Abstract
Inefficient thymic negative selection of self-specific T cells is associated with several autoimmune diseases, including type 1 diabetes (T1D). The factors that influence the efficacy of thymic negative selection, and the kinetics of thymic output of autoreactive T cells remain ill-defined. We investigated thymic production of β cell-specific T cells using a thymus transplantation model. Thymi from different aged NOD mice representing distinct stages of T1D, were implanted into NOD.scid recipients and the diabetogenicity of the resulting T cell pool examined. Strikingly, the development of diabetes-inducing β cell-specific CD4+ and CD8+ T cells was regulated in an age-dependent manner. NOD.scid recipients of newborn NOD thymi developed diabetes. However, recipients of thymi from 7 and 10 d-old NOD donor mice remained diabetes-free, and exhibited a progressive decline in islet infiltration and β cell-specific CD4+ and CD8+ T cells. A similar temporal decrease in autoimmune infiltration was detected in some but not all tissues of recipient mice implanted with thymi from NOD mice lacking expression of the autoimmune regulator transcription factor, which develop multi-organ T cell-mediated autoimmunity. In contrast, recipients of 10 d or older thymi lacked diabetogenic T cells but developed severe colitis marked by increased effector T cells reactive to intestinal microbiota. These results demonstrate that thymic development of autoreactive T cells is limited to a narrow time-window, and occurs in a reciprocal manner compared to colonic microbiota-responsive T cells in NOD mice.
INTRODUCTION
Events ongoing in the thymus play a critical role in shaping the repertoire of T cells (1, 2). Positive selection in the thymic cortex generates a pool of T cells restricted to self-MHC molecules. On the other hand, negative selection in the medulla of the thymus ensures that thymocytes reactive to self-antigens are purged via induction of apoptosis or anergy (3, 4). Medullary thymic epithelial cells (mTEC) (5-7) and dendritic cells (DC) (8-10) drive thymocyte negative selection by expressing and/or presenting self-antigens, respectively. A constellation of tissue-specific antigens (TSA) is expressed by mTEC (5, 11) and expression of many of these TSA is controlled by the autoimmune regulator (Aire) transcription factor (5, 12, 13). The parameters that influence the efficiency of thymic negative selection are ill-defined, but are believed to include the avidity of the interaction of thymocytes with mTEC and DC, intrinsic responses of thymocytes to apoptosis induction, and/or levels of thymic TSA expression and presentation (14-18).
Inefficient thymic negative selection has been associated with various T cell-mediated autoimmune diseases such as type 1 diabetes (T1D) (3, 19, 20). T1D in humans and rodent models, such as the NOD mouse, is characterized by the CD4+ and CD8+ T cell-mediated destruction of the insulin-producing β cells residing in the pancreatic islets of Langerhans (21). In NOD mice the diabetogenic response involves progressive insulitis in which T cells and other immune effectors infiltrate the islets over time. Insulitis is first detected at 3-4 wk of age and relatively few β cell autoantigens and epitopes are targeted by CD4+ and CD8+ T cells (22-25). By 12 wk of age, a late preclinical stage of T1D, the islets in NOD mice are heavily infiltrated, marked by effector T cells (Teff) targeting numerous β cell autoantigens and epitopes. Aberrant survival of islet resident Foxp3-expressing immunoregulatory CD4+ T cells (Foxp3+Treg) is then believed to promote a wave of robust β cell destruction and the onset of overt diabetes (26, 27). NOD mice also exhibit T cell autoimmunity to other tissues such as the thyroid (28, 29) and salivary gland (30), and low levels of colitis (31, 32) are detected suggesting general defects in mechanisms regulating autoimmune and inflammatory responses, respectively.
Currently, it is not known whether thymic production of autoreactive T cells in general, and diabetogenic T cells specifically, is a continuous versus time-limited process. The appearance of prevalent clones as autoimmunity progresses over time (33, 34) may for instance, reflect continued thymic production of autoreactive T cell clones albeit with distinct specificities (35). On the other hand, studies employing TCR transgenic mice specific for thymus-expressed neo-self antigens suggest that the efficiency of negative selection is reduced in younger animals (36, 37). A “window” may therefore exist early in life during which the development of autoreactive clones is enhanced, and the pool of anti-self T cells established. The latter has important implications for understanding the events that regulate thymic negative selection, in addition to establishing strategies to prevent T cell-mediated autoimmunity.
We investigated the ontogeny of autoreactive T cells using a thymus transplant approach. Immunodeficient NOD.scid recipients were implanted with thymus grafts from different aged NOD donor mice, and the pathogenicity of the resulting T cell pool assessed. Here we demonstrate that thymic production of organ-specific autoreactive Teff is limited to a 10 d period after birth, indicating that the efficacy of thymic negative selection is regulated in a temporal manner.
MATERIALS AND METHODS
Mice
NOD/LtJ, NOD.CB17-Prkdcscid/J (NOD.scid), and NOD.129S2(B6)-Airetm1.1Doi/DoiJ (NOD.Airenull) were originally purchased from The Jackson Laboratory (Bar Harbor). NOD.Cg-Tg(TcrαTcrβBDC2.5)1Doi/DoiJ (NOD.BDC2.5) mice have been previously described (38). NOD.BDC2.5 mice were bred with NOD.129P2(C)-Tcratm1Mjo/DoiJ (NOD.Cαnull) mice to generate NOD.BDC2.5.Cαnull mice. All mice were bred and maintained in specific pathogen-free facilities at the University of North Carolina at Chapel Hill. Mouse experiments were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Thymus transplantation and disease assessment
Thymic lobes from newborn (within 48 hr of birth) and various aged female NOD or NOD.Airenull mice were implanted under the kidney capsule of 6 wk-old female NOD.scid mice. NOD.scid recipients were monitored for diabetes by measuring blood glucose weekly; blood glucose levels ≥250 mg/dl (Abbott Diabetes Care Inc) for 2 consecutive measurements were scored as diabetic. The body weight of animals was measured weekly, and the development of weight loss was considered as the clinical onset of colitis.
