Abstract
Astrocyte Ca2+ signals in awake behaving mice are widespread, coordinated and differ fundamentally from the locally restricted Ca2+ transients observed ex vivo and in anesthetized animals. Here we show that the synchronized release of norepinephrine (NE) from locus coeruleus (LC) projections throughout the cerebral cortex mediate long-ranging Ca2+ signals by activation of astrocytic α1-adrenergic receptors. When LC output was triggered by either physiological sensory (whisker) stimulation or an air-puff startle response, astrocytes responded with fast Ca2+ transients that encompassed the entire imaged field (positioned over either frontal or parietal cortex). The application of adrenergic inhibitors, including α1-adrenergic antagonist prazosin, potently suppressed both evoked, as well as the frequently observed spontaneous astroglial Ca2+ signals. The LC-specific neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), which reduced cortical NE content by >90%, prevented nearly all astrocytic Ca2+ signals in awake mice. The observations indicate that in adult, unanesthetized mice, astrocytes do not respond directly to glutamatergic signaling evoked by sensory stimulation. Instead astrocytes appear to be the primary target for NE, with astrocytic Ca2+ signaling being triggered by the α1-adrenergic receptor. In turn, astrocytes may coordinate the broad effects of neuromodulators on neuronal activity.
Keywords: awake, astrocyte, norepinephrine, calcium, startle
1. Introduction
Ca2+ signaling represents the principle tool for cell-cell communication in astrocytes, similar to other electrically non-excitable cells. Dynamic changes in the intracellular calcium level ([Ca2+]i) of astrocytes during synaptic transmission have been intensively studied because these signals play a key role in bidirectional neuro-glial communication [1]. These responses have been shown to be driven by a wide number of factors, with complex spatiotemporal regulation [2]. In acute brain slice preparations from young pups the synaptic release of neurotransmitters glutamate can activate metabotropic receptors on astrocytes and trigger [Ca2+]i transients [3–6]. In turn, astrocytic [Ca2+]i transients modulate ongoing synaptic transmission through “glio-transmitter” release of substances such as ATP, glutamate and D-serine, in addition to stimulating active K+ re-uptake [7, 8]. Using microelectrode stimulation in brain slice it has also been shown that glutamatergic transmission can trigger highly localized Ca2+ increases involving a single astrocyte or subsets of astrocytic processes [4, 5]. In parallel, experiments in intact animals (in vivo) have demonstrated that astroglial [Ca2+]i transients can be elicited by a variety of sensory stimuli, including whisker stimulation, visual input, hind-limb shock and exposure to odor [9–12]. However, in contrast to slice preparations, in vivo sensory stimulation triggers widespread Ca2+ signaling that typically engages most astrocytes within an imaging field [11, 13–15]. This signaling pattern is even more pronounced in awake (unanesthetized) animals, such as those used in the current study, where both locomotion and sensory stimulation have been shown to elicit [Ca2+]i transients across hundreds of astroglia [14]. However, to our knowledge no study has previously asked why neuronal activity triggers localized astrocyte [Ca2+]i increases in acute brain slice and coordinated widespread [Ca2+]i increases in vivo.
Widespread astrocytic [Ca2+]i signaling has previously been observed following direct stimulation of the locus coeruleus (LC) in the brainstem [16]. It is therefore possible that the neuromodulator norepinephrine (NE) could contribute to the nearly global astrocytic signals observed with various forms of sensory stimulation in vivo. LC neurons project to large areas of cortex, cerebellum, and deeper structures where they release NE diffusely from numerous varicosities on their axons [17]. In the current study we directly examined whether NE plays a more prominent role than previously recognized in the widespread astrocytic Ca2+ signaling seen in vivo. To drive NE release we used the startle response - an involuntary reaction to an unexpected or salient event, such as a loud noise that involves a sudden increase in sympathetic tone, phasic activity of LC, and involuntary contraction of most skeletal muscles [18, 19]. Surprisingly, an analysis of awake behaving mice showed that most, if not all astrocytic Ca2+ signaling was mediated by activation of α1-AR, suggesting that NE is the principle pathway for neuro-glia signaling in the intact cortex. This finding is of critical importance, as it indicates that astrocytes do not respond to local neurotransmission, but are instead stimulated by the concerted release of NE or other neuromodulators [20, 21]. Since phasic LC discharges are typically observed under conditions that require a shift in attention, a key function of astrocytic Ca2+ signaling may be to facilitate the resetting of neural circuits to enhance refocusing on novel tasks. This conclusion is consistent with the recent observation that astrocytes in the adult human and mouse brain do not express Gq-coupled metabotropic glutamate (mGlu) receptors and that mGlu receptor agonists fail to mobilize astrocytic Ca2+ stores in both anesthetized and awake adult mice [22]. Thus, astrocytic Ca2+ increases in response to local synaptic activity may be a phenomenon restricted to developing brain tissue, perhaps analogous to changes in neuronal glutamate receptors expression during development [23].
