Abstract
Background
Whereas the motor dysfunction in Parkinson’s disease (PD) has been related to deficits in basal ganglia (BG) structures, neural correlates of cognitive changes remain to be fully defined. This study tested the hypothesis that cognitive changes in non-demented PD may be related to cortical gray matter (GM) loss.
Methods
High-resolution T1-weighted magnetic resonance images of the brain and comprehensive cognitive function tests were acquired in 40 right-handed, non-demented PD subjects and 40 matched controls. GM changes were assessed using voxel-based morphometry (VBM) in FSL. VBM and cognitive results were compared between PD and controls, and correlation analyses were performed between those brain areas and cognitive domains that showed significant group differences.
Results
PD patients demonstrated significant GM reduction localized predominantly in frontal and parieto-occipital regions. Patients also showed reduced performance in fine motor speed and set-shifting compared to controls. Fine motor speed and set-shifting were associated with GM volume in the frontal cortex in controls, whereas these domains were associated primarily with occipital GM regions in PD patients.
Conclusions
Non-demented PD subjects demonstrate cortical structural changes in frontal and parieto-occipital regions compared to controls. The association between typically recognized “frontal lobe” function and occipital lobe volume suggested a compensatory role of occipital lobe to primary fronto-striatal pathology in PD. Further longitudinal study of these changing structure-function relationships is needed to understand the neural bases of symptom progression in PD.
Keywords: Parkinson’s disease, Cognition, MRI, Voxel-based morphometry, Gray Matter Volume
Introduction
The pathological hallmark of Parkinson’s disease (PD) is the degeneration of dopamine neurons in the substantia nigra, with other neurons in cortex and subcortical nuclei also affected during the disease course. The characteristic motor impairments in PD are caused primarily by depletion of dopamine in the basal ganglia. Gradual and progressive cognitive symptoms also are common in PD, particularly in the domains of executive function, memory, spatial cognition, and psychomotor speed, and can lead to significant functional disability [1]. The exact mechanisms underlying the progression of cognitive impairments are unknown [2] but because the basal ganglia have extensive interconnections with cortical regions, PD cognitive symptoms have been ascribed to compromised information flow through the basal ganglia (i.e., dysfunction of cortico-striatal and/or striato-thalamo-cortical circuitry) [3,4]. Recent findings, however, support the presence of Lewy bodies and neurites in medial temporal cortex even at early clinical disease stages and in prefrontal and primary sensory areas at advanced disease stages [5]. These results raise the possibility that there may be direct cortical involvement in PD cognitive dysfunction.
Voxel-based morphometry (VBM) studies have begun to assess cortical gray matter (GM) changes in PD, although the results have been inconsistent [6] and the functional implications controversial. For example, some VBM studies have identified positive correlations of GM volume loss with cognitive impairments in executive and visuospatial functions [7], whereas other studies failed to detect any relationships [8].
The discrepancies in the literature may stem from methodological differences between studies. These include: varying disease duration, inclusion of older patients at advanced stages, and diverse handedness of participants (see [6] for a summary), all of which can influence brain imaging and cognitive test outcomes. For example, the pattern of cognitive dysfunction may change with disease progression [6]. In addition, multiple studies have reported accelerated cortical volume changes and cognitive decline after the age of 70 years [9], yet not many studies have been done in younger PD populations. Handedness may potentially confound cognitive functions that are mediated by different hemispheres in right and left-handers, masking reliable structure-function changes [10]. The use of abbreviated neuropsychological test batteries also may contribute further to the ambiguity of reported findings. Hence, an experimental design that considers duration of disease, age, handedness of participants, and multiple neurocognitive domains may help clarify the relationship between cortical GM loss and cognitive function in PD.
To address these issues, the current study was designed to test the following specific hypotheses: i) PD patients [inclusion criteria: non-demented, right-handed, at relatively early disease stages, age range <70 years] will show quantitative reductions in GM volume; ii) PD patients will exhibit a decline in the cognitive domains of processing speed, executive functions, spatial cognition, memory, and attention; and iii) reduced cognitive performance will correlate with reduced cortical GM volume.
Methods
Subjects
PD and controls were recruited for an ongoing study approved by the Institutional Review Board/Human Subjects Protection Office (IRB/HSPO) of the Penn State Hershey Medical Center. Written informed consent was obtained from all participants according to the IRB/HSPO guidelines.