Immunohistological analyses
Various tissues were fixed in 10% neutral buffered formalin (Fisher Scientific), paraffin embedded, and non-overlapping sections prepared and stained with hematoxylin and eosin (H&E) or Alcian blue. Severity of insulitis and colitis were graded as previously described (39, 40).
For thymus immunostaining, thymi were frozen in O.C.T. compound (Sakura Finetek USA) and 7μM sections cut. Sections were fixed and permeabilized in ice cold acetone/methanol for 5 min, then washed in PBS. Thymus sections were stained with UEA-1*biotin (Sigma Aldrich) and Troma-1 (anti-cytokeratin-8) (Developmental Studies Hybridoma Bank, University of Iowa) followed by Streptavidin*PE (eBiosciences) and AlexaFluor*488 goat anti-rat IgG (Invitrogen); each step was incubated 1hr at room temperature. Montage thymus images were taken using a Zeiss Axioplan 2 microscope 10x objective, and analyzed with Slidebook software (Intelligent Imaging Innovations).
T cell analyses
Cells isolated from the spleen, PLN, MLN and colon were stimulated with PMA (50ng/ml)/ionomycin (1μg/ml) in complete RPMI 1640 medium at 37°C for 4 to 5 hr and Brefeldin A was included in the culture for the last 2 hr of incubation. Cells were washed, stained with Abs specific for CD4 (GK1.5), CD8 (53-6.7), CD3 (2C11) and TCRβ (H57). After fixation and permeabilization using the Fixation/Permeabilization kit (eBioscience), cells were stained with Abs specific for intracellular IL-17 (TC11-18H10) and IFNγ (XMG1.2). Foxp3-expressing T cells were stained using an anti-mouse Foxp3 staining kit as per the manufacturer’s instructions (eBioscience). T cells were stained as previously described (41) with in house prepared soluble IAg7 multimers covalently linked to BDC mimetic or HEL peptides or H2Kd tetramers complexed with IGRP or HA peptides (42, 43). Data were acquired with CyAn flow cytometer (DakoCytomation) and analyzed using FlowJo (Tree Star Inc.) or Summit (DakoCytomation) software.
Single cell suspensions were prepared from NOD.scid thymus recipients 6 wks post-transplantation and 5-10×105 cells/well cultured in triplicate in complete RPMI1640 and 100μg/ml cecal bacterial lysate (CBL) (44) prepared from 6 wk-old NOD mice in 96-well round bottom plates at 37°C for 48 hr. The supernatants were harvested, and IFNγ and IL-17 measured using ELISA kits (eBiosciences) according to the manufacturer’s instructions.
For adoptive transfer experiments, splenocytes were harvested from NOD.scid thymus recipients 6 wk post-transplantation and CD4+ and CD8+ T cells purified by negative selection using mouse CD4 or CD8 T Cell Isolation Kits (Miltenyi Biotec). Female NOD.scid mice 6 wk of age were injected i.p. with 2×106 T cells per mouse, and then monitored for diabetes, body weight and rectal prolapse. In some experiments T cells were labeled prior to transfer with CellTrace Violet (Life Technologies) according to the manufacturer’s instructions.
Statistical analysis
Statistical tests were performed using prism 4.0 software (GraphPad). Body weight data were analyzed using the Two-way ANOVA. Kaplan-Meier Long-Rank Test was used to analyze the incidence of diabetes and colitis. Student’s t test and ANOVA were used for all other data.
RESULTS
Development of diabetes is restricted to a narrow postnatal thymic age
Thymic structural organization differs with ontogeny; the newborn thymus is characterized by small “islands” of medullary tissue whereas the medulla “coalesces” into a large, well organized structure with age (Fig. 1A). To assess the ontogeny of β cell-specific T cells, NOD.scid mice were engrafted under the kidney capsule with thymi from newborn and older NOD female mice, which represent different stages of T1D. Mature T cells were detected in the blood of thymus recipients as early as 1 wk post-implantation; by 4-6 wk ~40% of mononuclear cells consisted of CD4+ T cells in recipients of newborn and adult thymi (Fig. 1B). The reconstitution of CD8+ T cells, however, was delayed in adult versus newborn thymus recipients (Fig.1B).
