,8) = 9.28, P = 0.02; Fmorphology(1,8) = 92.61, P < 0.0001; Finteraction(1,8) = 8.53, P = 0.02).
Fig. 5.
Distribution of GFP+ immune cells in the CNS. Immunohistochemistry for GFP+ cells showed increased recruitment in HFD animals compared with Std Chow controls. Representative images are shown for the septum (Std Chow – A and C; HFD – B and D); hypothalamus (Std Chow – E and G; HFD – F and H) and cortex (Std Chow – I; HFD – J). There was a statistically significant increase in total GFP+ cells in the HFD animals due to an increase in cells of stellate morphology in the parenchyma. Data are presented as mean ± S.E.M. (n = 3). Two-way ANOVA was performed for statistical evaluation of the data. *P < 0.05 scale bars: Panels A, B, I and J = 100 µm; Panels C, D, G and H = 50 µm; Panels E and F = 75 µm.
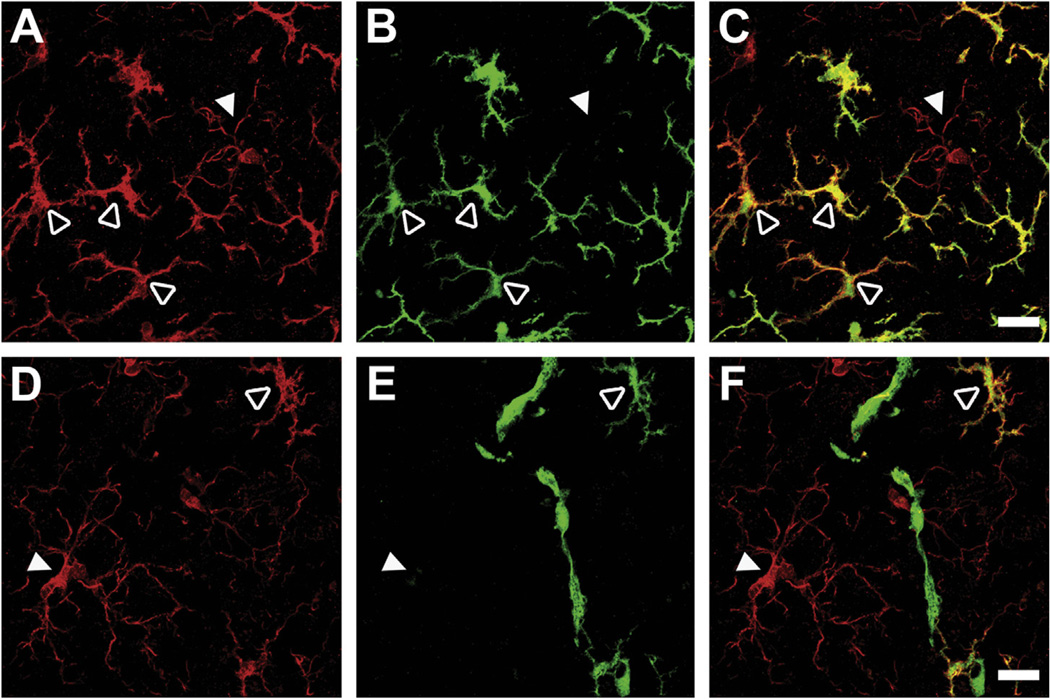
We next performed double-immunohistochemistry to further validate our previous finding using flow cytometry that >80% donor GFP+ cells in the brain were CD11b+CD45+, consistent with a microglia/macrophage phenotype. Immunostaining for the microglial marker ionized calcium-binding adapter molecule 1 (Iba1) revealed that most GFP+ recruited cells indeed co-localized with Iba1. Furthermore, donor GFP+ cells that were Iba1+ were morphologically distinct from brain-resident microglia (Fig. 6). Resident GFP− Iba1+ cells largely demonstrated a resting microglial morphology (Fig. 6, solid white arrowheads), whereas Iba1+ cells that were GFP+ exhibited a distinct stellate morphology with enlarged somata accompanied by retraction and thickening of processes (Fig. 6, open white arrowheads). In contrast, cells that were GFP+ but Iba1− were rare and typically found located close to the microvasculature with an elongated morphology (Fig. 6F) similar to what has been reported for perivascular macrophages (Vallieres and Sawchenko, 2003).
