Abstract
While premature infants have a high need for positive interactions, both infants and their mothers are challenged by the infant‘s biological immaturity. This randomized clinical trial of 198 premature infants born at 29–34 weeks gestation and their mothers examined the impact of the H-HOPE (Hospital to Home: Optimizing the Infant’s Environment) intervention on mother-premature infant interaction patterns at 6-weeks corrected age (CA). Mothers had at least 2 social environmental risk factors such as minority status or less than high school education. Mother-infant dyads were randomly assigned to the H-HOPE intervention group or an attention Control group. H-HOPE is an integrated intervention that included (1) twice-daily infant stimulation using the ATVV (auditory, tactile, visual, and vestibular-rocking stimulation) and (2) four maternal participatory guidance sessions plus two telephone calls by a nurse-community advocate team. Mother-infant interaction was assessed at 6-weeks CA using the Nursing Child Assessment Satellite Training–Feeding Scale (NCAST, 76 items) and the Dyadic Mutuality Code (DMC, 6-item contingency scale during a 5-minute play session). NCAST and DMC scores for the Control and H-HOPE groups were compared using t-tests, chi-square tests and multivariable analysis. Compared with the Control group (n = 76), the H-HOPE group (n = 66) had higher overall NCAST scores and higher maternal Social-Emotional Growth Fostering Subscale scores. The H-HOPE group also had significantly higher scores for the overall infant subscale and the Infant Clarity of Cues Subscale (p < 0.05). H-HOPE dyads were also more likely to have high responsiveness during play as measured by the DMC (67.6% versus 58.1% of controls). After adjustment for significant maternal and infant characteristics, H-HOPE dyads had marginally higher scores during feeding on overall mother-infant interaction (β = 2.03, p = .06) and significantly higher scores on the infant subscale (β = 0.75, p = .05) when compared to controls. In the adjusted analysis, H-HOPE dyads had increased odds of high versus low mutual responsiveness during play (OR = 2.37, 95% CI = 0.97, 5.80). Intervening with both mother and infant is a promising approach to help premature infants achieve the social interaction patterns essential for optimal development.
Keywords: Mother-infant interaction, social interactive behaviors, multisensory intervention, preterm infants, low-income mothers
1. Background
Preterm birth remains a major health concern in the US. Nearly half a million infants are born prematurely each year in the US (Martin et al., 2012). Despite declines in the preterm birth rates among all race and Hispanic origin groups, substantial disparities remain. The preterm birth rate for African-Americans is over 1.5 times the rate for non-Hispanic whites (17.1% vs. 10.8%), and the rate for Hispanics is 11.8%, slightly higher than for non-Hispanic whites (Martin et al., 2012). Although survival rates have increased among all racial and ethnic groups, premature birth places surviving infants at increased biological risk for difficulties in behavioral organization, feeding, social interaction, development and growth (Bendersky & Lewis, 1994; Boyle et al., 2012; Bradley et al., 1994; Burchinal, Roberts, Zeisel, Hennon, & Hooper, 2006; Conley & Bennett, 2000; Cserjesi et al., 2012; Kelly, 2012; Kerstjens et al., 2011; McGauhey, Starfield, Alexander, & Ensminger, 1991; Ruth, Roos, Hildes-Ripstein, & Brownell, 2012; Saigal, Szatmari, Rosenbaum, Campbell, & King, 1991; Williams et al., 2013).
Because preterm infants are at higher risk of social-emotional, language, mental and motor developmental delays, social interaction is especially important in optimizing these outcomes (Forcada-Guex, Pierrehumbert, Borghini, Moessinger, & Muller-Nix, 2006; McGroder, 2000; Smith, Landry, & Swank, 2000). For most infants, interaction with their mothers is the foundation that builds their capacity for presenting clear behavioral cues and responding during social interaction. Building positive mother-infant interaction requires sensitive maternal responses to infant cues, positive affect, maternal pauses during interaction and other maternal behaviors including cognitive and social-emotional growth fostering behaviors. These maternal behaviors have been shown to facilitate infant clarity of cues and responsiveness, leading to engagement and mutual responsiveness (Barnard, 1979; Barnard, 1997; Barnard, Hammond, Booth, Mitchell, & Spieker, 1989; Barnard, 1989; Cusson, 2003). These types of positive interactions help establish social competency and secure attachment, which are essential for later social, language, and cognitive development (Barnard, 1997; Kelly, Morisset, Barnard, Hammond, & Booth, 1996; Steelman, Assel, Swank, Smith, & Landry, 2002).