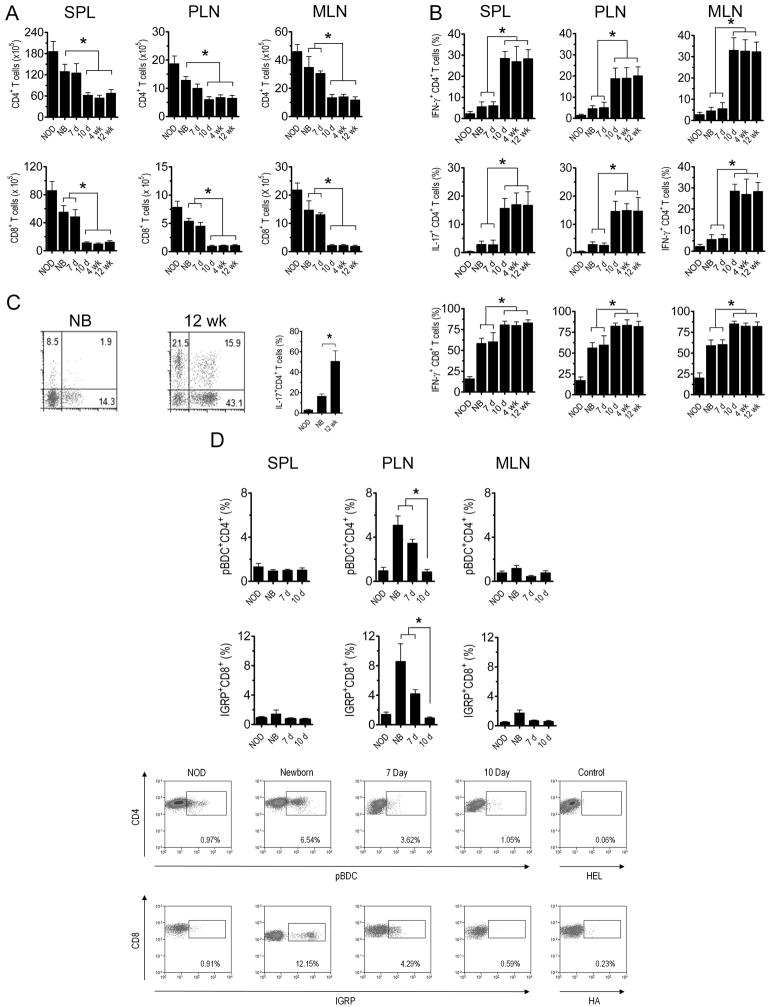
Figure 1. Reciprocal development of diabetes and colitis in NOD.scid recipients is dependent on the age of NOD donor thymus.
(A) Cytokeratin-8 (green, cortex) and UEA-1 (red, medulla) immunostaining of sections from newborn, 7 d-, 10 d-, and 4 wk-old NOD thymi; images were taken at 100X magnification. (B) NOD.scid mice were engrafted with thymi from newborn (NB) or 12 wk-old (n=10) NOD female donors and the frequency of CD4+ (left panel) and CD8+ (right panel; *p<10-4) T cells measured in peripheral blood. (C) Diabetes (left panel; *p<10-4 versus recipients of 7 d or older thymi; Kaplan-Meier Log Rank) and the frequency of insulitis (right panel; n=8; *p<10-4; Student’s t test) in thymus recipients; representative H&E staining of pancreatic sections of recipients of newborn (D) and 12 wk-old thymi (E). (F) Body weight (left panel; *p<10-4; 2-way ANOVA) and colitis scores (right panel; *p<10-4; Student’s t test) of recipients (n=8) of different aged thymi and control NOD.scid littermates. Representative Alcian blue stained colonic sections of recipients of newborn (G) and 12 wk-old (H) thymi. Representative H&E staining of thyroid (I,J) and salivary (K,L) glands from newborn (I,K) and 12 wk-old (J,L) thymus recipients. Error bars represent SEM.
Overt diabetes was detected in all NOD.scid mice receiving newborn thymi, and islets exhibited significant insulitis indicating T cell-mediated β cell destruction (Fig. 1C,D; Table I). Recipients of 7 and 10 d-old thymi remained diabetes-free; insulitis, however, was detected albeit at a reduced severity relative to newborn thymus recipients (Fig. 1C; Table I). In contrast, NOD.scid recipients of thymi from 2 wk and older NOD donors failed to develop both insulitis and diabetes (Fig. 1C,E; Table I). Similarly, the salivary gland and thyroid were infiltrated in recipients of newborn and 7 d thymi (Fig. 1I,K; Table I) but not 10 d or older thymi (Fig. 1J,L; Table I).
Table I.
T cell infiltration of organs in NOD.scid recipients of different aged NOD thymi.
| Pancreas | L. Intestine | Salivary Gland | Thyroid | |
|---|---|---|---|---|
| NB | 5/5 | 0/5 | 5/5 | 5/5 |
| 7 d | 5/6 | 0/6 | 6/6 | 6/6 |
| 10 d | 0/7 | 7/7 | 0/7 | 4/7 |
| 2 wk | 0/6 | 6/6 | 0/6 | 0/7 |
| 4 wk | 0/7 | 7/7 | 0/7 | 0/7 |
| 6 wk | 0/6 | 6/6 | 0/6 | 0/6 |
| 12 wk | 0/13 | 13/13 | 0/13 | 0/13 |
Thymi from various aged female NOD mice were transplanted under the kidney capsule of 6 week old NOD.scid recipients; L. Intestines denotes large intestines
Surprisingly, symptoms of colitis such as weight loss (Fig. 1F), diarrhea, and rectal prolapse were detected in NOD.scid mice implanted with 10 d and older thymi. Histologic examination of the gastrointestinal tract further revealed severe colitis (Fig. 1F; Table I) based on colonic hyperplasia, inflammation of the mucosal layer, significant infiltration of the lamina propria by mononuclear cells, and the depletion of goblet cells in the crypts (Fig. 1G,H). NOD.scid mice receiving newborn and 7 d thymi, however, exhibited only limited colitis and no weight loss or rectal prolapse (Fig. 1F; Table I).
Adoptive transfer experiments confirmed the organ-specific nature of pathogenic T cells developing in the thymus recipients. Splenocytes isolated from recipients of newborn and 7 d thymi readily transferred diabetes but not colitis to NOD.scid mice (Table II). In contrast, splenocytes from animals receiving 10 d or older thymi developed colitis but not diabetes (Table II). Together these findings demonstrate that thymic development of diabetogenic and colitogenic T cells are reciprocally regulated in an age-dependent manner.
Table II.