2. Materials and Methods
2.1 Animal preparation
Glt-1-eGFP BAC and C57BL6 wild-type mice were bred as described previously, and animals aged 8–12 weeks were used [24, 25]. Mouse preparation was modified from published protocols [13, 26]. Briefly, mice were anesthetized using isoflurane via nose cone (1.0–1.5% mixed with 1–2 L/min O2), head-restrained with a custom-made mini-frame, and habituated over 2 days in multiple 1-hour sessions. A 1.5 mm craniotomy (with the dura carefully removed) was opened over the somatosensory (barrel cortex: −0.7mm anterior/posterior (AP) to bregma, 3.5mm lateral (ML) to the midline) or frontal cortex (2.8mm AP, 1.6mm ML) and calcium indicator rhod-2 AM (Invitrogen, 0.5 mM) was loaded onto exposed cortex for 30–45 min before applying agarose (1.5%, type III-A, Sigma) and a coverslip. Animals were then head-restrained and placed on the stage located in a dark quiet room, with their feet resting on a metal cylindrical treadmill that rotates with very little friction. The animal can run freely on the upper surface of the treadmill with the skull immobilized with respect to the microscope objective. A subset of animals were restrained using a custom-made acrylic tube to reduce movement. N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) (Sigma-Aldrich) was administered i.p. at a concentration of 50mg/kg as described previously [16]. Animals were injected on days 1 and 3, and were imaged on days 4 or 5. All animal experiments were approved by the Animal Care and Use Committee of the University of Rochester.
2.2 Two-photon laser scanning microscopy
A Mai Tai laser (SpectraPhysics) attached to a confocal scanning system (Fluoview 300, Olympus) and an upright microscope (IX51W, Olympus) was used. Calcium transients were imaged in cortex 75–150 μm below the pial surface, as described previously, using a 20x (0.95NA, Olympus) lens [25]. Dual channel (rhod-2 and eGFP) frames were collected at approximately 1 Hz. Laser power was kept below 30mW to avoid phototoxicity. A calcium transient was defined as an event where the change in rhod-2 intensity (ΔF/F0) normalized to eGFP deviated >2 standard deviations (σ) from baseline with an increase of at least 25%. Amplitude was taken as the peak ΔF/F0 in this interval. Beginning and end were defined as ΔF/F0 being >50% the maximum increase in amplitude. Recordings were analyzed using previously described custom-made software (MatLab Inc.) and Image J (NIH) [25].
2.3 Electrocorticogram (ECoG) recordings, whisker stimulation, and startle response
Recordings were obtained from layer II somatosensory cortex using glass microelectrodes as described previously [11]. Briefly, signals were bandpass filtered at 0.1–2000 Hz and digitized at 9 kHz (Digidata 1440A by Axon Instruments). Recordings were analyzed offline using pClamp 10.2. Whisker stimulation was delivered as described previously using a picospritzer III (Parken Instrumentation) and Master 8 (A.M.P.I.). Stimuli consisted of 10 ms air pulses delivered at 3Hz for 30s [11]. For startle stimulation, a train of air pulses was directed at either the base of the tail or face of the animal (40–100ms duration each, 5–10 pulses) using a picospritzer III (Parken Instrumentation) and Master 8 (A.M.P.I.))
2.4 Drug application
Agonists (methoxamine (Sigma-Aldrich), dexmedetomidine (Tocris Biosciences), isoproterenol (Sigma-Aldrich), and carbachol (Sigma-Aldrich)) were micro-injected at a concentration of 200μM into the cortex using a fine glass electrode connected to a picospritzer (20 PSI, 10–20ms; Parker Instrumentation, Chicao, IL) and visualized by adding 100μM Alexa 488 (Invitrogen) to the aCSF pipette solution (145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 2mM Na2HPO4, and 1.0 mM MgCl2, adjusted to pH 7.4). The electrode was centered in the imaging field and all injections were done between 75–125μM below the pial surface. The injection radius was defined as all astrocytes exhibiting a >2σ increase over baseline in Alexa 488 fluorescence. Antagonists (200μM prazosin (Sigma-Aldrich), atipamezole (Tocris Biosciences), and propranolol (Sigma-Aldrich)) were added to the artificial cerebrospinal fluid bathing the craniotomy (approximate volume 500μL) and were allowed to diffuse through the agarose as described previously [14].
2.5 Collection of cortical homogenates and High-Pressured Liquid Chromatography (HPLC) measurements
Following calcium imaging, brains from WT and DSP-4 treated animals were dissected, and samples of the frontal cortex were diluted 10x in 5% TCA (MG Scientific). Samples were then sonicated on ice for 3 5s periods at a power of 0.5 with 30s in between to prevent overheating using a Sonicator 3000 by Misonix. Homogenates were then centrifuged at 14,000g for 15 minutes at 4 °C using a Microfuge 22R by Beckman Coulter. The supernatant was collected for catecholamine analysis. Briefly, 20μl samples were injected into an HPLC-EC system consisting of an ESA Model 584 pump, set at 0.200ml/min., an ESA MD-160, 1.5×250mm, 5μM column, and an ESA 5600A Coulochem II detector with an ESA 5041 analytical cell (+220V). MD-3MA mobile phase was used, consisting of 150mM ammonium acetate (Fisher Scientific), 140μM EDTA (Fisher Scientific), 15% Methanol (EM Science), and 5% Acetonitrile (Fisher Scientific) with a pH of 6.0.
2.6 Statistical analyses
All analysis was performed Prism (GraphPad Software) and all tests were two-tailed where significance was achieved at α = 0.05 level. Wherever necessary a Bonferroni correction for multiple testing was done.