While premature infants have a high need for positive interactions, establishing positive interaction patterns is challenging for both infants and their mothers because of the infant‘s biological immaturity. Premature infants have lower capacity for self-regulation, less alertness, hypersensitivity to stimulation, inefficient oral feeding and unclear behavioral cues that are difficult for parents to interpret (Feldman & Eidelman, 2006; Pickler et al., 2010; White-Traut, Nelson, Silvestri, Cunningham, & Patel, 1997; White-Traut, Nelson, Silvestri, Vasan, Littau, et al., 2002). Consequently, mothers may experience heightened levels of stress and anxiety related to their infant’s prematurity and a lack of knowledge and confidence regarding premature infant care, which can alter their early mothering experience (Brandon et al., 2011; Feldman & Eidelman, 2006; Howland, Pickler, McCain, Glaser, & Lewis, 2011; Miles, Holditch-Davis, & Schwartz, 2007).
Infant immaturity, maternal distress, and lack of knowledge related to the premature infant’s capacity for social interaction place the mother-infant dyad at risk for negative interaction patterns (Forcada-Guex, Borghini, Pierrehumbert, Ansermet, & Muller-Nix, 2011; Glascoe & Leew, 2010; Gravener et al., 2012; Holditch-Davis, Schwartz, Black, & Scher, 2007; Lee, Holditch-Davis, & Miles, 2007; McManus & Poehlmann, 2012; Treyvaud et al., 2011). A mother’s perceptions of her premature infant may be negatively altered, and these negative perceptions can limit a mother’s capacity to respond appropriately, causing disruptions in the development of appropriate mother-infant interaction (Brandon et al., 2011; Coyl, Roggman, & Newland, 2002; Miles et al., 2007; Nicolaou, Rosewell, Marlow, & Glazebrook, 2009; Pridham, Lin, & Brown, 2001). Initially, mothers respond to their preterm infants with overstimulation, resulting in negative infant responses, followed by maternal continued non-contingent overstimulation or decreased stimulation despite increasing infant readiness for social interaction (Forcada-Guex et al., 2006; Holditch-Davis, Miles, & Belyea, 2000; Magill-Evans & Harrison, 2001). Higher levels of maternal anxiety were also associated with premature infants being less facially responsive in interactions with their mothers (Schmucker et al., 2005). Over time, maternal lack of sensitivity and non-contingent behaviors during interactions lead to poorer infant growth and development (Feldman & Eidelman, 2006; Feldman, Keren, Gross-Rozval, & Tyano, 2004; Glascoe & Leew, 2010; Treyvaud et al., 2011; White-Traut & Norr, 2009).
Behavioral and developmental interventions are necessary to address the unique behaviors of premature infants and interactive capacities of the mother-infant dyad. To date, many interventions have addressed the needs of premature infants and mothers separately. Interventions for premature infants have largely concentrated on improving the development of the infant’s nervous system and have had positive outcomes including better neurobehavioral functioning, increased alertness, and increased arousal (Als et al., 2004; Burns, Cunningham, White-Traut, Silvestri, & Nelson, 1994; Lekskulchai & Cole, 2001; White-Traut et al., 1997; White-Traut, Nelson, Silvestri, Vasan, Littau, et al., 2002). Interventions for mothers of premature infants have focused on reducing maternal distress and improving maternal sensitivity to their premature infant, resulting in reduced maternal anxiety and improved mother-infant interactions (Bakermans-Kranenburg, van Ijzendoorn, & Juffer, 2003; Davis, Edwards, Mohay, & Wollin, 2003; Feeley, Zelkowitz, Westreich, & Dunkley, 2011; Kaaresen, Ronning, Ulvund, & Dahl, 2006; Parker, Zahr, Cole, & Brecht, 1992). Other interventions have been directed to mothers of premature infants with the aim of improving their capacity to recognize and respond