Fig. 6.
Morphology of GFP+ cells recruited to the CNS. Confocal microscopy of CNS sections from recipient mice confirmed that GFP+ cells expressed microglial marker Iba1 and had a stellate appearance (A–F, open arrowheads) compared with resident microglia which displayed a ramified (“resting”) phenotype (A–F, closed arrowheads). Representative images showing microglia in red (Iba1; A and D), GFP in green (B and E), and overlay in yellow-orange (C and F). Co-localization of GFP and Iba1 staining was used to distinguish recruited cells (Iba1+GFP+) from resident microglia (Iba1+GFP−) (upper panels). The few GFP+ cells that were negative for Iba1 were associated with blood vessels with an elongated morphology similar to perivascular macrophages (lower panels). Scale bars = 20 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
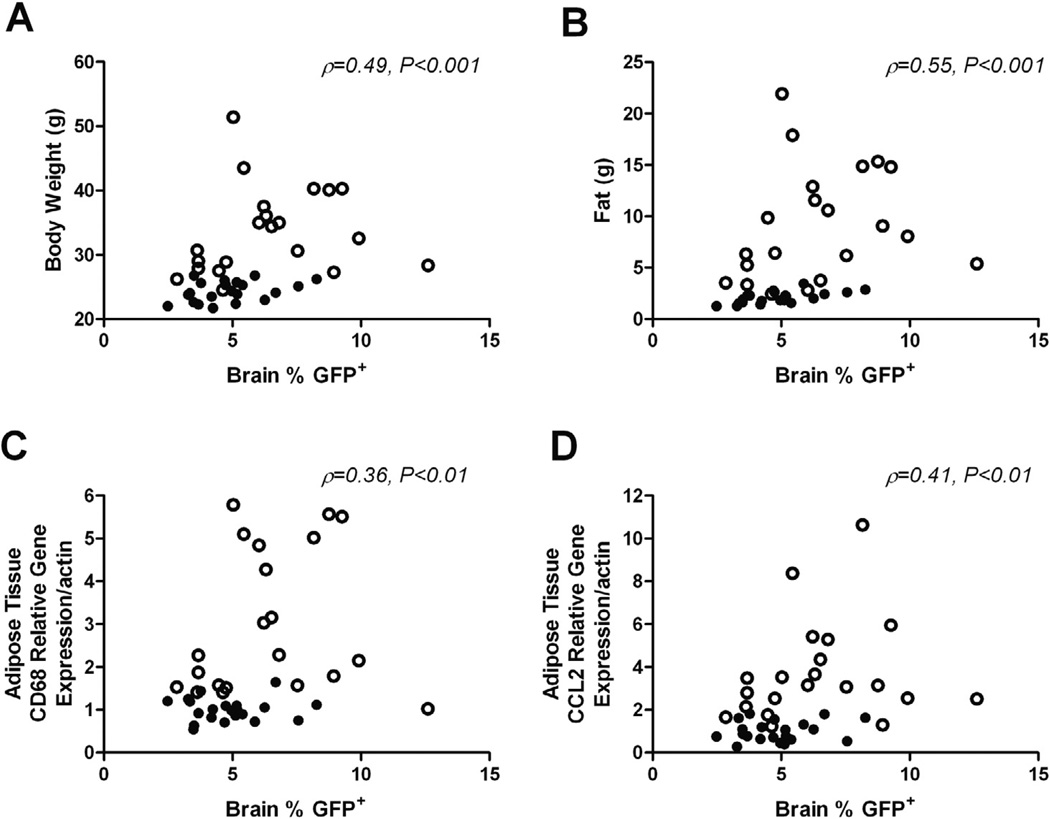
3.5. Body weight, adiposity and white adipose tissue inflammation positively correlate with the number of GFP+ immune cells in the CNS
Macrophage recruitment to white adipose tissue is an early event in obesity which drives the development of low-grade systemic inflammation in both humans and mice (Weisberg et al., 2003). Because macrophage content and inflammatory gene expression are highly correlated with the degree of obesity and metabolic dysfunction, we next looked at the relationship between measures of adiposity and macrophage inflammatory markers with the number of GFP+ peripheral immune cells recruited to the CNS across all groups. In this study, we found that the degree of GFP+ peripheral immune cell infiltrates in the CNS positively correlated with increases in body weight (Fig. 7A; ρ = 0.49, 95% CI = 0.21–0.70, P < 0.001) and fat mass (Fig. 7B; ρ = 0.55, 95% CI = 0.28–0.73, P < 0.001). Additionally, relative mRNA levels of CD68 (Fig. 7C; ρ = 0.36, 95% CI = 0.05–0.60, P < 0.01) and CCL2 (Fig. 7D; ρ = 0.41, 95% CI = 0.11–0.64, P < 0.01), markers of inflammation in white adipose tissue, were both positively correlated with the number of brain-infiltrating GFP+ immune cells.
