Abstract
In recent work, O’Reilly and colleagues demonstrate relatively intact interhemispheric functional connectivity in a macaque brain in the absence of major commissural fibers. This work adds to a growing body of literature challenging the notion that structural and functional brain connectivity metrics are related in a straightforward manner.
As the effort to fully characterize the human connectome gets underway, some long-held assumptions about the nature of brain connectivity have come to be challenged. Structural connectivity is defined as the existence of white matter tracts physically interconnecting brain regions and is typically measured in vivo in humans using diffusion weighted imaging. Functional connectivity is a statistical measure of correlation or covariation between functional MRI (fMRI) signals obtained from discrete brain regions. Although it has long been observed that functional connectivity can be detected between brain regions in the absence of direct structural connectivity [1], it is still tempting to assume that one can ascertain the nature of structural connections present by examining the strength of functional connections and vice versa.
In the healthy adult brain, structural connectivity (measures of white matter integrity) and functional connectivity (measures of coupling strength) seem to show positive correlations, in that regions of the brain that are highly structurally interconnected tend to exhibit strong patterns of functional connectivity. However, developmental neuroimaging studies show that such straightforward relationships do not appear to hold in younger children. Along anterior–posterior tracts, such as the cingulum bundle, it has been shown that, whereas structural connectivity is positively correlated with functional connectivity in adults, no significant correlation between these two measures exists in children [2]. Likewise, for other intrahemispheric tracts, such as the fronto-occipital fasciculus, strong structure–function correlations can be seen in adults, whereas no such relationship is observed in children [3]. These studies collectively demonstrate that structure–function relationships mature and become stronger with age [4], and are not static.
O’Reilly and colleagues collected resting-state fMRI data from macaque monkeys pre- and post-commissurrotomy to test whether abolishing direct structural links would result in reduced interhemispheric connectivity [5]. They performed complete sections of the corpus callosum, the major fiber bundle interconnecting the two hemispheres, with or without concomitant sectioning of the anterior commissure, which comprises a smaller set of interhemispheric fibers. They found that indeed, complete commissurotomy produced substantial decreases in functional connectivity between the two cerebral hemispheres. Surprisingly, they also report that in a monkey where the anterior commissure was spared, the animal exhibited near normal levels of functional connectivity between the otherwise disconnected hemispheres (Figure 1A). This work complements evidence from studies of human split-brain [6] and callosal agenesis [7] patients, where intact, bilateral resting-state functional connectivity has been reported in the absence of major commissural fibers (Figure 1B).
Figure 1.
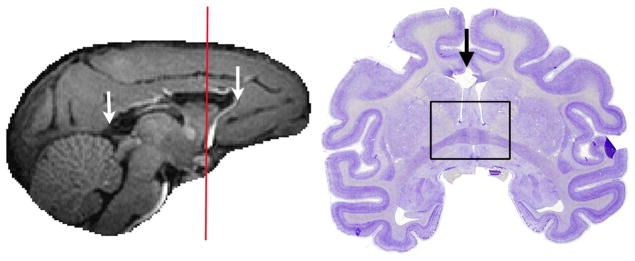
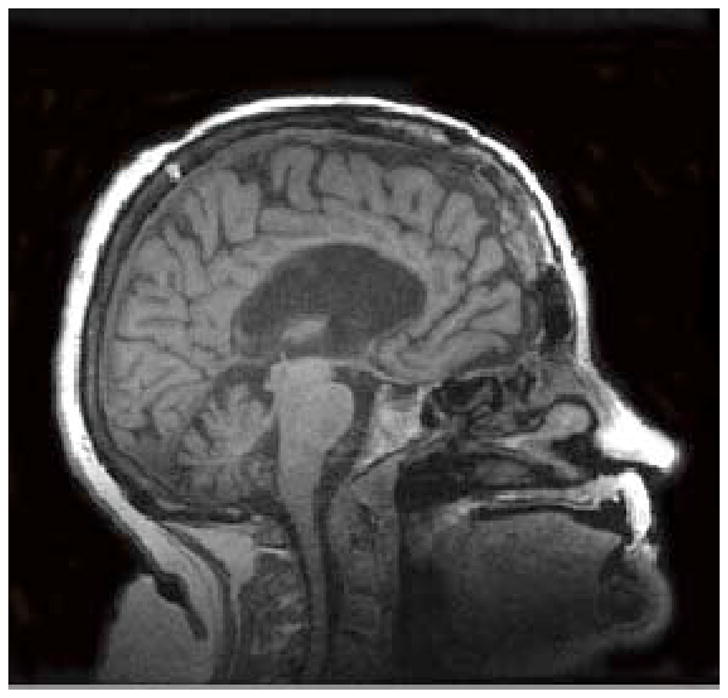
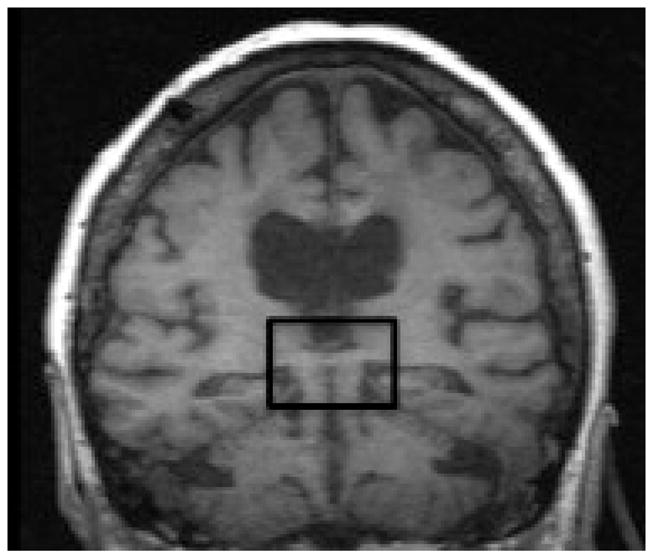
Degrees of interhemispheric disconnection. (A) Corpus callosum section with sparing of the anterior commissure in the macaque. Reproduced, with permission, from [5]. (B) Complete commissurotomy with sparing of the posterior commissure in the human [6]. In both macaque and human patients, relatively intact patterns of functional connectivity between the cerebral hemispheres can be observed, even after surgery that severed the major commissural fibers.
