Abstract
Objective
To identify determinants of HbA1c levels one year after the diagnosis of type 1 diabetes (T1D) in participants in the Pediatric Diabetes Consortium (PDC) T1D New Onset (NeOn) Study.
Research Design and Methods
Diabetes-specific as well as socioeconomic factors during the first year following diagnosis were analyzed in 857 participants (mean age 9.1 years, 51% female, 66% non-Hispanic White) not participating in an intervention study who had an HbA1c value at 12 months.
Results
Mean ± SD HbA1c at one year was 62 ± 16 mmol/mol (7.8% ± 1.5). In univariate and multivariate analyses, clinical center, non-Hispanic White race, private health insurance, living with both parents, higher frequency of self-monitoring of blood glucose (SMBG), and lower insulin requirements were associated with lower HbA1c concentrations at one year (p<0.01). No association was found with gender, age, Tanner stage, BMI, DKA at onset, number of positive autoantibodies or HbA1c at onset, or number of visits to diabetes physician during the first year.
Conclusions
White race, higher socioeconomic status, two-parent household, more frequent SMBG and low insulin requirements are associated with lower HbA1c concentration one year after the onset of T1D in children.
Keywords: Diabetes Mellitus, Type 1, Hemoglobin A1c
Introduction
Glucose control, as reflected by HbA1c, is one of the strongest predictors of chronic complications in adults (1) and children (2) with type 1 diabetes (T1D) including proliferative retinopathy (3), diabetic nephropathy (4), cardiovascular disease and age-adjusted mortality (5, 6) rates. Poor glucose control during the first few years of T1D in children predicts development of microalbuminuria (7) and retinopathy (8) in adulthood.
Although there are reports on factors associated with HbA1c in patients with established T1D (9–19), there are no published studies on the association of diabetes-specific and socioeconomic factors with HbA1c during the early stages of the disease in a representative sample of children who receive their care at pediatric diabetes centers in the United States.
The Pediatric Diabetes Consortium (PDC) T1D New Onset (NeOn) study aims to improve the care of children with diabetes through sharing of best practices (20). This large and geographically and ethnically diverse cohort of youth with T1D offered us the unique opportunity to study possible factors that may be associated with metabolic control one year after the diagnosis of T1D.
Study Design and Methods
Patients
The PDC T1D NeOn Study enrolled 1,052 patients with T1D between July 2009 and April 2011. The protocol was approved by the Institutional Review Board (IRB) at each of the 7 participating centers. Informed consent was obtained from participants 18 years of age and older and from parents of those less than 18 years of age. Assent was obtained from participants <18 years of age as required by local IRB regulations. To be eligible for enrollment in the study, patients had to be <19 years of age and managed at one of the 7 participating PDC centers within 3 months of diagnosis. A detailed description of PDC and the design of the study have been published previously (20). The analyses reported herein included data from 857 participants; 163 were excluded from this analysis due to no HbA1c measurement available at one year (319–455 days from diagnosis), and 32 were excluded due to participation in an intervention study.
Data Collection
Demographic, socioeconomic status (SES) and clinical characteristic data were collected from medical records and from interviews with the patient and/or parent. Follow-up visits were completed per usual care and all visits during the first year post-diagnosis were entered in standardized electronic case report forms for the study.
Body mass index (BMI) at diagnosis was computed from the closest height and weight within ± 14 days of diagnosis. BMI percentiles and Z scores adjusted for age and gender were calculated using 2000 CDC population growth chart data (21). If height and/or weight was missing within ± 14 days of diagnosis or if the participant was <2 years of age at the time of diagnosis, BMI percentile was not calculated.
Diabetes ketoacidosis (DKA) was defined according to DCCT criteria of pH <7.3 or HCO3 <15mEq/L and treatment in a healthcare facility (22). Tests for diabetes autoantibodies were included any time prior to diagnosis or within 91 days after diagnosis for insulinoma-associated autoantibody (IA-2) or glutamic acid decarboxylase (GADA) and within 14 days after diagnosis for insulin autoantibody (IAA).
