Abstract
Numerous methods of T cell depletion lead to impairment of learning and memory function in mice. While adoptive transfer of whole splenocytes rescues learning behavior impairments, the precise sub-population and antigenic specificity of the T cells mediating the rescue remains unknown. Using several transgenic mouse models in combination with adoptive transfers, we demonstrate the necessity of an antigen-specific CD4+ T cell compartment in normal spatial learning and memory, as measured by the Morris water maze (MWM). Moreover, transfer of a monoclonal T cell population reactive to the central nervous system (CNS) antigen, myelin oligodendrocyte glycoprotein (MOG), was sufficient to improve cognitive task performance in otherwise impaired OTII mice, raising the possibility that the antigen-specificity requirement of pro-cognitive T cells may be directed against CNS-derived self-antigens.
Introduction
The central nervous system (CNS) has traditionally been viewed as an immune privileged organ (Carson et al., 2006). T cell-CNS interactions have almost exclusively been studied under the severe pathological conditions of experimental autoimmune encephalomyelitis (Bettelli et al., 2006; Bhat et al., 2010; Kleiter et al., 2010; Steinman, 2010), where T cell presence in the CNS is solely a destructive phenomenon. However, data from several labs, including ours, has begun to identify homeostatic functions of T cells in the CNS, including roles in models of hippocampal dependent learning (Brynskikh et al., 2008; Kipnis et al., 2004; Kipnis et al., 2012; Wolf et al., 2009a; Wolf et al., 2009b; Ziv et al., 2006), stress responses (Cohen et al., 2006; Lewitus and Schwartz, 2009), and a protective function in models of neurodegeneration and CNS injury (Frenkel et al., 2005; Kipnis et al., 2002a; Kipnis et al., 2002b; Kipnis et al., 2001; Moalem et al., 1999; Serpe et al., 2003; Serpe et al., 1999; Walsh and Kipnis, 2011).
While the blood-brain barrier effectively precludes lymphocyte entry into the healthy brain parenchyma, the meninges, which envelopes the ventricular surfaces of the brain and spinal cord, is dynamically permissive to lymphocyte entry (Bartholomaus et al., 2009; Odoardi et al., 2012; Radjavi et al., 2013), and harbors diverse antigen presenting cell populations whose inflammatory phenotypes appear to be dictated by resident T cells (Derecki et al., 2010). Importantly, functional withdrawal of lymphocytes from the meningeal spaces, using either FTY720 or anti-VLA4, both resulted in impaired learning outcomes (Derecki et al., 2010). To expand on these findings, we sought to identify the subset and antigenic specificity of the T cells that modulate MWM task performance.
We show here that CD4+ T cells, but not CD8+ T cells nor B cells, are required for normal learning behavior, as measured by the MWM spatial navigation and memory task. T cell monoclonality against irrelevant chicken egg ovalbumin antigen in OTII mice resulted in cognitive impairment, which was reversed by intravenous injection of a monoclonal population of T cells recognizing the brain self-antigen myelin oligodendrocyte glycoprotein (MOG).
Results
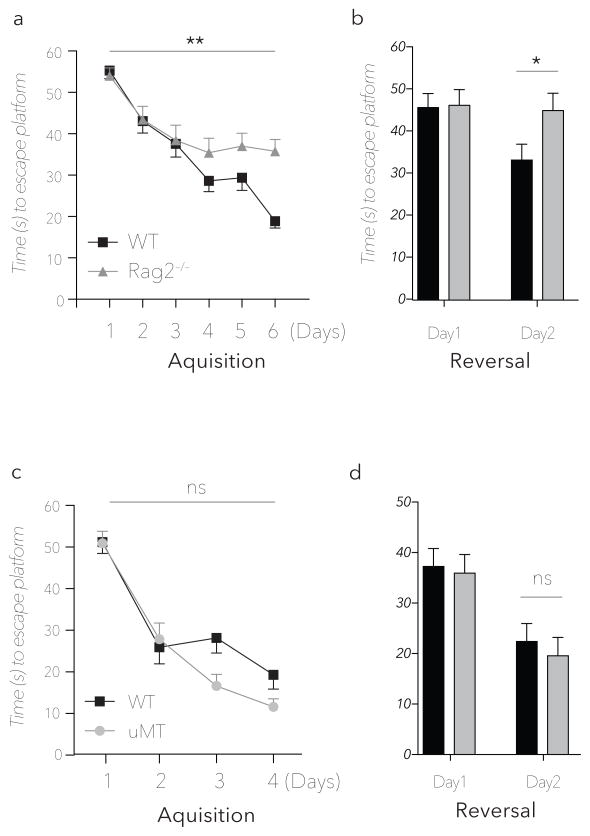
Previous studies have shown impaired learning and memory in lymphocyte deficient SCID, nude, and Rag1 knockout mice (Brynskikh et al., 2008; Kipnis et al., 2004; Wolf et al., 2009a; Ziv et al., 2006). To reinforce the conviction that this behavioral phenotype is caused by lymphocyte deficiency, and to eliminate confounding effects of Rag1 expression in the brain (Chun et al., 1991) on the cognitive impairment displayed in the MWM, we tested Rag2−/− knockout mice in the MWM paradigm. Consistent with SCID, nude and Rag1−/− mice, Rag2−/− showed a significant impairment in both the acquisition and reversal phase of the MWM (Fig. 1a, b). While the groups were significantly different in the acquisition phase, the major difference between the groups was detected on the second day of the reversal phase – in line with previous observations (Brynskikh et al., 2008; Ziv et al., 2006).