Disease incidence in NOD.scid mice adoptively transferred with splenocytes from different aged NOD thymus recipients.
| Diabetes | Colitis | |
|---|---|---|
| NB | 13/13 | 0/8 |
| 7 d | 5/6 | 0/6 |
| 10 d | 0/7 | 7/7 |
| 2 wk | 0/6 | 6/6 |
| 4 wk | 0/7 | 7/7 |
| 6 wk | 0/6 | 6/6 |
| 12 wk | 0/13 | 13/13 |
β cell-specific Teff are increased in the PLN of newborn thymus recipients
FACS analyses demonstrated that the number of CD4+ and CD8+ T cells was increased ~2 and ~3-fold, respectively, in the spleen, PLN, and MLN of recipients of newborn and 7 d old thymi versus 10 d and older thymi (Fig. 2A). Furthermore, Teff in these tissues (Fig. 2B), including the large intestine (Fig. 2C), consisted of IFNγ+ and/or IL-17+ CD4+ T cells, and IFNγ+ CD8+ T cells independent of thymus age. However, the frequency of IFNγ+ and IL-17+ CD4+ T cells and IFNγ+ CD8+ T cells was considerably elevated in recipients of 10 d or older thymi versus animals receiving newborn and 7 d-old thymi (Fig. 2B).
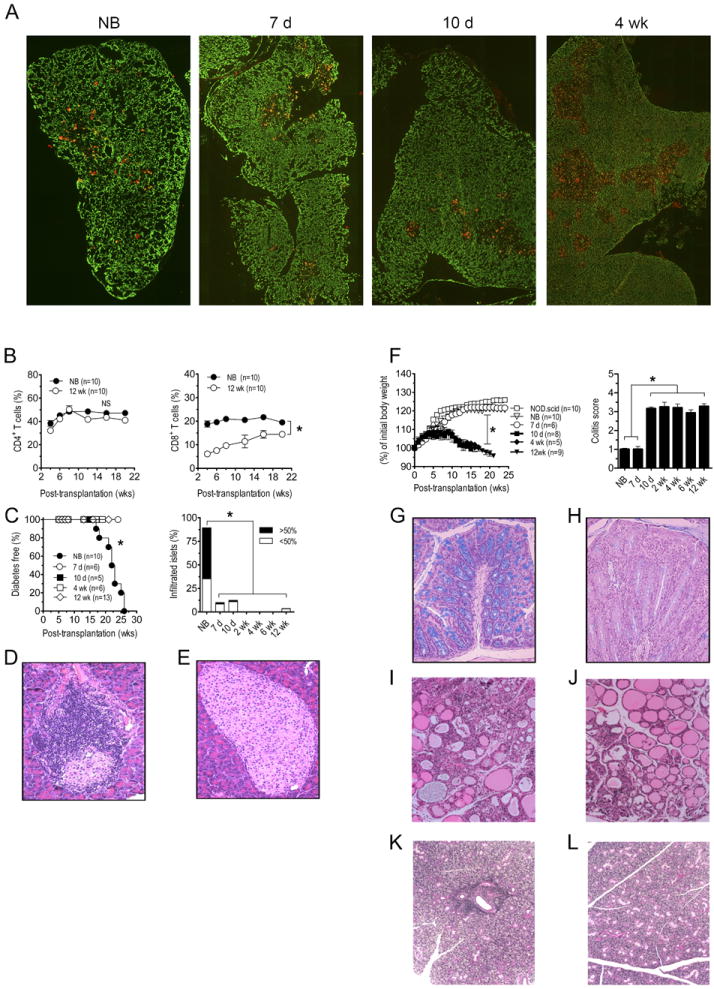
Figure 2. Distinct T cell phenotypes and specificities detected in recipients of different aged NOD donor thymi.
The spleen, PLN and MLN of different aged NOD thymus recipients 8 wk-post implantation and control 12 wk-old intact NOD female mice (NOD) were assessed via FACS for (A) CD3+CD4+ and CD3+CD8+ T cell numbers (n=7; *p<10-2; Student’s t test), (B) the frequency of intracellular IFNγ and IL-17 expressing CD4+ and CD8+ T cells (n=7; *p<0.005; Student’s t test). (C) Representative FACS plots (left and center) and frequency of IL-17 producing T cells isolated from the large intestines of newborn and 12 wk-old thymus recipients; gated on CD3+CD4+ T cells (n=7; *p<0.05; Student’s t test). (D) The frequency of IAg7-pBDC-binding CD4+ T cells and H2Kd-IGRP-binding CD8+ T cells with representative FACS plots from PLN isolated cells; gated on CD3+ and CD4+ or CD8+ T cells respectively (n=5; *p<0.05; Student’s t test). Error bars represent SEM.
The frequency of β cell-specific CD4+ and CD8+ T cells were measured in the spleen, PLN and MLN of newborn, 7 and 10 d thymus recipients. IAg7 and H2Kd multimers were used to detect CD4+ and CD8+ T cells that recognize a BDC mimetic peptide (IAg7-pBDC), and an islet-specific glucose-6-phosphatase catalytic subunit-related protein peptide (IGRP; H2Kd-IGRP), respectively. pBDC-specific CD4+ T cells (45) and IGRP-specific CD8+ T cells (24) are prevalent diabetogenic clonotypes in NOD mice. The highest frequency of CD4+ and CD8+ T cells staining with IAg7-pBDC and H2Kd-IGRP, respectively, were detected in the PLN of newborn thymus recipients (Fig. 2D). Notably, the frequency of IAg7-pBDC+ CD4+ T cells and H2Kd-IGRP+ CD8+ T cells progressively declined in the PLN of 7 d and 10 d thymus recipients (Fig. 2D).