Statistical Analysis
Least squares regression was used to assess the association of baseline (demographic, SES, and clinical) and one-year (total daily insulin dose, SMBG frequency, and number of visits to diabetes management providers [excluding visits during the first 3 weeks following diagnosis, which correspond to initial education in centers where patients are not routinely admitted at diagnosis]) factors with HbA1c at one year from T1D diagnosis. Initial models were constructed for each baseline factor individually. A multivariate model of baseline factors was then constructed using stepwise selection with p-values <0.10 required to be included in the model. Due to multiple comparisons, only factors with p-values <0.01 were considered statistically significant although factors with p-values <0.10 were included in the model to adjust for potential confounding. Interaction terms were tested for all variables included in the final multivariate model with a p-value <0.01 required to be included. A second multivariate model added the one-year factors to the significant baseline factors. Continuous variables were tested for linearity by adding a quadratic term to the model. If significant non-linearity was detected the variable was divided into categories and analyzed as discrete variables. Missing covariates were treated as a separate category for discrete variables and a missing value indicator was added to the model for continuous variables. All reported p-values are two-sided. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
At the time of diagnosis, the mean ± SD age of the 857 participants was 9.1 ± 4.1 years, 51% were female, 66% were Non-Hispanic White, and 68% had private health insurance. Mean ± SD HbA1c was 102 mmol/mol ± 25 (11.4% ± 2.3), 19% of participants were overweight or obese (BMI ≥85th percentile for age and gender) and 33% had DKA. Of the 510 participants who were tested for all three autoantibodies, 95% were positive for at least one (Table 1).
Table 1.
Participant Characteristics at Diagnosis (N=857a)
| % | |
|---|---|
| Gender: Female | 51% |
| Age (years) | |
| <5 | 19% |
| 5–<12 | 56% |
| 12–<19 | 25% |
| Mean ± SD | 9.1 ± 4.1 |
| Range | 0.7 − 18.8 |
| Tanner Stageb | |
| I | 67% |
| II | 11% |
| III | 7% |
| IV | 7% |
| V | 7% |
| BMI Percentilec | |
| <85th | 81% |
| 85th–<95th (Overweight) | 10% |
| ≥ 95th (Obese) | 9% |
| Race/Ethnicity | |
| White | 66% |
| Hispanic or Latino | 21% |
| Black/African American | 7% |
| Other/More than one race | 6% |
| Health Insurance | |
| Private | 68% |
| Military/CHP/Medicaid/Medicare | 30% |
| None | 2% |
| Parent Education | |
| High school or less | 32% |
| AA/BS/BA | 44% |
| MS/MA or professional degree | 23% |
| Family Income | |
| <$25,000 | 13% |
| $25,000−$49,999 | 19% |
| $50,000−$74,999 | 17% |
| $75,000−$99,999 | 14% |
| ≥ $100,000 | 37% |
| Family Structure | |
| Lives with both parents | 72% |
| HbA1c mmol/mol (%) | |
| <86 (<10%) | 28% |
| 86−<119 (10–<13%) | 45% |
| ≥ 119 (≥ 13%) | 27% |
| Mean ± SD | 102 ± 25 (11.4 ± 2.3%) |
| Range | 37−180 (5.5−18.6%) |
| Number of Positive Autoantibodiesd | |
| 0 | 5% |
| 1 | 20% |
| 2 | 39% |
| 3 | 35% |
| DKA | 33% |
Number of participants with missing data: Tanner stage (412), BMI percentile (361), race/ethnicity (20), health insurance (15), parent education (163), family income (280), family structure (1), HbA1c (39), DKA (27).
Imputed as stage 1 for girls < 8 years of age and boys < 10 years of age.
BMI percentile not calculated for n=36 participants less than 2 years of age.
Limited to those tested for all three auto antibodies (510).
One year after diagnosis of T1D, mean ± SD HbA1c was 62 mmol/mol ± 16 (7.8% ± 1.5) with a median insulin dose of 0.6 units/kg/day (interquartile range [IQR] 0.5 to 0.8). The median number of self-reported SMBG tests per day was 5 (IQR 4 to 6) and the median number of visits to diabetes providers during the first year of T1D was 4 (IQR 4 to 5). In cases where a blood glucose meter download was available (n=469), the median (and IQR) number of SMBG tests per day was similar to self-report. Insulin pump therapy was being used by 34% of participants at one year and 59% of all participants were taking three or more injections of insulin per day (Table 2).
Table 2.
Participant Characteristics at One Year (N=857a)
| % | |
|---|---|
| HbA1c mmol/mol (%) | |
| <53 (<7%) | 29% |
| 53−<64 (7–<8%) | 34% |
| 64−<75 (8–<9%) | 20% |
| ≥ 75 (≥ 9%) | 17% |
| Mean ± SD | 62 ± 16 (7.8 ± 1.5%) |
| Range | 31−140 (5.0−15.0%) |
| Insulin Delivery | |
| Pump | 34% |
| 1−2 injections/day | 7% |
| 3–4 injections/day | 45% |
| 5 or more injections/day | 14% |
| Median (IQR)b | 4 (3 to 4) |
| Rangeb | 1−12 |
| Insulin Dose (units/kg/day) | |
| <0.5 | 32% |
| 0.5–<0.8 | 44% |
| ≥ 0.8 | 25% |
| Median (IQR) | 0.6 (0.5 to 0.8) |
| Range | 0.02−4.6 |
| SMBG (# tests/day) | |
| 1−3 | 13% |
| 4–6 | 66% |
| ≥ 7 | 22% |
| Median (IQR) | 5 (4 to 6) |
| Range | 2−18 |
|
Visits to Diabetes Team Providers through 1 Year |
|
| 1−3 | 12% |
| 4–5 | 76% |
| ≥ 6 | 11% |
| Median (IQR) | 4 (4 to 5) |
| Range | 1−9 |
Number of participants with missing data: number of injections (305), insulin dose (124), blood glucose monitoring (360).
