Abstract
The number of memory phenotype CD8 T cells increases dramatically with aging in both humans and mice. However, the mechanism for this is unknown. The prevailing hypothesis is that memory T cells accumulate with aging as a result of lifelong antigenic stimulation. However, data supporting this supposition are lacking. In this study, we demonstrate that central memory CD8 T cells, which represent a large majority of memory CD8 T cells in aged mice, are not memory cells that develop in response to antigenic stimulation but are virtual memory cells that develop without antigenic stimulation. In addition to phenotypic evidence, we show that accumulation of central memory CD8 T cells is independent of CD4 T cells, CCR5 and CXCR3, all of which are known to be essential for antigen-driven development of central memory CD8 T cells. Thus, this study reveals a novel mechanism for aging-related changes in CD8 T cells.
Introduction
CD8 T cells play an important role in immunity against infection and tumor (1). These cells are a heterogeneous group of cells and can be divided into naive and memory subsets. Conventional memory phenotype (MP) CD8 T cells acquire their phenotype after antigenic stimulation in the periphery. In contrast, innate and virtual memory CD8 T cells develop without antigenic stimulation. Whereas innate memory CD8 T cells acquire their memory phenotype in response to IL-4 in the thymus (2), virtual memory (VM) CD8 T cells acquire their memory phenotype in response to IL-15 in the periphery (3-7).
Shortly after the characterization of naïve and memory T cells, it was realized that aging leads to the replacement of naive T cells by memory T cells. However, the mechanism for this is unclear. It has long been assumed that memory T cells accumulate with aging as a result of lifelong antigenic stimulation (8). However, recent data show that like conventional memory cells, the proportion of VM cells increases with aging (9). In this study, we analyzed the contribution of VM cells to aging-related accumulation of memory CD8 T cells by comparing strains of genetically engineered mice in which the formation of conventional MP CD8 T cell is either increased or decreased. All mice were on a C57BL6 background, which do not produce innate memory CD8 T cells (2), allowing us to focus on the role of conventional MP and VM CD8 T cells. Contrary to previous assumptions, we show that aging-related accumulation of central memory CD8 T cells is due to life-long accumulation of VM rather than conventional MP CD8 T cells.
Materials and Methods
Mice
Male C57BL/6 mice were obtained from the National Institute on Aging contract colony at Harlan Laboratories (Indianapolis, IN) or from the Jackson Laboratory (Bar Harbor, ME). CD4 deficient (B6.129S2-Cd4tm1Mak/J), CCR5 deficient (B6.129P2-Ccr5tm1Kuz/J) and CXCR3 deficient (B6.129P2-Cxcr3tm1Dgen/J) mice were obtained from the Jackson Laboratory. Male C57BL/6 congenic mice (CD45.1+CD45.2−) were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 F1 congenic mice (CD45.1+CD45.2+) were produced by crossing male C57BL/6 congenic (CD45.1+CD45.2−) with female C57BL/6 (CD45.1−CD45.2+) mice. The University of Michigan Committee on Use and Care of Animals (UCUCA) approved all animal studies.
Bone marrow stem cell adoptive transfer
Mixed bone marrow chimeras were generated by co-transferring bone marrow cells from CD45.1+CD45.2+ and CD45.1−CD45.2+ mice to CD45.1+CD45.2− congenic mice that were irradiated with a single dose of 7 Gy. Approximately 5 million bone marrow cells from each donor type were transferred to each recipient ~2 hours after the irradiation.
Flow Cytometry
Flow cytometric analysis was done as described (10). For peripheral blood analysis, 20 microliters of blood were collected via a tail vain nick. After lysing red blood cells, the entire sample were stained and subjected to flow cytometric analysis.
Statistical analysis
Single factor analysis of variance (ANOVA) was used for intergroup comparisons with P < 0.05 considered to indicate significance.
Results and Discussion
Central memory CD8 T cells accumulate in aged naive mice
Using CD44 and CD62L to identify central memory (CM) CD8 T cells (11), we found that more than half of the CD8 T cells in peripheral blood of aged (20 months) mice were CM CD8 T cells (Fig. 1a). Blood was examined because in tissues, CD62L expression by T cells may be transiently down regulated making it difficult to accurately identify all the CM CD8 T cells (12). However, large numbers of CM CD8 T cells were also found in the spleen, peripheral lymph node and bone marrow in aged mice (Fig. 2 and data not shown). Significant numbers of CM CD8 T cells were also detected in the blood of young (4 months) mice (Fig. 1a). However, the absolute number of CM CD8 T was twice as high in aged mice increasing from an average of about 200 cells per μl of blood in young to 400 cells in aged mice (Fig. 1b). These data indicated that substantial numbers of CM CD8 T cells develop in young naive mice but their numbers increase significantly with aging.