PD subjects were diagnosed by a specialist (XH) according to published criteria [11]. Except for two subjects who had very mild symptoms and were drug naïve, PD patients were treated with anti-parkinsonian medications. Patients were negative for other neurological history, hypothyroidism, vitamin B12 and folate deficiency, and kidney and liver disease. Only right-handed PD subjects less than 70 years of age with a Mini-Mental Status Examination (MMSE) Score ≥24, and who took neither a centrally acting acetylcholinesterase inhibitor nor memantine were selected for the study. Forty PD subjects met selection criteria and were included in the analysis.
Forty healthy individuals, matched generally with PD subjects for age, gender, and handedness, were randomly selected from a pool of controls that were part of the ongoing study. Controls were free from any history of neurologic or psychiatric disorder, including previous head injury.
For both motor and cognitive tests, PD subjects were assessed in a practically defined “off” state after withholding all medications overnight (~ 12 hours). Unified Parkinson’s Disease Rating Scale III (UPDRS) scores were recorded for all subjects and verified by a second rater from video recording of the original assessment. Disease severity was recorded using Hoehn and Yahr staging [12]. Levodopa equivalent daily dosage (LEDD) was calculated based on previously published criteria [13].
Magnetic Resonance Imaging
All images were acquired on a Siemens 3-Tesla TimTrio MRI with an 8-channel birdcage type Invivo coil. High-resolution T1-weighted (T1W) images (3D MPRAGE, TR=1540 ms, TE=2.3 ms, voxel spacing 1.0×1.0×1.0 mm, image resolution 256×256 mm2, 176 slices with no gap) were acquired for voxel-based morphometry analysis.
Voxel-based Morphometry Analysis
A voxel-based morphometry (VBM) analysis [14] was carried out with FMRIB Software Library (FSL) tools [15]. First, a study-specific template was created so that all images could be registered in the same stereotactic space (spatial normalization). Brain-extracted structural images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). GM images then were affine-registered to the GM ICBM-152 template and averaged to create an affine GM template. Next, GM images were reregistered to this affine GM template using non-linear registration and averaged to create the study-specific non-linear GM template in standard space. The individual GM images then were non-linearly registered to the study-specific template. After the normalization, the resulting GM images were modulated by multiplying with Jacobian determinants to correct for volume change induced by the nonlinear spatial normalization. Finally, the images were smoothed with an isotropic Gaussian kernel of 9 mm. For the statistical analysis, permutation-based non-parametric testing (5000 permutations) was used [16] with adjustment for depression. The following contrasts were tested: PD < control and PD > control. In the results, we only report the contrast PD < control because the contrast PD > control did not reach statistical significance. Group differences were considered significant at family-wise corrected (FWE) p < 0.05 after initial cluster thresholding of t> 2.3. Regions of interest (ROIs) were determined as cortical and subcortical areas with significant group differences. GM volumes were extracted from these ROIs for further statistical correlation analyses.
Cognitive Tests
All subjects were administered a standardized neuropsychological battery, along with the Hamilton Depression Scale (HAM-D) [17] and the Dementia Rating Scale, Second Edition (DRS-2 [18]). One fine motor function domain and seven cognitive domains were examined: (1) processing speed, executive function [(2) set-shifting and (3) spontaneous flexibility], (4) language, (5) learning/memory, (6) spatial cognition, and (7) attention/working memory. Domains were assessed by two or more tests except for the fine motor speed as follows.
Fine motor speed
The Grooved Pegboard Test (Lafayette Instrument Company, Lafayette, Indiana) measured manual dexterity for dominant and non-dominant hands. Average scores for dominant and non-dominant hands were calculated.
Processing Speed
The Color subtest from Delis-Kaplan Executive Function System (DKEFS [19]) Color-Word Interference Test (CWInt) and the Symbol Search subtest from the Wechsler Memory Scale, Third Edition (WMS-III [20]) provided measures of processing speed.
Executive Function: Set-shifting
The CWInt-Switch and CWInt-Inhibition subtests, including error scores, and the Visual Verbal Test (VVT) provided measures for cognitive set-shifting.
Executive Function: Spontaneous flexibility
The DKEFS Design Fluency Test (DesFlu) assessed spatial associative fluency, whereas the Verbal Fluency Test (VerbFlu) assessed verbal associative retrieval processes.
