Abstract
A versatile three-step, one-pot, sequential reaction protocol involving RCM, CM, and chemoselective hydrogenation is reported. This phosphate tether-mediated process occurs without intermediate isolation, is chemoselective and is governed by stereoelectronic properties innate to phosphate tethers, which ultimately act to preserve the integrity of the bisallylic, bicyclic phosphate for subsequent nucleophilic additions. Overall, this process can be used to efficiently generate advanced polyol synthons.
The development of reaction methods enabling the facile synthesis of complex structural motifs in minimum functional group manipulations is an important goal in organic synthesis. In this regard, sequential, one-pot reaction strategies have emerged as versatile approaches, due to their ability to form multiple bonds and stereocenters, while invoking step-, atom-, and green-economy.1 Several advantages associated with one-pot transformations exist, among the more notable, include: achievement of step economy – multiple transformations without isolating the intermediates, and higher efficiency – as only one workup/purification step is needed in a given sequence. Taken collectively, combination of several steps into a single pot integrates synthesis and purification to achieve an overall streamlined process.
Olefin-metathesis has emerged as an invaluable method for the formation of C=C bonds where catalysts show tremendous activity, selectivity, functional group tolerance and stability in both ring-closing metathesis (RCM) and cross-metathesis (CM).2 Recently, this versatility has been explored in several elegant one-pot reaction pathways,3 including: tandem RCM/hydrogenation,3a tandem RCM/Kharasch addition,3b tandem CM/intramolecular aza-Michael,3e and tandem RCM/CM/hydrogenation3f as outlined in Figure 1. Despite these successes, several challenges associated with one-pot reactions remain, including: (i) the development of suitable reaction conditions allowing compatibility of reactants, (ii) influence of excess reagents and byproducts generated from the previous reaction in a sequence, (iii) expansion of the number of compatible steps in the overall process and (iv) improvement of average and total yields.
Figure 1.
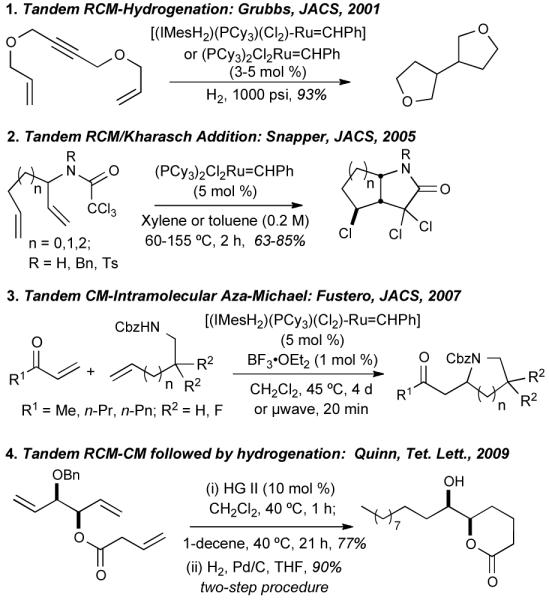
Tandem metathesis reactions
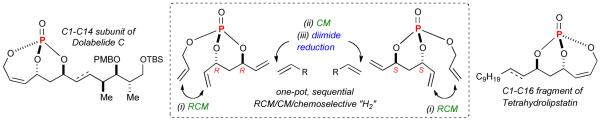
Interest in the development of phosphate-based methodologies has led us to investigate the potential of a phosphate tether to mediate a sequence of reactions cleanly, selectively and in one-pot. Previously, metathesis strategies incorporating multivalent activation of phosphate triesters for use in diastereoselective differentiation of 1,3-anti diol subunits4 have been developed for the total synthesis of tetrahydrolipstatin,5 dolabelide C6 and the formal total synthesis of salicylihalamides A and B.7 During the synthesis of tetrahydrolipstatin and dolabelide C, it was demonstrated that a stepwise sequence of RCM, CM, and chemoselective hydrogenation could be incorporated into a one-pot procedure to further streamline the synthetic route, albeit in non optimal conditions.5 Advantages of this one-pot, sequential method were many-fold, namely in terms of the reaction time, waste generation, and ease of purification. Moreover, several properties innate to phosphate tether-mediated processes, namely trivalent activation and stereoelectronic effects, were deemed ideal for further developing this method. In this regard, we herein report a versatile one-pot, sequential reaction protocol where three steps, namely RCM, CM and chemoselective hydrogenation are performed in a single pot without intermediate isolation to generate advanced polyol subunits with application to several 1,3-diol-containing natural products (Figure 2). To the best of our knowledge this is the first example of a chemoselective hydrogenation that is followed by an RCM/CM in a tandem reaction.