To examine temporal changes in antigen reactivity of colitogenic T cells, splenocytes prepared from thymus recipients were stimulated with CBL and IL-17 and IFNγ secretion measured. IL-17 and IFNγ secretion in response to CBL was substantially increased in cultures from animals receiving thymi from 10 d and older NOD donors (Fig. 3A). CBL also induced IL-17 and IFNγ secretion in cultures prepared from newborn and 7 d thymus recipients albeit at significantly reduced levels (Fig. 3A), despite increased T cell numbers (Fig. 2A). CD4+ T cells alone from adult thymus recipients were sufficient to transfer diabetes (Fig. 3B). The importance of T cell reactivity to colonic microbiota in the development of colitis was further demonstrated in NOD.scid recipients of thymi from 6 wk-old NOD.BDC2.5 versus NOD.BDC2.5.Cαnull donors. Severe colitis developed in NOD.scid recipients of NOD.BDC2.5 thymus (Fig. 3C), in which T cells expressed both the BDC2.5 clonotypic and endogenous TCR. On the other hand, severity of colitis was markedly reduced in NOD.scid recipients when the specificity of NOD.BDC2.5.Cαnull CD4+ T cells was restricted to the β cell autoantigen chromogranin A (46) (Fig. 3C). Recipients of NOD.BDC2.5 (or NOD.BDC2.5.Cαnull) thymi, however, developed diabetes (Fig. 3D) indicating that colitis per se did not block β cell autoimmunity. In sum these results demonstrate that increased thymic development of β cell-specific T cells is restricted to a 7 d window after birth. Furthermore development of colitogenic T cells specific for microbiota is significantly increased at, and maintained after 10 d of age in NOD mice.
Figure 3. Colitogenic T cells respond to intestinal microbial antigens.
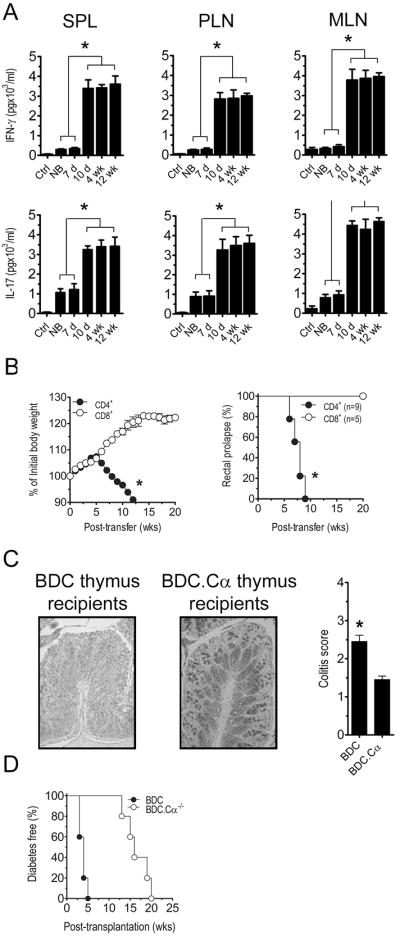
(A) Secretion of IFNγ and IL-17 by isolated T cells stimulated with CBL, as measured by ELISA, in the spleen, PLN, and MLN of recipients of different aged thymi 8 wk post-implantation (n=7; *p<10-4; Student’s t test). (B) Splenic CD4+ and CD8+ T cells isolated from NOD.scid thymus recipients 8 wk post-implantation, and adoptively transferred into NOD.scid mice, which were monitored for body weight loss (left panel; *p<0.05, 2-way ANOVA) and development of rectal prolapse (right panel; *p<0.05, 2-way ANOVA). (C) Representative colonic sections stained with Alcian blue, and colitis scores (*p<0.05; Student’s t test) and (D) diabetes incidence for recipients (n=5) of thymi from 6 wk-old NOD.BDC2.5 and NOD.BDC2.5Cαnull donors. Error bars represent SEM.
Thymus age-dependent development of diabetes is not due to changes in Foxp3+Treg and immunoregulation in the PLN of recipients
The above data indicated that thymic development of β cell-specific T cells declines with age resulting in reduced numbers of Teff to mediate diabetes. However, lack of β cell autoimmunity in recipients of post-newborn thymi may also be due to a reciprocal increase in Foxp3+Treg residing in the PLN to block expansion of diabetogenic Teff. To distinguish between these two possibilities, the frequency of Foxp3+Treg was assessed in the spleen, PLN and MLN of thymus recipients. Interestingly, PLN Foxp3+CD25+CD4+ T cells were increased in newborn and 7 d versus 10 d and older thymus recipients, whereas the frequency of spleen and MLN resident Foxp3+CD25+CD4+ T cells was similar independent of thymic age (Fig. 4A). To assess the immunoregulatory activity in the PLN, NOD.BDC2.5 CD4+ T cells were transferred into recipients of newborn and 4 wk thymi, and proliferation measured. No marked difference was detected in the level of NOD.BDC2.5 CD4+ T cell proliferation between the respective thymus recipients (Fig. 4B). These results indicate that the block in β cell autoimmunity in thymus recipients is not due to an increase in the Foxp3+Treg pool or immunoregulation in the PLN, but attributed to reduced numbers of β cell antigen-specific T cells.
Figure 4. Development of diabetes is not due to increased Foxp3+Treg frequency or suppression.