Figure 1. Central memory CD8 T cells accumulate in aged naive mice.
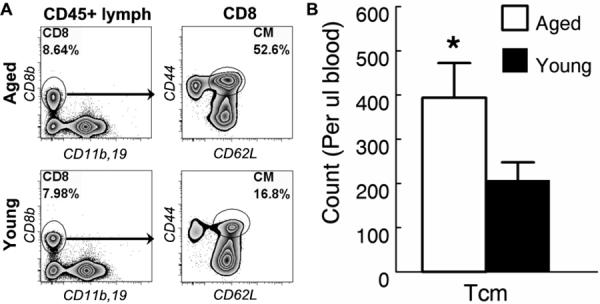
Twenty micro-liters of blood samples from young (4-6 mo.) and aged (20-22 mo.) naive mice were analyzed by flow cytometry. (A) CD8 T cells were identified by their expression of CD8β in CD11b/CD19 double-negative CD45+ lymphocytes. Gated CD8 T cells are shown to demonstrate that CD62L+CD44+ central memory (CM) CD8 T cells are found in both young and aged naive mice. (B) The mean and standard deviation of the number of CM CD8 T cells per micro-liter of blood are presented. Data are representative of 3 experiments (n = 5). * indicates statistical significance between young and aged mice with P <0.05.
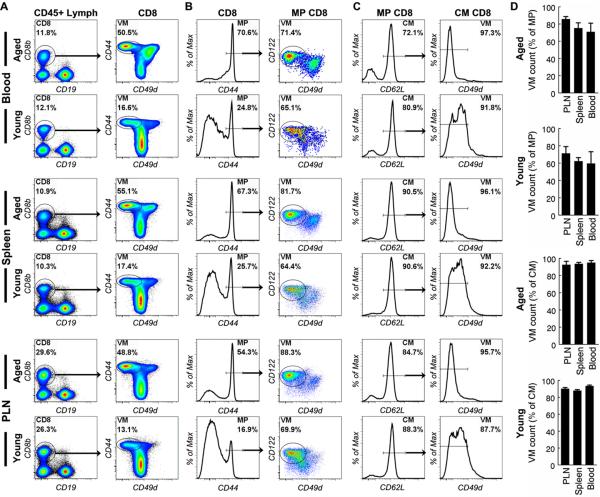
Figure 2. Central memory CD8 T cells in naive mice are virtual memory cells.
Cells from peripheral blood, spleen and lymph nodes (PLN) of aged and young naive mice were analyzed by flow cytometry. CD8 T cells were identified as in Fig. 1a. (A) Gated CD8 T cells are shown to demonstrate that VM cells are clearly separated from other cells. (B) Gated MP CD8 T cells are shown to demonstrate the proportion of VM cells. (C) Gated CM CD8 T cells are shown to demonstrate the proportion of VM cells. (D) The mean and standard deviation of the proportion of VM cells in MP and CM cell populations are presented. Data are representative of 3 experiments (n=3).
Central memory CD8 T cells in naive mice are virtual memory cells
CM CD8 T cells that develop after antigenic stimulation not only up-regulate expression of CD44 but also CD49d. In contrast, VM T cells do not up-regulate CD49d (3-5). Surprisingly, we found that in both aged and young mice, the expression levels of CD49d by most memory phenotype (CD44 high) CD8 T cells were lower than the levels expressed by naive (CD44 low) cells in all the organs tested, which include blood, spleen and peripheral lymph nodes (PLN) (Fig. 2), suggesting that most memory phenotype CD8 T cells are VM T cells. VM T cells are characterized by their expression of high levels of CD122 and low levels of CD49d (3). By using these two markers, we found that most memory phenotype CD8 T cells in aged mice were indeed VM T cells (Fig. 2b and 2d). Finally, using the CD62L marker to specifically analyze the central memory compartment, we found that nearly all (>90%) of the central memory CD8 T cells were VM cells in aged mice (Fig. 2c and 2d). Similar results were found in young (Fig. 2b to 2d) and mid aged (Fig. 4 and data not shown) mice. VM CD8 T cells in young mice appeared to express higher levels of CD49d than their counterparts in aged mice (Fig. 2c). The reason for this is not yet known. These data indicate that the vast majority of central memory CD8 T cells in aged mice are not memory cells that develop in response to antigenic stimulation but are CD122+CD62L+CD49dlo virtual memory cells that develop without antigenic stimulation.
