Abstract
Impulsive choice, a form of impulsivity, is associated with tobacco smoking in humans. Trait impulsivity may be a vulnerability factor for smoking, or smoking may lead to impulsive behaviors. We investigated the effects of 14-day nicotine exposure (6.32 mg/kg/day base, subcutaneous minipumps) and spontaneous nicotine withdrawal on impulsive choice in low impulsive (LI) and high impulsive (HI) rats. Impulsive choice was measured in the delayed reward task in which rats choose between a small immediate reward and a large delayed reward. HI and LI rats were selected from the highest and lowest quartiles of the group before exposure to nicotine. In non-selected rats, nicotine or nicotine withdrawal had no effect on impulsive choice. In LI rats, chronic nicotine exposure decreased preference for the large reward with larger effects at longer delays indicating increased impulsive choice. Impulsive choices for the smaller immediate rewards continued to be increased during nicotine withdrawal in LI rats. In HI rats, nicotine exposure and nicotine withdrawal had no effect on impulsive choice, although there was a tendency for decreased preference for the large reward at short delays. These results indicate that nicotine- and nicotine withdrawal-induced increases in impulsive choice depend on trait impulsivity with more pronounced increases in impulsive choice in LI compared to HI subjects. Increased impulsivity during nicotine exposure may strengthen the addictive properties of nicotine and contribute to compulsive nicotine use.
Keywords: Delayed reward, delay discounting, high and low impulsive rats, Wistar rats
1. Introduction
Impulsivity is defined as the predisposition to act prematurely without considering the future outcomes of actions. Impulsivity is a common symptom of several psychiatric disorders, such as attention-deficit/hyperactivity disorder, aggression, and personality disorders (Moeller et al., 2001). Furthermore, trait impulsivity in relatively healthy humans contributes to poor decision making. Impulsivity is not a unitary construct but rather refers to diverse forms of deficits in response inhibition at different stages of the behavior, such as preparation to respond, execution of the behavior, and the assessment of outcomes (Evenden, 1999). At the preparation phase, behaviors initiated without adequate sensory input result in “preparation” or “reflection” impulsivity (Dalley et al., 2011, Evenden, 1999). During the execution of behavior, a failure to inhibit a motor action or stop the initiated behavior causes “impulsive action” (Dalley, Everitt, 2011). Finally, making risky or inappropriate choices, such as preference for small immediate rewards and intolerability of delay associated with large rewards, is termed “impulsive choice,” also referred to as increased delay discounting (Dalley, Everitt, 2011).
Impulsive choice has been strongly associated with tobacco smoking and drug dependence in humans (Bickel et al., 1999, Bickel et al., 2008, Goldstein and Volkow, 2002, Perry and Carroll, 2008). Individuals with increased delay discounting begin the use of drugs, including nicotine, at an earlier age compared with less impulsive individuals (Kollins et al., 2005, Wulfert et al., 2002). Furthermore, tobacco smokers discounted future monetary rewards to a greater extent than non-smokers (Baker et al., 2003, Bickel, Odum, 1999, Dallery and Raiff, 2007, Heyman and Gibb, 2006, Mitchell, 2004). A recent meta-analysis of human studies that covered 57 articles and a total of 3329 subjects provided further evidence of increased impulsive choice in smokers and subjects with drug abuse (MacKillop et al., 2011). Nineteen of these studies investigated tobacco smokers, 15 of which found a significant increase in impulsive choices in the currently smoking group. Short-term nicotine abstinence also increased impulsive choices in smokers when the choice was related to smoking but not monetary choices (Mitchell, 2004).
Despite the considerable number of human studies, it remains unclear whether increased impulsivity, including impulsive choice, is a cause or consequence of nicotine dependence, or whether impulsivity and nicotine dependence are both consequences of a shared biological mechanism. Studies in humans cannot easily determine the direction of causality of these two behaviors (i.e., tobacco smoking and impulsivity), mainly because such evaluations necessitate long-term follow-up assessments that begin from the early years of adolescence and continue into adulthood. In this context, animal studies are important tools for understanding the neurobiological basis of the development of nicotine dependence in subjects that exhibit high or low levels of impulsivity before nicotine exposure.
A procedure that assesses impulsive choice is a delayed reward (i.e., delay discounting) task that has been used to evaluate cognitive impulsivity in both humans and experimental animals (Evenden and Ryan, 1996). In this task, impulsivity is defined and measured as the preference for a smaller immediate reinforcer over a larger delayed reward (Ainslie, 1975, Evenden, 1999). Acute nicotine administration increased impulsive choices in rats (Anderson and Diller, 2010, Dallery and Locey, 2005, Kelsey and Niraula, 2013, Kolokotroni et al., 2011), whereas exposure to chronic nicotine and nicotine withdrawal had mixed effects on impulsive choice behavior in rats (see Discussion for details). Differences in baseline trait impulsivity may play a role in differential responses to chronic nicotine exposure and nicotine withdrawal, a hypothesis that was explored in the present study.
The present study investigated the effects of chronic nicotine treatment and nicotine withdrawal on impulsive choice in a general population of Wistar rats, and rats selected for high and low baseline levels of impulsivity. Outbred Wistar rats were used in the present study because outbred rat strains best reflect the human population and are most suitable for the detection of individual differences because of a higher degree of genetic and phenotypic heterogeneity than inbred rat strains. A discrete-trial delayed reward task with predefined delay times for larger reinforcers was used in the present study to evaluate impulsive choice behavior. The rats were chronically exposed to nicotine via subcutaneous osmotic minipumps. Chronic nicotine administration via minipumps provides a stable nicotine blood concentration that mimics the regular nicotine exposure experienced by long-term tobacco smokers (Ulrich et al., 1997). Nicotine withdrawal was induced by removal of the osmotic minipumps. Control rats were treated with saline via osmotic minipumps. We hypothesized that exposure to chronic nicotine and nicotine withdrawal will have differential effects on impulsivity in subjects with high and low levels of trait impulsivity.
2. Materials and Methods
2.1. Animals
Male Wistar rats (Charles River, Raleigh, NC), weighing 200–225 g upon arrival in the laboratory, were housed two per cage on a 12 h/12 h reverse light/dark cycle (lights off at 8:00 AM). During behavioral training and testing, the rats were food-deprived and received 16 g/rat/day of food, including the food received in the experimental chamber. The rats were fed 1 h after the experimental session. Water was available ad libitum in the home cage. Behavioral tests were performed during the dark phase of the light/dark cycle. The animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. All experiments were approved by the Institutional Animal Care and Use Committee of the University of California San Diego.