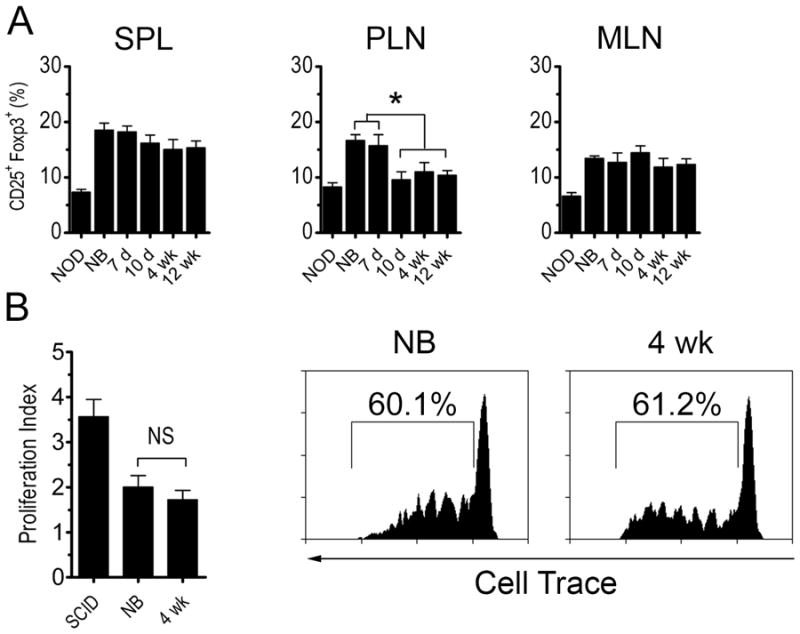
(A) Frequency of Foxp3+CD25+CD4+ T cells in recipients of different aged NOD thymi (n=7) 6 wk-post implantation or 12 wk-old intact NOD female mice (NOD). (B) In vivo proliferation of cell trace-labeled NOD.BDC2.5 CD4+ T cells 4 d post-transfer in the PLN of recipients of newborn and 4 wk-old NOD thymus 6 wk post-implantation or unmanipulated NOD.scid controls. Data is reported as proliferation index (left) with representative FACS histograms gated on CD3+CD4+cell trace+ T cells (right) (n=5). Error bars represent SEM.
The temporal development of autoreactive T cells occurs in the absence of Aire expression
Regulation of TSA expression by Aire may contribute to the temporal thymic development of autoreactive T precursors. To test this possibility the pathology of NOD.scid recipients transplanted with thymi from different aged NOD mice deficient in Aire expression (NOD.Airenull) was investigated. NOD.Airenull mice lack β cell autoimmunity but develop multi-organ T cell-mediated inflammation (47), which includes exocrine pancreatitis. Tissues normally targeted in NOD.Airenull mice were also infiltrated in NOD.scid recipients of newborn NOD.Airenull thymi (Fig. 5A, Table III). Strikingly, a progressive decline in T cell infiltration of the exocrine pancreas and salivary glands was detected in recipients of 7 and 10 d old NOD.Airenull thymi, and no infiltration of these tissues was observed in 4 wk-old thymus recipients (Fig. 5A, Table III). On the other hand, T cell infiltration continued to be detected in the eyes, ovaries, stomach and lungs of animals implanted with day 7 and older NOD.Airenull thymi (Fig. 5A,B). The severity of colitis in the recipients, however, was limited regardless of NOD.Airenull thymi age (Fig. 5B). These results demonstrate that temporal regulation of thymic development of autoreactive T cells can occur in the absence of Aire expression albeit for certain tissue-specificities and not others.
Figure 5. Temporal development of autoreactive T cells is independent of Aire in some organs.
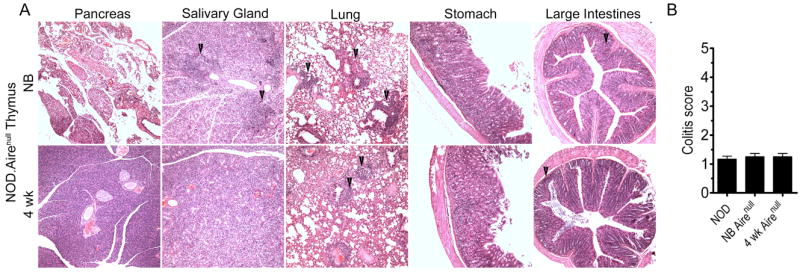
NOD.scid mice received thymi from different aged NOD.Airenull donors. (A) Representative H&E stained sections of organs; arrows highlight areas of infiltration. (B) colitis scores of different aged NOD.Airenull thymus recipients (n=5). Error bars represent SEM.
Table III.
Relative T cell infiltration of organs in NOD.scid recipients of different aged NOD.Airenull thymi.
| NB Airenull | 7 d Airenull | 10 d Airenull | 4 wk Airenull | |
|---|---|---|---|---|
| Pancreas | +++ | ++ | + | - |
| Ovary | +++ | +++ | +++ | ++ |
| L. Intestines | + | + | + | + |
| Stomach | ++ | ++ | ++ | ++ |
| Lung | ++ | ND | ND | ++ |
| Salivary Gland | ++ | ++ | + | - |
| Cecum | + | + | + | + |
| S. Intestines | + | + | + | + |
| Eyes | +++ | ND | ND | +++ |
Infiltration of various organs in NOD.scid recipients of various aged thyme from NOD.Airenull donors. “+++” = heavy/complete T cell infiltration; “++” = moderate T cell infiltration; “+” = mild T cell infiltration; “-“ indicates an absence of infiltration; ND = “not observed”; L. Intestines denotes large intestines; S. Intestines denotes small intestines
DISCUSSION
The dynamics of and the parameters that influence thymic output of autoreactive T cells are poorly understood. To address these issues a thymus transplant model system was employed. This approach provides a “snapshot” of the specificities of autoreactive T cells produced in the thymus at a given age.