Figure 2.
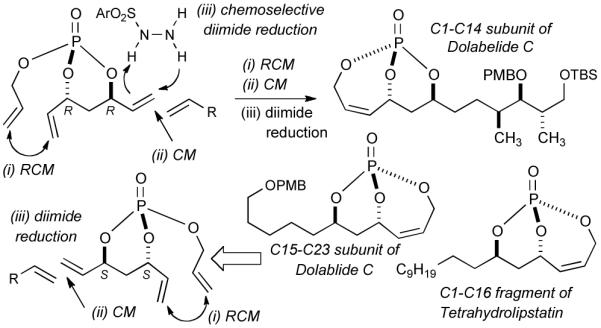
One-pot, sequential RCM/CM/chemoselective hydrogenation.
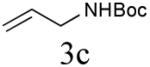
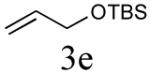
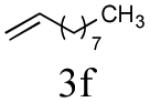
Initial studies focused on type I olefin cross partners during the CM event as outlined in Scheme 1 and Table 1. In accordance with olefin reactivity patterns reported by Grubbs, reactive olefin partners in CM steps are characterized as type I and type II olefins based on their propensity to undergo homo-dimerization and CM with other olefins partners.8 Previous studies suggested that bicyclic phosphate (R,R,RP)-2 behaves as a near type III olefin based on its ability to undergo efficient CM reaction with both type I and II olefins.9 Type III olefin character is ideal for CM reactions, especially in tandem processes such as described herein, thus enabling advancement of this method to more precious metathesis partners.
Scheme 1.
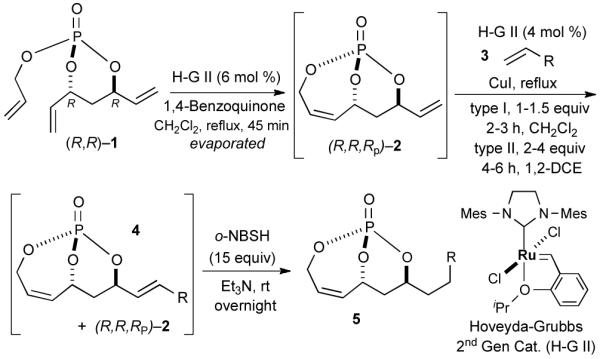
General protocol for RCM/CM/chemoselective hydrogenation
Table 1.
One-pot, sequential RCM/CM/chemoselective hydrogenation involving type I olefins.
| entry | olefin | yield %a (avg %) |
RCM-CM- chemoselective hydrogenation |
|---|---|---|---|
| 1 |
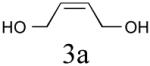
|
64%b (86%) |
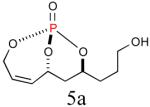
|
| 2 |
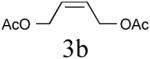
|
56% (82%) |
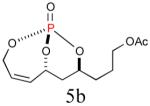
|
| 3 |
|
59%b (84%) |
|
| 4 |
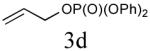
|
40% (74%) |
|
| 5 |
|
52% (80%) |
|
| 6 |
|
65% (87%) |
|
| 7 |
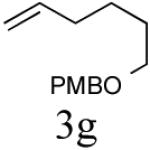
|
43%c (76%) |
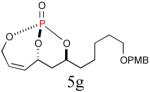
|
All reactions were performed using freshly distilled (over CaH2) FDT solvents
1,4-Benzoquinone is not used during RCM event.
Reaction was performed in CH2Cl2 purified by passing through basic Al2O3 and degassed by argon purging without any additives.