2.2. Apparatus
All of the tests was conducted in a set of 12 nine-hole operant boxes (Med Associates, St. Albans, VT). Each box consisted of a 25.5 cm width × 28.4 cm length × 28.7 cm height chamber placed in a sound-proof enclosure with a ventilator fan that provided air circulation and produced low levels of background noise. A 2.5 W, 24 V white house-light was positioned on one wall of the chamber and illuminated during each experimental session. Each testing chamber contained a curved wall with nine holes equipped with 3 W cue lights located at the rear panel and a photocell emitter and detector pair located at the entrance of each hole. Metal inserts covered every other hole, leaving open holes 1, 3, 5, 7, and 9. Food pellets (45 mg, Noyes Precision Pellets, New Brunswick, NJ) were delivered via a food dispenser into a pellet receptacle located in the center of the opposite wall. The pellet receptacle was also equipped with a cue light and photocell emitter and detector pair. Each apparatus was controlled by and provided data collected through a Med Associates (Med Associates, St. Albans, VT) interface to a computer. Behavioral training and baseline assessments in the delayed reward task were conducted 5 days per week (Monday-Friday), and behavioral testing during chronic nicotine/saline exposure and withdrawal was conducted daily (i.e., 7 days per week).
2.3. Delayed reward procedure
The delayed reward procedure used in the present study was similar to the procedure originally developed by Evenden and Ryan (1996) for two-lever boxes and modified by van Gaalen and colleagues (van Gaalen et al., 2006) for the five-hole chambers. In a discrete-trials choice procedure, the rats choose between one food pellet delivered immediately and four food pellets delivered after a delay.
On day 1, the rats were habituated to the chambers for 20 min. During habituation, the cue lights in holes 3 and 7 were illuminated, and food pellets were placed in each illuminated hole. On day 2, a 20-min session began with the illumination of the cue lights in holes 3 and 7, and one pellet was delivered into the pellet receptacle every 20 s, independent of the rats’ responses. On day 3, training on a fixed-ratio 1 (FR1) schedule of reinforcement was initiated. For the FR schedule, at the beginning of the session, the cue lights in holes 3 and 7 were illuminated, and nosepoking at either hole was rewarded with one pellet. The session was terminated after a maximum of 100 pellets were earned or 30 min elapsed, whichever occurred first. The intertrial interval (ITI) was 20 s, and the limited hold to make a response was 10 s. The rats were then trained to nosepoke into the hole in the center position (hole 5) to initiate a trial. A nosepoke in hole 5 resulted in the presentation of the cue lights in holes 3 and 7. Nosepoking in either illuminated hole during a 10 s limited hold period was rewarded with one pellet. If the rat did not respond within the limited hold period, then the house light was switched on for 5 s, and the same trial was initiated with the illumination of hole 5. The ITI was 20 s. Nosepoking in a non-illuminated hole was recorded but had no consequences. The session was terminated after a maximum of 100 pellets were earned or after 34 min elapsed, whichever occurred first. During the subsequent training sessions, the ITI was gradually increased from 20 to 100 s, and the session duration was also increased from 34 to 100 min. The duration of the final training and testing sessions was fixed at 100 min, together with increasing the ITI to 100 s. Thus, the maximal number of pellets obtained during a session decreased to 85 and 60 pellets when the ITI was increased to 70 and 100 s, respectively.
During the next phase, holes 3 and 7 were designated as small (one pellet) and large (four pellets) reward holes, respectively. The position associated with the small and large reward was the same for each individual subject and counterbalanced across rats. The hole opposite the initial preferred side was designated the large reward hole for each subject. The session was initiated with illumination of the cue light in hole 5. When the rat nosepoked in hole 5, the cue light was extinguished while the cue lights in holes 3 and 7 were illuminated. During a 10 s limited hold period, nosepoking in hole 3 or 7 was rewarded with one or four pellets, consistent with the size of the reward designated for each hole. If the rat did not respond within the limited hold period, then the house light was turned on for 5 s, and the same trial was initiated. The ITI was 100 s. Nosepoking in non-illuminated holes was recorded but had no consequence. The session was terminated after 60 trials or 100 min, whichever occurred first. The rats were trained under these conditions until they preferred the large reward for at least 50 trials. After reaching this criterion of performance, the delayed reward training was initiated.
During the delayed reward training, the session consisted of 60 trials divided into five blocks with 12 trials each. Each block began with two forced trials in which, after a nosepoke in hole 5, either hole 3 or 7 was illuminated in a counterbalanced order, and a response at the illuminated hole was rewarded with an immediate one pellet or delayed four pellets. No delay was applied during the first block. Beginning with the second block, delays for the large reward were increased per block as the following: 0, 1, 2, 4, and 8 s. Over the training sessions, the delays were gradually increased to 0, 10, 20, 40, and 60 s per block.
The ITI duration for all of the stages of delay discounting training was adjusted according to the delay duration (ITI duration = 100 s – [response latency + delay duration]). Thus, the delay duration was included in the ITI, and the trial duration was fixed at 100 s. The session duration was fixed at 100 min.
2.4. Osmotic minipump implantation and removal
The rats were anesthetized with an isoflurane/oxygen vapor mixture (1–2%), and an osmotic minipump (14-day 2ML2 [5 µl/h], Alzet Osmotic Pumps, Cupertino, CA) was inserted subcutaneously at the back of the animal parallel to the spine with the flow-moderator directed posteriorly. The wound was closed with 9 mm stainless steel wound clips (Becton Dickinson Primary Care Diagnostics, Sparks, MD), and antibacterial Bacitracin ointment was applied to the incision area. On day 14, the minipumps were surgically removed using the aforementioned procedure.
2.5. Experimental design
The experiment was performed in two replications; all of the groups were represented in each replication. Rats (n = 44) were trained in the delayed reward task until stable responding was achieved (< 20% variation in each block during the last three sessions). Then, rats were assigned to two treatment groups (n = 22/group) with equal levels of impulsivity under baseline conditions, defined as the mean percentage of delayed reward choices during the three longest (20, 40, and 60 s) delay blocks. Low impulsive (LI) and high impulsive (HI) rats in each treatment group were selected as the top and bottom 25% of the population, respectively (n = 5 per group). The rats were prepared with minipumps that contained either saline or nicotine hydrogen bitartrate (6.32 mg/kg/day, base; Sigma, St. Louis, MO) dissolved in sterile 0.9% saline. The effects of nicotine on impulsive choice were assessed for 14 days. The effects of spontaneous nicotine withdrawal on impulsive choice were assessed 6, 12, 24, and 48 h post-pump removal. Rats that did not exhibit stable performance (< 20% variation in each block during the last three sessions) were excluded from the data analyses (two rats from the nicotine group and one rat from the saline group).
