Abstract
The endogenous opioid system is expressed throughout the brain reinforcement circuitry, and plays a major role in reward processing, mood control and the development of addiction. This neuromodulator system is composed of three receptors, mu, delta and kappa, interacting with a family of opioid peptides derived from POMC (β-endorphin), preproenkephalin (pEnk) and preprodynorphin (pDyn) precursors. Knockout mice targeting each gene of the opioid system have been created almost two decades ago. Extending classical pharmacology, these mutant mice represent unique tools to tease apart the specific role of each opioid receptor and peptide in vivo, and a powerful approach to understand how the opioid system modulates behavioral effects of drugs of abuse. The present review summarizes these studies, with a focus on major drugs of abuse including morphine/heroin, cannabinoids, psychostimulants, nicotine or alcohol. Genetic data, altogether, set the mu receptor as the primary target for morphine and heroin. In addition, this receptor is essential to mediate rewarding properties of non-opioid drugs of abuse, with a demonstrated implication of β-endorphin for cocaine and nicotine. Delta receptor activity reduces levels of anxiety and depressive-like behaviors, and facilitates morphine-context association. PEnk is involved in these processes and delta/pEnk signaling likely regulates alcohol intake. The kappa receptor mainly interacts with pDyn peptides to limit drug reward, and mediate dysphoric effects of cannabinoids and nicotine. Kappa/dynorphin activity also increases sensitivity to cocaine reward under stressful conditions. The opioid system remains a prime candidate to develop successful therapies in addicted individuals, and understanding opioid-mediated processes at systems level, through emerging genetic and imaging technologies, represents the next challenging goal and a promising avenue in addiction research.
Keywords: opioid receptors, opioid peptides, knockout mice, drugs of abuse, addiction, reward
Introduction
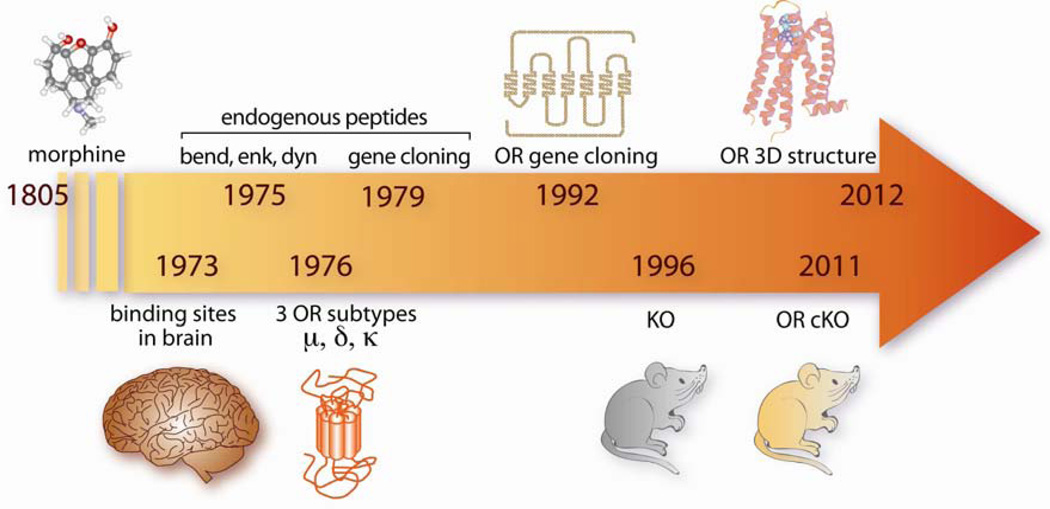
Opiates, including morphine, are potent analgesic compounds and represent major therapeutic drugs to treat severe pain. In addition, opiates induce strong euphoria and repeated exposure often leads to dependence and eventually opioid addiction. Milestones in discoveries of the opioid system are shown in Figure 1. Morphine, the most active component of opium, was isolated in 1805 by Serturner. Opioid receptors were described in 1973, based on opioid binding sites referred as mu, delta and kappa (Pert and Snyder, 1973; Simon et al., 1973; Terenius, 1973). Met- and Leu- enkephalins were characterized in 1975, and altogether three families of endogenous opioid peptides precursors (pre-proenkephalin pEnk, pre-prodynorphin pDyn and proopiomelanocortin POMC) were identified in the late 70’s (Goldstein et al., 1979; Guillemin et al., 1976; Hughes et al., 1975; Li and Chung, 1976). Genes encoding opioid peptide precursors were isolated in the early 80’s (pEnk (Comb et al., 1982; Gubler et al., 1982; Noda et al., 1982); pDyn (Kakidani et al., 1982); POMC (Nakanishi et al., 1979). The first opioid receptor gene, encoding delta receptors, isolated by expression cloning in 1992 (Evans et al., 1992; Kieffer et al., 1992), and the two other receptor genes were cloned by homology (Mestek et al., 1995; Simonin et al., 1994; Simonin et al., 1995). Opioid receptors belong to the superfamily of G-protein coupled receptors (Kieffer, 1995; Trigo et al., 2010), with coupling to Gi/Go proteins (Law et al., 2000), and their structure was solved at high-resolution by X-Ray crystallography (Granier et al., 2012; Manglik et al., 2012; Wu et al., 2012). The opioid system is broadly expressed in the nervous system, particularly within the neurocircuitry of addiction (Koob and Volkow, 2010). Both peptides and receptors are present in areas associated with reward, motivation, learning and stress (Le Merrer et al., 2009; Mansour et al., 1995), and therefore plays a key role in many aspects of addictive behaviors (see (Lutz and Kieffer, 2013).
Figure 1. Milestone discoveries in opioid research.
Opium is extracted from poppy seeds (Papaver somniferum) and consumed for several thousand years to relieve pain and produce euphoria. Morphine, the most active alkaloid extracted from opium, was the first opioid to be isolated (1805). Opiates act on the nervous system, where they specifically activate receptors (1973), which are normally stimulated by a family of endogenous neurotransmitters, β-endorphin, enkephalins and dynorphins (1975). Several opioid receptors subtypes were further described based on receptor pharmacology (1976). Gene cloning occurred in early 80’s for peptide precursors (1979) and early 90’s for opioid receptors (1992). Opioid receptors genes (Oprm1, Oprd1 and Oprk encoding mu-, delta- and kappa-opioid receptor; pomc, pEnk and pDyn encoding peptide precursors) were targeted in mice by homologous recombination, and mice lacking the mu receptor and enkephalins were available first (1996). Recently, refinement of in vivo targeted mutagenesis techniques led to the first conditional knockout mouse for the opioid system, with a delta receptor deletion restricted to primary afferent nociceptive neurons (2011). The 3D crystal structure of all three receptors was elucidated very recently (2012). OR: opioid receptor, KO: knockout mouse, cKO: conditional knockout mouse. Detailed references are in the text.
All the known drugs of abuse activate reinforcing brain circuitries (Koob and Volkow, 2010). These drugs, however, recruit distinct molecular targets in the brain and show notable differences in their pharmacological actions, which has led researchers and physicians to classify them into distinct groups. Opiates, acting directly at opioid receptors, produce sedative effects in addition to euphoria, and are therefore known as narcotics. In contrast, psychostimulants that include cocaine, amphetamine and methamphetamine, provide immediate euphoria with a feeling of intellectual and physical power, and indifference to pain and fatigue, mainly via direct stimulation of dopaminergic transmission. Nicotine, a major component of tobacco, is also considered a mild stimulant and α-nicotinic receptors constitute their molecular target. Relaxing and euphoric sensations searched by marijuana users arise from the stimulation of CB1 receptors by cannabinoids, including the most active component delta9-tetrahydrocannabinol (THC). Finally, a most widely abused licit drug is alcohol, targeting several receptors and ion channels in the brain and representing a major health problem (Hyman, 2008). It is now well established that the endogenous opioid system plays an important role in acute and chronic effects of all these drugs. The exact nature of opioid receptor or peptide involved has been clarified over the years, largely owing to genetic approaches, and this large set of data is overviewed here.
Drug abuse is a major threat to public health (Compton et al., 2007; Gustavsson et al., 2011). For 40 years, NIDA has supported extensive research towards understanding molecular bases of drug abuse (Everitt et al., 2008; Nestler, 2005; Pierce and Wolf, 2013), and developing innovative strategies for treatment (Heilig et al., 2011; Kalivas and Volkow, 2011; Koob et al., 2009; Pierce et al., 2012; Volkow and Skolnick, 2012). We are extremely grateful to NIDA for long-standing support to our efforts in developing genetic mouse models for opioid research. Knockout (KO) mice for the opioid system, developed by others and us, have been extensively studied and broadly shared within our research community. In this review, we have gathered data from these KO mice that have accumulated in the past fifteen years (for previous reviews see (Contet et al., 2004; Kieffer and Gaveriaux-Ruff, 2002), and enabled identification or clarification of the specific role of each component of the opioid system in drug reward and addiction. Note that the opioid system plays a central role in pain processing, but this particular aspect will not be reviewed here (see recent reviews in (Bodnar, 2012; Gaveriaux-Ruff and Kieffer, 2011; Woolf, 2011).
We will first summarize behavioral responses of null mutant mice to opiates, then overview reports investigating the effects of other drugs of abuse, including cannabinoids, psychostimulants (cocaine, MDMA, amphetamine), nicotine and alcohol in these mice, and finally conclude on the respective roles of opioid peptides and receptors, and perspectives of opioid research in the area of drug abuse. Whereas data from receptor KO mice have unambiguously clarified receptor roles in vivo, data from peptide KO mice are by essence more complex (low receptor selectivity) and the latter mutants still deserve further investigations.