3. Results
3.1. Cortical microinjection of adrenergic agonist evokes robust astrocyte calcium responses in awake behaving mice
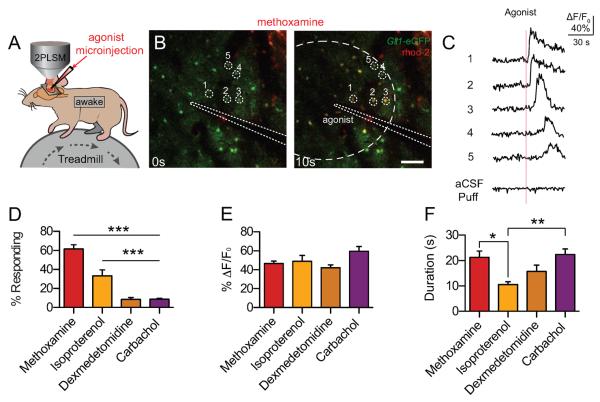
We first assessed whether astrocytes in awake mice respond to adrenergic agonists (Table 1). Prior to imaging, astrocytes were loaded with the fluorescent Ca2+-indicator dye, rhod-2 (AM), to visualize astrocytic Ca2+ increases in response to agonist microinjection. Agonists were injected using a fine glass electrode containing aCSF and the fluorescent tracer Alexa 488-cadaverine. All astrocytes were analyzed for increases in Alexa 488 fluorescence with all cells exhibiting a >2s increase in intensity being considered within the puff radius. (Fig. 1 A–C) Injection of the α1-AR agonist, methoxamine, resulted in the robust activation of a high proportion of astrocytes (60.94 ± 6.99%). The nonspecific β-AR agonist, isoproterenol, was also capable evoking responses in 36 ± 7.06% of contacted astrocytes (Fig. 1D). In contrast, only sporadic Ca2+ increases were seen in response to the α2-AR agonist, dexmedetomidine (8.87 ± 2.58%), and the nonspecific nicotinic/muscarinic acetylcholine receptor agonist, carbachol (8.83 ± 1.63%). Interestingly, responses to carbachol tended to be most pronounced near vascular endfeet. While the proportion of astrocytes responding varied substantially, responses across all agonists tended to show a similar amplitude and duration (Fig. 1E, F), with isoproterenol exhibiting shorter waves (10.5 ± 1.1s) than methoxamine (21.6 ± 2.4s) and carbachol (22.4 ±2.2s). These results suggest that astrocytes respond to activation of both α1- and β-AR with robust [Ca2+]i increases, and that these agonist-evoked responses exhibit a broadly similar profile.
Table 1.
Noradrenergic pharmacology
| Receptor | Coupled G-Protein | Agonist | Antagonist |
|---|---|---|---|
| α1-AR | Gq | Methoxamine | Prazosin |
| α2-AR | Gi | Dexmedetomidine | Atipamezole |
| β-AR | Gs | Isoproterenol | Propranolol |
Noradrenergic receptors targeted in this study; their associated G-protein; and the agonists and antagonists used.
Fig. 1.
Astrocytes respond to α1- and β-AR agonists with increases in intracellular Ca2+. (A) Cortical astrocytes loaded with rhod-2 (AM) were imaged in layers I/II to detect changes in [Ca2+]i. Agonists were injected using a microelectrode loaded with ACSF and the tracer Alexa 488. (B) Representative images of astrocytic [Ca2+]i responses to the α1-AR agonist, methoxamine, in awake Glt-1-eGFP transgenic mice. Astrocyte-specific loading of rhod-2 (AM) was confirmed by colocalization with Glt-1-eGFP. Scale bar = 100μM. (C) rhod-2 (AM) ΔF/F0 traces from (B), normalized to Glt-1-eGFP fluorescence. Representative aCSF-microinjection trace at the bottom. (D) Astrocytic [Ca2+]i responses to adrenergic and acetylcholinergic receptor agonists, measured by rhod-2 (AM) ΔF/F0. Bar graph showing the percent of astrocytes within the puff radius responding with a [Ca2+]i increase. *** p<0.001, one-way ANOVA, Bonferroni correction; [n= 25 trials in 6 animals (methoxamine); 14 trials in 4 animals (isoproterenol); 24 trials in 5 animals (dexmedetomidine); 23 trials in 5 animals (carbachol)] (E and F) Bar graphs showing average rhod-2 (AM) ΔF/F0 and response duration from trials with responding cells. ** p < 0.01; * p < 0.05, one-way ANOVA, Bonferroni correction; [n = 25 trials (methoxamine), 13 trials (isoproterenol), 17 trials (dexmedetomidine), 20 trials (carbachol).] Data are shown as mean ± SEM.
3.2. Sensory stimulation of awake mice triggers a startle response associated with widespread coordinated astrocyte calcium activity in the brain
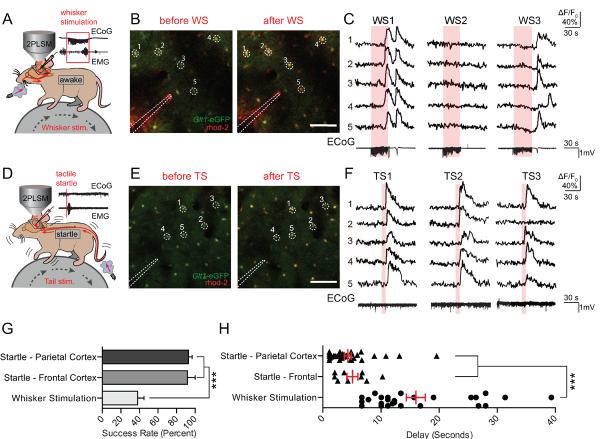
Previous work in our lab had demonstrated that astrocytes are capable of responding to physiological whisker stimulation under light anesthesia [11]. In order to test the response of astrocytes in the awake brain, whisker stimulation (3 Hz, 30 s) was used to drive Ca2+ responses. The local field potential (LFP) was recorded in conjunction with Ca2+ imaging to verify neuronal activation in the barrel cortex. Unexpectedly, while whisker stimulation could drive Ca2+ responses (Fig. 2A–C), activity was unreliable, with only 38.0 ± 6.9% of stimuli eliciting a broad response despite consistent increases in local glutamatergic transmission detected by the LFP recordings (Fig. 2 C, G).