2. 6. Statistical analyses
Behavioral outcome measures were preference for the large reward and the total number of omissions during choice trials. Impulsive choice was quantified using the area under the curve (AUC) because it provides a theoretically neutral measure of delayed discounting (Myerson et al., 2001). AUC was calculated as a sum of impulsive choices for all delays. The indifference point, the delay at which rats switched their preference over to the immediate, small reward (i.e., the delay on which the preference for large reward is 50%) was calculated using the hyperbolic function which best describes delay discounting with fixed delays (Cardinal, 2006, Green and Myerson, 2004, Mazur, 1987). We used the hyperbolic equation V = A/(1 + kD), where V is the preference for the large reward after a delay of D in seconds, A is the preference for the large reward at D = 0 s and the free parameter k describes how rapidly V declines with increasing delay Interpolation of mean indifference points was performed by fitting a logistic equation by non-linear regression using GraphPad Prism 5.0 software. The calculated k value represents the degree of discounting and 1/k value is used as indifference point. Only one indifference point was calculated for each rat at each specific time point of the experiment. The percentage of large reward choices was calculated for each block of 10 trials per each delay. Baseline impulsive choice was calculated as the average of each trial block during the last five days of testing under baseline conditions before nicotine/saline administration. The data were analyzed using a repeated-measures analysis of variance (ANOVA), with Delay, Day of nicotine/saline exposure, and Withdrawal hours as the within-subjects factors and Treatment (saline or nicotine) and Trait Impulsivity (HI and LI) as the between-subjects factors. Considering that baseline differences in impulsive choice behavior may impact the interpretation of the results, separate data analyses were performed on data from HI and LI rats to investigate the effects of nicotine/saline exposure and nicotine/saline withdrawal on delayed reward choice within each behavioral phenotype. The time-course analyses of delayed reward choice during chronic nicotine/saline exposure and nicotine/saline withdrawal were performed for each delay block. Post-hoc comparisons were conducted using the Newman-Keuls test. The level of significance was set at p < 0.05. The statistical analyses were performed using the SPSS version 17 software (Statistical Package for the Social Sciences, Chicago, IL).
3. Results
3.1. Baseline performance
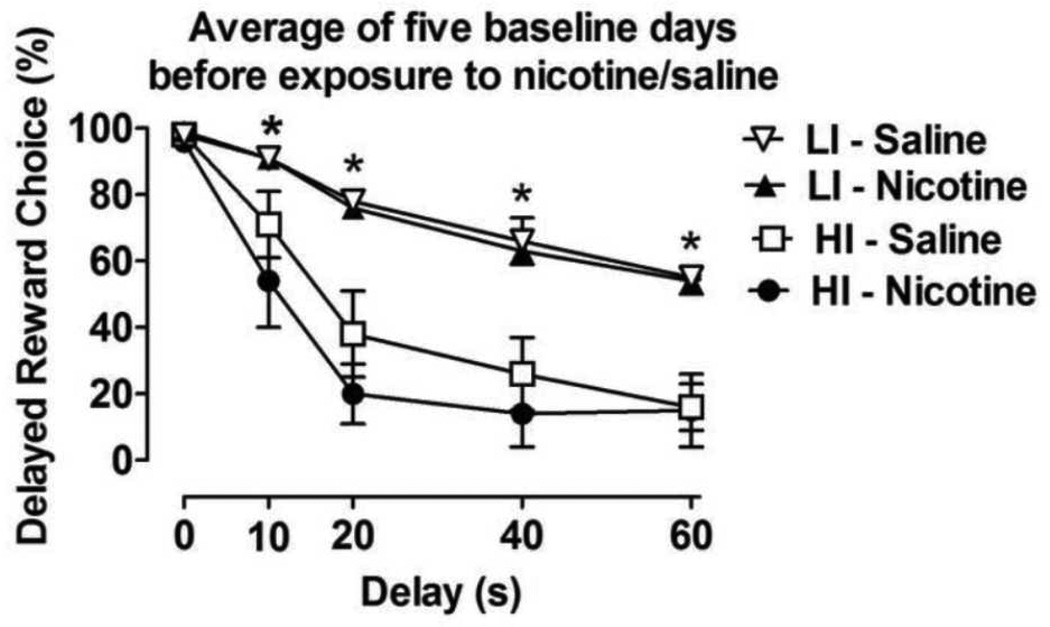
The mean baseline delayed reward choices of all of the rats (i.e., the general population) during the last 5 days before exposure to saline or nicotine were similar across all of the delay blocks and treatment conditions (Table 1). The ANOVA revealed a significant effect of Delay (F4,168 = 143.7, p < 0.0001) on choice behavior under baseline conditions, but no differences in choice behavior between the treatment groups before exposure to nicotine or saline. After the rats were selected for high and low levels of impulsivity, the ANOVA confirmed significant main effects of Trait Impulsivity (F3,16 = 14.8, p < 0.0001) and Delay (F4,64 = 84.4, p < 0.0001) and a significant Trait Impulsivity × Delay interaction (F12,64 = 3.8, p < 0.0001). No differences were found between the mean baseline delayed reward choices in the HI and LI rats assigned to the different treatment groups before exposure to nicotine or saline (Fig 1).
Table 1.
Percentage of delayed reward choices (mean ± SEM) in rats from the general rat population under baseline conditions and during exposure to chronic nicotine and nicotine withdrawal. The data are expressed as mean ± SEM. Baseline values represent the 5-day average percentage of delayed reward choices before exposure to nicotine/saline. Each treatment group had 22 rats.