Behavioral measures in the mouse
At present, behavioral paradigms to model distinct aspects of addiction (for a review see (Everitt et al., 2008; Koob et al., 2009) in rodents remain limited, particularly for mice (see Box). Several well-described behavioral models in rats have nevertheless been successfully adapted to mice, and largely applied to mutant animals. Among these, voluntary/operant testing (two-bottle choice, TBC and self-administration, SA) addresses some aspects of binge intoxication and/or excessive consumption, and conditioned place preference (CPP) examines drug reward. Withdrawal and the negative effect of drug abstinence can be revealed by conditioned place aversion (CPA) and drug-induced physical withdrawal, and preoccupation/anticipation can be tested by drug-, cue- or stress-induced reinstatement of CPP. Finally locomotor activation by drugs of abuse, and sensitization to this effect upon repeated treatment, are also typical responses studied in rodents although no human correlate exists for this behavior. Data from all these tests are summarized in Tables 1 to 6, and main findings are summarized below.
BOX: Behavioral measures in the mouse.
Behavioral responses examined in mutant mice (Tables 1 to 6) are briefly explained below.
Conditioned place preference (CPP) or aversion (CPA)
pavlovian conditioning based on capacity of the animal to associate the drug effect with the context. If the drug has rewarding effects, mice explore the drug-paired compartment more than the vehicle-paired compartment, and thus show a conditioned place preference (CPP). If the drug is aversive mice avoid the drug-paired box (Conditioned place aversion or CPA). Reinstatement can be measured after a CPP paradigm: drug priming or stress can reinstate preference for the initially drug-paired box after extinction. This test models drug-seeking behavior (Tzschentke, 2007).
Self-administration (SA)
operant paradigms model several elements of human drug consumption, and are therefore largely used in rodents. Drug SA in mice (except oral SA), however, is technically difficult, and studies remain scarce. In drug SA models, the animal works to obtain the drug and learns an action/outcome association. Various aspects are investigated: acquisition (under fixed ratio schedule); motivation (under progressive ratio schedule and determination of a breaking point, corresponding to the highest response possible for a single delivery); extinction (response rate after end of drug-delivery); reinstatement (as for CPP). In addition to rewarding effects of the drug, this model enables investigation of motivational aspects of drug intake (Sanchis-Segura and Spanagel, 2006).
Two-bottle choice
In this test, mostly used for measuring alcohol consumption, the animal has access to a water-containing bottle and an alcohol-containing bottle. This access is either continuous (24h/day) or intermittent (few hours a day or few days a week). The latter closely mimics binge drinking and can be used as a model of relapse by including phases of deprivation (Crabbe et al., 2011).
Locomotor effects and sensitization
Many drugs of abuse increase locomotor activity after acute treatment. Repeated administration of the drug, classically increases this locomotor response, a phenomenon referred to as sensitization that may reflect the transition from voluntary intake to compulsive use (Robinson and Berridge, 2008; Vanderschuren and Pierce, 2010), or vulnerability to drug addiction or drug-induced psychosis in humans (Loweth and Vezina, 2011).
Withdrawal
Chronic drug administration produces physical dependence, which is revealed after cessation of drug exposure. Spontaneous withdrawal is difficult to detect and quantify in animals, therefore physical withdrawal is typically precipitated by treatment with an antagonist, followed by scoring of withdrawal signs. The latter vary with the drug (ptosis, teeth chattering, tremor, paw tremor, wet-dog shakes, sniffing, jumping, diarrhea) and a global score is calculated to measure a general dependence index (Maldonado et al., 1996).
Table 1. Behavioral effects of morphine and heroin in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Behavioral tests are detailed in the Box. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO. CPP: Conditioned Place Preference; d: day; SA: self-administration; VTA: ventral tegmental area; FR: fixed ratio; PR: progressive ratio.
| Gene KO | Drug of abuse | Behavioral test | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|---|
| mu | morphine | CPP | 3 mg/kg, s.c. | abolished | Matthes et al., 1996 |
| CPP | 10 mg/kg, s.c. | abolished | Sora et al., 2001 | ||
| CPP | 10 mg/kg, s.c. | abolished | Nguyen et al., 2012a | ||
| CPP | 10 mg/kg, s.c. | abolished | Nguyen et al., 2012b | ||
| + challenge on d14 | 5 mg/kg, s.c. | abolished | |||
| SA | 2 or 4 mg/0.2mL, i.c.v. FR1 | lower than saline groups | Becker et al., 2000 | ||
| SA | 0.1 or 0.3 mg/kg/injection, i.v. FR4 | abolished | Sora et al., 2001 | ||
| VTA SA | 50 or 100 ng/infusion | abolished | David et al., 2008 | ||
| withdrawal | 20 to 100 mg/kg, i.p. (2x/d, 5d) | abolished | Matthes et al., 1996 | ||
| heroin | CPP | 1 mg/kg, i.p. | abolished | Contarino et al., 2002 | |
| delta | morphine | CPP preferred side | 10 mg/kg, s.c. | abolished | Chefer et al., 2009 |
| CPP non-preferred side | 10 mg/kg, s.c. | unchanged | |||
| CPP drug free state | 5 mg/kg, s.c. | abolished | Le Merrer et al., 2011 | ||
| CPP under morphine | 5 mg/kg, s.c. | unchanged | |||
| CPP without cue | 10 mg/kg, s.c. | abolished | Le Merrer et al., 2012 | ||
| CPP with cue | 5, 10 and 20 mg/kg, s.c. | unchanged/restored | |||
| SA | 0.25 or 0.5 mg/kg/infusion, i.v. FR1 | unchanged | Le Merrer et al., 2011 | ||
| 0.25 mg/kg/infusion, i.v. PR | unchanged | ||||
| 0.5 mg/kg/infusion, i.v. PR | increased | ||||
| VTA SA | 50 ng/infusion | unchanged | David et al., 2008 | ||
| withdrawal | 75 mg, pellet (3d) | unchanged | Nitsche et al., 2002 | ||
| kappa | morphine | CPP | 1 mg/kg, s.c. | unchanged | Simonin et al., 1998 |
| withdrawal | 20 to 100 mg/kg, i.p. (2x/d, 6d) | abolished | Simonin et al., 1998 | ||
| βend | morphine | CPP | 10 mg/kg, s.c. | increased | Skoubis et al., 2005 |
| CPP | 5 mg/kg, s.c. | unchanged | Niikura et al., 2008 | ||
| pEnk | morphine | CPP | 10 mg/kg, s.c. | unchanged | Skoubis et al., 2005 |
| withdrawal | 75 mg, pellet (3d) | increased | Nitsche et al., 2002 | ||
| withdrawal jumping | 20 mg/kg, s.c. (1 inj) | decreased | Shoblock & Maidment, 2007 | ||
| 100 mg/kg, s.c. (2d) | abolished | ||||
| pDyn | morphine | CPP | 5 mg/kg, s.c. | unchanged | Zimmer et al., 2001 |
| CPP | 3.5 mg/kg, s.c. | unchanged | Mizoguchi et al., 2010 | ||
| withdrawal | 20 to 100 mg/kg, i.p. (2x/d, 5d) | unchanged | Zimmer et al., 2001 | ||
Table 6. Drugs of abuse locomotor effects in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Measures of locomotor stimulation and sensitization are detailed in the Box. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO; d: day; inj: injection.
| Gene KO | Drug of abuse | Locomotor stimulation | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|---|
| mu | morphine | locomotion | 2.3 mg/kg, i.p. | abolished | Tian et al., 1997 |
| locomotion | 5 or 10 mg/kg, s.c. | decreased/saline | Becker et al., 2000 | ||
| locomotion | 10 mg/kg, s.c. | abolished | Sora et al., 2001 | ||
| locomotion | 10 or 20 mg/kg, s.c. | abolished | Chefer et al., 2003 | ||
| locomotor sensitization (6d inj) | 10 mg/kg, s.c. d1 | abolished locomotion | Yoo et al., 2003, 2006 | ||
| + challenge on day 12 | 10 mg/kg, s.c. d12 | abolished | |||
| heroin | locomotion | 3 mg/kg, i.p. | abolished | Contarino et al., 2002 | |
| THC | locomotor tolerance (2x/d, 5d) | 20 mg/kg, i.p. | unchanged | Ghozland et al., 2002 | |
| cocaine | locomotion | 20 or 40 mg/kg, i.p. | unchanged | Becker et al., 2002 | |
| locomotion | 30 mg/kg, i.p. | unchanged | Contarino et al., 2002 | ||
| locomotion | 15 mg/kg, i.p. | abolished | Yoo et al., 2003, 2006 | ||
| locomotion | 10 mg/kg, i.p. | unchanged | Chefer et al., 2004 | ||
| 20 mg/kg, i.p. | decreased | ||||
| locomotion | 20 mg/kg, s.c. | unchanged | Hall et al., 2004 | ||
| locomotion | 3, 10, 20, or 30 mg/kg i.p. | unchanged | Lesscher et al., 2005 | ||
| locomotor sensitization (6d inj) | 15 mg/kg, i.p. | Yoo et al., 2003, 2006 | |||
| + challenge on day 12 | 15 mg/kg, i.p. | decreased | |||
| locomotor sensitization (10d inj) | 15 mg/kg, i.p. | Hummel et al., 2004 | |||
| + challenge on day 17 | 15 mg/kg, i.p. | decreased in 129S6xC57BL/6J increased in C57BL/6J |
|||
| locomotor sensitization (5d inj) | 20 mg/kg, s.c. | unchanged | Hall et al., 2004 | ||
| locomotor sensitization (11d inj) | 20 mg/kg, i.p. | Lesscher et al., 2005 | |||
| + challenge on day 14 | 10 mg/kg, i.p. | unchanged | |||
| methamphetamine | locomotion | 1.25 mg/kg, i.p. | unchanged | Shen et al., 2010 | |
| 2.5 mg/kg, i.p. | decreased | ||||
| 10 mg/kg, i.p. | unchanged | ||||
| locomotor sensitization (7d inj) | 0.62 mg/kg, i.p. | abolished | |||
| nicotine | locomotion | 0.7, 1 or 3 mg/kg, s.c. | unchanged | Berrendero et al., 2002 | |
| locomotor sensitization (2x/d, 7d) | 0,05 mg/kg, s.c. d1 | no effect (WT and KO) | Yoo et al., 2004 | ||
| 0,05 mg/kg, s.c. d7 | abolished | ||||
| + challenge on day 11 | 0,05 mg/kg, s.c. | abolished | |||
| locomotor sensitization (2x/d, 7d) | 0,05 mg/kg, s.c. d1 | no effect (WT and KO) | Yoo et al., 2005 | ||
| 0,05 mg/kg, s.c. d7 | abolished | ||||
| alcohol | locomotion | 0.75, 1.25 or 1.75 g/kg, i.p. | abolished | Ghozland et al., 2005 | |
| locomotion | 0.5 or 1.2 g/kg, i.p. | decreased (trend) | Hall et al., 2001 | ||