Limited to those not on an insulin pump (552).
Several baseline (i.e., at diagnosis) characteristics were significantly associated with lower HbA1c at one year in univariate analyses: clinical site, white race, private health insurance, higher household income, higher parent education level, living with both parents, and absence of DKA at diagnosis (p-value <0.01 for each, Table 3). In the multivariate analysis of the baseline characteristics, clinical site, race, health insurance status and family structure remained significant. The effects of family income and parent education were confounded with the other SES factors so that possible independent effects of these factors cannot be confirmed nor ruled out. In the multivariate analysis, there was no detectable association with gender, age, Tanner stage, BMI, number of positive anti-islet autoantibodies, DKA at onset or HbA1c at onset. Positivity for each of the autoantibodies, i.e., GADA, IAA or IA-2, was not associated with one-year HbA1c (p-value >0.10; data not shown).
Table 3.
Factors at Diagnosis Associated with HbA1c at One Year (N±857a)
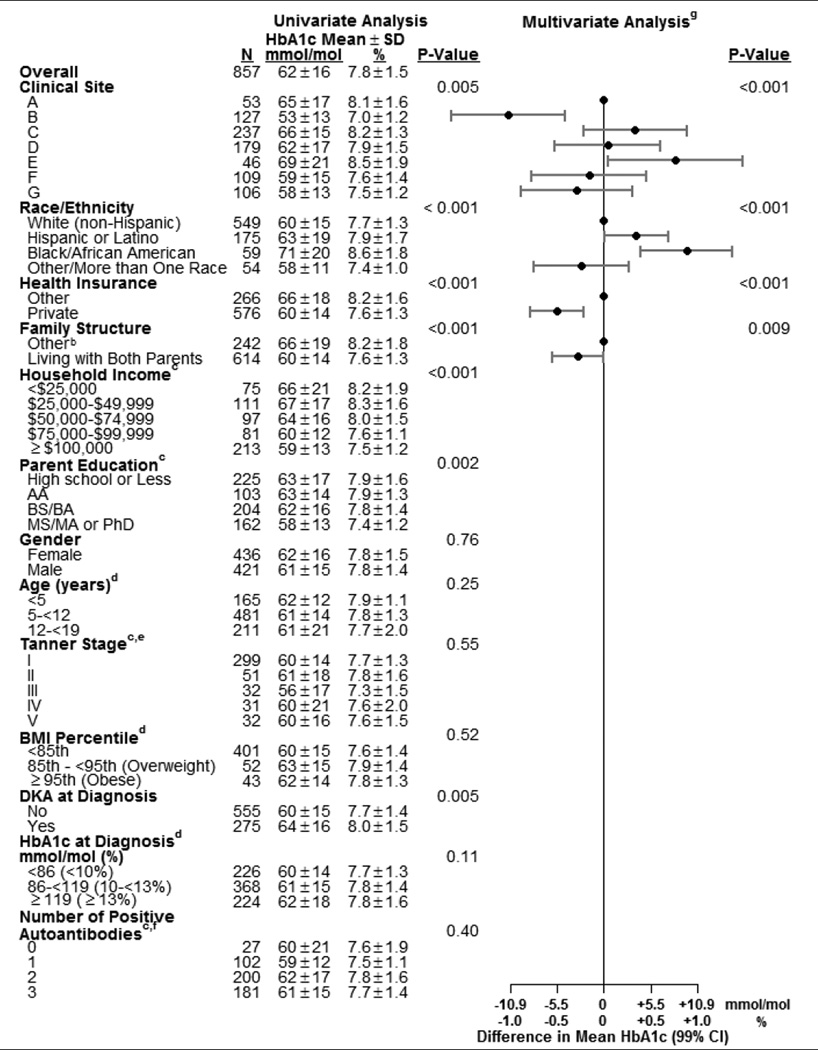 |
Number of participants with missing data: race/ethnicity (20), health insurance (15), family structure (1), household income (280), parent education (163), Tanner stage (412), BMI percentile (361), DKA (27) and HbA1c (39).