| Day | Exposure | Delay (s) | ||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | ||
| Baseline | Saline | 94.2 ± 3.9 | 78.9 ± 3.9 | 61.5 ± 5.3 | 48.4 ± 5.1 | 42.8 ± 4.8 |
| Nicotine | 97.4 ± 0.6 | 78.2 ± 4.5 | 60.5 ± 5.6 | 49.0 ± 5.1 | 43.8 ± 4.6 | |
| Chronic nicotine exposure (days) | ||||||
| Day 1 | Saline | 92.7 ± 4.1 | 82.7 ± 5.2 | 61.4 ± 6.3 | 50.5 ± 5.8 | 41.8 ± 5.0 |
| Nicotine | 90.0 ± 5.1 | 78.2 ± 5.6 | 66.2 ± 7.0 | 52.5 ± 6.8 | 44.7 ± 5.4 | |
| Day 2 | Saline | 99.0 ± 0.6 | 80.0 ± 5.0 | 65.4 ± 6.0 | 49.5 ± 5.6 | 35.7 ± 4.7 |
| Nicotine | 97.7 ± 1.1 | 75.9 ± 6.1 | 62.2 ± 7.2 | 50.4 ± 6.2 | 42.6 ± 5.4 | |
| Day 3 | Saline | 100.0 ± 0.0 | 81.3 ± 5.0 | 59.0 ± 6.4 | 50.4 ± 5.9 | 42.3 ± 5.7 |
| Nicotine | 97.7 ± 0.9 | 80.9 ± 5.6 | 57.2 ± 6.1 | 50.9 ± 6.4 | 40.7 ± 6.2 | |
| Day 4 | Saline | 98.6 ± 0.7 | 81.5 ± 4.2 | 62.2 ± 6.3 | 51.3 ± 6.0 | 42.9 ± 5.3 |
| Nicotine | 98.1 ± 0.8 | 78.6 ± 5.5 | 63.1 ± 6.4 | 54.5 ± 6.1 | 43.4 ± 5.6 | |
| Day 5 | Saline | 96.8 ± 1.5 | 81.8 ± 4.4 | 62.2 ± 6.5 | 51.8 ± 6.5 | 39.0 ± 5.3 |
| Nicotine | 96.3 ± 2.7 | 77.2 ± 5.9 | 62.7 ± 6.5 | 47.7 ± 5.4 | 41.2 ± 5.0 | |
| Day 6 | Saline | 97.7 ± 0.9 | 80.0 ± 4.9 | 63.1 ± 5.5 | 51.8 ± 5.9 | 40.0 ± 5.0 |
| Nicotine | 98.6 ± 0.7 | 79.5 ± 5.9 | 61.3 ± 6.5 | 48.6 ± 5.6 | 43.9 ± 5.1 | |
| Day 7 | Saline | 95.9 ± 1.9 | 81.3 ± 4.8 | 65.0 ± 5.9 | 50.0 ± 5.9 | 36.8 ± 4.8 |
| Nicotine | 94.5 ± 2.3 | 75.4 ± 5.8 | 58.1 ± 5.4 | 42.2 ± 5.2 | 37.7 ± 4.8 | |
| Day 8 | Saline | 97.2 ± 1.8 | 82.7 ± 4.9 | 61.8 ± 6.1 | 48.6 ± 5.3 | 38.6 ± 5.2 |
| Nicotine | 96.8 ± 1.3 | 79.0 ± 5.1 | 58.6 ± 6.5 | 49.5 ± 5.6 | 36.6 ± 4.8 | |
| Day 9 | Saline | 98.6 ± 0.7 | 80.9 ± 4.5 | 63.1 ± 5.7 | 46.3 ± 5.0 | 36.8 ± 5.0 |
| Nicotine | 97.2 ± 1.6 | 75.9 ± 5.7 | 64.5 ± 7.0 | 49.0 ± 5.7 | 39.0 ± 6.2 | |
| Day 10 | Saline | 97.7 ± 1.3 | 85.4 ± 4.4 | 63.6 ± 5.9 | 46.3 ± 6.1 | 38.1 ± 5.2 |
| Nicotine | 98.1 ± 1.0 | 74.0 ± 6.2 | 60.9 ± 7.2 | 45.4 ± 5.7 | 36.3 ± 4.9 | |
| Day 11 | Saline | 98.1 ± 0.8 | 81.8 ± 5.6 | 62.7 ± 6.7 | 45.0 ± 5.5 | 36.3 ± 5.0 |
| Nicotine | 98.6 ± 0.9 | 75.0 ± 5.9 | 57.2 ± 6.8 | 43.1 ± 5.0 | 40.6 ± 5.3 | |
| Day 12 | Saline | 97.7 ± 1.1 | 82.7 ± 5.3 | 57.2 ± 6.8 | 45.0 ± 6.0 | 39.8 ± 5.2 |
| Nicotine | 99.5 ± 0.4 | 82.7 ± 4.2 | 56.8 ± 6.2 | 48.6 ± 5.5 | 37.7 ± 5.5 | |
| Day 13 | Saline | 100.0 ± 0.0 | 76.3 ± 4.3 | 64.0 ± 6.8 | 43.1 ± 5.3 | 33.7 ± 4.4 |
| Nicotine | 98.6 ± 0.9 | 78.1 ± 5.5 | 61.8 ± 6.6 | 45.4 ± 5.2 | 37.5 ± 5.1 | |
| Spontaneous nicotine withdrawal (h) | ||||||
| 6th h | Saline | 98.6 ± 0.7 | 79.5 ± 4.9 | 64.5 ± 6.9 | 43.6 ± 5.5 | 35.0 ± 5.0 |
| Nicotine | 93.1 ± 3.2 | 70.4 ± 5.5 | 54.0 ± 6.4 | 40.0 ± 5.3 | 35.6 ± 5.6 | |
| 12th h | Saline | 98.6 ± 0.7 | 77.7 ± 5.3 | 57.7 ± 6.8 | 44.5 ± 5.8 | 33.9 ± 5.2 |
| Nicotine | 97.7 ± 1.1 | 75.4 ± 5.6 | 59.5 ± 7.2 | 44.5 ± 6.4 | 29.7 ± 6.0 | |
| 24th h | Saline | 97.2 ± 1.6 | 79.5 ± 5.5 | 60.0 ± 7.1 | 45.4 ± 5.5 | 39.1 ± 6.3 |
| Nicotine | 100.0 ± 0.0 | 79.0 ± 4.8 | 59.0 ± 7.2 | 49.5 ± 6.1 | 40.3 ± 5.4 | |
| 48th h | Saline | 99.0 ± 0.6 | 78.6 ± 5.8 | 59.0 ± 6.8 | 45.9 ± 5.9 | 35.0 ± 5.3 |
| Nicotine | 100.0 ± 0.0 | 76.8 ± 4.8 | 58.1 ± 6.8 | 46.3 ± 5.2 | 41.5 ± 5.3 | |
Figure 1.
Baseline choice behavior in high impulsive (HI) and low impulsive (LI) rats before assignment to saline and nicotine treatment groups. The data are expressed as mean ± SEM of the last 5 baseline days before exposure to nicotine or saline. n = 5 per group, selected as the highest and lowest 25% percentiles from the general population of rats. *p < 0.05, statistically significant differences between LI and HI rats.