| delta | morphine | locomotion | 10 or 20 mg/kg, s.c. | unchanged | Chefer et al., 2003 |
| locomotor sensitization (5d inj) | 20 mg/kg, s.c. | unchanged, faster | Chefer et al., 2009 | ||
| challenge on day +7 | 5 mg/kg, s.c. | increased | |||
| challenge on day +33 | 5 mg/kg, s.c. | unchanged | |||
| locomotor tolerance (3d) | 25 mg pellet, s.c. | decreased | |||
| THC | locomotor tolerance (2x/d, 5d) | 20 mg/kg, i.p. | unchanged | Ghozland et al., 2002 | |
| cocaine | locomotion | 10 mg/kg, i.p. | increased | Chefer et al., 2004 | |
| 20 mg/kg, i.p. | unchanged | ||||
| nicotine | locomotion | 0.35, 1.05 or 2.10 mg/kg, s.c. | unchanged | Berrendero et al., 2012 | |
| mu delta | THC | locomotion | 20 mg/kg, i.p. | unchanged | Castane et al., 2003 |
| cocaine | locomotion | 5 or 15 mg/kg, i.p. | unchanged | Chefer et al., 2005 | |
| locomotor sensitization (5d inj) | 15 mg/kg, i.p. d1 | increased locomotion | |||
| + challenge on day 8 | 15 mg/kg, i.p. d8 | abolished | |||
| kappa | THC | locomotor tolerance (2x/d, 5d) | 20 mg/kg, i.p. | decreased | Ghozland et al., 2002 |
| cocaine | locomotion | 5 or 15 mg/kg, i.p. | unchanged | Chefer et al., 2005 | |
| locomotor sensitization (5d inj) | 15 mg/kg, i.p. d1 | increased locomotion | |||
| + challenge on day 8 | 15 mg/kg, i.p. d8 | abolished | |||
| βend | cocaine | locomotion | 15, 30, or 60 mg/kg, i.p. | decreased | Marquez et al., 2008 |
| nicotine | locomotion (horizontal) | 1 or 3 mg/kg, s.c. | unchanged | Trigo et al., 2009 | |
| locomotion (vertical) | 1 mg/kg, s.c. | increased | |||
| 3 mg/kg, s.c. | unchanged | ||||
| alcohol | locomotor sensitization (12d inj) | 2 g/kg, i.p. | Sharpe et al., 2009 | ||
| + challenge on day 13 or 14 | 1.2 g/kg, i.p. | unchanged | |||
| pEnk | THC | locomotion | 20 mg/kg, i.p. | unchanged | Valverde et al., 2000 |
| nicotine | locomotion | 1, 3 or 6 mg/kg, s.c. | unchanged | Berrendero et al., 2005 | |
| pDyn | morphine | locomotion | 5 mg/kg, s.c. | unchanged | Zimmer et al., 2001 |
| locomotion | 4.2 mg/kg, s.c. | increased | Mizoguchi et al., 2010 | ||
| 5 mg/kg, s.c. | unchanged | ||||
| THC | locomotion | 20 mg/kg, i.p. | unchanged | Zimmer et al., 2001 | |
| cocaine | locomotion | 10 or 15 mg/kg, i.p. | decreased | Chefer et al., 2006 | |
| locomotor sensitization (14d inj) | 15 mg/kg, i.p. d1 | unchanged | Bailey et al., 2007 | ||
| 15 mg/kg, i.p. d3, 7 and 14 | increased | ||||
| nicotine | locomotion | 1, 3 or 6 mg/kg, s.c. | unchanged | Galeote et al., 2009 | |
| alcohol | locomotion | 2 g/kg, i.p. | unchanged | Nguyen et al., 2012c | |
Opioid system and opiate drugs
Morphine reward and withdrawal data are shown for the six KO lines in Table 1. Locomotor effects of morphine are presented in Table 6 together with stimulant effects of other drugs of abuse. Genetic studies have definitely established that the mu opioid receptor is required for therapeutic effects as well as unwanted effects of morphine (see (Contet et al., 2004). Hence, morphine (Matthes et al., 1996; Nguyen et al., 2012a; Nguyen et al., 2012b; Sora et al., 2001) and heroin (Contarino 2002) CPP were abolished in mu KO mice at all the tested doses. Intravenous as well as intra-VTA infusions of the drug observed in wild type animals were also abolished in mutants (Sora 2001); David 2008). In another study, mu KO mice self-administered morphine at levels lower than control mice self-administering saline, perhaps unmasking a kappa/dynorphin-mediated aversive state in these mutants (Becker et al., 2000). Locomotor responses to morphine (Tian 1997; Sora 2001; Chefer 2003; Yoo 2003 and 2006, Becker 2000) and heroin administration (Contarino et al., 2002) were eliminated in mu KO animals (see Table 6). Together all the data demonstrate that mu receptors indeed represent the primary in vivo molecular target for both most clinically useful (morphine) and most largely abused (heroin) opiates.
The role of delta receptor in reward is debated. Delta KO mice developed a place preference when morphine was paired with the initially non-preferred compartment, but failed to do so when paired to the preferred side of the apparatus (Chefer and Shippenberg, 2009). The authors interpreted this result as a ceiling effect in the biased CPP protocol that was used more than a decrease of rewarding properties of morphine. In another study, using unbiased CPP, delta KO animals did not develop place preference to morphine (Le Merrer et al., 2011). In the same study, mutant mice showed impaired place conditioning to lithium, an aversive stimulus, and showed normal motivation to obtain morphine in a SA paradigm (Le Merrer et al., 2011). Together with a previous study showing intact intra-VTA SA in delta KO mice (David et al., 2008), the data concur to indicate that morphine reward and motivation to obtain the drug are intact in these animals, however drug-context association is impaired. A subsequent study showed that internal or external non-spatial cues (circadian, drug, auditory) predicting drug or food reward restored morphine CPP in delta KO mice, suggesting that only contextual learning is impaired in these mice (Le Merrer et al., 2012). Considering locomotor effects, the stimulant effect of acute morphine was unchanged in delta KO mice (Chefer et al., 2003). However, sensitization or tolerance to this effect, observed upon distinct regimen of chronic morphine administration, were enhanced and reduced respectively (Chefer and Shippenberg, 2009), indicating a role for delta receptors in these adaptive responses to chronic morphine. Otherwise, physical dependence was unchanged in delta KO mice (Nitsche et al., 2002). In conclusion, the delta receptor does not directly mediate morphine reward and likely facilitates contextual learning. Also, as many other systems, this receptor contributes to chronic morphine-induced neuroplasticity. Mechanisms underlying a potential cross talk between delta receptor activity and mu opioid receptor signaling in vivo remain unclear (see (Pradhan et al., 2011; Stockton and Devi, 2012).
β-endorphin KO animals compared with wild-type controls spent equal (Niikura et al., 2008) or more (Skoubis et al., 2005) time in the drug-paired compartment, depending on the dose and paradigm used. No modification of morphine CPP could be detected in proenkephalin (pEnk) KO mice (Skoubis et al., 2005), and physical dependence was either decreased (Shoblock and Maidment, 2007) or enhanced in these mice (Nitsche et al., 2002). These results suggest paradoxical negative modulatory roles for the two endogenous peptides in morphine reward (βend) and withdrawal (pEnk), or that compensatory mechanisms have developed in knockout animals.
Morphine CPP was unchanged in mice lacking the kappa opioid receptor (Simonin et al., 1998), as well as dynorphin (Mizoguchi et al., 2010; Zimmer et al., 2001). Prodynorphin KO mice showed unchanged (Mizoguchi et al., 2010; Zimmer et al., 2001) or increased hyperlocomotor activity upon morphine administration (Mizoguchi et al., 2010), suggesting that dynorphin opposes mu receptor signaling for the control of locomotor effects. Several signs of naloxone-induced withdrawal were decreased in morphine-dependent kappa KO mice (Simonin et al., 1998), an effect that could not be observed in pDyn mutants (Zimmer et al., 2001). A tonic role for the kappa/dynorphin system is therefore detected in dependent animals, at receptor level, in agreement with pharmacological studies suggesting protective role of kappa receptor blockade in morphine dependence (Wee and Koob, 2010). Involvement of this antireward system (Koob and Le Moal, 2008) is overall better detected in knockout mice under conditions of stress (Bruchas et al., 2010) and in response to non-opioid drugs of abuse (see below).
Opioid system and cannabinoids
Both pharmacological studies and genetic approaches provide considerable evidence suggesting that cannabinoid and opioid systems interact bi-directionally to regulate both neurochemical effects of drug and behavioral responses (Trigo et al., 2010; Vigano et al., 2005). Although mechanisms underlying functional interactions remain unclear, receptors from the two systems show overlapping distribution in various brain structures, and potential heterodimer formation between CB1 and mu opioid receptors has been suggested from in vitro studies (Maldonado et al., 2011; Solinas et al., 2008). Data summarizing cannabinoid effects in KO mice for the opioid system are shown in Table 2. THC-induced CPP was unchanged in delta or kappa KO mice (Ghozland et al., 2002), but was abolished in mu KO mutants (Ghozland et al., 2002) and the double mu-delta KO line (Castane et al., 2003), suggesting that mu receptors mediate rewarding properties of THC. Interestingly conditioned place aversion (CPA), typically observed at a high dose of THC in wild-type mice, was abolished in both pDyn (Zimmer et al., 2001) and kappa KO mice (Ghozland et al., 2002). The latter observations indicate that the kappa/dynorphin system mediates aversive effects of THC, another facet of cannabinoid effects. This was further supported by facilitated self-administration of WIN, a cannabinoid agonist, in pDyn KO mice (Mendizabal et al., 2006). It has long been established that mu and kappa receptors oppositely regulate hedonic homeostasis (Spanagel et al., 1992) and it is therefore possible that the same opposing activities of the two opioid receptors mediate the well-know dual euphoric/aversive effects of cannabinoids. Notably, the delta receptor does not seem involved in all these THC effects, at least from knockout mice analysis (Ghozland et al., 2002).