to their infant’s unique behavioral cues, resulting in improved sensitivity and responsiveness among mothers (Kang et al., 1995; Pridham et al., 2005; Ravn et al., 2011; Schroeder & Pridham, 2006). However, no previous interventions have simultaneously focused on the needs of both mothers and premature infants to improve the quality of their interactions. Guided by the Transactional Model (Sameroff & MacKenzie, 2003), we developed the H-HOPE intervention (Hospital to Home: Optimizing the Infant’s Environment). H-HOPE integrates an infant remediation intervention (the ATVV intervention) with maternal re-education and redefinition offered via participatory guidance by a nurse-community advocate team (NAT) (White-Traut & Norr, 2009). In this study, we targeted clinically stable premature infants born at 29–34 weeks (considered at biologic risk due to prematurity but without severe biologic risk factors) who also had at least two social-environmental risk factors. These infants are sufficiently intact neurologically to achieve optimal development with intervention (Bendersky & Lewis, 1994; Burchinal et al., 2006; Nelson & Halverson, 1996; White-Traut, Berbaum, Lessen, McFarlin, & Cardenas, 2005). However, in the presence of multiple social/environmental risk factors such as poverty and racial and ethnic discrimination, the mother and infant are likely to be challenged by prematurity and infants have poorer long term health and development (Ruth et al., 2012; Williams et al., 2013). Thus, this target population is particularly likely to benefit from H-HOPE Intervention. While the intervention should also be effective for families with fewer social/environmental risks, these families need for intervention is less.
The purpose of this paper is to examine the impact of H-HOPE on mother-premature infant interaction patterns during feeding and play at 6-weeks corrected age (CA).
2. Methods
2.1. Design
The study employed a balanced two-group randomized trial design (Hinkelmann, 1994; Keppel, 1982). After baseline data collection, 198 premature infants born at 29–34 gestational age (GA), with at least 2 social-environmental risk factors, were randomly assigned to the H-HOPE intervention group or the Parent Education Program attention control group. Mother-infant interactions during feeding and play were assessed at a follow-up visit when infants were approximately 6-weeks CA, during which infant development and growth was also assessed and a maternal interview was administered in the preferred language of the mother (English or Spanish).
2.2. Setting and Sample
The research was conducted in two inner-city Midwestern community hospitals with neonatal intensive care units (NICUs). One hospital is a level II and the second is a level III facility.
Infants met eligibility criteria if they were born between 29 and 34 weeks gestation and had no other major health problems. Infants may have previously received ventilator support or other medical therapies for maintenance (e.g. IV therapy, or oxygen therapy via nasal cannula). However, they had to be deemed clinically stable at the time of enrollment. Their mothers met eligibility criteria if they had at least 2 social-environmental risk factors such as minority status, less then high school education, less than 18 years of age, history of current mental illness e.g. depression, family income less than 185% of the federal poverty guidelines, more than one child under 24 months, four or more children under 4 years of age in the home, or resided in a disadvantaged neighborhood. Infant exclusion criteria included the presence of congenital anomalies, necrotizing enterocolitis, brain injury, chronic lung disease, history of prenatal illicit drug exposure or positive toxicology screen. Mothers were excluded if they were illicit drug users, not the legal guardian of the infant or HIV positive.