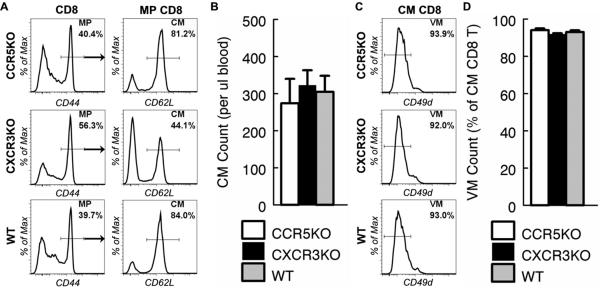
Figure 4. Accumulation of central memory CD8 T cells in naive mice is independent of CCR5 and CXCR3.
(A to D) Peripheral blood samples from CCR5 deficient (CCR5KO), CXCR3 deficient (CXCR3KO) and control WT mice were collected at 14 months of age and analyzed by flow cytometry. (A) CD8 T cells were identified as in Fig. 1a. Gated CD8 and MP CD8 T cells are shown to demonstrate the proportion of MP and CM CD8 T cells. (B) The mean and standard deviation of the number of CM CD8 T cells per micro-liter of blood are presented. Data are representative of 2 experiments (n = 2). (C) Gated CM CD8 T cells are shown to demonstrate the proportion of virtual memory (VM) CD8 T cells. (D) The mean and standard deviation of the proportion of VM CD8 T cells in CM CD8 T cells are presented. Data are representative of 2 experiments (n = 2).
Development of central memory CD8 T cells in naive mice is independent of CD4 T cells
CD4 T cells play an essential role in CM CD8 T cell development after antigenic stimulation (13). Therefore, if antigen stimulation were critical to CM CD8 development then CM CD8 T cells would be absent from CD4 T cell deficient mice. Surprisingly, the absolute number of CM CD8 T cells was significantly higher in CD4KO mice than in age-matched WT mice (Fig. 3b). Although the proportion of MP, CM and VM CD8 T cells was unchanged by CD4 deficiency, the proportion of CD8 T cells that express intermediate levels of CD44 was increased in CD4KO mice as compared to WT mice (Figure 3a and 3b). However, we found that the CD44 high cells in CD4KO mice expressed the same high levels of CXCR3 as in WT mice suggesting that these cells had indeed acquired a memory phenotype (Fig. 3a). These data suggest that development of CM CD8 T cells in naive mice is independent of CD4 T cells, and by implication also independent of antigenic stimulation.
Figure 3. Central memory CD8 T cell development in naive mice is independent of CD4 T cells.
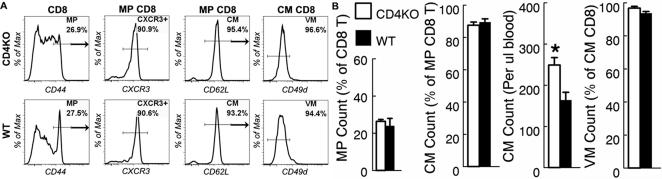
Peripheral blood samples from CD4 deficient (CD4KO) and control WT mice were collected at 6 months of age and analyzed by flow cytometry. CD8 T cells were identified as in Fig. 1a. (A) Gated CD8 T cells, MP and CM CD8 T cells are shown to demonstrate the proportion of MP, CXCR3+, CM and VM CD8 T cells respectively. (B) The mean and standard deviation of the proportion of MP cells in CD8 T cells, the proportion of CM cells in MP CD8 T cells, the number of CM cells per micro-liter of blood and the proportion of VM cells in CM CD8 T cells are presented. * indicates statistical significance between CD4KO and WT mice with P <0.05. Data are representative of two experiments (n = 2).
Development of central memory CD8 T cell in naive mice is independent of CCR5 and CXCR3
Although it has been shown that CD8 T cell development is normal in CD4KO mice, the total number of peripheral CD8 T cells is significantly increased (14) and the proportion of CD8 T cells that express intermediate levels of CD44 is increased in CD4KO mice as compared to WT mice (Figure 3a). Since it is not clear if CD4 deficiency might lead to development of CD4-independent antigen-induced CM CD8 T cells, we employed alternative approaches to test the role of antigenic stimulation in the development of CM CD8 T cells. Studies have shown that CD4 T cell help for CD8 T cell activation is mediated by CCR5 and its ligands. Consequently, development of memory CD8 T cells after infection or vaccination is significantly impaired without CCR5 expression by CD8 T cells (15). If CM CD8 T cells were antigen-stimulation dependent, they would fail to accumulate with age in CCR5KO mice. To test this, we aged CCR5KO mice to 14 months of age and analyzed their CD8 T cells. Surprisingly, the number of CM CD8 T cells in CCR5KO mice was as high as in aged-matched WT mice (Fig. 4a and 4b). In addition, like WT mice, nearly all CM CD8 T cells in CCR5KO mice were phenotypically VM cells (Fig. 4c and 4d).