3.4. Chronic nicotine exposure
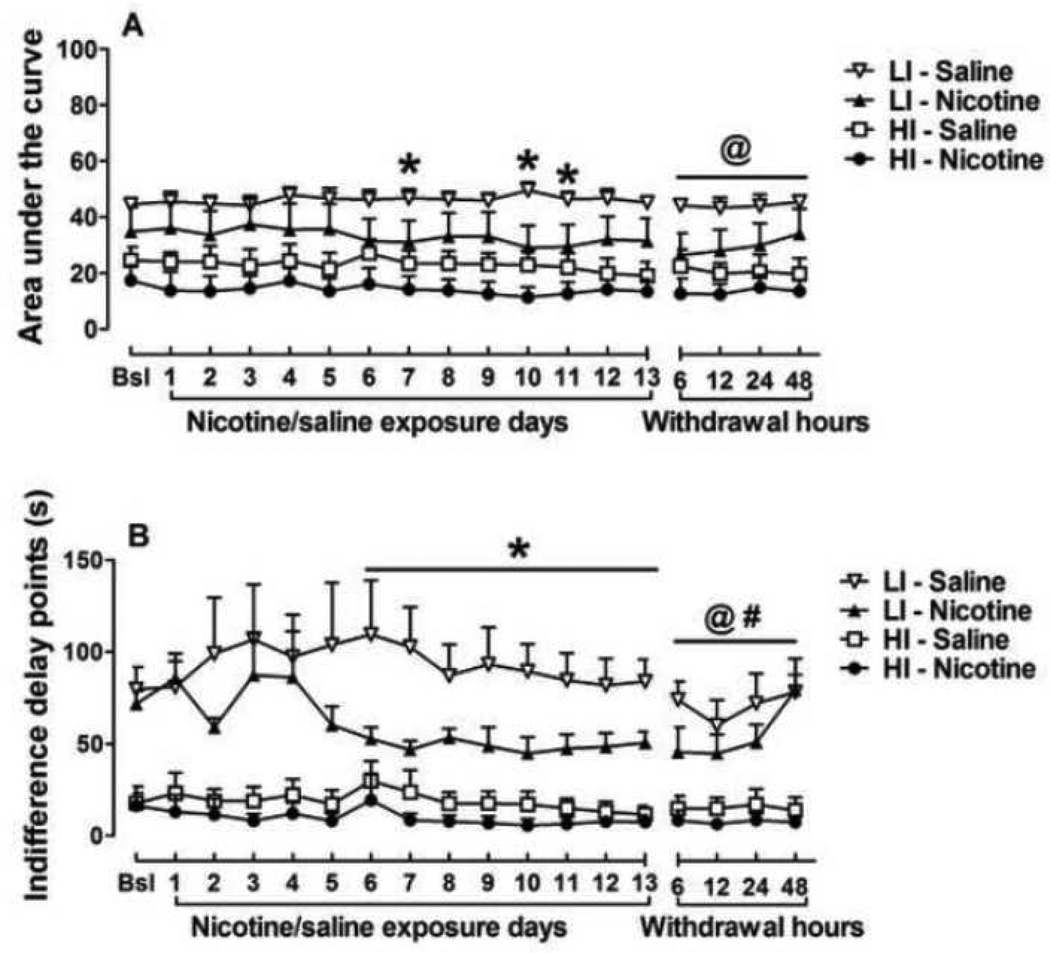
During the first block with no delay, all rats from all experimental groups chose the large reward (data not shown). ANOVAs revealed no effect of 13-day chronic nicotine exposure on choice behavior in the general rat population of (Table 1) or in rats with high and low levels of trait impulsivity (Fig 2). The area under the curve (AUC) was calculated for HI and LI rats during chronic (13 days) nicotine exposure (Fig. 3a). The ANOVAs on AUC data revealed significant main effects of Treatment (F1,16 = 4.2, p < 0.05) and Trait Impulsivity (F1,16 = 15.4, p < 0.001), but no Treatment × Trait Impulsivity interaction. A separate 2-way ANOVA on the LI group data showed a Treatment × Days interaction effect (F12,96 = 1.9, p < 0.05) with nicotine-treated LI rats showing increased impulsive choice compared to saline-treated LI rats on days 7, 10 and 11 of chronic nicotine exposure (Newman-Keuls test, p < 0.05). No significant main effect of Treatment was detected in HI rats.
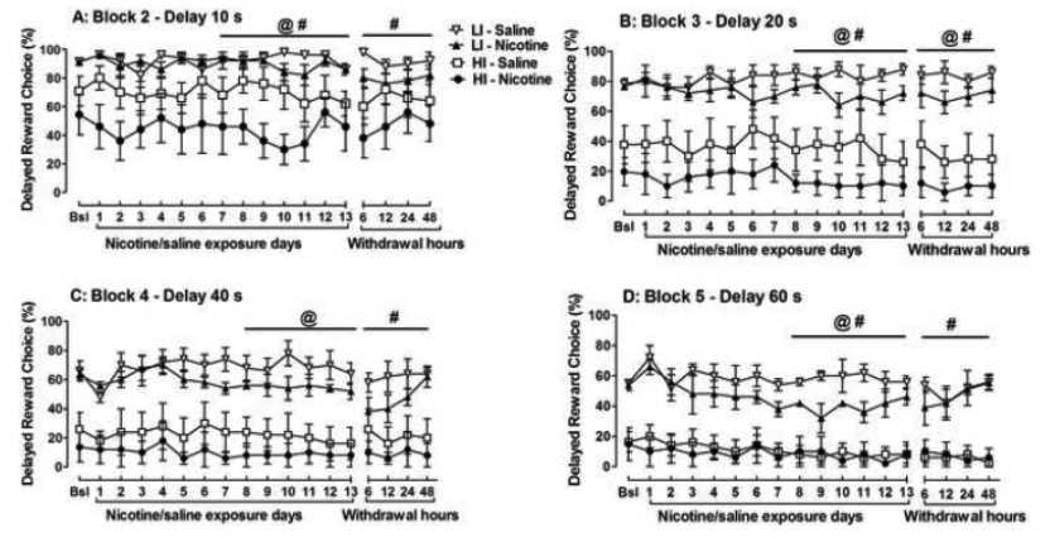
Figure 2.
Time course of delayed reward choice (%) at delays of 10 s (A), 20 s (B), 40 s (C), and 60 s (D) in high impulsive (HI) and low impulsive (LI) rats during chronic nicotine/saline exposure and nicotine/saline withdrawal. The data are expressed as mean ± SEM. Baseline values represent the 5-day average percentage of delayed reward choices before exposure to nicotine/saline. Five rats in each treatment group were selected as the highest and lowest 25% percentiles from the general population of rats. Bsl, baseline. Statistically significant effects of the factors Treatment (@, p < 0.05) and Trait Impulsivity (#, p < 0.05) were indicated in the ANOVAs.
Figure 3.