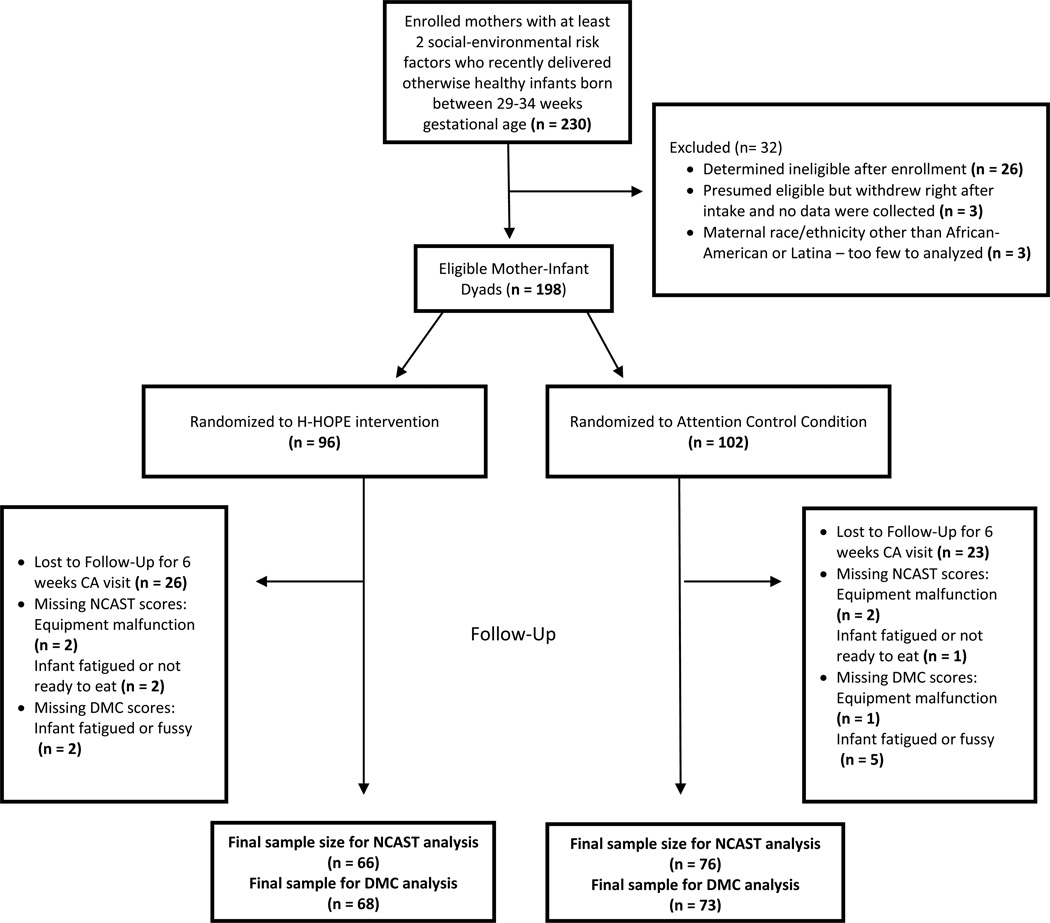
Of the 198 mother-infant dyads enrolled in the study, 149 (75.3%) were retained for the 6 week CA visit, with 142 having valid data for mother-infant interaction during feeding and 141 having valid data for mother-infant interaction during play. Reasons for missing outcome data among some dyads completing the visit included: infant fatigue or fussiness after completing the infant developmental assessments, infants not being ready to eat, and lack of audio on some videos of mother-infant feedings. See Figure 1 for more details. The only difference between dyads who returned and did not return for the six week CA follow-up visit was gestational age, with those returning born at a significantly younger gestational age on average compared to those not returning (mean = 32.4 and 32.8 weeks, respectively).
Figure 1.
Enrollment, Randomization and Final Sample Size
2.3. The H-HOPE Intervention and Attention Control Conditions
2.3.1. H-HOPE Intervention
The infant-directed ATTV component of H-HOPE provides 10 minutes of auditory (infant directed motherese voice), tactile (moderate touch stroking or massage) and visual (eye to eye) stimulation, followed by 5 minutes of vestibular stimulation (horizontal rocking) (Burns et al., 1994). Throughout the ATVV, stimulation is offered with sensitivity and responsiveness to infant behavioral cues and the stimuli are presented in a gradual progression: auditory only for the first 30 seconds, followed by combined auditory and tactile stimuli for 10 minutes, with visual stimulation added as the infant becomes alert. The vestibular stimuli are then added and the tactile component withdrawn for the remaining 5 minutes. The ATVV begins when the infant reaches 32 weeks PMA or at entry into the study for infants born at 33–34 weeks. The intervention is administered twice daily prior to feeding by the mother or by the research nurse when the mother is unable to visit the hospital (Burns et al., 1994; White-Traut & Nelson, 1988; White-Traut, Nelson, Silvestri, Vasan, Littau, et al., 2002; White-Traut & Pate, 1987). After hospital discharge, the mother continues the ATVV twice daily until the infant reached one month corrected age. Research staff and the mothers were taught the ATVV intervention to criterion of > 90% agreement. During inpatient ATVV administration, inter-rater reliability with the ATVV Checklist was maintained above 90% for the mothers and the research staff. The Nurse-Advocate team (NAT), which was responsible for implementing the intervention with mothers, confirmed on a daily basis the mothers’ plans to come into the hospital to administer the ATVV. When mothers indicated that they could not come in, a nurse on the research team arranged to be at the hospital to administer the massage.