Figure 1. Rag2−/− and μMT strain performance in the MWM spatial learning and memory task.
Representative experiments are shown of at least two to three independent, blinded experiments. (a) Acquisition and (b) reversal phases of Rag2−/− and wild type mice (n = 9mice/group **=P < 0.01 *=P < 0.05; Repeated measures ANOVA with Bonferonni post-hoc analysis). MWM Acquisition (c) and reversal (d) latency times for wild type and B cell deficient μMT mice (n = 8mice/group, P > 0.5; Repeated measures ANOVA with Bonferonni post-hoc analysis).
Splenocytes have been shown to improve learning behavior of immune deficient mice (Brynskikh et al., 2008; Kipnis et al., 2004), however, the role of B cell deficiency has not yet been addressed. Importantly, the MWM impairment in Rag2−/− mice is not attributable to B-cell deficiency as B cell-deficient (μMT) mice demonstrated normal latencies comparable to wild type counterparts (Fig. 1c, d), suggesting that the relevant lymphocytes are contained within the T cell compartment.
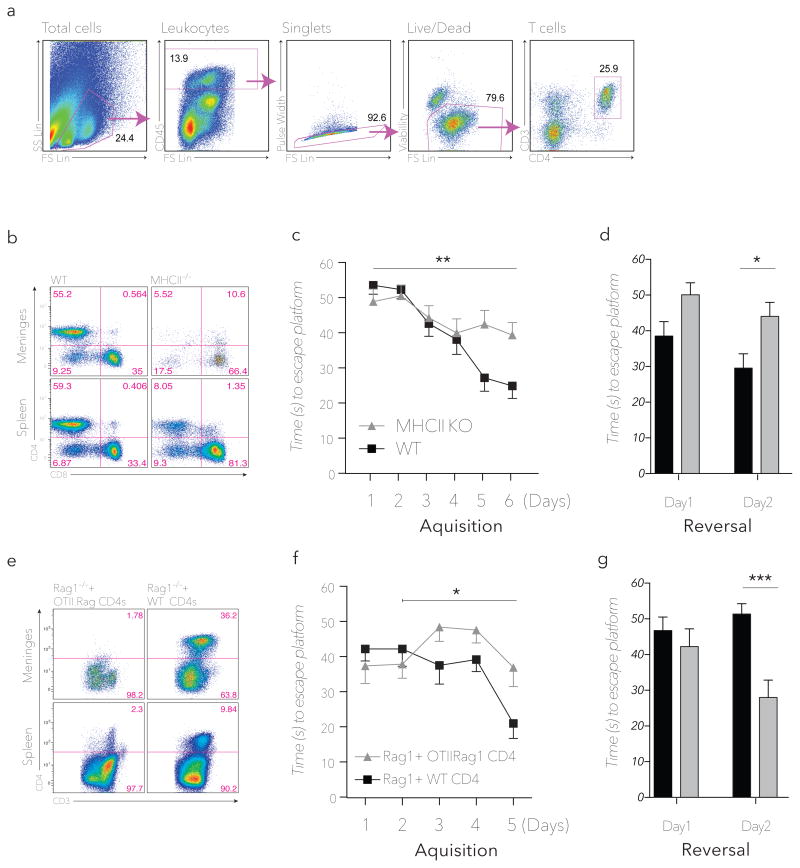
To differentiate between the contributions of CD4+ and CD8+ T cells we repeated the above experiments using MHCII-knockout mice, which are severely deficient in CD4+ T cells owing to ablation of CD4/MHCII interactions (Madsen et al., 1999), while their CD8+ T cell compartment remains functionally intact (Fig. 2a, b). MHCII−/− mice had significantly impaired performance on the MWM task compared to matched wild type controls (Fig. 2c, d), suggesting that CD4/MHCII functionality is required for normal task performance. Adoptive transfer of CD4+ T cells isolated from OTII/Rag1−/− donors into Rag1−/− recipients led to partial reconstitution of splenic but not meningeal T cells, whereas adoptive transfer of CD4+ T cells derived from wild type donors resulted in substantial reconstitution of both splenic and meningeal T-cell compartments (Fig. 2e). Finally, we showed that the MWM performance of Rag1−/− mice could be partially rescued after adoptive transfer of negatively selected CD4+ T cells derived from wild type donors but not with those from OTII/Rag1−/− donor mice (Fig. 2f, g), collectively suggesting that some degree of antigenic specificity of CD4+ T cells is required for both meningeal homing and the pro-cognitive effects of these T cells.