Language
The Boston Naming Test (BNT [21]) provided a standardized measure of semantic knowledge and word retrieval. The CWInt-Word subtest assessed word reading speed and accuracy.
Learning/Memory
Multi-trial visuospatial learning and short-term memory were measured with the Brief Visuospatial Memory Test-Revised (BVMT-R [22]), whereas verbal learning and memory were assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R [23]).
Spatial Cognition
Visuospatial perception was evaluated with Benton’s Judgment of Line Orientation (JoLO) and the DRS2-Construction subtest provided a quantitative measure of constructional praxis.
Attention
Digit Span provided a measure of immediate attention span, with Spatial Span being a non-verbal analogue [20]. The Letter-Number Sequencing Test required greater mental manipulation of stimulus materials.
Statistical Analyses
Comparisons of demographic information and clinical characteristics (i.e., age, education, HAM-D, MMSE, and UPDRS III) were completed using simple one-way analysis of variance (ANOVA). The sex ratio between groups was tested using Fisher’s Exact Test.
Cognitive test results were converted to standardized z-scores. One-way analysis of covariance (ANCOVA) was used to examine group differences in each cognitive domain and in individual cognitive tests, using HAM-D scores as a covariate. To explore any correlations between neurocognitive tests and cortical GM volume, Spearman’s partial correlation analyses were performed in both PD and controls between cognitive test scores and GM volume only for those brain areas and cognitive domains that showed significant group differences with adjustment for depression. Based on the directional hypothesis (iii) proposed, one-tailed p-values (α = 0.05) were used. Since there were multiple brain areas and cognitive tests compared between PD and controls, the Benjamini-Hochberg method was used to control the false discovery rate (FDR) at the 0.05 level [24]. For the cognitive tests and correlation analyses, we reported raw p-values and indicated whether the tests were significant at a FDR level of 0.05. Z-scores that were 3 SD (standard deviations) greater/less than the group mean of each domain were treated as outliers and considered for exclusion from the analysis to avoid any distortion of the data. According to this criterion, three controls and one PD patient showed fine motor speed scores more than 3 SD lower than the group mean [mean(SD) = −0.39(1.34) for controls and mean(SD) = −3.75 (3.59) for PD patients, N=40] and their data were excluded from both the one-way ANCOVA and the correlation analyses for the fine motor speed domain. All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
Demographics
There were no significant differences in age, MMSE, sex ratio, education, or total DRS-2 scores between PD and controls (Table 1). PD subjects had significantly higher UPDRS III and HAM-D scores. Average disease duration for PD subjects was 3.1 years, with a median of 2 years. Average Hoehn and Yahr staging was 1.8, with 15 subjects having stage 1 disease, 22 subjects having stage 2 disease, and 3 subjects having stage 3 disease. Twelve PD subjects had HAM-D scores ≥10, consistent with depressive symptoms [14], but none reached the cut-off score for dementia based on the DRS-2 (≥10) [15].
Table 1.
Demographic information and general characteristics of the study subjects.
| Information | Controls (n=40) |
PD (n=40) |
P - value |
|---|---|---|---|
| Age (years) | 58 ± 6 | 59 ± 7 | 0.85 |
| Sex (male/female)† | 19/21 | 21/19 | 0.82 |
| Education (years) | 16 ± 3 | 15 ± 3 | 0.41 |
| MMSE score | 29.6 ± 0.8 | 29.4 ± 1 | 0.32 |
| Disease durationzzz (yrs.) | 0 | 3.1 ± 3.2 | n.a. |
| Levodopa Equivalent Dose (mg/day) |
0 | 322.1 ± 244.4 | n.a. |
| UPDRS-III | 0.9 ± 2.1 | 17.7 ± 10.7 | <0.0001* |
| Hoehn & Yahr stage | n.a. | 1.8 ± 0.7 | n.a. |
| Hamilton Depression Score | 3.8 ± 2.1 | 7.4 ± 3.9 | <0.0001* |
| Dementia Rating Scale II | 12.9 ± 1.7 | 12.9 ± 1.7 | 0.95 |
Demographic information is shown as mean ± standard deviation. P-values were derived from a simple one-way analysis of variance comparing the two groups, except for
that was derived using Fisher’s Exact test.