“Other” could be lives with mother, lives with father, splits time with mother and father, lives with legal guardian who is not parent, lives away at school or other.
Analyzed as an ordinal variable.
Analyzed as a continuous variable. Categories are for display purposes in this table.
Missing values imputed as stage I for girls < 8 years of age and boys < 10 years of age.
Limited to those tested for all three auto antibodies (510).
The multivariate model contains all factors with an adjusted p-value <0.10 to account for potential confounding, but only p-values <0.01 are considered statistically significant in this analysis. Factors with blank entries in the multivariate columns were excluded from the model because p ≥0.10.
In the multivariate model for one-year factors, adjusting for the significant baseline factors indicated above, lower one-year HbA1c was associated with higher frequency of SMBG testing and a lower total daily insulin dose per kg of body weight (both p-value <0.001; Table 4, Figure 1).
Table 4.
Factors at One Year Associated with HbA1c at One Year (N±857a)
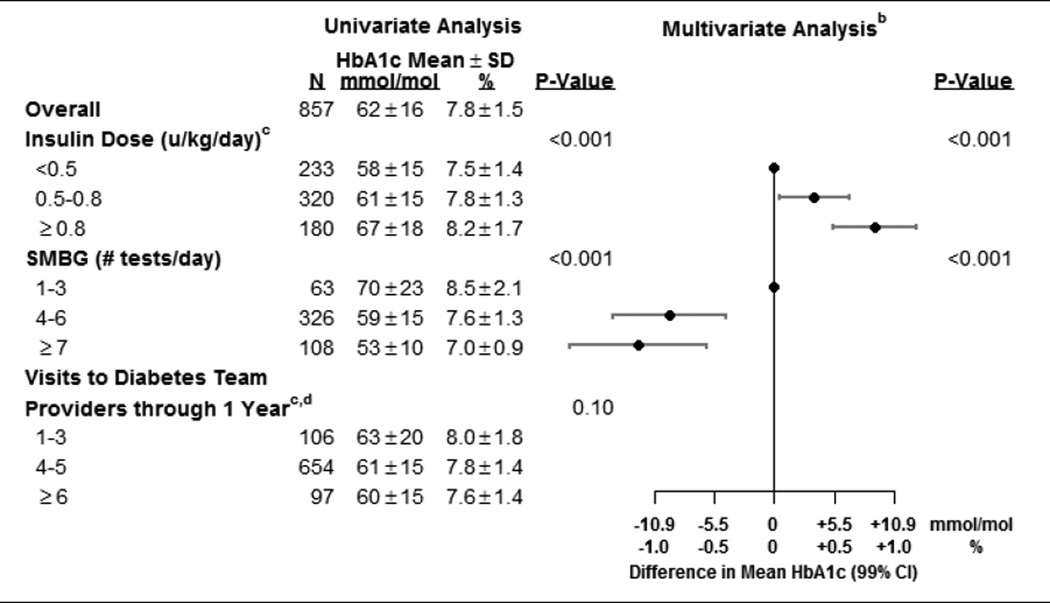 |
Number of participants with missing data: insulin dose (124) and blood glucose monitoring (360).
Also adjusted for all factors (p<0.10) from diagnosis model in Table 3.
Analyzed as continuous variables. Categories are for display purposes in this table.
Visits during the first 3 weeks following diagnosis were excluded due to differences among centers in initial diabetes education.