Figure 2. CD4+ T cells are required for cognitive performance in the MWM spatial learning and memory task.
(a) Representative flow cytometry gating strategy for meningeal CD4+ T cells beginning with meningeal single cell suspensions. Same gating strategy is applied for all meningeal samples and similar strategy is applied for other tissues throughout the manuscript. (b) CD4+/CD8+ FACS analysis of meningeal (upper panel) and splenic (lower panel) single-cell suspensions from wild type and MHCII−/− mice (at least 3 pairs of mice were analyzed). (c) MWM Acquisition and (d) reversal latency time for wild type and MHCII−/− mice (n = 8mice/group **= P < 0.01, *= P < 0.05; Repeated measures ANOVA with Bonferonni post-hoc analysis). (e) CD4+/CD3+ FACS analysis of meningeal (upper panels) and splenic (lower panels) single-cell suspensions from Rag1−/− mice receiving 3×105 purified CD4+ T cells from wild type (right) or OTII.Rag1−/− (left) donors (at least 3 pairs of mice were analyzed). (f) MWM Acquisition and (g) reversal latency time of Rag1−/− mice receiving 3×105 purified CD4+ T cells from wild type (right) or OTII.Rag1−/− (left) donors (n = 4mice/group*= P < 0.05, ***=P < 0.001; Repeated measures ANOVA with Bonferonni post-hoc analysis, significance in acquisition between groups only achieved between days 2–5).
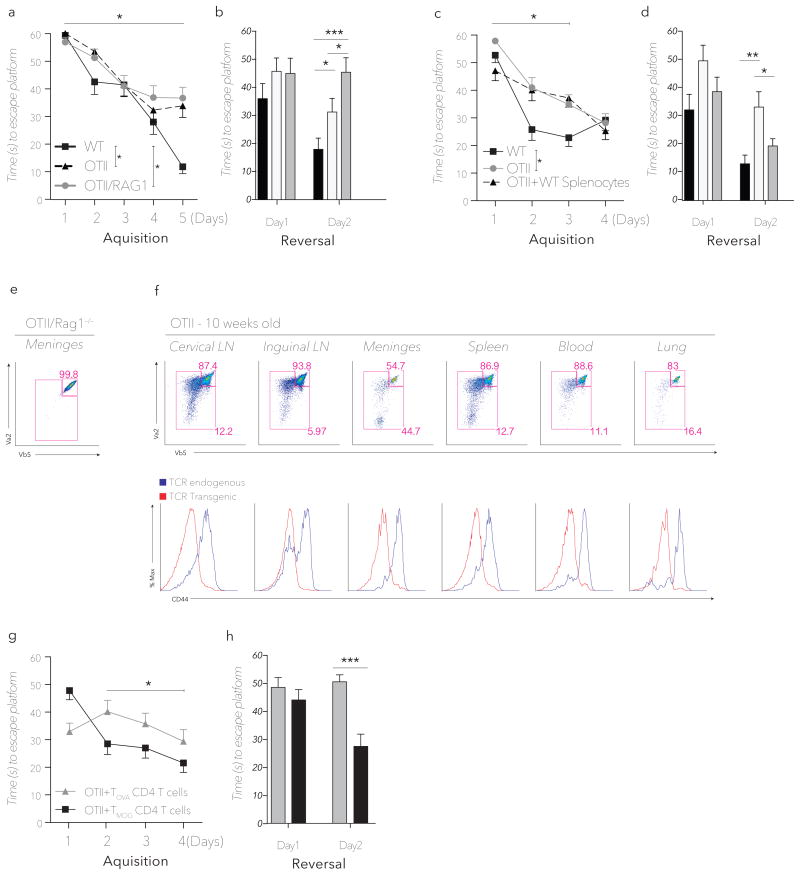
To address the antigen-specificity requirement of CD4+ T cells in mediating normal MWM task performance, we tested OTII and OTII.Rag1−/− mice along with their wild type controls, in the MWM. Emphasized in the reversal phase, OTII mice showed impaired learning (Fig. 3a, b), suggesting that their limited T cell repertoire cannot fully support normal learning behavior. Interestingly, when OTII mice were crossed to Rag1−/− mice yielding a completely monoclonal T cell repertoire, learning behavior was further impaired, most prominently in the reversal phase (Fig. 3a, b). The cognitive impairment of OTII mice could be reversed, however, by the transfer of wild type splenocytes four weeks prior to training. While the effect of wild type splenocyte transfer into OTII mice is not noticeable in the acquisition phase, a significant difference between OTII control mice and OTII mice injected with wild type splenocytes is obtained in the reversal phase (Fig. 3c, d). These results demonstrate that a limited T cell repertoire is associated with impaired learning – a phenotype partially remedied by transfer of wild type splenocytes.