Fig. 7.
Infiltration of GFP+ peripheral immune cells into the CNS is positively correlated with measures of adiposity and white adipose tissue inflammation. The relationship between the percentage of GFP+ cells in the brains of chimeric mice with body weight (A), fat mass (B) and adipose tissue CD68 (C) and CCL2 (D) mRNA expression, across all groups. Data were analyzed using Spearman correlation. ρ and P values for associations indicated on graphs (n = 10–11/diet/timepoint). ○, HF; ●, Std Chow.
4. Discussion
We have demonstrated that high-fat diet induced obesity enhances recruitment of bone marrow-derived monocytes to the CNS. Radiation bone marrow chimera strategies have been highly effective for studying the role of leukocytes in the pathophysiology of obesity (Coenen et al., 2009; De Taeye et al., 2007; Furuhashi et al., 2008; Kowalski et al., 2011; Nicholls et al., 2011; Orr et al., 2012; Saberi et al., 2009; Xu et al., 2012), including one of the initial studies demonstrating recruitment of macrophages into adipose tissue with obesity (Weisberg et al., 2003). In agreement with these studies, our results indicate that bone marrow chimeras fed a high-fat diet exhibited the normal physiological response to high-fat feeding including increased body weight gain, adipose tissue macrophage recruitment and white adipose tissue inflammation. Furthermore, we found that the number of CNS-infiltrating monocytes was positively correlated with body weight, fat mass and markers of inflammation in adipose tissue (CD68 and CCL2 gene expression). These data point to a relationship between adiposity and adipose tissue inflammation and the recruitment of immune cells to the CNS.
Obesity has previously been shown to be associated with microglial activation as assessed by immunohistochemistry (Drake et al., 2011; Thaler et al., 2012; Yi et al., 2012a) and positron emission tomography (Drake et al., 2011). In the normal/non-inflamed brain, microglia exist primarily in a resting state with low levels of CD45 expression, which increases to high levels upon activation by inflammatory stimuli such as lipopolysaccharide and during neuropathology (Akiyama et al., 1994; Nikodemova and Watters, 2012; Sedgwick et al., 1998). For this reason, it is not possible to unequivocally separate activated microglia and bone marrow-derived cells in the CNS by differences in CD45 expression alone. We observed a 30–35% increase in the ratio of CD11b+CD45hi to CD11b+CD45lo cells in our high-fat fed animals compared with the standard chow controls. This may reflect an increase in activation of resident microglia and/or the increase in bone marrow-derived cells in the CNS as non-CNS monocytes/macrophages constitutively express high levels of CD45; however, our finding that fewer than 10% of the CD11b+CD45hi cells were GFP− suggests that it is likely that the increased recruitment of bone-marrow derived cells to the CNS in the obese animals largely underlies the increase in the CD11b+CD45hi to CD11b+CD45lo cell ratio seen in our study.