All comparisons were considered statistically significant at p < 0.05. [MMSE = Mini Mental Status Examination; UPDRS = Unified Parkinson’s Disease Rating Scale III.]
VBM Analysis
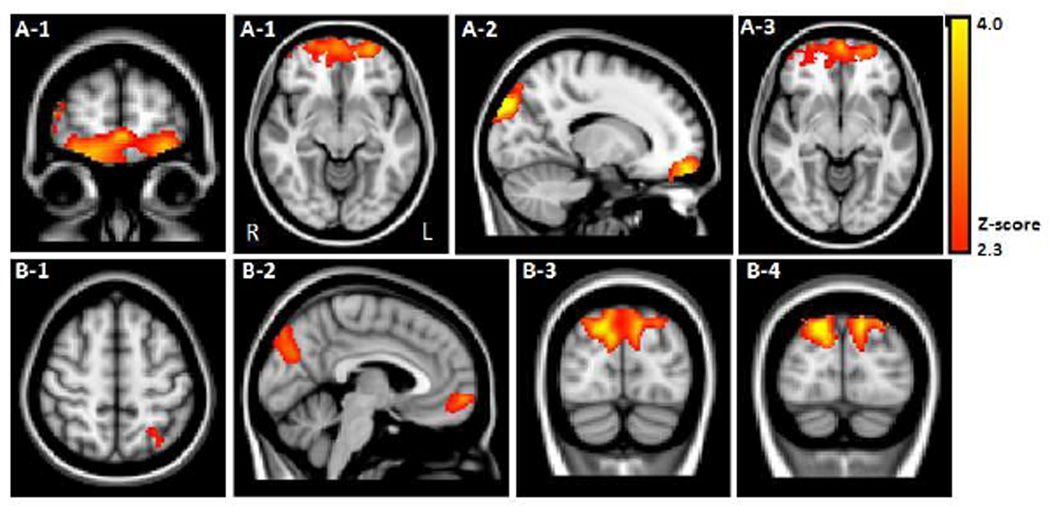
PD subjects demonstrated significant loss of GM volume compared to controls in the following frontal and parieto-occipital brain regions: bilateral frontal pole including the orbitofrontal cortex (BA 10, 11), bilateral medial frontal gyrus (BA 10, 32), left superior parietal cortex (BA 7), bilateral parieto-occipital junction (BA 7, 18, 19), bilateral lateral occipital cortex (BA 19), and bilateral superior occipital cortex (BA 19) [Table 2 and Figure 1. A–B].
Table 2.
Local peaks of clusters indicating significant gray matter loss in PD group compared to Controls. Results were considered significant at FWE (family-wise) corrected p < 0.05.
| Anatomical Region | MNI coordinatesa | Zmaxb | Mean Volumec | Volume Reduction (%) d |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Controls | PD | |||
| Frontal Cortex | |||||||
| R. Frontal Pole (BA 10,11) | 14 | 56 | −16 | 3.93 | 5431±629 | 5011±517 | 7.73 |
| L. Frontal Pole (BA 10,11) | −26 | 62 | −14 | 3.56 | 3384±418 | 3115 ±340 | 7.96 |
| R. Medial Frontal gyrus (BA 10, 32) | 8 | 50 | −10 | 2.43 | 2297±332 | 2113±232 | 8.01 |
| L. Medial Frontal gyrus (BA 10, 32) | −4 | 52 | −14 | 2.67 | 1846±271 | 1699±196 | 7.97 |
| Parieto-Occipital Cortex | |||||||
| L. Superior Parietal Lobe (BA 7) | −28 | −58 | 54 | 2.57 | 152±27 | 138±23 | 8.84 |
| R. Parieto-occipital junction (BA 18) | 2 | −80 | 28 | 3.06 | 4090±589 | 3525±439 | 13.81 |
| L. Parieto-occipital junction (BA 18) | −2 | −78 | 22 | 2.70 | 3139±518 | 2668±407 | 15.00 |
| R. Lateral Occipital Cortex (BA 19) | 24 | −86 | 38 | 3.25 | 2570±346 | 2205±273 | 14.20 |
| L. Lateral Occipital Cortex (BA 19) | −10 | −88 | 38 | 3.91 | 2140±254 | 1808±247 | 15.53 |
| R. Superior Occipital Cortex (BA 19) | 16 | −92 | 36 | 4.71 | 2382±330 | 2029±264 | 14.82 |
| L. Superior Occipital Cortex (BA 19) | −12 | −92 | 36 | 3.44 | 1621±241 | 1351±205 | 16.62 |
Coordinates of local maxima are given in MNI (Montreal Neurological Institute) standard space.