Table 2. Behavioral effects of cannabinoid in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Behavioral tests are detailed in the Box1. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO. THC: δ9-tetrahydrocannabinol; WIN: WIN 55,212-2; CPP: Conditioned Place Preference; CPA: Conditioned Place Aversion; d: day; SA: selfadministration; FR: fixed ratio.
| Gene KO | Drug of abuse | Behavioral test | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|---|
| mu | THC | CPP | 1 mg/kg, i.p. | abolished | Ghozland et al., 2002 |
| CPA | 5 mg/kg, i.p. | decreased | Ghozland et al., 2002 | ||
| withdrawal | 10 mg/kg, s.c. (5d) | unchanged | Lichtman et al., 2001 | ||
| 30 or 100 mg/kg, s.c. (5d) | decreased | ||||
| withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | unchanged | Ghozland et al., 2002 | ||
| delta | THC | CPP | 1 mg/kg, i.p. | unchanged | Ghozland et al., 2002 |
| CPA | 5 mg/kg, i.p. | unchanged | Ghozland et al., 2002 | ||
| withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | unchanged | Ghozland et al., 2002 | ||
| mu delta | THC | CPP | 1 mg/kg, i.p. | decreased | Castane et al., 2003 |
| withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | decreased | Castane et al., 2003 | ||
| kappa | THC | CPP | 1 mg/kg, i.p. | unchanged | Ghozland et al., 2002 |
| CPP without priming | 1 mg/kg, i.p. | present, absent in WT | |||
| CPA | 5 mg/kg, i.p. | abolished | Ghozland et al., 2002 | ||
| withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | unchanged | Ghozland et al., 2002 | ||
| pEnk | THC | withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | decreased | Valverde et al., 2000 |
| pDyn | THC | CPA | 5 mg/kg, i.p. | abolished | Zimmer et al., 2001 |
| withdrawal | 20 mg/kg, i.p. (2x/d, 6d) | decreased (trend) | Zimmer et al., 2001 | ||
| WIN | SA | 6.25 mg/kg/infusion, i.v. FR1 | increased | Mendizabal et al., 2005 | |
| 12.5 mg/kg/infusion, i.v. FR1 | abolished | ||||
THC withdrawal upon chronic THC treatment was reduced in pEnk KO mice (Valverde et al., 2000) and double mu-delta KO mice (Castane et al., 2003). Reduced THC withdrawal was also detected in mu KO animals, at high doses of THC (Lichtman et al., 2001). Single mutants for pDyn (Zimmer et al., 2001), mu, delta or kappa receptors (Ghozland et al., 2002) otherwise showed normal THC withdrawal. The data together suggest that an endogenous enkephalinergic tone, acting jointly at mu and delta receptors, contributes to the development of physical dependence to THC.
Opioid system and psychostimulants
Multiple studies have pointed out a role for opioid receptors and their endogenous ligands in psychostimulant - particularly cocaine- addiction (for a recent review, see (Yoo et al., 2012), and Table 3). Cocaine self-administration was dose-dependently reduced in mu KO mice (Mathon et al., 2005), and cocaine CPP was maintained (Contarino et al., 2002; Hall et al., 2004; Nguyen et al., 2012a) or decreased (Hall et al., 2004) depending on dose and experimental conditions (number of pairings, number and duration of conditioning sessions). These data indicate that mu receptors mediate, at least in part, cocaine reward. A rightward shift of the CPP dose-response curve was observed in both mu (Becker et al., 2002) and β-endorphin (Marquez et al., 2007) KO mice, suggesting decreased cocaine sensitivity in the two lines and a possible implication of mu/βend signaling in cocaine reinforcement. Place preference studies were also conducted in mu KO for amphetamine (Marquez et al., 2007) and MDMA (Robledo et al., 2004) but no phenotype could be detected.
Table 3. Behavioral effects of psychostimulant in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Behavioral tests are detailed in the Box. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO. CPP: Conditioned Place Preference; d: day; SA: self-administration; FR: fixed ratio.
| Gene KO | Drug of abuse | Behavioral test | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|---|
| mu | cocaine | CPP | 5-10 mg/kgi.p. | rightward shift | Becker et al, 2002 |
| CPP | 10 mg/kg, i.p. | unchanged | Contarino et al., 2002 | ||
| CPP | 5 mg/kg, s.c. | unchanged | Hall et al., 2004 | ||
| 10 mg/kg, s.c. | decreased | ||||
| CPP | 30 mg/kg, i.p. | unchanged | Nguyen et al., 2012a | ||
| SA | 0.4, 0.8 or 1.6 ug/inf, i.v. FR1 | decreased | Mathon et al., 2005 | ||
| MDMA | CPP | 10 mg/kg, i.p. | unchanged | Robledo et al., 2004 | |
| amphetamine | CPP | 1 mg/kg, i.p. | unchanged | Marquez et al., 2007 | |
| kappa | cocaine | CPP +/− forced swim stress | 15 mg/kg, s.c. | unchanged/no effect of stress | McLaughlin et al., 2006a |
| CPP | 15 mg/kg, s.c. | unchanged | Redila and Chavkin, 2008 | ||
| stress-induced reinstatement | abolished | ||||
| cocaine prime test | 15 mg/kg, s.c. | unchanged | |||
| βend | cocaine | CPP | 30-60 mg/kg i.p. | rightward shift | Marquez et al., 2008 |
| CPP | 30 mg/kg, i.p. | abolished | Nguyen et al., 2012a | ||
| pDyn | cocaine | CPP +/− forced swim stress | 15 mg/kg, s.c. | unchanged/no effect of stress | McLaughlin et al., 2003 |
| CPP + social defeat stress | 15 mg/kg, s.c. | decreased | McLaughlin et al., 2006b | ||
| CPP | 15 mg/kg, s.c. | unchanged | Redila and Chavkin, 2008 | ||
| stress-induced reinstatement | abolished | ||||
| cocaine prime test | 15 mg/kg, s.c. | decreased | |||
The rewarding properties of cocaine were examined using CPP in mice lacking either kappa receptors or preprodynorphin. Preference for the drug-paired compartment was maintained in both animal models (McLaughlin et al., 2006a; McLaughlin et al., 2003; Redila and Chavkin, 2008). In presence of stress, cocaine CPP is typically increased in wild type mice but remained unchanged in kappa and pDyn KO mice (forced-swim stress in (McLaughlin et al., 2006a; McLaughlin et al., 2003); social defeat stress in (McLaughlin et al., 2006b)), indicating that the kappa/dynorphin system contributes to the stress-mediated response. Within this line, stress-induced reinstatement of extinguished cocaine CPP was decreased in pDyn KO, although this was not observed in kappa KO mice (Redila and Chavkin, 2008).
Another well-known effect of psychostimulants is drug-induced hyperlocomotion (Table 6). In some reports, the locomotor response to cocaine was reduced in mu KO mice (Chefer et al., 2004; Yoo et al., 2006; Yoo et al., 2003) as well as in βend KO mice (Marquez et al., 2008), while in many other mu KO studies, this cocaine effect was unchanged (Becker et al., 2002; Chefer et al., 2004; Contarino et al., 2002; Hall et al., 2004; Lesscher et al., 2005). Furthermore, sensitization to locomotor effects of cocaine was reduced (Yoo et al., 2006; Yoo et al., 2003), maintained (Lesscher et al., 2005), or enhanced (Hummel et al., 2004), depending on the mouse genetic background (Hummel et al., 2004) and the pattern of drug exposure (administration regimen and timing of injections) (Allouche et al., 2013; Puig et al., 2012). In mu KO mice also, methamphetamine-induced locomotion, was decreased at one dose, maintained in lower and higher doses, and no behavioral sensitization was found (Shen et al., 2010), therefore altogether, evidence exist that mu receptor activity contributes to locomotor effects of cocaine, and the adaptive response to repeated exposure to the drug.
Cocaine-induced locomotion was also investigated in delta KO mice, showing an increased response to cocaine in these mutant animals (Chefer et al., 2004). Locomotion stimulation upon cocaine administration was maintained or increased (Chefer et al., 2005) in kappa KO animals depending on the dose, and maintained (Bailey et al., 2007) or decreased (Chefer and Shippenberg, 2006) in pDyn KO mice, indicating contrasting effects of the kappa/dynorphin system in this response. Similarly, locomotor sensitization was abolished in kappa KO mice (Chefer et al., 2005), and increased in pDyn KO animals (Bailey et al., 2007), suggesting a dissociation of kappa receptors and dynorphins in the locomotor stimulant effect of cocaine.
Opioid system and nicotine
Among psychostimulants, nicotine is the primary component of tobacco that maintains smoking habits. The drug acts as a nicotinic acetylcholine receptor agonist to produce relaxation and enhanced cognitive performance, and is strongly addictive. Pharmacological and genetic studies have provided evidence for a critical role for the opioid system in nicotine addiction (for recent reviews, see (Berrendero et al., 2010; Drews and Zimmer, 2010; Hadjiconstantinou and Neff, 2011; Tuesta et al., 2011), and knockout studies addressing nicotine reward and withdrawal are summarized in Table 4.