Area under the curve (AUC, A) and indifference points (B) for the preference of the large reward in high impulsive (HI) and low impulsive (LI) rats during chronic nicotine/saline exposure and nicotine/saline withdrawal. The data are expressed as mean ± SEM. *p < 0.05, statistically significant differences between LI rats treated with saline and nicotine. Statistically significant effects of the factors Treatment (@, p < 0.05) and Trait Impulsivity (#, p < 0.05) were indicated in the ANOVAs.
Further, we analyzed indifference delay points in HI and LI rats (Fig 3b). Similar to the AUC analyses reported above, ANOVA on indifference points revealed significant main effects of Treatment (F1,16 = 4.4, p < 0.05) and Trait Impulsivity (F1,16 = 35.8, p < 0.001), but no Treatment × Trait Impulsivity interaction. A separate 2-way ANOVA on the LI group data showed a significant effect of Treatment (F1,8 = 5.3, p < 0.05) but no Treatment × Days interaction. Nicotine-treated LI rats showed decreased indifference points (i.e., increased impulsive choice) compared to saline-treated LI rats during days 6–13 of chronic nicotine exposure (pairwise comparisons with Newman-Keuls test, p < 0.05). No significant main effect of Treatment was detected in HI rats.
In addition, changes in choice behavior HI and LI rats in response to chronic nicotine were assessed during days 1–7 and days 8–13 of nicotine exposure (Fig 2). Separate ANOVAs on days 1–7 of nicotine exposure confirmed no differences in choice behavior between HI and LI rats for any of the five delay blocks. In contrast, during days 8–13, differential effects of nicotine exposure were observed in HI and LI rats. Interestingly, these effects were delay-dependent and are reported below according to each delay block.
Delay Block 10 s: The ANOVA revealed significant main effects of Treatment (F1,16 = 5.3, p < 0.05) and Trait Impulsivity (F1,16 = 22.8, p < 0.0001), but no Treatment × Trait Impulsivity interaction. Nicotine exposure tended to decrease the percentage of choices for the delayed large reward in HI but not LI rats compared with the respective saline-treated groups (Fig 2a).
Delay Block 20 s: The ANOVA revealed significant main effects of Treatment (F1,16 = 5.4, p < 0.05) and Trait Impulsivity (F1,16 = 49.2, p < 0.0001,) but no Treatment × Trait Impulsivity interaction. Nicotine tended to decrease the percentage of choices for the delayed large reward in HI rats but not LI rats throughout chronic nicotine exposure compared with the respective saline-treated control groups (Fig 2b).
Delay Block 40 s: No significant differences were found between HI and LI rats in their response to nicotine (Fig 2c). The ANOVA revealed a significant main effect of Trait Impulsivity (F1,16 = 37.4, p < 0.0001) but no effect of Treatment or Day of exposure and no interactions.
Delay Block 60 s: The ANOVA revealed significant main effects of Treatment (F1,16 = 5.3, p < 0.05) and Trait Impulsivity (F1,16 = 73.2, p < 0.0001), but no Treatment × Trait Impulsivity interaction. Nicotine tended to decrease the percentage of choices for the large delayed reward in LI rats on days 8 – 11 but had no effect on choice behavior in HI rats (Fig 2d).
Omission errors during chronic nicotine exposure did not differ between treatment groups as indicated lack of significant effects of the factors of Trait Impulsivity, Treatment or Day of exposure and no interactions in the ANOVAs (data not shown).
3.5. Nicotine withdrawal
The ANOVAs revealed no effect of spontaneous nicotine withdrawal on choice behavior in the general rat population (Table 1) or in rats with high and low levels of trait impulsivity (Fig 2). The AUC was calculated for HI and LI rats during nicotine withdrawal (Fig 3a). The ANOVAs on AUC data revealed a significant main effect of Treatment (F1,16 = 4.3, p < 0.05), but no effect of Trait Impulsivity (F1,16 = 15.4, p < 0.001) and no Treatment × Trait Impulsivity interaction. Separate 2-way ANOVAs on the LI and HI groups’ data showed no significant main or interaction effects.
Further, we analyzed indifference delay points in HI and LI rats (Fig 3b). ANOVAs on indifference points data revealed significant main effects of Treatment (F1,16 = 4.4, p < 0.05) and Trait Impulsivity (F1,16 = 35.8, p < 0.001), but no Treatment × Trait Impulsivity interaction. A separate 2-way ANOVA on the LI group data showed a significant main effect of Trait Impulsivity (F1,16 = 37.4, p < 0.0001), but no effect of Treatment or Treatment × Days interaction. No significant effects were detected in HI rats.
Importantly, however, delay- and impulsivity-dependent changes in choice behavior were detected during nicotine withdrawal in HI and LI rats, and these effects are described in detail below.
Delay Block 10 s: The percentage of choices for large delayed rewards did not differ between HI and LI rats during nicotine withdrawal (Fig 2a). The ANOVA revealed a significant main effect of Trait Impulsivity (F1,16 = 10.7, p < 0.01), but no effect of Treatment or Withdrawal hours and no interactions.
Delay Block 20 s: The percentage of choices for large delayed rewards was decreased in both HI and LI rats during nicotine withdrawal (Fig 2b). An ANOVA revealed significant main effects of Treatment (F1,16 = 4.7, p < 0.05) and Trait Impulsivity (F1,16 = 44.5, p < 0.0001), but no Treatment × Trait Impulsivity interaction.
Delay Block 40 s: The percentage of choices for large delayed rewards did not differ between HI and LI rats during nicotine withdrawal (Fig 2c). The ANOVA revealed a significant main effect of Trait Impulsivity (F1,16 = 19.8, p < 0.0001), but no effect of Treatment or Withdrawal hours and no interactions.
Delay block 60 s: No differences were found between HI and LI rats in their response to nicotine withdrawal (Fig 2d). The ANOVA revealed a significant main effect of Trait Impulsivity (F1,16 = 92.7, p < 0.0001), but no effect of Treatment or Withdrawal hours and no interactions.
Omission errors during nicotine withdrawal did not differ between treatment groups as indicated by lack of significant effects of the factors Trait Impulsivity, Treatment or Day of exposure and no interactions in the ANOVAs (data not shown).