Fig. 2.
Astrocytes respond reliably to startle stimulation with widespread Ca2+ waves. (A) Astrocytic Ca2+ transients measured by rhod-2 (AM) were detected in response to 30s of 3Hz whisker stimulation. Representative LFP and EMG recordings are shown. (B) Representative images of rhod-2 (AM) fluorescence increases during whisker stimulation in a Glt-1-eGFP animal. Scale bar = 100μM. (C) Selected cells from (B). rhod-2 ΔF/F0 was normalized to Glt-1-eGFP fluorescence. ECoG traces corresponding to rhod-2 ΔF/F0 are shown below. (D) Air pulses were directed at the face or tail of the animal to elicit a startle response. Representive ECoG and EMG traces are shown with no apparent evoked ECoG response, and strong EMG activity – indicative of a startle response. (E and F) Representative images and corresponding rhod-2 dF/F0 traces show stable and repeatable astrocytic [Ca2+]i transients after startle stimulations. Bottom, representative ECoG traces from startle stimulation are shown. (G) Bar graph showing the response rate of cortical astrocytes to whisker and startle stimulation, averaged across animals. *** p<0.001, one-way ANOVA, Bonferroni correction; [n = 11 animals (parietal cortex startle and whisker stimulation), 4 animals (frontal cortex startle)] (H) Scatter diagram with superimposed mean and SEM for the delay from the onset of whisker/startle stimulation to the beginning of astrocytic rhod-2 ΔF/F0 responses. Average delay from each successful trial is shown. *** p<0.001, one-way ANOVA, Bonferroni correction; [n= 28 trials in 14 animals (whisker stimulation), 38 trials in 13 animals (parietal cortex startle), and 9 trials in 4 animals (frontal cortex startle)]. Data are shown as mean ± SEM.
Based on the above agonist studies, we decided to investigate the possibility that awake responses were dependent on widespread neuromodulatory release instead of local glutamatergic signaling. As startle stimulation is known to drive widespread NE release from the LC, we adapted a model of tactile startle, consisting of a brief (<1 s) air puff directed at the either the base of the tail or the animal's face. Startle stimulation was verified using an EMG placed on the trapezius muscle (Fig. 2D), and in some animals an LFP was recorded in the imaged cortex to preclude the possibility that the stimulation was evoking local neuronal activity (Fig. 2D–F). In contrast to whisker stimulation, startle reliably evoked widespread astrocytic Ca2+ waves, with stimuli eliciting waves in both the prefrontal (91.75 ± 8.25%) and parietal cortex (92.91 ± 3.77%) (Fig. 2G). In addition, while whisker stimulation evoked sporadic responses with widely variable delays [10.1–23.9s], both frontal and parietal responses to tactile startle occurred within several seconds of the stimulus ([2.0–7.2s] and [1.1–5.0s], respectively) (Fig. 2H). These results suggest that widespread, somatic Ca2+ responses in awake, head-restrained mice are not driven by local synaptic activity. Instead, reliable responses were evoked as a result of a sudden stressor, suggesting that activity is driven by the widespread release of neuromodulators.
3.3. Inhibition of LC-driven, α1-adrenergic receptor activity potently blocks astrocytic calcium signals
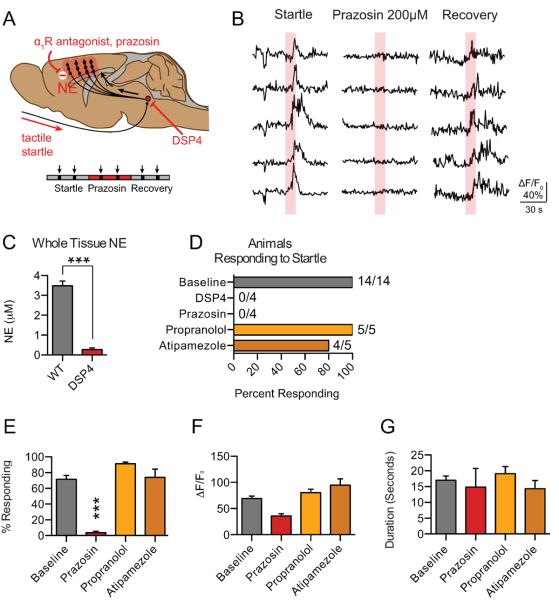
As startle stimulation has been shown to drive burst release of NE from LC neurons, we next sought to determine the role of NE in mediating astrocytic Ca2+ responses [27]. To test the involvement of the LC, we first used the LC-specific neurotoxin DSP4 to deplete cortical NE. Following treatment with DSP4, animals were imaged with startle stimulation to determine how pharmacological ablation of the LC affected astrocytic Ca2+ responses (Fig. 3D). After imaging, animals were sacrificed and the frontal cortex was dissected, homogenized, and analyzed by HPLC to verify decreased cortical NE concentration (0.27 ± 0.08μM in DSP4 treated animals versus 3.48 ± 0.24 μM in WT animals, Fig 3C). We next sought to determine the specific receptors involved in the astrocytic Ca2+ response to startle. To do so, specific antagonists to the α1-, α2-, and β-AR were added to the aCSF solution bathing the surface of the cortex. Consistent with our results using AR agonist micro-injection, startle-evoked responses were eliminated both by depletion of cortical NE with DSP4 and by local application of the α1-AR antagonist, prazosin (Fig. 3A, B, E). In contrast, antagonists to α2- and β-ARs neither altered the prevalence (Fig. 3E) nor the characteristic profile (Fig. 3F–H) of astrocytic responses to startle stimulation. These data suggests that astrocytic Ca2+ responses are dependent on α1-AR, and not β-AR, activation during the startle response.