Table 4. Behavioral effects of nicotine in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Behavioral tests are detailed in the Box. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO. CPP: Conditioned Place Preference; d: day; SA: self-administration; FR: fixed ratio; PR: progressive ratio.
| Gene KO | Behavioral test | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|
| mu | CPP | 0.5 or 0.7 mg/kg, s.c. | abolished | Berrendero et al., 2002 |
| CPP | 1 mg/kg, i.p. | abolished | Walters et al., 2005 | |
| 2 mg/kg, i.p. | unchanged | |||
| withdrawal | 10 mg/kg/d, minipump (6d) | decreased | Berrendero et al., 2002 | |
| delta | CPP | 0.17 mg/kg, s.c. | abolished | Berrendero et al., 2012 |
| SA | 15 µg/kg/infusion 10d, i.v. FR1 | unchanged | Berrendero et al., 2012 | |
| 30 µg/kg/infusion 10d, i.v. FR1 | decreased | |||
| 30 µg/kg/infusion, i.v. PR | decreased | |||
| withdrawal | 8.77 mg/kg/d, minipump (6d) | unchanged | Berrendero et al., 2012 | |
| βend | CPP | 0.5 mg/kg, s.c. | abolished | Trigo et al., 2009 |
| withdrawal | 10 mg/kg/d, minipump (6d) | unchanged | Trigo et al., 2009 | |
| pEnk | CPP | 0.5 mg/kg, s.c. | abolished | Berrendero et al., 2005 |
| withdrawal | 25 mg/kg/d, minipump (6d) | decreased | Berrendero et al., 2005 | |
| pDyn | CPP | 0.5 mg/kg, s.c. | unchanged | Galeote et al., 2009 |
| SA | 5.2–85.5 mg/kg/infusion, i.v. FR1 | leftward shift | Galeote et al., 2009 | |
| 5.2, 10.6, 21.3 or 85.5 mg/kg/infusion, i.v. PR | unchanged | |||
| 42.7 mg/kg/infusion, i.v. PR | decreased | |||
| withdrawal | 25 mg/kg/d, minipump (6d) | unchanged | Galeote et al., 2009 | |
Rewarding properties of nicotine were altered in pEnk, βend, mu and delta KO mice, as shown by decreased nicotine CPP in these mutant mice (Berrendero et al., 2002; Berrendero et al., 2005; Berrendero et al., 2012; Trigo et al., 2009; Walters et al., 2005). In agreement, enhanced extracellular dopamine induced by nicotine in the nucleus accumbens was attenuated in mice lacking pEnk (Berrendero et al., 2005) and delta receptors (Berrendero et al., 2012). Also, the acquisition of nicotine SA was decreased in delta KO mice (Berrendero et al., 2012), further substantiating the notion that delta/pEnk receptor signaling contributes to reinforcing properties of nicotine. In contrast, self-administration of a low nicotine dose was increased in pDyn KO mice (Galeote et al., 2009) suggesting that, as for THC, dynorphin may contribute to aversive effects of nicotine. It would be interesting to pursue similar experiments in kappa KO mice to confirm this hypothesis.
Mu/pEnk signaling seems involved in nicotine dependence. Withdrawal signs of chronically nicotine-treated pEnk (Berrendero et al., 2005) and mu KO (Berrendero et al., 2002) mice were attenuated, while no difference with wild-type controls was observed for pDyn (Galeote et al., 2009), delta (Berrendero et al., 2012) and βend (Trigo et al., 2009) KO mice. Finally, the mu receptor also contributes to nicotine-induced locomotor sensitization (Yoo et al., 2005; Yoo et al., 2004), see Table 6).
Opioid system and alcohol
Alcohol produces euphoria, among many other effects, and acts on several molecular targets in the brain. A recent analysis of 37 KO mouse lines has provided evidence that alcohol consumption is controlled by multiple physiological systems (Blednov et al., 2012). Among these, endogenous opioids represent an important neurobiological component of alcohol intake and dependence (Gianoulakis, 2009; Koob et al., 2003). Extensive research has implicated endogenous opioid peptide release in alcohol consumption, and naltrexone, a general opioid antagonist, showed some efficacy in the treatment of alcoholism (Koob et al., 2009). Knockout mice have provided key insights into opioid mechanisms underlying alcohol-related behaviors (see Table 5). Mice lacking mu opioid receptors did not self-administer alcohol under several conditions, including oral self-administration and the two-bottle choice, and did not display conditioned place preference to alcohol (Becker et al., 2002; Hall et al., 2001; Roberts et al., 2000), demonstrating that mu receptors are essential to consumption and motivation for alcohol. Mu receptor also plays a role in alcohol withdrawal as the absence of mu receptor accelerated the progression of physical signs of withdrawal (Ghozland et al., 2005). Finally, no locomotor stimulation was observed following alcohol administration in mu KO mice (Ghozland et al., 2005) and Table 6), and altogether data show a prominent role of mu receptors in many aspects of alcohol effects.
Table 5. Alcohol behavioral effects in opioid receptor and peptide knockout mice.
Data are shown for each knockout (gene KO) mouse line. Behavioral tests are detailed in the Box. Unchanged: no genotype effect; increased: KO shows higher response compared to wild-type (WT); decreased: KO shows lower response compared to WT; abolished: no response in KO. CPP: Conditioned Place Preference; d: day; SA: self-administration; TBC: two-bottle choice; FR: fixed ratio.
| Gene KO | Behavioral test | Drug of abuse dose, route | Genotype effect | Ref |
|---|---|---|---|---|
| mu | TBC limited access | 10% | unchanged | van Rijn and Whistler, 2009 |
| TBC | 10% | decreased | Becker et al., 2002 | |
| TBC | 2–32% | decreased (female) | Hall et al., 2001 | |
| TBC | 10% | decreased | Roberts et al., 2000 | |
| oral SA | 5–10% | abolished | ||
| oral SA following TBC | abolished | |||
| CPP | 2 or 4 g/kg | unchanged | Becker et al., 2002 | |
| CPP | 2 g/kg | abolished (female) | Hall et al., 2001 | |
| withdrawal | liquid diet 0.8–5% | earlier signs | Ghozland et al., 2005 | |
| delta | TBC limited access | 10% | increased | van Rijn et al., 2009, 2010 |
| oral SA | 5–10% | increased | Roberts et al., 2001 | |
| TBC following SA | 10% | increased | ||
| kappa | TBC limited access | 10% | decreased | van Rijn and Whistler, 2009 |
| TBC | 3–12% | decreased | Kovacs et al., 2005 | |
| βend | TBC | 7% | increased | Griesel et al., 1999 |
| TBC +/−mild foot shock | 8% | decreased/no effect of stress | Racz et al., 2008 | |
| SA | 75 mg/kg; 2hsession/9d; i.v. FR3 | aquisition in KO but not WT | Grahame et al., 1998 | |
| oral SA | 3–6% | unchanged | Hayward et al., 2004 | |
| withdrawal | forced drinking 16% | unchanged | Racz et al., 2008 | |
| pEnk | TBC | 2 to 10% | unchanged | Koenig et al., 2002 |
| TBC | 8% | unchanged | Racz et al., 2008 | |
| TBC + foot shock | decreased (male) | |||
| oral SA | 3–6% | unchanged | Hayward et al., 2004 | |
| CPP | 2 g/kg, i.p. | unchanged | Koenig et al., 2002 | |
| withdrawal | forced drinking 16% | unchanged | Racz et al., 2008 | |
| pDyn | TBC | 2–8% | increased | Femenia et al., 2012 |
| TBC | 3–12% | decreased (female) | Blednov et al., 2006 | |
| TBC | 8% | increased | Racz et al., 2012 | |
| TBC + foot shock | prolonged/WT | |||
| TBC | 4–10% | unchanged | Sperling et al., 2010 | |
| CPP | 2 g/kg, i.p. | increased | Femenia et al., 2012 | |
| CPP drug free state | 2 g/kg, i.p. | unchanged (female) | Nguyen et al., 2012c | |
| CPP priming | 2 g/kg, i.p. challenge 1 g/kg | increased (female) | ||
| CPP | 2 g/kg, i.p. | unchanged | Blednov et al., 2006 | |
| conditioned taste aversion | 2.5 g/kg, i.p. | unchanged | ||
| withdrawal | 4 g/kg, p.o. | increased | Femenia et al., 2012 | |
| 4 g/kg, i.p. | unchanged | Blednov et al., 2006 | ||
Opposing mu receptor mutants, delta KO mice showed increased alcohol consumption in TBC (Roberts et al., 2001; van Rijn et al., 2010; van Rijn and Whistler, 2009) and oral SA combined with TBC (Roberts et al., 2001) paradigms and their innate anxiety returned to wild-type levels after alcohol SA (Roberts et al., 2001). Given the important role of delta in reducing emotional responses (Filliol et al., 2000), increased alcohol intake in these mutants may reflect a self-medication approach to alleviate high levels of anxiety (for a recent review, see (Chu Sin Chung and Kieffer, in press). Interestingly, pEnk KO animals showed intact rewarding effect of alcohol and a normal pattern of alcohol consumption (Koenig and Olive, 2002), however alcohol drinking was modified in pEnk KO under stressful conditions. The latter observation supports a role for delta/pEnk signaling in regulating emotional responses that may impact on alcohol consumption. β-endorphin may also be involved since alcohol intake was reduced (Racz et al., 2008), unchanged (Hayward et al., 2004) or increased (Grahame et al., 1998; Grisel et al., 1999) in βend KO mice.
Paradoxically, mice lacking the kappa receptor showed reduced preference and alcohol consumption in TBC paradigms (Kovacs et al., 2005; van Rijn and Whistler, 2009), which contrast with increased reinforcing effects of other drugs of abuse in these mice. Using similar TBC testing, pDyn KO mice showed increased voluntary consumption (Femenia and Manzanares, 2012; Racz et al., 2012) suggesting that the kappa receptor and dynorphins regulate alcohol intake via distinct mechanisms. Alcohol CPP was unchanged (Blednov et al., 2006; Nguyen et al., 2012c; Sperling et al., 2010) or increased (Femenia and Manzanares, 2012) in mice lacking pDyn. The latter observation is in agreement with the TBC data and the reported aversive-like activity of dynorphin peptides. pDyn KO mice otherwise showed normal increase in stress-induced alcohol preference (Racz et al., 2012; Sperling et al., 2010), but developed stronger withdrawal signs after chronic alcohol (Femenia and Manzanares, 2012). As mu receptors therefore, pDyn influences several aspects of responses to alcohol, and future studies will examine whether kappa/Pdyn signaling indeed operates in alcohol abuse.