Z-scores of the local maxima.
Cluster extent is given in mean volume ± standard deviation (mm3).
PD patients’ relative gray matter volume reduction (in %), when compared to Controls. BA= Brodmann Area, R.: right, L.: left.
Figure 1.
VBM results contrasting PD < Control indicated GM volume loss in PD subjects in (A) Frontal cortex: bilateral frontal pole (A-1; BA 10, 11) encompassing the orbitofrontal cortex (A-2) and bilateral medial frontal gyrus (A-3; BA 10, 32); (B) Parieto-occipital cortex shown in axial, sagittal, and coronal planes: 1) left superior parietal cortex (BA 7), 2) bilateral parieto-occipital junction (BA 7, 18, 19), 3) bilateral lateral occipital cortex (BA 19), and 4) bilateral superior occipital cortex (BA 19).
Cognitive Tests
PD subjects differed significantly from controls in fine motor speed [F(1,73) = 20.36, p < 0.0001] and set-shifting [F(1,77) =8.65, p = 0.004] after FDR correction. Processing speed, spontaneous flexibility, language, learning/ memory, spatial cognition, and attention domains failed to show group differences following FDR correction (for individual subtests see Table 3).
Table 3.
Neuropsychological test results for Controls and PD subjects.
| Test | Controls (n =40) |
PD (n =40) |
P-value |
|---|---|---|---|
| Fine motor speed | |||
| Grooved Pegboard† | −0.14 ± 0.78 | −3.26 ± 3.05 | < 0.0001§ |
| Processing speed | |||
| Mean z-score | 0.67 ± 0.59 | 0.37 ± 0.79 | 0.196 |
| CWInt Color | 0.52 ± 0.75 | 0.23 ± 1.01 | 0.814 |
| Symbol Search | 0.82 ± 0.74 | 0.50 ± 0.86 | 0.046 |
| Executive function: Set-shifting | |||
| Mean z-score | 0.43 ± 0.34 | −0.03 ± 0.71 | 0.004§ |
| CWInt Inhibition | 0.56 ± 0.63 | 0.18 ± 1.06 | 0.274 |
| CWInt Inhibition Errors | 0.38 ± 0.48 | −0.36 ± 1.07 | 0.0005 |
| CWInt Switch | 0.56 ± 0.85 | 0.49 ± 0.84 | 0.391 |
| CWInt Switch Errors | 0.51 ± 0.57 | 0.13 ± 0.80 | 0.043 |
| VVT Total | 0.35 ± 0.72 | −0.15 ± 1.15 | 0.017 |
| VVT Switch | 0.22 ± 0.66 | −0.44 ± 1.23 | 0.006 |
| Executive function: Spontaneous flexibility | |||
| Mean z-score | 0.28 ± 0.55 | 0.01 ± 0.68 | 0.042 |
| DesFlu Switch | 0.63 ± 0.81 | 0.44 ± 0.86 | 0.547 |
| DesFlu Total Correct | 0.60 ± 0.99 | 0.21 ± 0.94 | 0.116 |
| DesFlu Total Design | 0.88 ± 1.24 | 0.42 ± 1.33 | 0.178 |
| DesFlu Design Accuracy | −0.58 ± 1.06 | −0.57 ± 1.04 | 0.697 |
| VerbFlu Letter | −0.12 ± 0.83 | −0.27 ± 1.20 | 0.239 |
| VerbFlu Category | 0.25 ± 0.93 | −0.18 ± 0.94 | 0.021 |
| Language | |||
| Mean z-score | 0.49 ± 0.57 | 0.22 ± 0.77 | 0.340 |
| CWInt Word | 0.38 ± 0.79 | 0.30 ± 1.01 | 0.659 |
| BNT | 0.61 ± 0.87 | 0.13 ± 1.14 | 0.101 |
| Spatial cognition | |||
| Mean z-score | 0.09 ± 0.53 | −0.21 ± 0.77 | 0.040 |
| JoLO | 0.18 ± 1.06 | −0.35 ± 1.56 | 0.079 |
| DRS2 Construction | 0 | −0.08 ± 0.27 | 0.046 |
| Memory | |||
| Mean z-score | −0.15 ± 1.02 | −0.46 ± 1.07 | 0.126 |
| BVMT-R Total Learning | −0.10 ± 1.30 | −0.43± 1.30 | 0.304 |
| BVMT-R Delayed Recall | 0.12 ± 1.44 | −0.22± 1.35 | 0.343 |
| BVMT-R Discrimination | −0.28 ± 1.63 | −1.04 ± 2.35 | 0.329 |
| Index* | |||
| HVLT-R Total Learning | −0.08 ± 1.28 | −0.40 ± 1.27 | 0.231 |
| HVLT-R Delayed Recall | −0.44 ± 1.00 | −0.35 ± 1.05 | 0.562 |