The initial RCM reaction was carried out using Hoveyda-Grubbs catalyst (6 mol %), after which the type I olefin cross partner and additional catalyst (4 mol %) were added with simultaneous evaporation of CH2Cl2 to reach an optimal concentration of 0.05 M for CM. The reaction was continued for 2–3 h. Of notable importance is the fact that RCM must be completed before the CM partner is added (i.e. sequential addition) as experimental combination of all the components (i.e., triene (R,R)-1, olefin cross partner 3 and metathesis catalyst) for a tandem RCM/CM reaction did not yield promising results, but rather produced a mixture of RCM and several CM byproducts. Presumably, these byproducts result from deleterious CM events as RCM precursor (R,R)-1 contains two type II CM partners and one type I olefin.
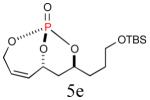
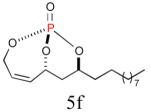
The aforementioned results indicate that the RCM reaction needs to go to completion prior to the addition of olefin CM partner. In addition, and in accord with literature precedence,10 CM with the more reactive Hoveyda-Grubbs catalyst produced better yields compared to Grubbs second-generation catalyst [(IMesH2)(PCy3)(Cl2)-Ru=CHPh] as demonstrated in our earlier studies.9 Moreover, detailed freeze-degas-thaw (FDT) solvent studies with and without various additives11 showed that a combination of factors can drastically improve yields.12 Subsequent chemoselective diimide reduction at room temperature was next carried out by simple addition of o-nitrobenzenesulfonyl hydrazine (o-NBSH) to the crude reaction mixture.13 Purification after hydrogenation step showed product formation along with hydrogenated (R,R,RP)-2. This one-pot, sequential procedure with type I olefins generated the desired products in 40-65% overall yield with 74–87% average yield over three steps.
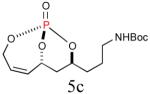
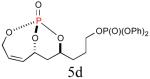
Since the endocyclic olefin is doubly deactivated due to the presence of bisallylic phosphate moieties, the chemoselective, diimide reduction of the exocyclic olefin is most likely governed by electronic parameters rather than steric considerations. While successful chemoselective reductions of the doubly deactivated exocyclic olefin in entries 2 and 4 (Table 1) would at first glance seem to contradict this trend, it is known that innate stereoelectronic factors within the bicyclic phosphate framework impart greater electron withdrawing properties at the constrained P=O in 5d compared with the acyclic, exocyclic P=O in 5d. This fact is further substantiated by comparison of the 31P chemical shifts for each system, where the endocyclic P=O appears further downfield than the exocyclic P=O (−3.24 ppm vs. −11.31 ppm, respectively in 5d, Scheme 3).14
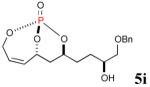
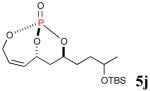
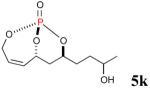
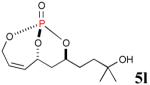
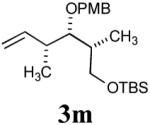
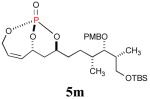
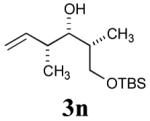
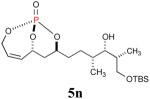
The reaction sequence with type II olefins was also carried out using a similar protocol as with type I CM partners (Table 2). However, solvent manipulation in the CM event [switched from CH2Cl2 to 1,2-dichloroethane (1,2-DCE)] was required to obtain desirable yields since high temperature conditions are more efficient with type II olefins CM partners. Subsequent diimide reduction in DCE was successful using a variety of olefinic CM partners. Of particular note, are entries 3j and 3l (Table 2), possessing sterically encumbered olefins, which further substantiates the aforementioned electronic viewpoint model for chemoselective reduction, vide supra. This one-pot procedure with type II olefins produced the desired product in 30–85% overall yield with 67–95% avg. yield over three steps.
Table 2.