4. Discussion
The present study demonstrated that neither chronic nicotine exposure nor nicotine withdrawal had any effect on impulsive choice in Wistar rats from the general population. When rats were divided based on levels of baseline impulsivity, chronic nicotine and nicotine withdrawal increased impulsive choice in a delay- and impulsivity-dependent manner. Specifically, LI rats showed decreased preference for the large reward during chronic nicotine exposure and nicotine withdrawal as reflected in the analyses of indifference points, AUC data, and raw data values at each delay. In HI rats, nicotine exposure had no effect on preference for the large reward, although there was a tendency for increased impulsive choice at the shorter delay blocks (10 and 20 s), but not at the longer delays. These findings are consistent with recent reports showing that the noncompetitive N-methyl-D-aspartate receptor antagonist ketamine selectively increased impulsivity in LI, but not HI, rats (Cottone et al., 2013). Exposure to chronic nicotine or nicotine withdrawal had no effect on the number of omissions made in either LI or HI rats.
Previous studies that investigated the effects of chronic nicotine exposure on impulsive choice in experimental animals have provided contradictory findings. Consistent with our findings, impulsive choice was dose-dependently increased in Long-Evans rats after nicotine injections [0.35 mg/kg once a day for 65 days (Dallery and Locey, 2005); 0.8 mg/kg twice a day for 6 days (Kelsey and Niraula, 2013)]. In contrast, in another study, chronic daily nicotine injections (1 mg/kg for 30 days) had no effects on delayed reward choices in either Lewis or Fisher 344 rats, although Lewis and Fisher rats emitted different baseline impulsive choice responses and exhibited differential sensitivity to the effects of acute nicotine on impulsive choice (Anderson and Diller, 2010). Importantly, the increases in impulsive choice were evident when nicotine was administered chronically either via bolus injections (Dallery and Locey, 2005, Kelsey and Niraula, 2013) or at a high dose with a constant rate of delivery via minipumps (the present study). Furthermore, increases in impulsivity after chronic nicotine exposure were detected in the delayed reward task with predefined delays (present study) and an adjusting-delay task in which the delays to obtain the larger reinforcer were adjusted based on the subject’s choice until an equilibrium was reached, at which point the subject was indifferent between the two alternatives (Dallery and Locey, 2005, Kelsey and Niraula, 2013). Thus, independent of rat strains or procedural task differences, nicotine dose and treatment duration appear to be important factors that affect nicotine-induced increases in impulsive choice behavior.
The observed nicotine-induced increases in impulsivity were transient and dissipated by day 13 of nicotine exposure in both HI and LI rats, possibly reflecting the development of tolerance to nicotine. Consistent with our findings, increased impulsive choice induced by acute nicotine (0.1-1 mg/kg) dissipated after chronic daily nicotine injections in both Lewis and Fisher rats (Anderson and Diller, 2010). Furthermore, our previous work demonstrated that chronic nicotine exposure transiently increased motor impulsivity (i.e., impulsive action), reflected by premature responses in the 5-choice serial reaction time task (Amitai and Markou, 2009, Semenova et al., 2007).
In human studies, current smokers were more impulsive when they were allowed to smoke regularly (Baker, Johnson, 2003, Heyman and Gibb, 2006, MacKillop, Amlung, 2011, Ohmura et al., 2005). However, in a study that followed a cohort (n = 947) of subjects from age 15 to 21 and measured smoking and delay discounting rates every year during this period, the results showed that delayed discounting did not change across time (Audrain-McGovern et al., 2009). Thus, baseline delay discounting appears to promote smoking initiation, but smoking does not significantly alter delay discounting. Similarly, in the present study, nicotine transiently increased impulsive choice responses.
The increased impulsive choice for cigarettes only, but not other reinforcers, was reported in human smokers during early withdrawal. Specifically, at 24 h of nicotine withdrawal, subjects chose the immediate reward when the immediate reward was a cigarette instead of a delayed monetary reward (Mitchell, 2004). Interestingly, however, when small or large reward alternatives were monetary rewards, abstinent smokers chose the delayed large money reward, indicating no effect of nicotine withdrawal on impulsive choice (Mitchell, 2004). In another study, smokers made more impulsive choices for both monetary and cigarette rewards after 13 h of withdrawal (Field et al., 2006). In the present study, impulsive choice continued to be increased during spontaneous nicotine withdrawal in LI, but not HI, rats with the largest effect at the 6–12 h withdrawal time points (Fig. 3). These findings are consistent with our previous work showing the short-lasting effect of nicotine withdrawal on the affective and somatic aspects of spontaneous nicotine withdrawal (Epping-Jordan et al., 1998, Harrison et al., 2001, Liechti and Markou, 2007, Semenova and Markou, 2003, Skjei and Markou, 2003). In contrast, increased impulsive choice behavior during nicotine withdrawal in Long-Evans rats on day 14 post-nicotine (Dallery and Locey, 2005) and Lewis rats on day 10 post-nicotine but not in Fisher 344 rats (Anderson and Diller, 2010). The long-lasting effects observed in these studies may be related to the conditioned effects of nicotine rather than the direct effects of nicotine withdrawal on impulsivity. Exposure to cues or contexts associated with nicotine contributes to the maintenance of tobacco smoking in humans and increased nicotine-seeking behavior in animals (Balfour et al., 2000, Caggiula et al., 2001, Chaudhri et al., 2006, Chiamulera, 2005, Rose et al., 1993). Therefore, exposure to the chamber previously associated with nicotine injections, but not nicotine withdrawal, may have elicited increased impulsive responding (Anderson and Diller, 2010, Dallery and Locey, 2005).
Upregulation of high-affinity nicotinic acetylcholine receptors (nAChRs) is observed after chronic cigarette smoking in humans (Benwell et al., 1988, Breese et al., 1997, Perry et al., 1999) and after chronic nicotine exposure in experimental animals (Collins et al., 1990, Marks et al., 1983, Rowell and Li, 1997, Sanderson et al., 1993, Ulrich, Hargreaves, 1997). Decreases in nAChR function also occur with chronic nicotine exposure (Dani and Heinemann, 1996, Gentry and Lukas, 2002, Gentry et al., 2003, Marks et al., 1993, Marks et al., 2004, Wonnacott, 1990, Zambrano et al., 2012), which may compensate for nAChR upregulation. Furthermore, decreased nAChR-mediated dopamine release in the striatum was observed after termination of chronic nicotine exposure (Jacobs et al., 2002, Marks, Grady, 1993), indicating decreases in nAChR function.
Impulsivity, including impulsive choice, is partly mediated by the mesocorticolimbic dopamine system, among other systems (Pattij and Vanderschuren, 2008). The role of mesocorticolimbic dopamine in impulsive choice is suggested by data showing decreased impulsive choice behavior after manipulations that increase dopamine transmission (Fernando et al., 2012, van Gaalen, van Koten, 2006), and data demonstrating increased impulsive choice behavior after lesions of the dopamine-rich nucleus accumbens (NAc) core (Cardinal et al., 2001). Dopamine release in the medial prefrontal cortex and NAc core and shell subregions was significantly reduced in HI rats compared with LI rats selected in the delayed reward task (Diergaarde et al., 2008). Thus, increased baseline impulsive choice responses appear to be associated with reduced dopamine activity in the shell and core regions of the NAc and the medial prefrontal cortex.