Discussion and concluding remarks
Knockout studies have highlighted very distinct roles for each component of the opioid system in drug reward and dependence: the mu receptor is a convergent molecular target mediating rewarding properties of all drugs of abuse, the kappa receptor opposes mu receptor signaling in the control of hedonic homeostasis, and also mediates aversive effects of cannabinoids and nicotine, and the delta receptor most likely modulates drug consumption indirectly, by improving emotional states or facilitating drug-context association (see (Lutz and Kieffer, 2012, 2013). Confronting data from receptor KO and peptide KO mice is a difficult task, since ideally behavioral responses of the six knockout lines should be examined in parallel, using the same experimental setting. This was performed with the three receptor lines for some responses, but was never achieved for the six lines together. Also studies from constitutive gene deletions have sometimes yielded results which are discordant with behavioral pharmacology, often attributed to compensatory mechanisms that may develop in genetically modified animals (Kieffer and Gaveriaux-Ruff, 2002; Portugal and Gould, 2008). Altogether however, data analysis across the literature allows identification of potential endogenous receptor/peptide systems operating in drug reinforcement processes, and reveals differing mechanisms across the distinct classes of drugs of abuse (Figures 2 and 3).
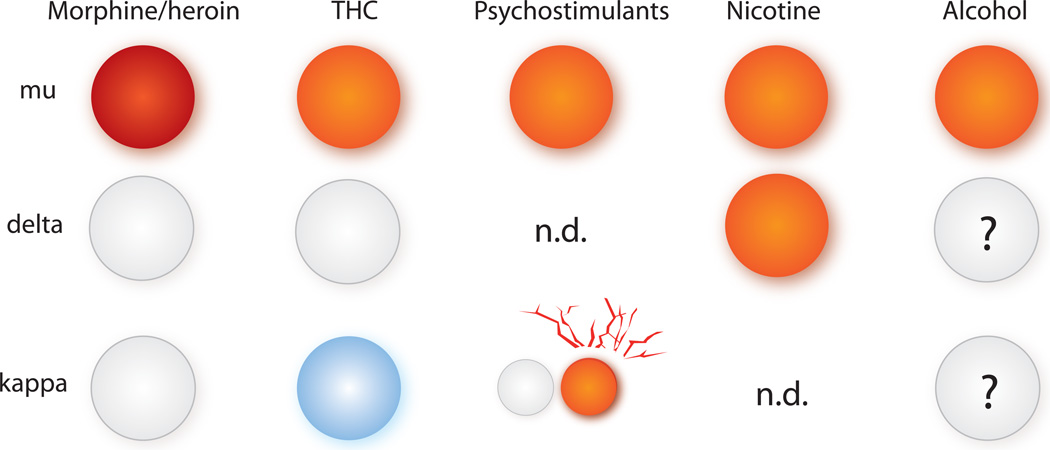
Figure 2. Involvement of opioid receptors in drug reward.
The scheme summarizes data from receptor KO mice and highlights the role of each receptor in drug reward. The mu opioid receptor mediates rewarding properties of both opioid and non-opioid drugs of abuse. With the exception of nicotine, the delta receptor does not seem involved in drug reward. The kappa receptor mediates dysphoric effects of THC and favors cocaine reward after stress (red lines). The role of delta and kappa receptor in alcohol intake is under investigation (see text). Circles indicate euphoria (red/orange), no effect (white) or dysphoria (blue); n.d: not determined in receptor KO mice.
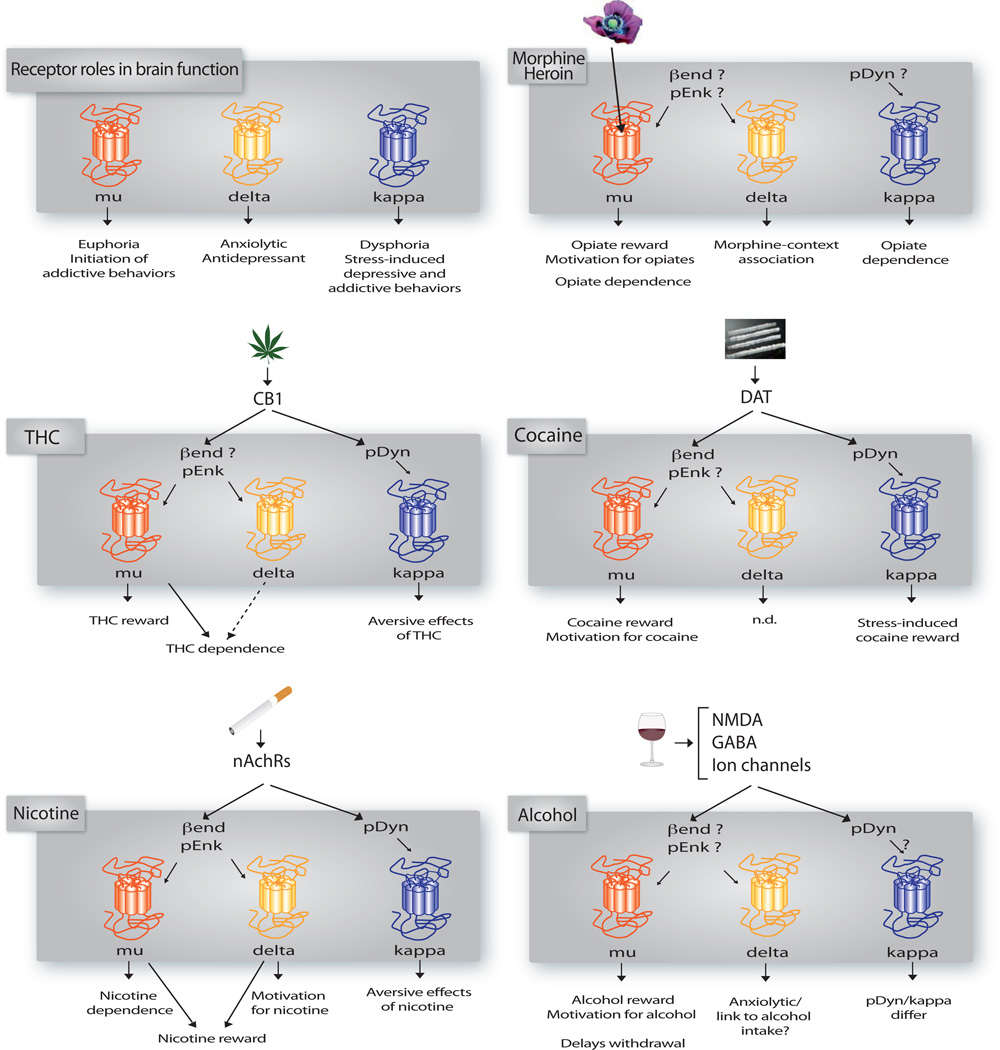
Figure 3. Distinct roles of opioid receptors and peptides in addiction-related effects of drugs of abuse.
The upper left scheme summarizes known roles of opioid receptors in brain functions related to hedonic homeostasis and mood (from (Lutz and Kieffer, 2012). In the five other panels, we propose mechanisms implicating opioid receptors and/or peptides in addiction liability of each class of drugs of abuse, as inferred from both receptor and peptide knockout mouse data reviewed here. “Reward” and “drug-context association” refer to CPP data, “aversive effects” to CPA data, “motivation for the drug” to SA experiments, and “dependence” to scores of physical withdrawal under antagonist treatment. Data from locomotor studies are not included (see summary in Table 6). Opiates: peptide KO mice show paradoxical (β-end/reward, pEnk/withdrawal) or no (pDyn/withdrawal) phenotype. THC: β-end KO mice not tested; cocaine: pEnk KO mice not tested; nicotine: β-end KO mice tested for reward but not withdrawal; alcohol: β-end KO mice show contrasting phenotypes and pEnk show a phenotype under stress.
Altogether, data from peptide KO mice, combined with those from receptor KO mice, concur to substantiate involvement of a kappa/dynorphin system in dysphoric states associated to drugs of abuse, although this may not apply to alcohol. Data also suggest a role for mu/Bend signaling in cocaine and nicotine reward, and implication of delta/pEnk signaling to regulate alcohol intake.
Role of mu signaling in drug reward
Mu receptor is essential for rewarding effects of opiates as well as non-opiate drugs (cannabinoids, psychostimulants and alcohol). Both pEnk and βend (Roth-Deri et al., 2008) are involved in rewarding effects of non-opioid drugs of abuse, with a demonstrated implication of βend for cocaine and alcohol, whereas nicotine or cannabinoid reward has been little explored so far for the two peptides.
Role of kappa signaling in drug aversion
The important role of kappa/dynorphin in dysphoric effects of drugs of abuse has been reviewed recently (Shippenberg et al., 2007; Wee and Koob, 2010). The set of data summarized here supports the notion that kappa receptors mainly interact with pDyn-derived peptides to limit drug reward and mediate dysphoric aspects for some drugs (cannabinoids, nicotine). Moreover, and only under stressful conditions, kappa/dynorphin activity increases sensitivity to cocaine reward. The kappa/dynorphin partnership regulating alcohol intake, however, requires further studies.
Role of delta signaling in drug reward
Data indicate that delta receptor activity reduces levels of anxiety and depressive-like behaviors, and that enkephalin is involved in this process (Chu Sin Chung and Kieffer, in press; Lutz and Kieffer, 2012; Pradhan et al., 2011), and it is likely that delta/pEnk signaling also regulates alcohol intake through similar mechanisms.