Fig. 3.
Astrocytic Ca2+ responses are dependent upon activation of the α1-AR. (A) Schematic illustrating diffuse NE release from the LC across the cortex in response to startle stimulation. LC-specific neurotoxin DSP4 and prazosin are shown blocking astrocytic Ca2+ responses, as measured by rhod-2 ΔF/F0. (B) Representative traces of astrocytic rhod-2 ΔF/F0 before, during, and after application of the α1-AR antagonist prazosin. (C) Bar graphs of cortical NE content in whole brain homogenate determined by HPLC showing depletion following DSP-4 injection. *** p<0.001, unpaired, two-tailed t-test [n=5 animals per group]. (D) Histogram presenting the percent of animals exhibiting at least one response to startle stimulation (>1/3 of cells exhibiting a rhod-2 ΔF/F0 response) for DSP4 and adrenergic antagonist treated animals. (E) Bar graph showing the average percent of cells responding with an increase in rhod-2 ΔF/F0 in the imaging field *** p<0.001, paired t-test; [prazosin: 20 trials baseline, 16 trials with drug in 4 animals; atipamezole: n = 13 trials baseline, 13 trials with drug in 5 animals; propranolol: 14 trials baseline, 14 trials with drug in 5 animals.] (F and G) Bar graphs showing the average peak amplitude increase in astrocytic rhod-2 ΔF/F0 and the duration of the response, presented as the average responses from each trial with at least 1 cell responding. paired t-test; [n = 13 trials baseline, 4 trials with drug (prazosin); 13 trials baseline, 11 trials with drug (atipamezole); 14 trials baseline, 14 trials with drug (propranolol)] Baseline data are presented as a pooled average. Data are shown as mean ± SEM.
3.4. Spontaneous Ca2+ waves are dependent upon LC-driven α1-AR activity
Widespread spontaneous astrocytic Ca2+ waves are also frequently observed in awake behaving mice [13–15]. Based on our findings that astrocytic Ca2+ responses to startle stimulation are dependent on LC release of NE driving the α1-AR, we next sought to determine the role of NE in mediating spontaneous activity. To do so, long duration scans (15 minutes) were taken at a scan rate of 0.33 Hz to minimize phototoxicity. Ca2+ responses were quantified as above, with waves being defined as a coordinated response of >1/3 of the visible cells. Large-scale waves were found to occur at a frequency of 0.233 ± 0.06 waves/minute (Fig. 4) in awake, restrained mice. Interestingly, both treatment with DSP4 and the application of prazosin abolished almost all of these coordinated waves (Fig. 4). These data strongly suggest that all widespread astrocytic Ca2+ waves in the awake adult cortex are driven by LC/NE-dependent activation of the α1-AR.
Fig. 4.
Spontaneous astrocytic Ca2+ activity in awake mice is dependent upon activation of the α1-AR. (A) Representative astrocytic rhod-2 fluorescence traces show frequent widespread and coordinated astrocytic (Ca2+)i waves in awake animals, which were almost completely abrogated by the α1-AR antagonist, prazosin, and LC toxin, DSP4. (B) Bar graph showing that prazosin and DSP4 treatment causes a significant decrease in the frequency of coordinated astrocytic [Ca2+]i waves (quantified by counting each time >1/3 of cells responded within the imaging field) ** p<0.01, * p<0.05, paired t-test (Control versus prazosin), unpaired t-test (control versus DSP4); [n=4 animals (prazosin, control), 5 animals (DSP4)] Data are shown as mean ± SEM.
4. Discussion
Using awake behaving mice we found that widespread release of the neuromodulator NE, and not local synaptic release of glutamate, is the primary mediator of cortical astrocytic Ca2+ signals. Our data indicate that the highly coordinated nature of astrocyte Ca2+ signaling in vivo is a direct result of the widespread release of NE from LC projections. The use of unanesthetized animals was critical for the observations reported here, as general anesthetics have been shown to suppress both evoked and spontaneous astrocytic Ca2+ transients [10, 14, 15], and are known to also suppress NE release from LC neurons [28].
This study built off of previous studies on the mechanisms underlying astrocytic Ca2+ signaling. While early work identified a myriad of receptors capable of eliciting IP3-dependent astrocytic [Ca2+]i transients, these studies consistently demonstrated astrocytic responses to α1-AR activation in a diverse set of brain regions – with positive responses being shown in both cultured striatal [29, 30], cerebral cortex [31], and hippocampal astrocytes [32], as well as in slice preparations from the hippocampus [32, 33], hypothalamus [34], and cerebellum [35]. Coupled to this lack of regional selectivity, the late expression of these receptors (after p8 [33]) fits well with more recent data showing that astrocytic responses to glutamate may be a developmental phenomenon [22]. Building off of earlier work in our lab showing that astrocytes are capable of responding through the α1-AR receptor in vivo [16], the present study sought to determine if the apparent global expression of the α1-AR receptor in glial cells throughout the brain was the primary mechanism driving astrocytic calcium activity in adulthood.