Figure 3. Impaired MWM performance of TCR restricted mice is rescued by transfer of CNS specific CD4+ T cells.
(a) MWM Acquisition and (b) reversal latency time of OTII, OTII.Rag1−/− and wild type mice (n = 8mice/group *, P < 0.05, *** P < 0.001; Repeated measures ANOVA with Tukey post-hoc analysis). (c) MWM Acquisition and (d) reversal latency time of wild type and OTII mice, either untreated or reconstituted with wild type splenocytes four weeks prior to training. (n = 7mice/group *=P < 0.05, **=P < 0.01; Repeated measures ANOVA with Tukey post-hoc analysis, significance in acquisition only achieved between days 1–3). (e) Transgenic TCR usage in splenic CD4+ T cells (the presented panel is after gating strategy for CD4+ T cells, as described in Fig. 1) of OTII.Rag1−/− mice showing 1:1 Vβ5:Vα2 expression indicative of pure monoclonality of the CD4+ compartment. (f) Upper panel, FACS analysis of CD4+ gated T cells for the expression of OTII transgenic TCR chains in the indicated tissues of 10-week-old mice. Upper right gate encompasses the bulk of monoclonal transgenic T cells (Vβ5hi/Vα2hi), while the larger left gate represents the population of T cells bearing endogenous TCRs. Lower panel; CD44 expression on transgenic OTII (red) and endogenous post-revisional (blue) TCR bearing CD4+ T cells in the indicated tissues of OTII mice. (g) MWM Acquisition and (h) reversal latency time of OTII mice receiving 2×106 purified MOG or OVA specific CD4+ T cells (n = 5mice/group *, P < 0.05 ***, P < 0.001; Repeated measures ANOVA with Bonferonni post-hoc analysis significance in acquisition only achieved between days 2–4).
We recently suggested that meningeal space is the site at which T cells exert their effect on learning behavior (Derecki et al., 2010). To further demonstrate antigen specificity is required to achieve T cell-mediated benefits on learning behavior, we exploited a unique feature of the OTII mouse model, namely post-thymic T cell receptor (TCR) rearrangement (McMahan and Fink, 1998; Sharma et al., 2008). In models bearing specific TCR-β chains (including Vβ5 of OTII mice), a process of extrathymic Rag re-expression and fresh VDJ recombination as well as intrathymic receptor editing occurs at low frequency, so that by 10 weeks of age ~10%–15% of the formerly monoclonal TCR repertoire now bare an additional set of endogenous TCRs (McMahan and Fink, 1998; Sharma et al., 2008). In contrast, OTII.Rag−/− mice (lacking endogenous TCR rearrangement owing to Rag1 ablation) remain purely monoclonal, with near-perfect 1:1 surface expression of Vα2/Vβ5 TCR chains (Fig. 3e). The fact that OTII.Rag1−/− mice exhibit cognitive impairment beyond that of OTII mice (Fig. 3b), possibly suggests that antigen-specific post-revisional T cells may contribute to learning behavior. We therefore hypothesized that if the meningeal compartment were indeed selective for antigen-specific CD4+ T cells, the dual TCR-bearing T-cell compartment would be enriched preferentially in the meninges over other tissues of OTII mice. Consistent with this hypothesis, the meninges of OTII mice showed more than a four-fold enrichment of the endogenous TCR subset relative to CD4+ T cells in the blood (Fig. 3f), supporting the notion of an active T-cell selection process based on (or correlated with) TCR specificity in the meninges. Interestingly, similar enrichment for antigen-specific T cells appeared in the CNS and meninges-draining deep cervical lymph nodes, relative to non-draining inguinal lymph nodes (Fig. 3f). As expected, post-revisional T cells expressed higher CD44 levels than transgenic OTII T cells (Fig. 3f). Further studies are required to address whether antigen-specificity or high levels of CD44 expression or both, result in preferential recruitment of post-revisional T cells to meningeal areas.