| HVLT-R Discrimination | −0.19 ± 1.04 | −0.35 ± 1.04 | 0.034 |
| Index* | |||
| Attention | |||
| Mean z-score | 0.40 ± 0.56 | 0.21 ± 0.59 | 0.096 |
| Letter Number Sequencing | 0.28 ± 0.67 | 0.12 ± 0.73 | 0.117 |
| Spatial Span | 0.53 ± 0.88 | 0.38 ± 0.98 | 0.372 |
| Digit Span | 0.40 ± 0.85 | 0.13 ± 0.77 | 0.235 |
Neuropsychological test results showing mean ± standard deviation. All test scores were converted to standard z-scores. Higher z-scores indicate better performance. One-way analysis of covariance (ANCOVA) was conducted with Group as the independent variable and each cognitive domain as the dependent variable, with the Hamilton Depression score as a covariate. Individual neuropsychological tests also were tested using one-way analysis of covariance (ANCOVA) with Hamilton Depression score as a covariate. The analysis results of eight domain variables were considered significant at FDR (false discovery rate) corrected p < 0.05. We report raw p-values and
indicates the domains which were significant after FDR correction.
[CWInt = Color-Word Interference Test; VVT = Visual Verbal Test; DesFlu = Design Fluency; VerbFlu = Verbal Fluency; BNT = Boston Naming Test; JoLO = Judgment of Line Orientation; DRS2 = Mattis Dementia Rating Scale 2; BVMT-R = Brief Visuospatial Memory Test-Revised; HVLT-R = Hopkins Verbal Learning Test-Revised.]
For the Grooved Pegboard test, outliers were excluded from the analysis and the number of subjects (N) was 37 and 39 for controls and PD patients, respectively.
Discrimination index scores were calculated by number of Hits –number of False Alarms.
Correlation Analysis of GM Volume with Relevant Neuropsychological Tests
Spearman’s partial correlation analyses with adjustment for depression were conducted between the brain region volumes (frontal and parieto-occipital areas) and behavioral task scores (fine motor and set-shifting domains) that showed significant group differences. Correlation analyses with the frontal and superior parietal cortex volume were considered primary because these brain areas are more likely to be involved in fine motor [25] and set-shifting tasks [26], with results considered significant at a FDR of 0.05. Correlation analyses between the occipital cortex volume and behavioral task scores were deemed secondary, exploratory analyses and thus no multiple testing corrections were made.
For the primary frontal and parietal volume analyses, controls showed significant positive correlations between fine motor speed and bilateral frontal pole [R=0.322, p=0.028 for left and R=0.359, p=0.016 for right, 1-tailed, N= 37] and bilateral medial frontal gyrus [R=0.339, p=0.022 for left and R=0.395, p=0.009 for right, 1-tailed, N= 37]. The set-shifting domain was positively correlated with bilateral medial frontal gyrus [R=0.296, p=0.034 for left and R=0.328, p=0.021 for right, 1-tailed, N= 40], right frontal pole [R=0.285, p=0.040, 1-tailed, N= 40], and left superior parietal cortex [R=0.402, p=0.006, 1-tailed, N= 40], all of which remained significant after FDR correction. In PD patients, there were no significant correlations of frontal and parietal cortex volume with either behavioral task (ps > 0.36).