To directly test whether astrocytic Ca2+ signaling in awake mice was a result of LC activity, we took advantage of prior studies showing that LC displays phasic activity during the startle response [16, 18, 36, 37]. We found that tactile startle, elicited by puffing air at the tail or side of the animal, triggered Ca2+ increases in astroglia in the frontal cortex and parietal cortex of awake mice. In response to cortical micro-injection of α1-, α2- and nonselective β- AR agonists astrocytes promptly mobilized [Ca2+]i, confirming previous studies showing that astrocytes express the relevant G-protein coupled receptors (Gq Gi and Gs, respectively) [16, 38–40]. However, only the α1-adrenergic receptor blocker prazosin consistently suppressed astrocytic Ca2+ signaling evoked by the startle response. The key role of NE in eliciting astrocytes signals was further confirmed by the observation that mice treated with DSP4, an LC-specific neurotoxin, exhibited a marked decrease in cortical NE content and a concurrent attenuation of startle-induced astrocytic Ca2+ signaling [41].
Adrenergic neurons located in LC have long-ranging and highly branched unmyelinated projections throughout the cortex, cerebellum, and subcortical nuclei [38, 42]. Through volume transmission of NE from varicosities on these many projections the LC is believed to regulate arousal and state dependent activity such as attention and working memory [17, 43]. LC neurons display phasic discharges in response to novel or noxious sensory input such as the startle response, which consist of a burst of two to three action potentials followed by a longer lasting quiescent period of several hundred milliseconds [36, 44]. Phasic LC discharges have been linked to sudden changes in attention, and are critical for resetting cortical activity and rapidly shifting attention in response to unexpected events [43]. Additionally, during mildly aroused states LC neurons fire tonically at a frequency of around 2–4 Hz, and this continuous release of NE is believed to enhance sustained task performance [43, 45]. The LC is thus thought to optimize neuronal activity by altering receptive fields, increasing signal-to-noise and strengthening plasticity of synaptic neurotransmission [43]. Several lines of evidence indicate that astrocytes might mediate some of these adrenergic effects on neuronal signaling by virtue of their strategic location and numerous key homeostatic functions [16, 46–48]. Astrocyte [Ca2+]i mobilization can rapidly modulate synaptic function through release of gliotransmitters, effects on potassium buffering, changes in neurotransmitter recycling, and facilitation of neuro-vascular or neuro-metabolic coupling [1, 40]. The current study finds that the majority of evoked and spontaneous astrocytic Ca2+ increases in awake mice are mediated by the activation of α1-adrenergic receptors in response to stress-dependent LC firing.
Only a handful of studies have examined astrocytic Ca2+ signaling in awake behaving mice to date. In contrast to previous observations in anesthetized animals, these reports consistently describe the occurrence of widespread coordinated astroglial Ca2+ increases [13–15]. These Ca2+ signaling events were both temporally and spatially linked to voluntary motor activity and sensory stimulation, although their correlation was much weaker and less region-specific than accompanying neuronal signals [13, 14]. In cerebellum, Ca2+ transients in Bergmann glia often occurred after initiation of movement and were blocked by general anesthesia, TTX, and the broad-spectrum glutamate receptor antagonist D-γ-glutamylglycine (DγGG) [14]. The time course of the Ca2+ increases evoked during locomotion or by sensory stimulation in awake intact mice closely mimics the rises in astrocytic Ca2+ previously observed with direct LC stimulation or shown in our study using the startle response [13–16]. Conversely, the characteristic Ca2+ waves observed ex vivo or in young animals propagate slowly across the tissue, perhaps indicating they are driven by a different mechanism such as auto-/para-crine ATP or glutamate release [14, 15, 22]. We therefore speculate that the widespread release of NE from the LC is what mediates the coordinated astroglial Ca2+ signals observed in awake, adult mice.
An unexpected finding in this study was that sensory stimulation (whisker air puffing) only unreliably triggered Ca2+ signaling in awake mice. The success rate of whisker stimulation-induced Ca2+ signaling in awake mice was less than half that of startle stimulation. The delay by which whisker stimulation triggered increases in Ca2+ was also highly variable in awake mice [10–24s] and tended to occur at periods where the animal was moving and/or noticeably agitated (data not shown). In contrast, the delay in response to a startle stimulus or in anesthetized animals was more tightly restricted in for calcium waves in both frontal [2–7s] and parietal [1–5s] cortex, consistent with the delay seen in previous studies of anesthetized animals [11]. This may reflect that whisker stimulation often is perceived as a normal physiological stimulus, while the startle puff is perceived as noxious or novel, eliciting a phasic burst of LC activity in awake mice. While studies have shown that NE levels are decreased in sleep and anesthesia [28], startle responses are intact in lightly anesthetized animals [49]. We therefore suggest two possible explanations for the unreliable nature of our awake-whisker stimulation responses. First, as startle is intact under light anesthesia, and as startle is a highly adaptive response – habituating extremely rapidly – it is possible that whisker stimulation in untrained, anesthetized mice is sufficient to repeatedly induce a startle response, but not in awake mice. Second, astrocyte Ca2+ signals in anesthetized animals correlate closely with the neuronal response, and are primarily elicited by stimulation frequencies around 5 Hz [10]. Although our study does not directly address what pathway mediates astroglial Ca2+ signals in anesthetized mice, it is therefore tempting to speculate that these high frequencies of whisker stimulation represent a larger neurometabolic strain to the anesthetized cortical tissue and consequently elicit a hypoxic astrocytic Ca2+ response in vascular micro-watershed [50, 51]. In contrast to anesthetized animals, ours and others studies indicate that awake behaving mice often also exhibit spontaneous Ca2+ waves that do not appear directly linked to sensory input or neuronal activation [13–15]. However, this study shows that spontaneous Ca2+ increases are also potently inhibited by the α1-receptor antagonist prazosin, suggesting that the majority of astrocytic Ca2+ increases in awake mice require α1-receptor activation.