One-pot, sequential RCM/CM/chemoselective hydrogenation involving type II olefins
| entry | olefin | yield %a (avg %) |
RCM-CM-chemoselective hydrogenation |
|---|---|---|---|
| 1 |
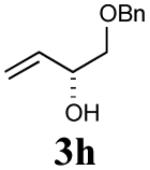
|
41% (75%) |
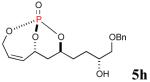
|
| 2 |
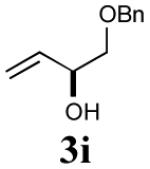
|
35% (71%) |
|
| 3 |
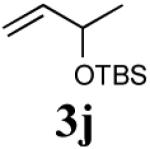
|
69% (89%) |
|
| 4 |
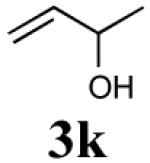
|
48% (78%) |
|
| 5 |
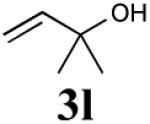
|
72% (90%) |
|
| 6 |
|
30%b (67%) |
|
| 7 |
|
54% (81%) |
|
| 8 |
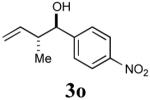
|
85% (95%) |
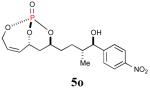
|
| 9 |
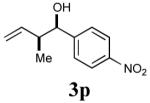
|
79% (92%) |
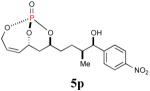
|
All reactions were performed using freshly distilled (over CaH2) FDT solvents
Reaction was performed in CH2Cl2, 1,2-DCE purified by passing through basic Al2O3 and degassed by argon purging.
In conclusion, an efficient one-pot, sequential RCM/CM/chemoselective hydrogenation protocol has been developed. This procedure enables the synthesis of advanced substrates in a streamlined manner. Based on observations, it is noteworthy to mention that the CM event is deemed as the key factor in determination of overall yield. Further efforts in this area are in progress and will be reported in due course.
Supplementary Material
Figure 3.
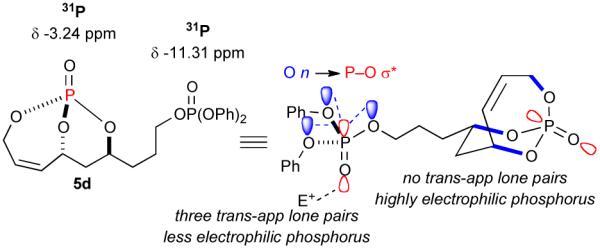
Stereoelectronic effects governing chemoselective hydrogenation
Acknowledgment
This investigation was generously supported by partial funds provided by the National Institute of General Medical Sciences (NIH RO1 GM077309-05), the NSF-REU program (CHE-1004897) for summer fellowship support (G.S.) and Research & Graduate Studies at KU. The authors thank Dr. Justin Douglas and Sarah Neuenswander for assistance with NMR measurements, Dr. Todd Williams for HRMS analysis at The University of Kansas, and Materia, Inc. for supplying metathesis catalyst and helpful suggestions.
Footnotes
Supporting Information Available Experimental details and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For a review on step economy, see: Wender PA, Verma VA. Acc. Chem. Soc. 2008;41:40–49. doi: 10.1021/ar700155p. For reviews on atom economy, see: Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. Trost BM. Angew. Chem., Int. Ed. 1995;34:259–281. For reviews on protecting group free synthesis, see: Young SI, Baran PS. Nat. Chem. 2009;1:193–205. doi: 10.1038/nchem.216. Hoffmann RW. Synthesis. 2006:3531–3541. For reviews on domino/cascade reactions, see: Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551–564. doi: 10.1039/b209440c. Tietze LF, Brasche G, Gericke KM. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem., Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. Enders D, Grondal C, Hüttl MRM. Angew. Chem., Int. Ed. 2007;46:1570–1581. doi: 10.1002/anie.200603129. Grondal C, Jeanty M, Enders D. Nat. Chem. 2010;2:167–178. doi: 10.1038/nchem.539. Ishikawa H, Honma M, Hayashi Y. Angew. Chem., Int. Ed. 2011;50:2824–2827. doi: 10.1002/anie.201006204.