Clinical perspectives
Many pharmacotherapies to treat addiction have been developed in the past decades, but have often shown modest efficacy or acted on sub-populations of patients (Potenza et al., 2011; Volkow and Skolnick, 2012). Clinical studies also showed reduced relapse rate in patients receiving behavioral therapy (alcohol), and in general individual differences, including genetic vulnerability, need be considered (Heilig et al., 2011).The question of whether novel opioid compounds could lead to more efficient treatments is under intense investigations. Naltrexone, a general opioid antagonist, was the first opioid medication with FDA approval to reduce the level or frequency of drug intake (Pettinati and Rabinowitz, 2006). Methadone treatment, targeting mu receptors, was a pioneering substitution approach to treat heroin addiction, and a recent report describing eight compounds effective in the treatment of alcohol (acamprosate, naltrexone), opioid (buprenorphine, methadone, naloxone) and nicotine (nicotine, varenicline, bupropion) addiction, shows that mu receptors remain a prime target in most successful treatments for addiction (Pierce et al., 2012). Delta agonists may be efficient to limit disruption of emotional responses in addicted individuals (Lutz and Kieffer, 2012). Delta drugs have been developed to treat chronic pain and depression, and are currently being tested in the clinic, but their use in indications related to drug abuse has not been considered, as yet (Gaveriaux-Ruff and Kieffer, 2011). Preclinical research has definitely established that kappa receptor activity plays a role in addiction-related behaviors, with a prodepressant-like activity (see review (Lutz and Kieffer, 2013). Kappa antagonists are therefore promising candidates for pharmacotherapies in stress- and addiction-related disorders, and may attenuate compulsive drug intake (Wee and Koob, 2010) or specific symptoms of depressive disorders, depending on the administration time point (Knoll and Carlezon, 2010). Finally, considering the growing evidence of comorbidity between addiction and depression, possible improvement of addiction therapies may arise from the combination of substitution treatments (mu agonists such as methadone, or partial agonists such as buprenorphine) with kappa antagonists or delta agonists, for treating patients with comorbid conditions (Lutz and Kieffer, 2013).
Further development of delta and kappa opioid drugs will join the growing body of studies addressing other targets, such as gamma-aminobutyric acid receptors and voltage-gated ion channels. These drugs will likely complete other non-pharmacological therapies, including transcranial magnetic stimulation or behavioral, cognitive therapies and group therapies considered very effective in long-term treatments (Addolorato et al., 2012; Volkow and Skolnick, 2012).
Future directions – addressing the neural circuit by genetic approaches
Conditional knockout
Conventional knockout approaches have proved valuable to tease apart respective contributions of opioid receptor and peptides in several aspects of drug abuse. Further important developments in addiction research involve investigation of molecular mechanisms operating at the level of neuronal circuits underlying the distinct aspects of addiction (Koob and Volkow, 2010). Therefore, genetic approaches targeted at specific brain sites or neuronal populations are required (Fowler and Kenny, 2012; Gaveriaux-Ruff and Kieffer, 2007; Heldt and Ressler, 2009), among which conditional gene knockout using the Cre/loxP system has received great attention (Nagy, 2000). In the addiction field, several studies using this technology have provided invaluable insights into circuit mechanisms of drug reward. Site-specific deletion of α4-containing nAChR (McGranahan et al., 2011) as well as NMDA receptor NR1 subunit (Wang et al., 2010) has revealed involvement of NMDA receptors expressed in dopaminergic neurons in nicotine reward. Mice lacking CREB specifically in the cerebral cortex were tested for cocaine self-administration and showed a role for CREB in mediating cocaine reinforcement in this brain structure (McPherson et al., 2010). A comprehensive analysis of behavioral and autonomic effects of THC in several conditional lines has revealed implication of the CB1 receptor expressed at the level of forebrain glutamatergic neurons (CB1CamKIIa-Cre mice), cortical glutamatergic neurons (CB1NEX-Cre mice) and dopaminergic neurons (CB1Drd1a-Cre mice), but not GABAergic neurons (CB1Dlx5/6-Cre mice) (Monory et al., 2007). Also a conditional knockout approach using Pet1-Cre mice, targeting the transcription factor Lmx1b in developing serotonergic neurons of the hindbrain, showed that central serotonergic neurons modulate supraspinal pain but are not involved in morphine reward (Zhao et al., 2007). So far, only one conditional line has been reported for opioid receptors and peptides, demonstrating a key role of delta receptors expressed in primary nociceptive neurons in delta analgesia and the control of chronic pain (Gaveriaux-Ruff et al., 2011). It is expected that conditional lines for the opioid system, targeting the neurocircuitry of addiction, will be instrumental to understand circuit mechanisms underlying opioid-mediated drug effects and plasticity.
Optogenetics and brain imaging
More recently, a novel area of investigation has emerged with the development of optogenetic approaches to manipulate specific neuronal populations in live animals (Fowler and Kenny, 2012). For example, light-mediated phasic activation of dopaminergic neurons in the VTA produced a place preference in a CPP paradigm (Tsai et al., 2009) and the specific light-activation of cholinergic neurons from nucleus accumbens reduced cocaine reward (Witten et al., 2010). The specific manipulation of mu, delta or kappa receptor expressing neurons will be of great interest towards understanding neuronal connectivity and plasticity while addiction develops. Within this line, non-invasive neuroimaging and functional connectivity techniques, now developed in small rodents, offer promises in translational medicine (Dalley et al., 2009; Jasinska et al., 2013), and neuroimaging of opioid receptor and peptide genetic mutants may provide invaluable information towards understanding the human disease.
New animal models
Behavioral testing in mice is limited, however new models have been developed to better characterize several stages of the addiction cycle, or protracted abstinence and relapse (for example (Goeldner et al., 2011) ; for review see (O'Brien and Gardner, 2005; Spanagel, 2003) Animal research is expanding in this direction for brain disorders in general (Ahmed, 2010; Berton et al., 2012; Nestler and Hyman, 2010). Also, automated multidimensional systems now enable recording behavior of mice living in social groups to characterize novelty-seeking trait, anxiety, impulsivity, compulsivity and motivation, and such systems can be successfully applied to study behavioral adaptations to drugs of abuse (Radwanska and Kaczmarek, 2011). Also, drosophila or zebra fish are model organisms that allow rapid genetic screens and are being developed in the context of drug abuse (Kaun et al., 2012; Klee et al., 2012; Stewart et al., 2011).
Ultimately, the combination of emerging technologies at molecular, circuit and behavioral levels holds enormous potential to discover novel mechanisms operating at integrated level. The opioid system remains a prime candidate to develop successful therapies in addicted individuals, and understanding opioid-mediated processes at systems levels represents a challenging goal in addiction research.
Highlight for review.
The opioid system is central for reward processing and the development of addiction.
Here we review 15 years data from knockout mice for opioid receptors and peptides.
Mu receptors mediate opiate reward; mu/βend mediate reward for other drugs of abuse.
Delta receptors reduce anxiety and delta/pEnk signaling regulates alcohol intake.
Kappa/pDyn signalling mediates dysphoria and increases drug reward under stress.
Acknowledgments
We would like to thank Sandra Bour for her help with figure preparation and Dominique Massotte for critical reading of the manuscript. This work was supported by CNRS, INSERM, and Université de Strasbourg. We also thank the Mouse Clinical Institute (ICS, Illkirch, France), the European Union (Grant No. GENADDICT/FP6 005166), and the National Institutes of Health (National Institute of Drug Addiction, grant #05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Leggio L, Hopf FW, Diana M, Bonci A. Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology. 2012;37:163–177. doi: 10.1038/npp.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Allouche S, Le Marec T, Noble F, Marie N. Different patterns of administration modulate propensity of methadone and buprenorphine to promote locomotor sensitization in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:286–291. doi: 10.1016/j.pnpbp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Bailey A, Yoo JH, Racz I, Zimmer A, Kitchen I. Preprodynorphin mediates locomotion and D2 dopamine and mu-opioid receptor changes induced by chronic 'binge' cocaine administration. J Neurochem. 2007;102:1817–1830. doi: 10.1111/j.1471-4159.2007.04661.x. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh HH, Hollt V. Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:584–589. doi: 10.1007/s002100000244. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, Maldonado R. Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology. 2012;37:2332–2344. doi: 10.1038/npp.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Robledo P, Trigo JM, Martin-Garcia E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Hahn CG, Thase ME. Are we getting closer to valid translational models for major depression? Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Mayfield RD, Belknap J, Harris RA. Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav. 2012;11:424–435. doi: 10.1111/j.1601-183X.2012.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2011. Peptides. 2012;38:463–522. doi: 10.1016/j.peptides.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Robledo P, Matifas A, Kieffer BL, Maldonado R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. Eur J Neurosci. 2003;17:155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS. Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci. 2003;18:1915–1922. doi: 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS. Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience. 2004;127:497–503. doi: 10.1016/j.neuroscience.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Paradoxical effects of prodynorphin gene deletion on basal and cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens. Eur J Neurosci. 2006;23:229–238. doi: 10.1111/j.1460-9568.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Sin Chung P, Kieffer BL. Delta opioid receptors in brain function and diseases. Pharmacol Ther. doi: 10.1016/j.pharmthera.2013.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M, Seeburg PH, Adelman J, Eiden L, Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982;295:663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2002;446:103–109. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Aigbirhio FI, Brichard L, Richards HK, Hong YT, Baron JC, Everitt BJ, Robbins TW. Modelling human drug abuse and addiction with dedicated small animal positron emission tomography. Neuropharmacology. 2009;56(Suppl 1):9–17. doi: 10.1016/j.neuropharm.2008.05.029. [DOI] [PubMed] [Google Scholar]
- David V, Matifas A, Gavello-Baudy S, Decorte L, Kieffer BL, Cazala P. Brain regional Fos expression elicited by the activation of mu- but not delta-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology. 2008;33:1746–1759. doi: 10.1038/sj.npp.1301529. [DOI] [PubMed] [Google Scholar]