We demonstrate that thymic production of β cell-specific T cells is regulated in a temporal manner in NOD mice. Insulitis and diabetes developed in recipients of NOD newborn thymi (Fig. 1C,D), which corresponded with an increased frequency of PLN-resident pBDC-specific CD4+ and IGRP-specific CD8+ T cells (Fig. 2D), 2 major clonotypes associated with the progression of β cell autoimmunity in NOD mice. These results are consistent with findings demonstrating that NOD mice develop diabetes with normal kinetics and incidence despite thymectomy 3 d after birth, indicating that a sufficient pool of diabetogenic T cells is established early in ontogeny (48). Interestingly, autoimmunity has been reported in immunodeficient children with congenital athymia receiving a human infant thymus transplant (49). Noteworthy was the progressive decline in insulitis (Fig. 1C) and the frequency of β cell-specific T cells in recipients of 7 and 10 d old NOD thymi (Fig. 2D). This reduction in β cell-specific T cells was not due to a reciprocal increase in the pool of PLN-resident Foxp3+Treg or enhanced tissue-specific immunoregulation that would be expected to block the expansion of diabetogenic Teff (Fig. 4). Furthermore, colitis per se had no suppressive effect on β cell autoimmunity. For instance, both diabetes and colitis were detected in NOD.scid mice receiving NOD.BDC2.5 thymi (Fig. 3C,D) or a mixture of splenocytes from colitogenic and diabetic donor animals (Fig. S1), showing that the progression of the 2 pathologies is independent and not mutually exclusive. Together these findings indicate that the lack of insulitis and diabetes, and reduced frequency of diabetogenic T cells in recipients of post-newborn thymi is the result of diminished thymic production of β cell-specific T cells. Importantly, thyroiditis and sialitis detected in newborn thymus recipients were also reduced in recipients of 7 and 10 d-old thymi (Fig. 1I-L, Table I) demonstrating that thymic production of autoreactive T cells in general is regulated in an age-dependent manner.
To explain the temporal decline in autoreactive T cell production we favor a model in which the efficacy of thymic negative selection increases during postnatal life. Several mutually nonexclusive possibilities may account for this effect. Reduced expression of TSA due to limiting Aire expression may lead to inefficient thymic negative selection of autoreactive T cells in the neonatal thymus (12, 16, 18). Indeed, both the frequency of Aire-expressing mTEC, and mRNA expression of Aire-dependent TSA genes such as Ins2 are reduced in thymi from newborn versus older NOD mice (R.T. & C.J.K; unpublished data). Furthermore, Guerau-de-Arellano and colleagues reported that induced expression of Aire and corresponding TSA by mTEC during embryonic life and up to 21 d after birth was critical to block the multi-organ autoimmunity typical of NOD.Airenull mice (50). Our observation that recipients developed significant infiltration of the ovaries, stomach, lungs and eyes implanted with newborn and older NOD.Airenull thymi (Fig. 5, Table III) supports a role for Aire in the temporal development of these tissue-specific T cells. Strikingly, however, exocrine pancreatitis and sialadenitis failed to develop in recipients of thymi from 10 d or older NOD.Airenull donors (Fig. 5, Table III) suggesting that Aire-dependent TSA expression alone does not account for the observed temporal production of autoreactive T cells specific for these tissues. Age-dependent changes in the stimulatory capacity of the thymic APC pool, due to the number, composition and/or maturation status of mTEC and thymic DC may contribute to the efficiency of thymic negative selection (18, 50-52). Alternatively, the development of autoreactive T cells may reflect intrinsic properties of T cell progenitors residing in the thymus during ontogeny. For example, studies have shown that hematopoietic stem cells that seed the thymus at various stages of ontogeny give rise to T cells with distinct properties and antigen specificity (53-56). The latter may influence the affinity and/or cross-reactivity or promiscuity of TCR specific for TSA-derived epitopes that are either Aire- dependent or -independent. Finally, major changes in the structural organization of the medulla seen during postnatal life may impact the efficiency of negative selection. The rudimentary thymus of newborn animals (Fig. 1A) may limit thymocyte interactions with medullary resident APC thereby reducing the efficiency of negative selection, particularly if given TSA are expressed and presented at relatively low levels. Efforts are ongoing to delineate what is likely to be a complex interplay between multiple events that regulate the temporal efficiency of thymic negative selection.
An interesting observation made in this study was that thymic development of colitogenic T cells was also temporally regulated. Negligible colitis was detected in recipients of newborn and 7 d NOD thymi (Fig. 1F). However, severe colitis developed in recipients of thymi from NOD donors 10 d of age and older (Fig. 1F) which was marked by an increased number and frequency of IL-17 and IFNγ producing CD4+ T cells specific for CBL (Fig. 2B). Unlike β cell-specific T cells, which were selectively increased in the PLN (Fig. 2D), CBL reactivity was readily detected in all tissues examined (Fig. 3A), likely reflecting systemic trafficking of a relatively large pool of colitogenic Teff. Recognition of commensal microbiota antigen was necessary for colitis; recipients of thymi from NOD.BDC2.5.Cαnull donors which express chromogranin A-specific TCR (46) failed to develop significant colitis (Fig. 3B). In contrast, increased colitis was detected in animals receiving thymi from adult NOD.BDC2.5 mice, which co-express transgenic and endogenous TCR (Fig. 3B). These findings demonstrate that in addition to autoreactive T cells, the development of T cells specific for exogenous (e.g. microbial) antigens is regulated temporally, but in a reciprocal relationship to autoimmune T cells. In this instance, production of T cells specific for microbial antigens is enhanced after postnatal life, suggesting an increase in the efficiency of thymocyte positive selection. In addition, colitis was detected in NOD.scid recipients of thymi from 12 wk-old C57BL/6 mice congenic for H2g7 (Fig. S2), suggesting that thymic development of colitogenic T cells is independent of the NOD genotype. These results further support the prevailing concept that chronic immune-mediated colitis is driven by microbial-responsive T cells rather than autoimmune responses (57).
Since T cell reconstitution occurred under identical conditions in NOD.scid recipients, lymphopenic expansion cannot explain the temporal development of autoreactive and colitogenic T cell repertoires. It is likely, however, that lymphopenia favored the differentiation of pathogenic Teff driving autoimmunity and colitis. Development of autoreactive T cells that is largely restricted to early ontogeny further underscores the role of peripheral mechanisms in maintaining life-long self-tolerance. Our findings may also in part explain the long-lasting and robust tolerance typically induced by administration of self-antigen to neonates (58-60). Here, deletion early in ontogeny would be expected to permanently purge the corresponding autoreactive clonotype(s) from the immune system.