- 2.Grubbs RH. Handbook of Metathesis. Wiley-VCH; Weinheim: 2003. [Google Scholar]
- 3.For tandem, metathesis/hydrogenation, see: Louie J, Bielawski CW, Grubbs RH. J. Am. Chem. Soc. 2001;123:11312–11313. doi: 10.1021/ja016431e. For CM/Wittig olefination, see: Murelli RP, Snapper ML. Org. Lett. 2007;9:1749–1752. doi: 10.1021/ol070445t. For RCM/oxidation, see: Scholte AA, An M-H, Snapper ML. Org. Lett. 2006;8:4759–4762. doi: 10.1021/ol061837n. For RCM/Kharasch addition, see: Seigal BA, Fajardo C, Snapper ML. J. Am. Chem. Soc. 2005;127:16329–16332. doi: 10.1021/ja055806j. For tandem CM/intramolecular aza Michael, see: Fustero S, Jimenez D, Sanchez Rosello M, del Pozo C. J. Am. Chem. Soc. 2007;129:6700–6701. doi: 10.1021/ja0709829. For tandem RCM/CM and hydrogenation, see: Quinn KL, Curto JM, McGrath KP, Biddick NA. Tetrahedron Lett. 2009;50:7121–7123. Quinn KJ, Isaacs AK, Arvary RA. Org. Lett. 2004;6:4143–4145. doi: 10.1021/ol040047f. Virolleaud M-A, Bressy C, Piva O. Tetrahedron Lett. 2003;44:8081–8084. Virolleaud M-A, Piva O. Tetrahedron Lett. 2007;48:1417–1420. For CM/hydrogenation/cyclization, see: Cossy J, Bargiggia F, BouzBouz S. Org. Lett. 2003;5:459–462. doi: 10.1021/ol027347m. For tandem CM/amidation, see: Ferrie L, BouzBouz S, Cossy J. Org. Lett. 2009;11:5446–5448. doi: 10.1021/ol9021386. For other tandem metathesis processes, see: Ferrie L, BouzBouz S, Cossy J. Org. Lett. 2009;11:5446–5448. doi: 10.1021/ol9021386. Riache N, Blond A, Nay B. Tetrahedron. 2008;64:10853–10859. Cross F, Pelotier B, Piva O. Eur. J. Org. Chem. 2010:5063–5070. O’Leary-Steele C, Pedersen PJ, James T, Lanyon-Hogg T, Leach S, Hayes J, Nelson A. Chem. Eur. J. 2010;16:9563–9571. doi: 10.1002/chem.201000707.
- 4.(a) Whitehead A, McReynolds MD, Moore JD, Hanson PR. Org. Lett. 2005;7:3375–3378. doi: 10.1021/ol0512886. [DOI] [PubMed] [Google Scholar]; (b) Thomas CD, McParland JM, Hanson PR. Eur. J. Org. Chem. 2009:5487–5500. doi: 10.1002/ejoc.200900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venukadasula PKM, Chegondi R, Maitra S, Hanson PR. Org. Lett. 2010;12:1556–1559. doi: 10.1021/ol1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson PR, Chegondi R, Nguyen J, Thomas CD, Waetzig JD, Whitehead A. J. Org. Chem. 2011;76:4358–4370. doi: 10.1021/jo2003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chegondi R, Tan MML, Hanson PR. J. Org. Chem. 2011;76:3909–3916. doi: 10.1021/jo200337v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 9.Waetzig JW, Hanson PR. Org. Lett. 2006;8:1673–1676. doi: 10.1021/ol0602809. [DOI] [PubMed] [Google Scholar]
- 10.For a review, see:• Hoveyda AH, Gillingham DG, Van Veldhuizen JJ, Kataoka O, Garber SB, Kingsbury JS, Harrity JPA. Org. Biol. Chem. 2004;2:8–23. doi: 10.1039/b311496c. and references cited therein. Cossy J, BouzBouz S, Hoveyda AH. J. Organomet. Chem. 2001;624:327–332. Dewi P, Randl S, Blechert S. Tetrahedron Lett. 2005;46:577–580.
- 11.1,4-Benzoquinone is generally used to suppress any Ru-H generated during the metathesis event. CuI is generally used in conjunction with Grubbs 2nd Gen catalyst to scavenge the phosphine and keep open coordination site at Ru to enhance the rate of metathesis reaction; Voigtritter K, Ghorai S, Lipshutz BH. J. Org. Chem. 2011;76:4697–4702. doi: 10.1021/jo200360s.
- 12.After the CM reaction, the reaction also contained some unreacted bicylic phosphate (R,R,RP-2). Optimization studies substantially lowered this unwarranted result.
- 13.(a) Myers AG, Zheng B, Movassaghi M. J. Org. Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]; (b) O’Doherty GA, Haukaas MH. Org. Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x. [DOI] [PubMed] [Google Scholar]; (c) Buszek KR, Brown N. J. Org. Chem. 2007;72:3125–3128. doi: 10.1021/jo0622173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