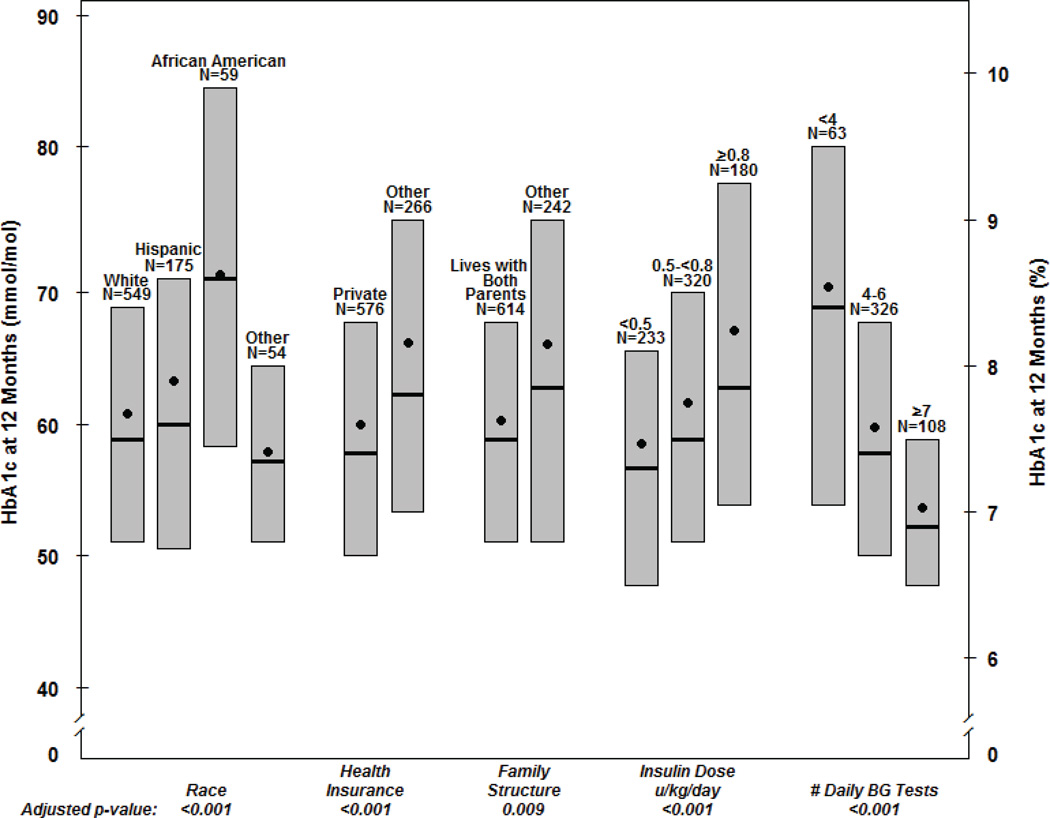
Figure 1. Factors Associated with HbA1c at One Year (N=857a).
Bottom and top of each box denote the 25th and 75th percentiles. Horizontal line inside each box denotes the median and the dot denotes the mean.
a. Number of participants with missing data: race/ethnicity (20), health insurance (15), family structure (1), insulin dose (124), # daily BG tests (360).
DISCUSSION
This study describes a large cohort of racially diverse children from seven pediatric diabetes treatment centers across the United States and reports characteristics associated with glycemic control one year following diagnosis of T1D. We observed that race/ethnicity, socioeconomic factors, frequency of SMBG and lower dose of insulin were independently associated with HbA1c one year after diagnosis of T1D in children.
There have been few previous studies analyzing factors associated with HbA1c at the early stages of T1D in children. In a study of 275 European children, Mortensen et al. identified ethnic minority, higher HbA1c at diagnosis and positivity for GAD65 autoantibodies as predictors of higher HbA1c one year after diagnosis (23). Our study found that the association between race/ethnicity and one-year HbA1c was independent of SES, frequency of SMBG, pump use and insulin dose. Higher levels of HbA1c have been previously reported in African-American adults with and without diabetes (24) and minority youth with diabetes (9) compared with their white counterparts. Whether this difference reflects higher glycemia, or higher rates of glycosylation, is currently unknown (25, 26). It is also possible that the distribution of socioeconomic factors is narrower for minorities thus not allowing studies to completely rule out confounding. It is concerning, however, that African-Americans also have higher mortality rates and risk of diabetic complications than whites (27).
Several socioeconomic factors were significant in univariate analysis, but because of colinearity, were not included in the multivariate model. Health insurance, the SES factor that was included in the multivariate analysis, was independently associated with HbA1c at one year. Our analysis does not allow us to completely rule out a possible independent effect of family income, structure or education. Socioeconomic factors were not analyzed in Mortensen’s study (23) but analyses in established, long-duration T1D have demonstrated a strong impact of health insurance type (28, 29), family structure (12–14, 18), education (18), family income (19, 30) and other social circumstances (e.g. immigration) (17, 31, 32) on glucose control. Although health insurance type may impact the choice of insulin delivery modality (28), in our analysis both factors were analyzed as independent variables associated with HbA1c.
Higher frequency of SMBG was associated with lower one-year HbA1c after adjustment for the other variables in the model. The association between frequency of SMBG and glucose control has been reported in patients with established T1D in several (15, 16, 18, 19, 33–35) but not all (14, 36) studies. The disagreement among studies might be explained by the strong and complex relationships between SMBG and other factors, such as use of insulin pumps or family structure and support (14), which also affect HbA1c. Our and others’ studies (18) suggest that frequency of SMBG is associated with HbA1c independently of those factors, and thus frequent BG monitoring should be encouraged.