For the secondary occipital volume analyses, PD patients did show positive correlations between fine motor speed and left parieto-occipital junction [R=0.301, p=0.033, 1-tailed, N= 39], left superior occipital cortex [R=0.360, p=0.013, 1-tailed, N= 39], and left lateral occipital cortex [R=0.401, p=0.006, 1-tailed, N= 39]. The set-shifting domain also was correlated positively with right lateral occipital cortex in PD patients [R=0.345, p=0.016, 1-tailed, N= 40], whereas there were no such correlations for controls (0.477 > ps > 0.052).
Discussion
This study examined the relationship between GM volume and cognitive functions in early PD patients without dementia. The results revealed that cortical GM volume was reduced in early PD compared to controls and this loss was related to PD cognitive status. Specifically, early stage, non-demented PD patients demonstrated lower GM volume predominantly in frontal and parieto-occipital regions, which ranged from 7.73% (right frontal pole) to 16.62% (left superior occipital cortex) volume reduction compared to controls. Patients also showed lower performance on fine motor and set-shifting tasks, representing on average a 93% and 10% performance decrement, respectively, when compared to controls. Interestingly, scores on these tasks correlated significantly with occipital GM volumes in PD subjects rather than with the expected frontal lobe regions identified in controls.
Previous studies have reported GM volume loss in PD patients of various age ranges and disease stages [7,27]. The current results expand the knowledge by showing that GM volume loss can be found even in early-stage PD patients who are considerably younger (mean age = 59 years with maximum age <70 years) than those typically reported in the literature (i.e., mean age = 69.6 years) [27]. It is possible that the observed GM changes are due to primary cortical GM pathology [5] that may contain Lewy bodies and neurites even in early clinical stage patients. Since many of the cortical areas have direct connections with the striatum [3,4], it also is possible that the cortical changes may reflect progressive striato-thalamo-cortical circuitry dysfunction in PD, although changes in subcortical structures were not detected using the restrictive FWE correction in the present study. It is noteworthy that PD patients had relatively greater volume reduction in occipital regions than frontal and parietal cortex compared to controls. The exact cause of these differences is not clear but may arise because elderly controls already have sustained some GM loss in fronto-parietal regions as part of the normal aging process, whereas the occipital volume are relatively preserved with normal aging [28].
In addition to GM loss, PD patients had reduced performance on the neuropsychological tests compared to controls, particularly in the fine motor speed and set-shifting domains. Impaired fine motor speed may have been exaggerated by testing patients in a practically defined “off” state. Future studies comparing “off” and “on” states in the same population may help to clarify this issue. In contrast to a recent study [27], the current analysis did not find group differences in spontaneous flexibility, spatial cognition, learning/memory, or attention/working memory tests after correcting for multiple comparisons. This may be due partly to PD patients in the current study being considerably younger than in other studies (vide supra) [27] and in earlier disease stages. PD patients also were under treatment for their disease, although they were tested after an overnight (12 hr) medication withdrawal. It is possible that some of the null effects listed above might be masked by residual medication effects, since some PD medications have long half-lives (e.g., monoamine oxidase inhibitors). Nevertheless, there were some trends toward reduced spontaneous flexibility, spatial cognition, and attention in the current sample of PD subjects. These trends may become more apparent with disease progression.
The correlations observed between cortical GM volume loss and decline in certain functional areas extend the understanding of possible pathophysiological substrates for PD symptoms. As expected, initial examination of controls indicated that fine motor function and set-shifting were associated with fronto-parietal regions. These results are consistent with previous studies revealing a predominant role of the prefrontal cortex as part of fronto-striatal circuits in mediating normal fine motor and set-shifting behaviors, as well as the involvement of parietal cortex in set-shifting domain [26]. Considering the relatively smaller volume reduction in frontal areas, the lack of correlation between the fine motor/set-shifting and frontal lobe volume in PD implies that frontal atrophy may not be the determining factor for the “frontal lobe” dysfunction in PD. Indeed, it is well known that chemical changes (i.e. dopamine deficiency) may contribute to dysfunction on these tasks due to disruption in cortical-striatal processing [29].