Immunohistochemistry revealed that 93% of the bone marrow-derived cells in the CNS were found in the parenchyma, but were not associated with vessels and had a distinct stellate morphology characterized by enlarged somata accompanied by retraction and thickening of processes similar to what has previously been reported (Djukic et al., 2006; Simard and Rivest, 2004; Vallieres and Sawchenko, 2003). This is in contrast to resident microglia, which primarily had the ramified morphology characteristic of resting cells (Nimmerjahn et al., 2005). While we have not proven it definitively by co-staining for activation markers, these morphological changes typically correlate with alterations in microglial action towards a macrophage-like phenotype including increased motility, migration, phagocytosis and cytokine production; however, it is not uncommon that microglial function changes independent of morphology (Ransohoff and Perry, 2009). Activated microglial morphology may alternatively indicate a lack of maturation of infiltrating cells following recruitment to the CNS. Undifferentiated microglia have an amoeboid morphology similar to activated microglia and thus have the ability to distribute across different CNS sites where they transition into a mature morphological ramified/resting phenotype (Perry and Gordon, 1988; Rezaie et al., 1999).
In support of the idea that bone marrow-derived monocytes behave like macrophages in the CNS, Simard et al. (2006) demonstrated that bone marrow-derived monocytes/macrophages enter the CNS and phagocytose and degrade amyloid more effectively than resident microglia in a mouse model of Alzheimer’s disease. The function of the bone marrow-derived monocytes and their potential contribution to disease pathology in obesity remains to be elucidated. High-fat feeding has been shown to be associated with increased apoptosis of hypothalamic neurons (Moraes et al., 2009); thus, it is feasible that bone marrow-derived monocytes are recruited to the CNS in obesity in response to this neuronal apoptosis and that they function like macrophages to clear neuronal debris. However, the finding that the increase in GFP+ infiltrating cells was seen throughout the brain, and not exclusively in the hypothalamus, suggests that other mechanisms are likely to be involved. In the peripheral vasculature, obesity and high-fat feeding are associated with endothelial cell “activation” characterized by changes in expression of proinflammatory cytokines and cell adhesion molecules (for review see (Rutkowski et al., 2009)), which is thought to be mediated, at least in part, by the activation of the toll-like receptor-4 pathway by diet-derived saturated fatty acids (Kim et al., 2007). It is feasible that a similar mechanism may be causing changes in cerebral endothelial cells and thus contributing to the increased recruitment of monocytes to the CNS. Other potential mechanisms include obesity associated changes in CNS vascularity (Yi et al., 2012b), blood–brain barrier permeability (Nerurkar et al., 2011) and/or elevated CNS expression of chemokines such as MCP-1.
Due to their common cell surface markers it is difficult to accurately distinguish between resident microglia and peripheral immune cells recruited to the CNS during disease. To overcome this we used a radiation bone marrow chimera model, using GFP-labeled bone marrow to unequivocally distinguish between resident microglia and bone marrow-derived monocytes. The same strategy has also been used in a number of other disease models (Djukic et al., 2006; Getts et al., 2008; Kokovay and Cunningham, 2005; Malm et al., 2005; Priller et al., 2006; Simard et al., 2006); however, it remains controversial whether the irradiation associated with the procedure influences recruitment of myeloid cells to the brain (Ajami et al., 2007; Mildner et al., 2007). Nonetheless, in our study both groups received equivalent treatment prior to the diet switch indicating that, in common with other neuroinflammatory conditions, diet-induced obesity increases recruitment of bone marrow-derived monocytes to the brain within this experimental paradigm.
In summary, our study demonstrates increased recruitment of bone marrow-derived monocytes into the CNS during high-fat diet induced obesity. Future work is needed to determine the physiological significance of immune cell recruitment to the CNS during obesity and whether these cells are recruited as a consequence of obesity-induced neuroinflammation and/or contribute to the neuropathology associated with obesity.