We found that lower dose of insulin was independently associated with HbA1c at one year. In patients who retain residual endogenous insulin secretion, the dose of exogenous insulin adjusted for body weight is a measure of both beta-cell function and insulin resistance. In this fashion, insulin dose has been used to define the partial remission period (37, 38). The long known association between greater beta-cell function and lower HbA1c (39) provides the rationale for trials that aim to preserve beta-cell function and prolong the partial remission period in an effort to prevent complications of diabetes.
Clinical center was independently associated with HbA1c concentrations after adjustment for other variables. Differences in the therapeutic approaches by different centers may explain this finding, highlighting the importance of networks that promote sharing clinical practices among centers.
In our study, age was not associated with HbA1c. This is contrary to studies in established T1D that have found that age, and in particular late adolescence, is associated with higher HbA1c (11, 14, 16, 31). A likely explanation for this discrepancy is that we studied children and adolescents only one year after onset and therefore before the deterioration in compliance with diabetes tasks and glucose control that are typical during adolescence (40, 41). Alternatively, the challenges of managing diabetes in teenagers may have been offset by the accelerated rate of autoimmune destruction of beta-cells in younger patients, compared with adolescents. Mortensen et al. (23) also did not find an association with age.
Several studies have demonstrated that the influence of glucose control on risk of complications persists for many years (5). HbA1c in the first five years of childhood-onset T1D predicts microalbuminuria (7) and retinopathy (8) on follow-up. HbA1c at onset of diabetes correlates with glucose control in subsequent years (36, 42) and predicted microalbuminuria 11 years later (6). Our study sought to understand the factors associated with metabolic control one year after T1D diagnosis in children. By addressing characteristics that are associated with HbA1c at the early stages of disease, long-term chronic complications could be prevented in patients with childhood-onset T1D.
Although the list of predictors that our study was able to assess was comprehensive, one limitation is that we did not explore psychological factors. Previous literature indicates that diabetes-specific conflict (15, 34), self-care behavior, eating disturbances, depression (11), and family behavior factors (12, 43) influence glucose control in established T1D likely through their effect on adherence to treatment (40). It is likely that these factors also affect HbA1c at the early stages of disease. Missing data for some of the factors studied at one year of onset is another limitation of the study.
In summary, we studied a large sample of children with T1D and analyzed a comprehensive series of variables at diagnosis and during the first year of T1D. We found that sociodemographic factors, SMBG frequency and low insulin requirements are associated with HbA1c at one year of childhood-onset T1D. Further investigation is needed to identify potentially modifiable factors that mediate these associations.
Acknowledgements
The Pediatric Diabetes Consortium and its activities are supported by the Jaeb Center for Health Research Foundation through an unrestrictive grant from Novo Nordisk. The University of Michigan Consortium center is supported by the Michigan Diabetes Research and Training Center from the National Institute of Diabetes and Digestive and Kidney Diseases (DK020572).
Abbreviations
- HbA1c
Hemoglobin A1c
- T1D
type 1 diabetes
- PDC
Pediatric Diabetes Consortium
- NeOn
New Onset Study
- SMBG
self-monitoring of blood glucose
- BMI
body mass index
- DKA
diabetic ketoacidosis
- IRB
Institutional Review Board
- SES
socioeconomic status
- CDC
Centers for Disease Control and Prevention
- DCCT
The Diabetes Control and Complications Trial
- IA-2
insulinoma-associated autoantibody
- GADA
glutamic acid decarboxylase
- IAA
insulin autoantibody
- SD
standard deviation
- BG
blood glucose
The Pediatric Diabetes Consortium Study Group
Clinical Centers: (Listed clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Baylor College of Medicine, Houston, TX: Morey Haymond, MD (PI); Maria J. Redondo, MD, PhD (I); Krishna Hassan, MD (C); Kathy Shippy, RN, CCRP (C); Chris George (C); Mariam Pontifes (C); (2) Children’s Hospital of Los Angeles, Los Angeles, CA: Jamie Wood, MD (PI); Brian Ichihara, BA (C); Megan Lipton, MA, CCRP (C); Marisa Cohen, MPH (C); (3) Stanford University, Stanford, CA: Bruce Buckingham, MD (PI); Breanne Harris, BS (C); Satya Shanmugham, BS (C); (4) Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Georgeanna J. Klingensmith, MD (PI); Eric Cruz, BA (C); Heidi Haro, BA, BS (C); Maria King, BA (C); Katherine Manseau (C); (5) University of Florida, Gainesville, FL: Desmond Schatz, MD (PI); Janet Silverstein, MD (I); Michael J. Haller, MD (I); Erica Dougherty, BS (C); (6) Yale University, New Haven, CT: William V. Tamborlane, MD (I); Eda Cengiz, MD (PI); Melody Martin, CCRP (C); Amy Steffen, BA (C); Lori Carria, MS (C); Darryll Cappiello (C); (7) University of Michigan, Ann Arbor, MI: Joyce M. Lee, MD, MPH (PI); Surair Bashir (C); Ashley Eason (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Crystal G. Connor, MS, MPH; Beth Stevens.