The mother directed component of H-HOPE consisted of redefinition and re-education for mothers along with education and social support through individualized participatory guidance (White-Traut & Norr, 2009). This component of the intervention was provided by the Nurse/Advocate Team (NAT). The NAT intervention team always included at least one fluent Spanish speaker and one African-American. Both attended every in-hospital and at-home visit. The NAT component was provided during 2 in-hospital visits, 2 home visits and 2 phone calls after discharge. At every contact, the NAT asked about and responded to the mother’s concerns about herself and her infant. The content of each visit built upon the content presented in the prior visit. After all visits and phone calls, referrals were made immediately for urgent care, mental health, potential abuse, or social service. During the first hospital visit, the NAT taught the mother the ATVV, including how to recognize her infant’s behavioral states and pre-feeding, engagement and disengagement behavioral cues (redefinition). The NAT and mother discussed preparing for the transition to home. At both hospital visits, mothers gave a return demonstration of the ATVV to document continued reliability. During the second hospital visit, the NAT reviewed infant behavioral states and cues and taught the mother how to modulate her baby’s behavior and modify her own behavior in response to her baby’s behavioral cues. A pamphlet, “How to Soothe a Fussy Baby” was introduced and provided to research participants. The content of the prepared pamphlet was specifically adapted from materials in the Medical Foster Parent Handbook, unpublished (Stroemple, 1992).
At each home visit, the NAT discussed feeding and weighing the infant and assessed the mother’s stressors, supports and signs of depression. The nurse discussed maternal and infant signs and symptoms that require immediate attention while the advocate reviewed emergency contact numbers and procedures. The mother demonstrated the ATVV and described her infant’s state, modulation of state, and behavioral cues. The nurse observed a feeding and offered participatory guidance regarding the contribution of mother and infant to the interaction, infant behavioral state and cues, how to modulate infant state, and the mother’s use of soothing behaviors based on “Keys to Caregiving” content (Barnard & Kelly, 1990; Sumner & Spietz, 1994). “Keys to Caregiving” has been used during the infant’s hospitalization and at home to support caregiver engagement in the care of their neonates. Specifically, topics include describing the language of the newborn, what infant behaviors mean in the context of feeding and mother-infant interaction, and teaching caregivers how to support infant sleep and wakefulness states. At the second home visit, the NAT introduced maturational changes in cues and contingency behavior and parent social interaction responsibilities during feeding. Two telephone contacts made by the advocate between home visits provided mothers with an opportunity to discuss any new concerns, and reinforce the concepts introduced at each prior visit.
Fidelity of the intervention. We assessed two aspects of the fidelity of the intervention for both hospital and home visits: the “dose” of the intervention, and the degree to which the intervention was delivered according to the protocol. Of the women assigned to the H-HOPE group, 63% participated in both of the visits in the hospital and 93% participated in both home visits. The main reason that a hospital visit was missed was that the infant was discharged more rapidly than expected. Because the NAT was not located at the hospital sites and did not have 7-day coverage, the team did not always learn about the plan to discharge the infant in time to complete the visit. All but three of the women who missed one visit in the hospital received both home visits.
We also tracked the number of times each infant received the ATVV. The study protocol called for administration of the ATVV twice per day for five days per week. While the infant was in the NICU, the date and time of each ATVV was recorded in a bedside log. Sometimes the ATVV did not occur twice daily because the mothers told the NAT that they would visit the baby and administer the massage but then were not able to. By the time the research team learned that the mother did not visit, the research team was not always available to administer the ATVV. Approximately 70% of infants were administered the ATVV twice per day on at least half of the days of their hospital stay. Of the remaining infants, 87% were administered the ATVV at least once per day on over half of their days in the hospital. After hospital discharge, mothers were asked to keep daily logs of the number of times per day the ATVV was administered at home. Fifty-seven of the 66 (86.4%) women in the H-HOPE group returned ATVV logs at the follow-up visit, and most of these logs (73%) had been completed for all days between the infant's hospital discharge and the follow-up visit (mean = 75 days, SD = 11, range = 49–99). Logs indicated that mothers (or other family members) administered the ATVV 1.5 times per day (SD = 0.80) during this two to three month time period.