Interestingly, reduced fine motor speed and set shifting (typical “frontal lobe” functions) in PD were both associated with reduced volume predominantly in occipital cortical areas—the areas where we found the most volume change in PD compared to controls. These significant associations may represent the compensatory role of occipital cortex for primary fronto-striatal pathology. Namely, the PD subjects with lowest volume in occipital cortex are less likely to take over the “typical frontal lobe” function in PD and vice versa. Indeed, a previous neuroimaging study revealed that during sequential finger movements, PD patients showed less activity in frontal cortex but increased activity in parietal and lateral premotor cortices compared to controls, suggesting functional changes of brain regions in PD [30].
In summary, the current results support the hypothesis that early stage PD subjects sustain cortical GM volume loss and demonstrate reduced performance in select cognitive functions. Furthermore, the cognitive decline correlated with measured loss in cortical GM volume. These results suggest that in addition to striato-thalamo-cortical circuitry dysfunction, there may be cortical anatomical changes and altered functional networks, which may underlie PD cognitive symptoms. A longitudinal study of this cohort is ongoing to clarify the effects of disease progression and potentially modifiable neuronal and cognitive factors.
Acknowledgements
This work was supported by grants NS060722 (XH), and the HMC GCRC (NIH M01RR10732) and GCRC Construction Grant (C06RR016499). We thank all the participants in the study, as well as their caregivers and the controls, and acknowledge the critical support of the study coordinator, Ms. Brittany Jones, and the MRI technical expertise from Mr. Jeffery Vesek.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors report no conflicts of interest.
Statistical analysis: Performed by Drs. Eun-Young Lee, Suman Sen, Michele L. Shaffer, and Lan Kong.
Author Roles
Eun-Young Lee: Data analysis and interpretation; statistical analysis; primary drafting and critical revision of the manuscript.
Suman Sen: Conception and design of the study; data acquisition, analysis and interpretation; statistical analysis; primary drafting and critical revision of the manuscript.
Paul J. Eslinger: Conception and design of the study; data acquisition, analysis, and interpretation; drafting and critical revision of the manuscript.
Daymond Wagner: Data acquisition and interpretation; critical revision of the manuscript.
Michele L. Shaffer: Statistical analysis of the data; critical revision of the manuscript.
Lan Kong: Statistical analysis of the data; critical revision of the manuscript.
Mechelle M. Lewis: Data acquisition; critical revision of the manuscript; administrative support for the study.
Guangwei Du: Data acquisition; critical revision of the manuscript.
Xuemei Huang: Conception and design of the study; data acquisition and interpretation; drafting and critical revision of the manuscript; funding of the study; administration and supervision of the project.
References
- 1.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1300. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 2.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 3.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur J Neurol. 2012;19:199–206. doi: 10.1111/j.1468-1331.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 7.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord. 2009;24:1193–1199. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- 8.Dalaker TO, Zivadinov R, Larsen JP, Beyer MK, Cox JL, Alves G, et al. Gray matter correlations of cognition in incident Parkinson's disease. Mov Disord. 2010;25:629–633. doi: 10.1002/mds.22867. [DOI] [PubMed] [Google Scholar]
- 9.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 10.Gunstad J, Spitznagel MB, Luyster F, Cohen RA, Paul RH. Handedness and cognition across the healthy lifespan. Int J Neurosci. 2007;117:477–485. doi: 10.1080/00207450600773483. [DOI] [PubMed] [Google Scholar]
- 11.Calne D, Snow B, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurica PJ, Leitten CL, Mattis S. Dementia. Ratings Scale-2. Professional Manual. Odessa FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 19.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 20.Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 21.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 22.Benedict R. Brief Visuospatial Memory Test, Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 23.Brandt J, Benedict R. Hopkins verbal learning test, Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Series B (Methodological) 1995:289–300. [Google Scholar]
- 25.Marchand WR, Lee JN, Thatcher JW, Hsu EW, Rashkin E, Suchy Y, et al. Putamen coactivation during motor task execution. Neuroreport. 2008;19:957–960. doi: 10.1097/WNR.0b013e328302c873. [DOI] [PubMed] [Google Scholar]
- 26.Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural Bases of Set-Shifting Deficits in Parkinson's Disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibarretxe-Bilbao N, Junque C, Marti MJ, Tolosa E. Brain structural MRI correlates of cognitive dysfunctions in Parkinson's disease. J Neurol Sci. 2011;310:70–74. doi: 10.1016/j.jns.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 28.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 30.Samuel M, Ceballos-Baumann A, Blin J, Uema T, Boecker H, Passingham R, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]