References
- 1.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336:367–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrivarhaug T, Fosmark DS, Stene LC, et al. Low cumulative incidence of proliferative retinopathy in childhood-onset type 1 diabetes: a 24-year follow-up study. Diabetologia. 2006;49:2281–2290. doi: 10.1007/s00125-006-0364-7. [DOI] [PubMed] [Google Scholar]
- 4.Skrivarhaug T, Bangstad H-J, Stene LC, Sandvik L, Hanssen KF, Joner G. Low risk of overt nephropathy after 24 yr of childhood-onset type 1 diabetes mellitus (T1DM) in Norway. Pediatr Diabetes. 2006;7:239–246. doi: 10.1111/j.1399-5448.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 5.Stadler M, Auinger M, Anderwald C, et al. Long-Term Mortality and Incidence of Renal Dialysis and Transplantation in Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2006;91:3814–3820. doi: 10.1210/jc.2006-1058. [DOI] [PubMed] [Google Scholar]
- 6.Giordano C, Amato MC, Ciresi A, et al. Predictors of microvascular complications in type 1 diabetic patients at onset: The role of metabolic memory. Eur J Intern Med. 2011;22:266–274. doi: 10.1016/j.ejim.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Rudberg S, Ullman E, Dahlquist G. Relationship between early metabolic control and the development of microalbuminuria--a longitudinal study in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:1309–1314. doi: 10.1007/BF00400811. [DOI] [PubMed] [Google Scholar]
- 8.Svensson M, Eriksson JW, Dahlquist G. Early Glycemic Control, Age at Onset, and Development of Microvascular Complications in Childhood-Onset Type 1 Diabetes: A population-based study in northern Sweden. Diabetes Care. 2004;27:955–962. doi: 10.2337/diacare.27.4.955. [DOI] [PubMed] [Google Scholar]
- 9.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic Control in Youth with Diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668–672. doi: 10.1016/j.jpeds.2009.05.025. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of Insulin Regimens and Impact on Outcomes in Youth with Type 1 Diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:183–189. doi: 10.1016/j.jpeds.2009.01.063. e1. [DOI] [PubMed] [Google Scholar]
- 11.Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of Metabolic Control among Adolescents with Diabetes: A 4-Year Longitudinal Study. J Pediatr Psychol. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meunier J, Dorchy H, Luminet O. Does family cohesiveness and parental alexithymia predict glycaemic control in children and adolescents with diabetes? Diabet Metab. 2008;34:473–481. doi: 10.1016/j.diabet.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Swift EE, Chen R, Hershberger A, Holmes CS. Demographic risk factors, mediators, and moderators in youths’ diabetes metabolic control. Ann Behav Med. 2006;32:39–49. doi: 10.1207/s15324796abm3201_5. [DOI] [PubMed] [Google Scholar]
- 14.Urbach SL, LaFranchi S, Lambert L, Lapidus JA, Daneman D, Becker TM. Predictors of glucose control in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:69–74. doi: 10.1111/j.1399-543X.2005.00104.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreland EC, Tovar A, Zuehlke JB, Butler DA, Milaszewski K, Laffell LM. The impact of physiological, therapeutic and psychosocial variables on glycemic control in youth with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1533–1544. doi: 10.1515/jpem.2004.17.11.1533. [DOI] [PubMed] [Google Scholar]
- 16.Haller MJ, Stalvey MS, Silverstein JH. Predictors of Control of Diabetes: Monitoring May be the Key. J Pediatr. 2004;144:660–661. doi: 10.1016/j.jpeds.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 17.Scottish Study Group for the Care of the Young Diabetic. Factors Influencing Glycemic Control in Young People With Type 1 Diabetes in Scotland: A population-based study (DIABAUD2) Diabetes Care. 2001;24:239–244. doi: 10.2337/diacare.24.2.239. [DOI] [PubMed] [Google Scholar]
- 18.Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139:197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 19.Rosilio M, Cotton JB, Wieliczko MC, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes The French Pediatric Diabetes Group. Diabetes Care. 1998;21:1146–1153. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric Diabetes Consortium. The Pediatric Diabetes Consortium: Improving Care of Children with Type 1 Diabetes Through Collaborative Research. Diabetes Technol Ther. 2010;12:685–688. doi: 10.1089/dia.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski R, Ogden C, Grummer-Strawn L, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 22.The Diabetes Control and Complications Trial Research Group. The Diabetes Control and Complications Trial (DCCT) Design and methodologic considerations for the feasibility phase. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 23.Mortensen HB, Swift PG, Holl RW, et al. Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218–226. doi: 10.1111/j.1399-5448.2009.00566.x. [DOI] [PubMed] [Google Scholar]