To assess whether the H-HOPE intervention was administered as intended, a research team member observed the Nurse Advocate Team (NAT) during selected hospital and home visits. The fidelity assessment guide developed prior to initiation of the study captured both coverage of content and the use of a facilitative teaching process. All visits that were observed had high fidelity, using participatory guidance for teaching and covering all content with individualized attention to each mother’s needs.
2.3.2. The Attention Control Condition: Parent Education Program - Infant Safety Education
The Attention Control Condition was designed to provide a similar amount of contact with the mother and staff attention, but with content distinctly different from the H-HOPE intervention. In addition to receiving the standard of care, mothers of infants assigned to the Attention Control Condition received educational content that included premature infant care and car safety and four phone calls after the infant’s discharge to home. During each phone call, mothers received information on infant care including bathing, sleep positions and sleep habits, holding the baby, and safety of infant equipment.
2.4. Outcome Measures
2.4.1 Mother-Infant Interaction during Feeding
The Nursing Child Assessment Satellite Training–Feeding Scale (NCAST-Feeding) assessed maternal and infant behaviors during a breast or bottle feeding at 6-weeks corrected age (Sumner, 1994). The Feeding Scale (76 items) consists of six subscales: (a) maternal - sensitivity to cues; (b) maternal response to distress; (c) maternal social -emotional growth-fostering; (d) maternal cognitive growth fostering; (e) infant clarity of cues; and (f) infant responsiveness to caregiver. Items were summed to yield a mother score, an infant score, and a total score. Infant clarity of cues includes alert behaviors and responses such as looking at the mother when the mother is looking at the baby, vocalizing to the mother, either initiation of a vocalization or responding to the mothers’ vocalization, or touching the mother. An example of an unclear behavioral cue includes lack of signaling readiness to eat, a lack of alertness during the feeding, or lack of movement during the feeding.
The NCAST has been widely used in parenting research and has well-established reliability and validity for term and preterm infants. For premature infants, the infant subscales are significantly correlated to later IQ (Barnard, 1989). Internal consistency is high for the total score (a = .86), the mother total score (α =.83) and for the infant total score (α =.73) (Barnard, 1989). The feeding interactions were video-recorded and later judged by a coder. Prior to data collection, two coders were re-trained to criterion of > 90% agreement. Inter-rater reliability between the primary and a secondary coder was assessed using NCAST manual guidelines throughout the study period and remained above 95% agreement. Both coders were blinded to the infant’s group assignment.
2.4.2 Mother-Infant Interaction During Play
Mother-infant interaction during play was measured via the Dyadic Mutuality Code (DMC) at 6-weeks CA (Censullo, no date; Coffman, 1991). The DMC measures levels of mutuality in infant-adult interaction, e.g., the partners coordinate repetitive sequences of behavior as they move as a joint unit to higher levels of energy, heightened positive affects, and increased mutual attention (Censullo, Bowler, Lester, & Brazelton, 1987; Lester, Hoffman, & Brazelton, 1985). The DMC consists of six items that represent key components of mutuality: mutual attention, positive affect, mutual turn-taking, maternal pauses, infant clarity of cues, and maternal sensitivity to cues and responsiveness (each scored as 1 if none or very brief and 2 if half the time or more), for a total score (range 6–12). Mothers were given standardized instructions for the 5-minute play session. For analysis, scores were categorized into low (6–8), moderate (9–10), or high responsivity (11–12). DMC reliability and construct and concurrent validity have been established using healthy term and preterm infants (Censullo, no date; Coffman, 1991; Lester et al., 1985; Tronick, Als, & Brazelton, 1980) and in high risk infants (Tronick et al., 1980). Inter-rater reliability was .89 for the total score, with item reliabilities from .82 to .96. Two coders were trained to criterion of > 85% by the developer of the DMC instrument. Like the NCAST, the play sessions were video-recorded and later judged by a coder. Inter-rater reliability between the primary and a secondary coder remained above 98% agreement. Both coders were blinded to the infant’s group assignment.