While the focus of the present study has been on determining the mechanisms driving widespread astrocytic Ca2+ waves, transient increases in [Ca2+]i in astrocytic processes and microdomains have been reported [4, 11, 52]. While our imaging was limited to ~1Hz, and therefore unable to detect transients lasting less than one second, these spontaneous Ca2+ transients have been shown to occur despite inhibition of excitatory postsynaptic activity, presynaptic release, GABA receptor activity, P2Y, and NMDA receptor activity [11, 15, 53, 54]. As such, these responses may be reflective of basic homeostatic mechanisms instead of direct responses to neuronal activity. Supporting this hypothesis, channels shown to be involved in transient events include the store-operated channel, TRPC1, which is responsible for replenishing intracellular stores [55] as well as the mechanosensitive TRPA1 channel [52]. This suggests that Ca2+ transients in microdomains may serve a housekeeping role in contrast to the broad neuromodulatory role investigated in this paper.
The observations reported here are of fundamental importance because they suggest that astrocytic Ca2+ signals in the intact, adult brain are primarily governed by the widespread release of neuromodulators, not local synaptic activity. Although we here identified NE as the primary mediator of astrocytic Ca2+ signaling in cortex, it is possible that other neuromodulators, including dopamine and acetylcholine may also mediate astrocytic Ca2+ signaling in other brain regions [20, 21]. The slow time frame of the action of neuromodulators (in the order of seconds) fits well with the temporal aspects of astrocytic Ca2+ signaling. Our observations suggest that astrocytes, rather than modulating individual synapses, participate in the classical neuromodulator functions such as tuning network activity and the basal responsiveness of neural circuits. Our study confirms and extends the observation that glutamate does not trigger Ca2+ signaling in the adult mouse cortex [22]. Current models of neuroglia signaling are largely based on data collected in hippocampal slices prepared from rodent pups and the conclusions derived from these studies may therefore be restricted to the developing brain. Combined, our observations call for a critical re-appraisal of what elicits astrocytic Ca2+ signaling and thereby of how astrocytes participate in complex neural computation.
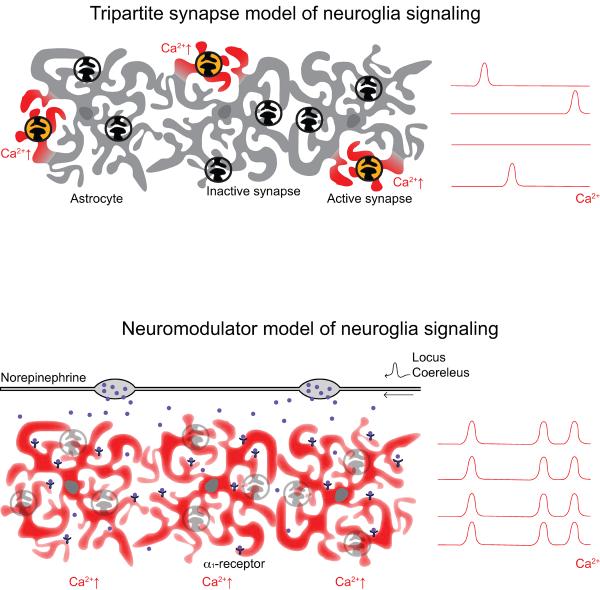
Fig. 5.
Diagram illustrating two possible mechanisms driving astrocytic Ca2+ responses in awake, head-restrained mice. (A) The tripartite synapse model: Here, astrocytes respond to local neuronal activity and neurotransmitter release with spotty, asynchronous increases in [Ca2+]i. (B) The neuromodulator model: Following novel or noxious behavioral stimuli, neuromodulators are released throughout the cortex to rapidly alter network connectivity. Our data suggests that in the startle response, the release of NE from the LC drives a coordinated increase in astrocytic [Ca2+]i in multiple brain regions. This suggests that coordinated astrocytic calcium transients in the awake brain are integrally related to widespread neuromodulation in response to behaviorally salient stimuli – placing them at the center of the network response to these stimuli.