A strength of the current study is that the post-lesion measurements were conducted eight months after the experimental manipulation, so that acute postoperative effects on functional connectivity did not dominate the results. Previous studies in humans that reported dramatic reductions of interhemispheric connectivity following sectioning of the corpus callosum may have missed out on the opportunity to observe compensatory reorganization of functional connections that might take place in the months following surgery [8]. A further advance implemented by O’Reilly was the use of multidimensional scaling to visualize global changes in functional connectivity patterns post-surgery. This approach was used to illustrate the effects of corpus callosum lesion on the pattern of pairwise interhemispheric correlations, which occupied spaces farther apart as a result of the surgery.
The current results from O’Reilly and colleagues address two critical outstanding questions:
How malleable are functional connections in terms of their relationships with structural connections?
What is the functional significance of resting-state blood-oxygen-level dependent (BOLD) signals that give rise to functional connectivity?
In discussing the surprising findings of preserved interhemispheric functional connections in the macaque with the spared anterior commissure, the authors mention that the number and directness of structural connections may not be the deciding factor in enabling functional connections. In a case study of a human commissurotomy patient, it was also observed that sparing of small commissural fibers (in that case, the posterior commissure) seems sufficient to support normal levels of interhemispheric functional connectivity [6]. An alternative possible explanation for preserved interhemispheric connectivity is the existence of subcortical inputs, including those from the brainstem, that work to maintain interhemispheric coherence in the absence of direct structural links. A relevant study published several years ago reports data from a minimally conscious patient who suffered acute brainstem ischaemia [9]. This patient exhibited an absence of interhemispheric functional connectivity, which the authors interpreted as suggesting a role for ascending neurotransmitter systems in maintaining coherent low-frequency oscillations in bilateral homologous cortical regions.
As for the question of the functional significance of resting-state BOLD signals, evidence from the current work by O’Reilly et al. points in favor of a role for functional connectivity in maintaining the integrity of functional brain systems. It has previously been suggested that spontaneous activity serves to organize and coordinate neural activity [10]. Results from the macaque lesion study conducted by O’Reilly et al. provide further evidence that functional connections can dynamically reconfigure after structural connections are removed, in order to maintain previous levels of interhemispheric coordination, provided some residual or alternative pathways are present.
In sum, the study by O’Reilly and colleagues, together with the accumulating data from human patients, demonstrates the plasticity, flexibility, and reorganization capabilities of functional connections in the brain. It is quite clear that functional connectivity is not a static measure and can change as a result of development, as well as insult or injury to the brain. With all of these studies, important insights may be gained from long-term MRI follow-ups. Examining animals and human patients with different degrees of commissural disconnection at various stages pre- and post-surgery, as well as patients with brainstem and other subcortical lesions, may help us arrive at a better understanding of the complex relationships between structural and functional connectivity in the brain.
Acknowledgments
This work was supported by grant number K01MH092288 from the National Institute of Mental Health to L.Q.U.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Supekar K, et al. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uddin LQ, et al. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagmann P, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reilly JX, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci U S A. 2013;110:13982–13987. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uddin LQ, et al. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport. 2008;19:703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyszka JM, et al. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31:15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvador R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]