2.5. Covariates
Infant characteristics were abstracted from the medical record and included sex, gestational age (GA) at birth, birthweight, five minute Apgar score, pre-discharge morbidity measured using the Problem-Oriented Perinatal Risk Assessment System (POPRAS) score(Davidson & Hobel, 1978), and chronological age at the follow-up visit. The POPRAS score is a standard and widely used summary of all health problems experienced by each preterm infant from birth through discharge
Maternal baseline characteristics were obtained during a face-to-face interview with the mother at enrollment, except for parity which was abstracted from medical records. Age (calculated from the mother’s birthdate), race/ethnicity (African-American or Latina), education level, if working full-time or part-time prior to delivery, and living situation (with baby’s father, with mother or other adult, alone) were self-reported. Education was categorized as low for women 20 or older who did not have a high school degree or GED and for women <20 who did not finish high school or were not currently still in school.
Depression was ascertained using a composite variable consisting of responses to the Postpartum Depression Screening Scale (PDSS), the Center for Epidemiologic Studies – Depression Scale (CES-D) and women’s self-report of a prior diagnosis of a mental health condition, such as depression (Beck & Gable, 2001; Radloff, 1977; Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977). Women who screened high on the PDSS (≥ 60), high on the CES-D (≥ 16) or reported a prior diagnosis, were classified as depressed. Maternal trait anxiety was assessed using the trait subscale of the State-Trait Anxiety Inventory (STAI) (Spielberger & Gorsuch, 1983). Given the highly right-skewed distribution of the scores in our sample, trait anxiety was dichotomized into high (score of 40 or higher) and low/moderate (<40) as previously used in studies of prenatal and postnatal anxiety (Barnett & Parker, 1985; Britton, 2008; Grant, McMahon, & Austin, 2008).
Neighborhood disadvantage was derived using 5-year estimates (2005–2009) from the American Community Survey at the census tract level (US Census Bureau, 2011). Mothers’ addresses were geocoded to census tracts. The Index of Neighborhood Disadvantage Score (Ross & Mirowsky, 2001) was calculated for each census tract by subtracting the sum of the percent of mother-headed households and the percent living below the poverty line (two indicators of neighborhood disadvantage) from the sum of the percent of adults >24 years who are college educated and the percent of households in the census tract that were owner occupied (2 indicators of neighborhood advantage). Women’s neighborhoods were considered disadvantaged if the INDS was greater than zero.
2.6. Procedure
The research was approved by the Institutional Review Boards from the university and the two clinical sites. After informed consent was obtained, mothers and infants were randomly assigned to the H-HOPE or Attention Control group. Random assignment was conducted using two computer generated lists of random numbers, one for each site, so that approximately half the infants at each site were in the experimental group. The intervention began in the hospital after the baseline feeding assessment. For infants born at 32 weeks GA or less, the baseline assessment occurred when the infant reached 32 weeks PMA and began oral feeding. For infants born at 33–34 weeks GA, baseline assessment began shortly after recruitment since most infants born at this GA were oral feeding at birth. The respective interventions were administered as described above. Mother-infant interaction was assessed when the infants reached 6 weeks corrected age.
2.7. Statistical Methods
Descriptive statistics were calculated overall and by group (H-HOPE vs. Control) and chi-square tests and t-tests were used to examine group equivalence at baseline. Mean scores and standard deviations for the overall NCAST and subscales were calculated by group, using t-tests to assess group differences. A chi-square test for trend was used to compare the proportion of mother-infant dyads in the H-HOPE vs. control group that demonstrated low, moderate and high responsivity according to the DMC.
Multivariable analyses were conducted to assess the effect of the H-HOPE intervention on mother-infant interactions during feeding and play controlling for covariates. Initial models included all of the infant and mother variables in Tables 1 and 2. To choose the final models, manual backward selection was used to select only the significant (p < 0.05) covariates from those listed in Tables 1 and 2. Multivariable linear regression models were conducted for mother-infant interaction during feeding, with the overall NCAST score, maternal subscore, and infant subscore as separate outcomes. A generalized logit model was conducted to assess the effect of H-HOPE on mother-infant responsivity during play. Odds ratios (OR) and 95% confidence intervals were calculated contrasting high vs. low and moderate vs. low responsivity.
Table 1.