In the absence of candidate antigens, no unbiased and robust assay exists for determining antigenic specificities of CD4+ T cell populations. Therefore, to examine whether T cells recognizing CNS-specific self-antigens can improve learning behavior, we injected OTII mice with a clonal population of MOG-specific CD4+ T cells (Tmog) derived from 2D2 mice bearing TCR transgenes directed against MOG35–55 antigen. The MWM performance of OTII mice receiving Tmog cells was significantly improved eight weeks post-transfer (Fig. 3g, h), suggesting that at least in animals with clonally restricted T cell repertoires, CD4+ T cells directed against CNS-derived self-antigens may be sufficient to rescue MWM task performance.
Discussion
We show here that mice deficient in CD4+ T cells or mice with clonally restricted T cell repertoires exhibit impaired learning behavior as measured using the MWM spatial learning and memory task. Moreover, we demonstrated that adoptive transfer of CD4+ T cells directed against the CNS-specific MOG antigen is sufficient to partially rescue the impaired MWM performance of OTII mice, suggesting that the relevant physiological CD4+ T cell population may be directed against CNS self-antigens.
The role of T cells in learning behavior and CNS homeostasis has been described over the last decade by several laboratories (reviewed in (Kipnis et al., 2012)). Studies from our lab have demonstrated that the T cells affecting learning behavior are located in the meningeal spaces (Derecki et al., 2010), and that the repertoire of meningeal T cell immunity is controlled by the CNS draining deep cervical lymph nodes (Radjavi et al., 2013). Specifically, surgical removal of the deep cervical lymph nodes resulted in dysregulated meningeal T cell immunity that correlated with cognitive impairment in the MWM task (Radjavi et al., 2013).
Previous published works have suggested that mice with an absent or limited T cell repertoire are impaired in the MWM. It has been shown that DO11.10 mice are impaired in learning behavior compared with their wild type counterparts, while transgenic mice bearing MBP-specific T cell receptors are superior to their wild type controls (Ziv et al., 2006). However, these studies were limited by the background of strains used in the assay, as DO11.10 mice were on Balb/c background (albino mice are blind and poor learners of visual tasks), whereas TMBP transgenic mice were on C57Bl/6J background. More recent work by Baruch et al., similarly suggests that pro-cognitive T cells residing in the choroid plexus bear TCRs directed to CNS antigens (Baruch et al., 2013). Taken together, these studies along with our own, support a model in which T cells directed against CNS self-antigens may have productive homeostatic function measurable in the MWM task.
Our present study provides additional evidence that autoimmune T cell may contribute to normal brain function. Although the data available thus far is insufficient to claim that the endogenous pro-cognitive T cells in wild type animals are specific to CNS self-antigens, we do provide evidence that CD44+ antigen-specific T cells comprise a substantial portion of the healthy meningeal repertoire – a location, we argue, is conducive to influencing learning behavior. We go on to demonstrate that a restricted or monoclonal T cell repertoire is associated with cognitive impairment and when this repertoire is supplemented by either polyclonal wild type T cells, or monoclonal T cells reactive to brain antigen MOG, improved learning behavior is attained.
While this work focuses on the effect of meningeal immunity on learning and memory, it is conceivable that when productive meningeal immunity is deregulated, these same pro-cognitive T cells, may be anatomically positioned to infiltrate the CNS and induce autoimmunity. Further understanding of the meningeal T cell repertoire will not only elucidate the emerging homeostatic functions of T cells, but may also shed new light on etiology and pathogenesis of neuroinflammatory diseases, and possibly lead to development of new therapeutic modalities that target the immune system for the benefit of the CNS.
Methods
Animals
Male adult mice on the C57BL background were purchased either from the Jackson Laboratory (C57BL/6J, OTII, μMT, Rag2−/−, MHCII−/−), or Taconic (OTII.Rag1) and housed in temperature and humidity controlled rooms with 12 hour light/dark cycle, with standard diet ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Virginia.