- Drews E, Zimmer A. Modulation of alcohol and nicotine responses through the endogenous opioid system. Prog Neurobiol. 2010;90:1–15. doi: 10.1016/j.pneurobio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Manzanares J. Increased ethanol intake in prodynorphin knockout mice is associated to changes in opioid receptor function and dopamine transmission. Addict Biol. 2012;17:322–337. doi: 10.1111/j.1369-1600.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Utility of genetically modified mice for understanding the neurobiology of substance use disorders. Hum Genet. 2012;131:941–957. doi: 10.1007/s00439-011-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacol Ther. 2007;113:619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, Maldonado R, Kieffer BL. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Chu K, Kieffer BL, Roberts AJ. Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology. 2005;49:493–501. doi: 10.1016/j.neuropharm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Low MJ, Cunningham CL. Intravenous self-administration of ethanol in beta-endorphin-deficient mice. Alcohol Clin Exp Res. 1998;22:1093–1098. [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS, Grahame NJ, Rubinstein M, Belknap JK, Crabbe JC, Low MJ. Ethanol oral self-administration is increased in mutant mice with decreased beta-endorphin expression. Brain Res. 1999;835:62–67. doi: 10.1016/s0006-8993(99)01384-0. [DOI] [PubMed] [Google Scholar]
- Gubler U, Seeburg P, Hoffman BJ, Gage LP, Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982;295:206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Ling N, Burgus R. [Endorphins, hypothalamic and neurohypophysial peptides with morphinomimetic activity: isolation and molecular structure of alpha-endorphin] C R Acad Sci Hebd Seances Acad Sci D. 1976;282:783–785. [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jonsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, den Bergh PV, van Os J, Vos P, Xu W, Wittchen HU, Jonsson B, Olesen J. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60:1209–1220. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Hall FS, Goeb M, Li XF, Sora I, Uhl GR. mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res Mol Brain Res. 2004;121:123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Hansen ST, Pintar JE, Low MJ. Operant self-administration of ethanol in C57BL/6 mice lacking beta-endorphin and enkephalin. Pharmacol Biochem Behav. 2004;79:171–181. doi: 10.1016/j.pbb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. The Use of Lentiviral Vectors and Cre/loxP to Investigate the Function of Genes in Complex Behaviors. Front Mol Neurosci. 2009;2:22. doi: 10.3389/neuro.02.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Hummel M, Ansonoff MA, Pintar JE, Unterwald EM. Genetic and pharmacological manipulation of mu opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neuroscience. 2004;125:211–220. doi: 10.1016/j.neuroscience.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Hyman SE. A glimmer of light for neuropsychiatric disorders. Nature. 2008;455:890–893. doi: 10.1038/nature07454. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakidani H, Furutani Y, Takahashi H, Noda M, Morimoto Y, Hirose T, Asai M, Inayama S, Nakanishi S, Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982;298:245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Klee EW, Schneider H, Clark KJ, Cousin MA, Ebbert JO, Hooten WM, Karpyak VM, Warner DO, Ekker SC. Zebrafish: a model for the study of addiction genetics. Hum Genet. 2012;131:977–1008. doi: 10.1007/s00439-011-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. Ethanol consumption patterns and conditioned place preference in mice lacking preproenkephalin. Neurosci Lett. 2002;325:75–78. doi: 10.1016/s0304-3940(02)00242-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kenneth Lloyd G, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Kieffer BL, Heyser CJ, Katner SN, Ciccocioppo R, Weiss F. Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent Dev Alcohol. 2003;16:263–281. doi: 10.1007/0-306-47939-7_19. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O'Brien D, Wang R, Vinod KY, Saito M, Simonin F, Kieffer BL, Vadasz C. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29:730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Hordijk M, Bondar NP, Alekseyenko OV, Burbach JP, van Ree JM, Gerrits MA. Mu-opioid receptors are not involved in acute cocaine-induced locomotor activity nor in development of cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2005;30:278–285. doi: 10.1038/sj.npp.1300529. [DOI] [PubMed] [Google Scholar]
- Li CH, Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976;73:1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- Loweth JA, Vezina P. Sensitization. In: Olmstead MC, editor. Animal models of drug addiction. Saskatoon, SK, Canada: Humana Press; 2011. pp. 191–205. [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2012;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi: 10.1016/j.neuroscience.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Kieffer BL, Lutfy K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. doi: 10.1016/j.neuropharm.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM. Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CS, Mantamadiotis T, Tan SS, Lawrence AJ. Deletion of CREB1 from the dorsal telencephalon reduces motivational properties of cocaine. Cereb Cortex. 2010;20:941–952. doi: 10.1093/cercor/bhp159. [DOI] [PubMed] [Google Scholar]
- Mendizabal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology. 2006;31:1957–1966. doi: 10.1038/sj.npp.1300957. [DOI] [PubMed] [Google Scholar]
- Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Watanabe C, Osada S, Yoshioka M, Aoki Y, Natsui S, Yonezawa A, Kanno S, Ishikawa M, Sakurada T, Sakurada S. Lack of a rewarding effect and a locomotor-enhancing effect of the selective mu-opioid receptor agonist amidino-TAPA. Psychopharmacology (Berl) 2010;212:215–225. doi: 10.1007/s00213-010-1946-0. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Kieffer B, Friedman TC, Lutfy K. The rewarding action of acute cocaine is reduced in beta-endorphin deficient but not in mu opioid receptor knockout mice. Eur J Pharmacol. 2012a;686:50–54. doi: 10.1016/j.ejphar.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Lutfy K. The role of mu opioid receptors in psychomotor stimulation and conditioned place preference induced by morphine-6-glucuronide. Eur J Pharmacol. 2012b;682:86–91. doi: 10.1016/j.ejphar.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K, Tseng A, Marquez P, Hamid A, Lutfy K. The role of endogenous dynorphin in ethanol-induced state-dependent CPP. Behav Brain Res. 2012c;227:58–63. doi: 10.1016/j.bbr.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Narita M, Okutsu D, Tsurukawa Y, Nanjo K, Kurahashi K, Kobayashi Y, Suzuki T. Implication of endogenous beta-endorphin in the inhibition of the morphine-induced rewarding effect by the direct activation of spinal protein kinase C in mice. Neurosci Lett. 2008;433:54–58. doi: 10.1016/j.neulet.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Furutani Y, Takahashi H, Toyosato M, Hirose T, Inayama S, Nakanishi S, Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982;295:202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont) 2006;3:14–16. [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, O'Brien CP, Kenny PJ, Vanderschuren LJ. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med. 2012;2:a012880. doi: 10.1101/cshperspect.a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 2013;3:a012021. doi: 10.1101/cshperspect.a012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Noble F, Benturquia N. Short- and long-lasting behavioral and neurochemical adaptations: relationship with patterns of cocaine administration and expectation of drug effects in rats. Transl Psychiatry. 2012;2:e175. doi: 10.1038/tp.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Markert A, Mauer D, Stoffel-Wagner B, Zimmer A. Long-term ethanol effects on acute stress responses: modulation by dynorphin. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Osby U, Gunnar A, Maier W, Bilkei-Gorzo A, Nothen M, Zimmer A. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Kaczmarek L. Characterization of an alcohol addiction-prone phenotype in mice. Addict Biol. 2011;17:601–612. doi: 10.1111/j.1369-1600.2011.00394.x. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–1256. [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Mendizabal V, Ortuno J, de la Torre R, Kieffer BL, Maldonado R. The rewarding properties of MDMA are preserved in mice lacking mu-opioid receptors. Eur J Neurosci. 2004;20:853–858. doi: 10.1111/j.1460-9568.2004.03532.x. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Shen X, Purser C, Tien LT, Chiu CT, Paul IA, Baker R, Loh HH, Ho IK, Ma T. mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010;88:2294–2302. doi: 10.1002/jnr.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973;70:1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Befort K, Gaveriaux-Ruff C, Matthes H, Nappey V, Lannes B, Micheletti G, Kieffer B. The human delta-opioid receptor: genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol Pharmacol. 1994;46:1015–1021. [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. Embo J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol. 2003;17:507–518. doi: 10.1016/s1521-6918(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff AV. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci. 2011;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- Stockton SD, Jr, Devi LA. Functional relevance of mu-delta opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:167–172. doi: 10.1016/j.drugalcdep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L. Characteristics of the"receptor" for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol (Copenh) 1973;33:377–384. doi: 10.1111/j.1600-0773.1973.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Zimmer A, Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology. 2009;56:1147–1153. doi: 10.1016/j.neuropharm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valverde O, Maldonado R, Valjent E, Zimmer AM, Zimmer A. Cannabinoid withdrawal syndrome is reduced in pre-proenkephalin knock-out mice. J Neurosci. 2000;20:9284–9289. doi: 10.1523/JNEUROSCI.20-24-09284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology. 2012;37:290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang LP, Li F, Shen X, Tsien JZ. Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine-conditioned place preference. PLoS One. 2010;5:e8616. doi: 10.1371/journal.pone.0008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. What is this thing called pain? J Clin Invest. 2011;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Cho JH, Lee SY, Lee S, Loh HH, Ho IK, Jang CG. Differential effects of morphine- and cocaine-induced nNOS immunoreactivity in the dentate gyrus of hippocampus of mice lacking mu-opioid receptors. Neurosci Lett. 2006;395:98–102. doi: 10.1016/j.neulet.2005.10.089. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Cho JH, Lee SY, Loh HH, Ho IK, Jang CG. Reduced nNOS expression induced by repeated nicotine treatment in mu-opioid receptor knockout mice. Neurosci Lett. 2005;380:70–74. doi: 10.1016/j.neulet.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Kitchen I, Bailey A. The endogenous opioid system in cocaine addiction: what lessons have opioid peptide and receptor knockout mice taught us? Br J Pharmacol. 2012;166:1993–2014. doi: 10.1111/j.1476-5381.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG. Loss of nicotine-induced behavioral sensitization in micro-opioid receptor knockout mice. Synapse. 2004;51:219–223. doi: 10.1002/syn.10303. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Yang EM, Lee SY, Loh HH, Ho IK, Jang CG. Differential effects of morphine and cocaine on locomotor activity and sensitization in mu-opioid receptor knockout mice. Neurosci Lett. 2003;344:37–40. doi: 10.1016/s0304-3940(03)00410-5. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RWt, Chen ZF. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci U S A. 2007;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R. Absence of delta-9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci. 2001;21:9499–9505. doi: 10.1523/JNEUROSCI.21-23-09499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]