In conclusion, our results demonstrate that thymic development of T cells specific for self and foreign antigens is tightly regulated over a short ontogenic time window. These findings also indicate that the pool of β cell (and other tissue)-specific T cells is to a large extent established early in ontogeny. A number of coordinated events within the thymus are likely to contribute to the temporal development of autoreactive and bacterial antigen-responsive T cells in a reciprocal manner. Exploiting the use of the thymus transplant model provides a novel approach to better define these events.
Supplementary Material
Acknowledgments
The Troma-1 hybridoma developed by Philippe Brulet and Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
This work was supported by grants from the National Institutes of Health (R01 AI083269, R01 DK53347, P30 DK34987) and the Juvenile Diabetes Research Foundation (33-2008-412). N.S. and C.K were supported by a NIH training grant (5T32 AI07273).
References
- 1.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med. 2002;196:1175–1188. doi: 10.1084/jem.20020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 5.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 6.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 8.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 9.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 12.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Kyewski B, Peterson P. Aire, master of many trades. Cell. 2010;140:24–26. doi: 10.1016/j.cell.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 15.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 17.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–213. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 18.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 21.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 28.Bernard NF, Ertug F, Margolese H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes. 1992;41:40–46. doi: 10.2337/diab.41.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Damotte D, Colomb E, Cailleau C, Brousse N, Charreire J, Carnaud C. Analysis of susceptibility of NOD mice to spontaneous and experimentally induced thyroiditis. Eur J Immunol. 1997;27:2854–2862. doi: 10.1002/eji.1830271117. [DOI] [PubMed] [Google Scholar]
- 30.Thompson C, Jacobsen H, Pomeranz Krummel D, Nagai K, Cooke A. Non-depleting anti-CD4 antibody not only prevents onset but resolves sialadenitis in NOD mice. Autoimmunity. 2004;37:549–554. doi: 10.1080/08916930400021352. [DOI] [PubMed] [Google Scholar]
- 31.Alam C, Valkonen S, Palagani V, Jalava J, Eerola E, Hanninen A. Inflammatory tendencies and overproduction of IL-17 in the colon of young NOD mice are counteracted with diet change. Diabetes. 2010;59:2237–2246. doi: 10.2337/db10-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara M, Nagayama Y, Ichikawa T, Yu L, Eisenbarth GS, Abiru N. The effect of regulatory T-cell depletion on the spectrum of organ-specific autoimmune diseases in nonobese diabetic mice at different ages. Autoimmunity. 2011;44:504–510. doi: 10.3109/08916934.2010.548839. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 34.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 36.Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamurthy B, Chee J, Jhala G, Fynch S, Graham KL, Santamaria P, Morahan G, Allison J, Izon D, Thomas HE, Kay TW. Complete diabetes protection despite delayed thymic tolerance in NOD8.3 TCR transgenic mice due to antigen-induced extrathymic deletion of T cells. Diabetes. 2012;61:425–435. doi: 10.2337/db11-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 39.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 41.Yi Z, Diz R, Martin AJ, Morillon YM, Kline DE, Li L, Wang B, Tisch R. Long-term remission of diabetes in NOD mice is induced by nondepleting anti-CD4 and anti-CD8 antibodies. Diabetes. 2012;61:2871–2880. doi: 10.2337/db12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, He Q, Garland A, Yi Z, Aybar LT, Kepler TB, Frelinger JA, Wang B, Tisch R. beta cell-specific CD4+ T cell clonotypes in peripheral blood and the pancreatic islets are distinct. J Immunol. 2009;183:7585–7591. doi: 10.4049/jimmunol.0901587. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Yi Z, Wang B, Tisch R. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol. 2009;183:4809–4816. doi: 10.4049/jimmunol.0901616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 46.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gagnerault MC, Lanvin O, Pasquier V, Garcia C, Damotte D, Lucas B, Lepault F. Autoimmunity during thymectomy-induced lymphopenia: role of thymus ablation and initial effector T cell activation timing in nonobese diabetic mice. J Immunol. 2009;183:4913–4920. doi: 10.4049/jimmunol.0901954. [DOI] [PubMed] [Google Scholar]
- 49.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135:236–246. doi: 10.1016/j.clim.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fornari TA, Donate PB, Macedo C, Marques MM, Magalhaes DA, Passos GA. Age-related deregulation of Aire and peripheral tissue antigen genes in the thymic stroma of non-obese diabetic (NOD) mice is associated with autoimmune type 1 diabetes mellitus (DM-1) Mol Cell Biochem. 2010;342:21–28. doi: 10.1007/s11010-010-0464-z. [DOI] [PubMed] [Google Scholar]
- 52.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 53.Bogue M, Candeias S, Benoist C, Mathis D. A special repertoire of alpha:beta T cells in neonatal mice. Embo J. 1991;10:3647–3654. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH, Weissman IL. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 55.Kikuchi K, Kondo M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci U S A. 2006;103:17852–17857. doi: 10.1073/pnas.0603368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 58.Billingham RE, Brent L. Acquired tolerance of foreign cells in newborn animals. Proc R Soc Lond B Biol Sci. 1956;146:78–90. doi: 10.1098/rspb.1956.0073. [DOI] [PubMed] [Google Scholar]
- 59.Posselt AM, Barker CF, Tomaszewski JE, Markmann JF, Choti MA, Naji A. Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science. 1990;249:1293–1295. doi: 10.1126/science.2119056. [DOI] [PubMed] [Google Scholar]
- 60.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.