Upon infection or vaccination, antigenic stimulation leads to activation of CD8 T cells and population expansion followed by population contraction (11). Recent studies showed that the extent of CD8 T cell population contraction after infection or vaccination depends on the extent of antigenic stimulation, which in turn is regulated by CXCR3. In the absence of CXCR3 expression by CD8 T cells, population contraction is attenuated, leading to massive accumulation of memory cells (16). We therefore surmised that numbers of antigenic stimulation dependent CM CD8 T cells would be significantly higher in CXCR3 deficient than age-matched WT naive mice. To test this, we aged CXCR3KO mice to 14 months and analyzed their CD8 T cells. We found that unlike CCR5KO mice, the proportions of MP and CM CD8 T cells were altered in CXCR3KO mice (Fig. 4a). Remarkably, despite this alteration, similar numbers of CM CD8 T cells were found in CXCR3KO and age-matched WT naive mice (Fig. 4b). These data suggest that CM CD8 T cells differ from other MP CD8 T cells in that their accumulation is independent of CXCR3. Indeed, like WT mice, nearly all of the CM CD8 T cells in CXCR3KO mice were phenotypically VM cells (Fig. 4c and 4d). Together, these data indicate that CM CD8 T cell development in naive mice is independent of CCR5 and CXCR3, which in turn suggests that CM CD8 T cell development in naive mice is independent of antigenic stimulation.
It has generally been assumed that MP CD8 T cells accumulate with aging as a result of lifelong antigenic stimulation. Contrary to this supposition, our findings suggest that the increase in the number of CM CD8 T cells can be largely accounted for by the accumulation of VM T cells. Together with previous studies in young mice (3-5), the data suggest that the unique ability of VM T cells to utilize IL-4 and IL-15 and to proliferate extensively under steady state conditions allows this cell population to expand over time. Although effecter (CD62L−) memory cells represent a minority of CD8 T cells, these cells also accumulate with aging (Fig. 1, Fig. 2 and data not shown). Antigenic challenge from commensal microbes is likely to be important in this process in mice housed in specific pathogen-free conditions because very few effecter memory cells are VM cells (ref. (5, 9, 17) and B. Chiu, unpublished observations). The fact that effecter memory CD8 T cells accumulated much more rapidly in CXCR3KO mice than in WT mice supports this notion.
It is not yet clear if VM T cells also develop in humans and have a survival advantage over other T cell populations. If they do, it is likely that VM T cells also dominate the CD8 T cell memory population, which accumulates in aged humans. Unlike specific pathogen-free laboratory mice, humans may be exposed to various infectious agents potentially driving the accumulation of conventional antigen-stimulated CD8 T cell memory. However, studies have shown that infection fails to reduce the VM T cell population in mice (4).
In summary, we provide compelling evidence that the aging-related preponderance of central memory CD8 T cells is not a result of life-long antigenic stimulation but is due to accumulation of virtual memory T cells. Thus, our study reveals a novel mechanism for aging-related changes in CD8 T cells and calls for a re-evaluation of the effect of aging on CD8 T cells and CD8 T cell-mediated immune responses.
Acknowledgments
This work was supported by NIH-NIAID grant AI43460 and in part by the Department of Veterans Affairs.
Abbreviations used in this paper
- NP
naive phenotype
- MP
memory phenotype
- CM
central memory
- VM
virtual memory
- DC
dendritic cell
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, Kedl RM. CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haluszczak C, Akue AD, Hamilton SE, Johnson LDS, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 7.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu BC, Shang XZ, Stolberg VR, Komuniecki E, Chensue SW. Population analysis of CD4+ T cell chemokine receptor transcript expression during in vivo type-1 (mycobacterial) and type-2 (schistosomal) immune responses. J Leukoc Biol. 2002;72:363–372. [PubMed] [Google Scholar]
- 11.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 12.Chao CC, Jensen R, Dailey MO. Mechanisms of L-selectin regulation by activated T cells. J Immunol. 1997;159:1686–1694. [PubMed] [Google Scholar]
- 13.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 15.Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadiere C, Amigorena S, Fetler L. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. 2007;8:921. doi: 10.1038/ni1495. [DOI] [PubMed] [Google Scholar]
- 16.Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, Tomura M, Sugihara K, Takamura S, Kakimi K, Matsushima K. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208:1605–1620. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]