Acknowledgments
We would like to thank Bob Matthews of the Vanderbilt Shared Imaging Resource for assistance with confocal microscopy. This work was supported by a pilot and feasibility grants to KLJE from Vanderbilt Digestive Disease Research Center (DDRC; NIH P30 DK058404) and Vanderbilt Diabetes Research Training Center (DRTC; NIH P30 DK020593) and start-up funds from Vanderbilt University. LBB was supported in part via the Molecular Endocrinology Training Grant (NIH T32 DK007563). Flow Cytometry Core Services were also supported via a Vanderbilt DDRC Core Scholarship. Vanderbilt Mouse Metabolic Phenotyping Center is supported in part by NIH Grant U24 DK059637.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Ikeda K, Katoh M, McGeer EG, McGeer PL. Expression of MRP14, 27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J. Neuroimmunol. 1994;50(2):195–201. doi: 10.1016/0165-5728(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J. Comp. Neurol. 2013;521(6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS data brief, No 82. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res. Rev. 2007;53(2):344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52(2):318–328. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007;293(3):E713–E725. doi: 10.1152/ajpendo.00194.2007. [DOI] [PubMed] [Google Scholar]
- Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129(Pt 9):2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 2011;25(6):1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Zhang L, Nair A, Dasuri K, Francis J, Fernandez-Kim SO, Bruce-Keller AJ, Keller JN. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic. Biol. Med. 2013;56:226–233. doi: 10.1016/j.freeradbiomed.2012.08.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 2008;118(7):2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 2008;205(10):2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132(Pt 4):889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 2007;100(11):1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Cunningham LA. Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson’s disease. Neurobiol. Dis. 2005;19(3):471–478. doi: 10.1016/j.nbd.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, Bobik A, Lancaster GI, Febbraio MA. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 2011;54(4):888–899. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69(9):1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 2005;18(1):134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA, Prada PO, Saad MJ, Velloso LA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61(6):1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4(4):e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, Engelhardt B, Hallenbeck JM, Lonser RR, Ohlfest JR, Prat A, Scarpa M, Smeyne RJ, Drewes LR, Neuwelt EA. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013;33(1):13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar PV, Johns LM, Buesa LM, Kipyakwai G, Volper E, Sato R, Shah P, Feher D, Williams PG, Nerurkar VR. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J. Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A, Febbraio M, Lancaster GI, Febbraio MA. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes. 2011;60(4):1100–1110. doi: 10.2337/db10-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J. Neuroinflammation. 2012;9:147. doi: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407(3):313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61(11):2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2001. [Google Scholar]
- Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Priller J, Prinz M, Heikenwalder M, Zeller N, Schwarz P, Heppner FL, Aguzzi A. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J. Neurosci. 2006;26(45):11753–11762. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Patel K, Male DK. Microglia in the human fetal spinal cord–patterns of distribution, morphology and phenotype. Brain Res. Dev. Brain Res. 1999;115(1):71–81. doi: 10.1016/s0165-3806(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276(20):5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10(5):419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. USA. 1991;88(16):7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Ford AL, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J. Immunol. 1998;160(11):5320–5330. [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18(9):998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J. Neurosci. 2003;23(12):5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Morinaga H, Oh D, Li P, Chen A, Talukdar S, Mamane Y, Mancini JA, Nawrocki AR, Lazarowski E, Olefsky JM, Kim JJ. GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity. J. Immunol. 2012;189(4):1992–1999. doi: 10.4049/jimmunol.1103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Al-Massadi O, Donelan E, Lehti M, Weber J, Ress C, Trivedi C, Muller TD, Woods SC, Hofmann SM. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol. Behav. 2012a;106(4):485–490. doi: 10.1016/j.physbeh.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Yi CX, Gericke M, Kruger M, Alkemade A, Kabra DG, Hanske S, Filosa J, Pfluger O, Bingham N, Woods SC, Herman J, Kalsbeek A, Baumann M, Lang R, Stern JE, Bechmann I, Tschop MH. High calorie diet triggers hypothalamic angiopathy. Mol. Metab. 2012b;1(1–2):95–100. doi: 10.1016/j.molmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GX, Li J, Ventura E, Rostami A. Parenchymal microglia of naive adult C57BL/6J mice express high levels of B7.1, B7.2, and MHC class II. Exp. Mol. Pathol. 2002;73(1):35–45. doi: 10.1006/exmp.2002.2441. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]