Acknowledgements
This work was supported by the US National Institutes of Health (grants NS075177 and NS078304 to M.N.), Research Council of Norway (STORFORSK, NevroNor, and FUGE grants), Nordic Center of Excellence Program, Letten Foundation, and Fulbright Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nedergaard M, Verkhratsky A. Artifact versus reality--how astrocytes contribute to synaptic events. Glia. 2012;60:1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- [3].Benedetti B, Matyash V, Kettenmann H. Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex. J Physiol. 2011;589:1159–1172. doi: 10.1113/jphysiol.2010.203224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Castro MA, Chuquet J, Liaudet N, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- [5].Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- [6].Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- [8].Wang F, Smith NA, Xu Q, et al. Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hartl S, Heil JE, Hirsekorn A, Lohr C. A novel neurotransmitter-independent communication pathway between axons and glial cells. Eur J Neurosci. 2007;25:945–956. doi: 10.1111/j.1460-9568.2007.05351.x. [DOI] [PubMed] [Google Scholar]
- [10].Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- [11].Wang X, Lou N, Xu Q, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- [12].Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thrane AS, Rangroo Thrane V, Zeppenfeld D, et al. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A. 2012;109:18974–18979. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adams LM, Geyer MA. Effects of 6-hydroxydopamine lesions of locus coeruleus on startle in rats. Psychopharmacology (Berl) 1981;73:394–398. doi: 10.1007/BF00426474. [DOI] [PubMed] [Google Scholar]
- [19].Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Navarrete M, Perea G, Fernandez de Sevilla D, et al. Astrocytes mediate in vivo cholinerg-icinduced synaptic plasticity. PLoS Biol. 2012;10:e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takata N, Mishima T, Hisatsune C, et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun W, McConnell E, Pare JF, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- [24].Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thrane AS, Rappold PM, Fujita T, et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- [27].Szabadi E. Modulation of physiological reflexes by pain: role of the locus coeruleus. Front Integr Neurosci. 2012;6:94. doi: 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kushikata T, Yoshida H, Kudo M, Kudo T, Kudo T, Hirota K. Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br J Anaesth. 2011;107:924–929. doi: 10.1093/bja/aer303. [DOI] [PubMed] [Google Scholar]
- [29].Delumeau JC, Marin P, Cordier J, Glowinski J, Premont J. Synergistic effects in the alpha 1- and beta 1-adrenergic regulations of intracellular calcium levels in striatal astrocytes. Cell Mol Neurobiol. 1991;11:263–276. doi: 10.1007/BF00769039. [DOI] [PubMed] [Google Scholar]
- [30].Delumeau JC, Tence M, Marin P, Cordier J, Glowinski J, Premont J. Synergistic Regulation of Cytosolic Ca2+ Concentration by Adenosine and alpha1-Adrenergic Agonists in Mouse Striatal Astrocytes. Eur J Neurosci. 1991;3:539–550. doi: 10.1111/j.1460-9568.1991.tb00841.x. [DOI] [PubMed] [Google Scholar]
- [31].Shao Y, McCarthy KD. Receptor-mediated calcium signals in astroglia: multiple receptors, common stores and all-or-nothing responses. Cell Calcium. 1995;17:187–196. doi: 10.1016/0143-4160(95)90033-0. [DOI] [PubMed] [Google Scholar]
- [32].Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shelton MK, McCarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem. 2000;74:555–563. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- [34].Espallergues J, Solovieva O, Techer V, et al. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience. 2007;148:712–723. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- [35].Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by alpha1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci. 1996;8:1198–1208. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- [36].Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- [37].Tsuruoka M, Tamaki J, Maeda M, Hayashi B, Inoue T. The nucleus locus coeruleus/subcoeruleus contributes to antinociception during freezing behavior following the air-puff startle in rats. Brain Res. 2011;1393:52–61. doi: 10.1016/j.brainres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [38].Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- [39].Gupta MK, Papay RS, Jurgens CW, et al. alpha1-Adrenergic receptors regulate neurogenesis and gliogenesis. Mol Pharmacol. 2009;76:314–326. doi: 10.1124/mol.109.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. 2010;57:411–420. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dudley MW, Howard BD, Cho AK. The interaction of the beta-haloethyl benzylamines, xylamine, and DSP-4 with catecholaminergic neurons. Annu Rev Pharmacol Toxicol. 1990;30:387–403. doi: 10.1146/annurev.pa.30.040190.002131. [DOI] [PubMed] [Google Scholar]
- [42].Maeda T, Abe T, Shimizu N. Histochemical demonstration of anomatic monoamine in the locus coeruleus of the mammalian brain. Nature. 1960;188:326–327. doi: 10.1038/188326b0. [DOI] [PubMed] [Google Scholar]
- [43].Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- [44].Motaghi S, Sheibani V, Farazifard R, Joneidi H. Electrical stimulation of locus coeruleus strengthens the surround inhibition in layer V barrel cortex in rat. Neurosci Lett. 2006;401:280–284. doi: 10.1016/j.neulet.2006.03.034. [DOI] [PubMed] [Google Scholar]
- [45].Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- [46].Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012;32:2135–2145. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hertz L, Chen Y, Gibbs ME, Zang P, Peng L. Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr Drug Targets CNS Neurol Disord. 2004;3:239–267. doi: 10.2174/1568007043337535. [DOI] [PubMed] [Google Scholar]
- [48].Paspalas CD, Papadopoulos GC. Ultrastructural relationships between noradrenergic nerve fibers and non-neuronal elements in the rat cerebral cortex. Glia. 1996;17:133–146. doi: 10.1002/(SICI)1098-1136(199606)17:2<133::AID-GLIA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [49].Chan MH, Chiu PH, Lin CY, Chen HH. Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr Res. 2012;136:96–103. doi: 10.1016/j.schres.2012.01.024. [DOI] [PubMed] [Google Scholar]
- [50].Mathiesen C, Brazhe A, Thomsen K, Lauritzen M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab. 2013;33:161–169. doi: 10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- [52].Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- [54].Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- [55].Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]