Nicotine increases dopamine release in the NAc through nAChRs located on dopaminergic neurons in the ventral tegmental area in animal studies (Clarke, 1993, Fu et al., 2000, Mameli-Engvall et al., 2006, Nisell et al., 1994, Pidoplichko et al., 1997). Similarly, brain imaging studies in humans demonstrated that smoking increased dopamine release in the ventral striatum in tobacco-dependent smokers (Brody, 2006). Interestingly, smokers with genes associated with low resting dopamine tone had greater smoking-induced (phasic) dopamine release than smokers with alternative genotypes (Brody et al., 2006). Based on these findings, one may hypothesize that smokers with low resting dopamine tone may have increased impulsivity, and smoking may attenuate increased impulsivity by increasing dopamine levels. In contrast, in smokers with high resting dopamine tone, smoking may further increase dopamine levels and directly or indirectly activate other pathways (e.g., adrenergic or serotonergic), leading to increased impulsivity. Several other neurotransmitter systems, such as glutamate, γ-aminobutyric acid (GABA), norepinephrine, and serotonin, contribute to impulsive choice (Dalley et al., 2008) and various effects of nicotine (D'Souza and Markou, 2011). However, the impact of these systems on the effects of nicotine on impulsivity in HI and LI subjects has not yet been determined.
5. Conclusions
The present study showed that baseline levels of impulsivity, assessed in the delayed reward task, are important determinants of the effects of chronic nicotine and nicotine withdrawal on impulsivity. Specifically, subjects that make few impulsive choices under baseline conditions exhibit more pronounced nicotine- and nicotine withdrawal-induced increases in impulsivity than subjects that make more impulsive choices under baseline conditions. However, nicotine-induced increases in impulsivity in HI subjects may not be detectable in the delayed reward procedure when the reinforcer is a non-drug reinforcer, as shown in humans (Mitchell, 2004). In conclusion, increased impulsivity induced by chronic nicotine exposure and withdrawal may contribute to compulsive drug use, manifested as a loss of control over drug use, and strengthen the addictive properties of nicotine.
Highlights.
Chronic nicotine exposure increased impulsive choice in low impulsive rats.
Impulsive choice continued to be increased during nicotine withdrawal in low impulsive rats.
Chronic nicotine exposure or nicotine withdrawal had no effect on impulsive choice in high impulsive rats or non-selected rats.
Acknowledgements
The authors would like to thank Mrs. Jessica Benedict for excellent technical assistance with data collection, and Mr. Michael Arends for outstanding editorial assistance. This work was funded by NIH grant R01DA11946 to AM, and Tobacco-Related Disease Research Program (TRDRP) grant 18KT-0022 from the State of California to SS. HK was supported by a postdoctoral fellowship from The Scientific and Technological Research Council of Turkey (TUBITAK-BIDEP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
HK, SS and AM designed this project. HK and SS performed the experiments, data analyses and wrote the manuscript. AM provided input for manuscript writing. All authors discussed the results and commented on the manuscript.
Conflict of interest
The NIH, TUBITAK-BIDEP, and TRDRP had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication. SS and HK have nothing to disclose. AM has received contract research support from Bristol-Myers Squibb Co., Forest Laboratories and Astra-Zeneca, and honoraria/consulting fees from AbbVie during the past 3 years. There are no actual or potential financial conflicts of interest.
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl) 2009;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010;21:754–764. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Rodgers K, Cuevas J, Wileyto EP. Young adult smoking: what factors differentiate ex-smokers, smoking cessation treatment seekers and nontreatment seekers? Addict Behav. 2009;34:1036–1041. doi: 10.1016/j.addbeh.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Kowal BP, Gatchalian KM. Cigarette smokers discount past and future rewards symmetrically and more than controls: is discounting a measure of impulsivity? Drug Alcohol Depend. 2008;96:256–262. doi: 10.1016/j.drugalcdep.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural networks : the official journal of the International Neural Network Society. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chiamulera C. Cue reactivity in nicotine and tobacco dependence: a "multiple-action" model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Brain Res Rev. 2005;48:74–97. doi: 10.1016/j.brainresrev.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Clarke PB. Nicotinic receptors in mammalian brain: localization and relation to cholinergic innervation. Progress in brain research. 1993;98:77–83. doi: 10.1016/s0079-6123(08)62383-3. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology (Berl) 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2011;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology (Berl) 2007;190:485–496. doi: 10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, et al. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology (Berl) 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Wilkins LH, Jr, Lukas RJ. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human alpha4beta2- and alpha4beta4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;304:206–216. doi: 10.1124/jpet.102.041756. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behav Pharmacol. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Jacobs I, Anderson DJ, Surowy CS, Puttfarcken PS. Differential regulation of nicotinic receptor-mediated neurotransmitter release following chronic (-)-nicotine administration. Neuropharmacology. 2002;43:847–856. doi: 10.1016/s0028-3908(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Niraula A. Effects of acute and sub-chronic nicotine on impulsive choice in rats in a probabilistic delay-discounting task. Psychopharmacology (Berl) 2013;227:385–392. doi: 10.1007/s00213-013-2984-1. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) 2011;217:455–473. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Mazur J. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantative analyses of behaviour: the effects of delay and intervening events on reinforcement value. Hillsdale: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6:819–828. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the experimental analysis of behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav. 1993;44:891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Sanderson EM, Drasdo AL, McCrea K, Wonnacott S. Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res. 1993;617:349–352. doi: 10.1016/0006-8993(93)91104-z. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Ulrich YM, Hargreaves KM, Flores CM. A comparison of multiple injections versus continuous infusion of nicotine for producing up-regulation of neuronal [3H]-epibatidine binding sites. Neuropharmacology. 1997;36:1119–1125. doi: 10.1016/s0028-3908(97)00107-x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J Pers. 2002;70:533–552. doi: 10.1111/1467-6494.05013. [DOI] [PubMed] [Google Scholar]
- Zambrano CA, Salamander RM, Collins AC, Grady SR, Marks MJ. Regulation of the Distribution and Function of [125I]Epibatidine Binding Sites by Chronic Nicotine in Mouse Embryonic Neuronal Cultures. J Pharmacol Exp Ther. 2012;342:245–254. doi: 10.1124/jpet.112.192542. [DOI] [PMC free article] [PubMed] [Google Scholar]