Behavior
Morris water maze experiments were conducted in a 100cm diameter pool, maintained between 21 and 22°C. Non-toxic washable tempera paint was added to conceal a 10cm wide platform submerged 1cm below the water line. Bold visible signs were placed on the walls of the testing room (but within 100cm of the tank center) to serve as visual navigation cues, and a curtain was placed to between the tank and the experimenter to minimize use of the experimenter as a visual cue. On day1 mice were placed at a random point along the periphery of the pool and given up to 60 seconds to find the escape platform. If the mouse failed to find the platform within 60 seconds the mouse was lifted and placed on the platform by the experimenter for a total of 30 seconds for Day1, and 10 seconds for subsequent days. This procedure was repeated two more times, for a total of three “acquisition” trials, each trial starting from a unique position along the pools perimeter, and each mouse given 20–30 minutes of rest between trials. This procedure was carried out for four consecutive days or until wild type mice reached the platform within 20 seconds, in a phase collectively termed “acquisition”. On the day following the last acquisition, the platform was removed and mice were given a single trial lasting 60s without any available escape. The following day the platform is reintroduced to a novel quadrant of the pool, and mice were again given 3 trials per day for 2–3 days in order to find the escape platform in the “reversal” phase of the MWM. All Data was recorded using EthoVision tracking system, statistical analysis was performed with repeated measures ANOVA and the Bonferroni/Tukey post-hoc test. All groups were blinded to the experimenter and run in shuffled orders, with each experiment repeated at least once.
Flow cytometry
Mice were thoroughly perfused with heperanized PBS for 5 minutes. Tissues were passed through 70uM nylon screens and single cell suspensions were stained for extracellular markers (eBiosciences). Dissection of the meninges was carried out as described by Derecki et al (Derecki et al., 2010). All samples were run on ADP Cyan (Dako) and analyzed with Flowjo (Tree Star).
Adoptive transfer
In adoptive transfer experiments, cultured T cells, negatively selected CD4 T cells or single cell suspensions of RBC lysed Donor splenocytes, were transferred i.v. into recipient animals in 200uL PBS. MOG specific CD4 T cells were a gift from Avraham Ben-Nun (Weizmann Institute of Science, Israel) and were cultured as described previously (Krishnamoorthy et al., 2009).
Highlights.
Mice with deficient or clonally restricted CD4+ T cell repertoires display an impaired learning phenotype that is rescued by transfer of a monoclonal T cell population directed against CNS self-antigen.
Acknowledgments
We thank the members of the Kipnis lab for their valuable comments during multiple discussions of this work. This work was primarily supported by a grant from the National Institute on Aging, NIH (AG034113 award to J. K).
Footnotes
Disclosure of Conflicts of Interest
All authors disclose no conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002a;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Yoles E, Ben-Nun A, Schwartz M. Myelin specific Th1 cells are necessary for post-traumatic protective autoimmunity. J Neuroimmunol. 2002b;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter I, Song J, Lukas D, Hasan M, Neumann B, Croxford AL, Pedre X, Hovelmeyer N, Yogev N, Mildner A, Prinz M, Wiese E, Reifenberg K, Bittner S, Wiendl H, Steinman L, Becker C, Bogdahn U, Neurath MF, Steinbrecher A, Waisman A. Smad7 in T cells drives T helper 1 responses in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2010;133:1067–1081. doi: 10.1093/brain/awq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, Lassmann H, Dornmair K, Kurschus FC, Liblau RS, Wekerle H. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nature medicine. 2009;15:626–632. doi: 10.1038/nm.1975. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan CJ, Fink PJ. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637–647. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flugel-Koch C, Flugel A. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Derecki NC, Kipnis J. Dynamics of the Meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.79. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Ju AC, Kung JT, Fu SM, Ju ST. Rapid and selective expansion of nonclonotypic T cells in regulatory T cell-deficient, foreign antigen-specific TCR-transgenic scurfy mice: antigen-dependent expansion and TCR analysis. Journal of immunology. 2008;181:6934–6941. doi: 10.4049/jimmunol.181.10.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11:41–44. doi: 10.1038/ni.1803. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Kipnis J. Regulatory T cells in CNS injury: the simple, the complex and the confused. Trends in Molecular Medicine. 2011;17:541–547. doi: 10.1016/j.molmed.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009a;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. Faseb J. 2009b;23:3121–3128. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]