- 24.Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-Independent, Black-White Differences in Hemoglobin A1c Levels A Cross-sectional Analysis of 2 Studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial Differences in Glycemic Markers: A Cross-sectional Analysis of Community-Based Data. Ann Intern Med. 2011;154:303–309. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamps JL, Hempe JM, Chalew SA. Racial Disparity in A1C Independent of Mean Blood Glucose in Children With Type 1 Diabetes. Diabetes Care. 2010;33:1025–1027. doi: 10.2337/dc09-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. Cause-Specific Mortality Trends in a Large Population-Based Cohort With Long-Standing Childhood-Onset Type 1 Diabetes. Diabetes. 2010;59:3216–3222. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wintergerst KA, Hinkle KM, Barnes CN, Omoruyi AO, Foster MB. The impact of health insurance coverage on pediatric diabetes management. Diabetes Res Clin Pract. 2010;90:40–44. doi: 10.1016/j.diabres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Beck JK, Lewis TV, Logan KJ, Harrison DL, Gardner AW, Copeland KC. Intensive vs conventional insulin management initiated at diagnosis in children with diabetes: Should payer source influence the choice of therapy? Pediatr Diabetes. 2009;10:368–373. doi: 10.1111/j.1399-5448.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 30.Gallegos-Macias AR, Macias SR, Kaufman E, Skipper B, Kalishman N. Relationship between glycemic control, ethnicity and socioeconomic status in Hispanic and white non-Hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes. 2003;4:19–23. doi: 10.1034/j.1399-5448.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilf-Miron R, Peled R, Yaari E, et al. Disparities in diabetes care: role of the patient’s socio-demographic characteristics. BMC Public Health. 2010;10:729. doi: 10.1186/1471-2458-10-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbauer J, Dost A, Karges B, et al. Improved Metabolic Control in Children and Adolescents With Type 1 Diabetes: A trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35:80–86. doi: 10.2337/dc11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11–17. doi: 10.1111/j.1399-5448.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 34.Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Prediction of Adolescents’ Glycemic Control 1 Year After Diabetes-Specific Family Conflict. The Mediating Role of Blood Glucose Monitoring Adherence. Arch Pediatri Adolesc Med. 2011;165:624–629. doi: 10.1001/archpediatrics.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorchy H, Roggemans MP, Willems D. Glycated hemoglobin and related factors in diabetic children and adolescents under 18 years of age: a Belgian experience. Diabetes Care. 1997;20:2–6. doi: 10.2337/diacare.20.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Shalitin S, Phillip M. Which factors predict glycemic control in children diagnosed with type 1 diabetes before 6.5 years of age? Acta Diabetol. 2012;49:355–362. doi: 10.1007/s00592-011-0321-x. [DOI] [PubMed] [Google Scholar]
- 37.Muhammad BJ, Swift PG, Raymond NT, Botha JL. Partial remission phase of diabetes in children younger than age 10 years. Arch Dis Child. 1999;80:367–369. doi: 10.1136/adc.80.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kordonouri O, Danne T, Enders I, Weber B. Does the long-term clinical course of type I diabetes mellitus differ in patients with prepubertal and pubertal onset? Results of the Berlin Retinopathy Study. Eur J Pediatr. 1998;157:202–207. doi: 10.1007/s004310050796. [DOI] [PubMed] [Google Scholar]
- 39.Sochett EB, Daneman D, Clarson C, Ehrlich RM. Factors affecting and patterns of residual insulin secretion during the first year of type 1 (insulin-dependent) diabetes mellitus in children. Diabetiologia. 1987;30:453–459. doi: 10.1007/BF00279611. [DOI] [PubMed] [Google Scholar]
- 40.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson AM, Hauser ST, Wolfsdorf JI, et al. Psychologic predictors of compliance in children with recent onset of diabetes mellitus. J Pediatr. 1987;110:805–811. doi: 10.1016/s0022-3476(87)80030-6. [DOI] [PubMed] [Google Scholar]
- 42.Jorde R, Sundsfjord J. Intra-individual variability and longitudinal changes in glycaemic control in patients with Type 1 diabetes mellitus. Diabet Med. 2000;17:451–456. doi: 10.1046/j.1464-5491.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 43.Lewin AB, Heidgerken AD, Geffken GR, et al. The Relation Between Family Factors and Metabolic Control: The Role of Diabetes Adherence. J Pediatr Psychol. 2006;31:174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]