Infant Characteristics of the Sample by Group
| H-HOPE (n = 66) %, mean(SD) |
Attention Control (n = 76) %, mean(SD) |
||
|---|---|---|---|
| Sex | |||
| Female | 53.0 | 42.1 | |
| Male | 47.0 | 57.9 | |
| Gestational Age at birth | 32.2 (1.7) | 32.5 (1.6) | |
| Birth Weight | 1816 (374) | 1865 (435) | |
| Apgar score 5 min | 8.3 (1.0) | 8.2 (1.2) | |
| Infant Morbidity During Initial Hospitalization (POPRAS) | 69.8 (21.2) | 71.7 (18.8) | |
| Chronological age at 6 week | 13.6 (1.8) | 13.2 (1.9) | |
Table 2.
Maternal Characteristics of the Sample by Group
| Maternal Characteristics | H-HOPE (n = 66) %, mean(SD) |
Attention Control (n = 76) %, mean(SD) |
|
|---|---|---|---|
| Age | 25.26 (6.56) | 26.38 (6.55) | |
| Parity | |||
| Multiparous | 60.6 | 57.9 | |
| Primiparous | 39.4 | 42.1 | |
| Race/Ethnicity | |||
| African-American | 48.5 | 51.3 | |
| Latina | 51.5 | 48.7 | |
| Educationa | |||
| Low | 26.2 | 19.7 | |
| Appropriate for Age | 73.8 | 80.3 | |
| Work Status before Delivery | |||
| Didn’t work | 61.5 | 61.8 | |
| Worked | 38.5 | 38.2 | |
| Living Situation | |||
| With Baby's Father | 55.4 | 61.8 | |
| With Mother or Other Adult | 32.3 | 25.0 | |
| Single | 12.3 | 13.2 | |
| Depressed | |||
| Not depressed | 60.0 | 54.1 | |
| depressed | 40.0 | 45.9 | |
| Trait Anxiety | |||
| Low/Moderate | 69.2 | 78.1 | |
| High | 30.8 | 21.9 | |
Education is considered appropriate for age if woman is 20 or older and has a high school degree or GED, or if a women is younger and has a high school degree or is still enrolled in school
3. Results
3.1. Sample characteristics
Of the 142 mother-infant dyads in this analysis, there were no significant differences in baseline characteristics between the control and H-HOPE groups for any infant or maternal characteristics (See Tables 1 and 2). The sample included 47% female and 53% male infants overall. The average age of the infants was 32.4 weeks gestation at birth (SD = 1.6), and the mean birth weight was 1844 grams (SD = 407). The mean Apgar Score at 5 minutes was 8.3 (SD= 1.1). Infant health status at birth and throughout the infant’s hospital stay was assessed by the Problem-Oriented Perinatal Risk Assessment System (POPRAS). The infants’ mean POPRAS Score was 70.8 (SD= 19.9) (scores ranged from 36 to 136). At the six week CA follow up visit, mean chronological age was 13.4 weeks (SD = 1.9).
Maternal ethnic/racial background was evenly distributed (50% Latina and 50% African American). Mean maternal age was 26 (SD = 6.6). The sample consisted of 41% primiparous mothers; 77% had an education appropriate for their age. The sample was largely homogeneous with respect to income level, with almost 90% of the sample having income <185% of the federal poverty level (FPL) guidelines (as measured by direct reporting of income and household size or, for those who could not report income, participation in the Women, Infants and Children Nutrition program which has an income eligibility level of <185% FPL in Illinois). In addition, 38% of mothers worked before the delivery, 59% lived with the baby’s father, 43% screened high for depressive symptoms, and 26% had high trait anxiety.
3.2. Mother-Infant Interaction During Feeding by Intervention Group
The mean total NCAST score was marginally (p < 0.10) higher for the H-HOPE group compared to the control group. The maternal subscale score for the H-HOPE group showed a mean score slightly higher than the control group, but these differences did not reach significance. The infant subscale score, specifically the infant clarity of cues subscale, was significantly higher for the H-HOPE compared to the control group (Table 3).
Table 3.
Association between Intervention Group and Mother-Infant Interaction Outcomes
| H-HOPE (n = 66) mean (SD) |
Control (n = 76) mean (SD) |
||
| Total NCAST Score * | 64.3 (5.2) | 62.5 (7.0) | |
| Maternal Score | 44.4 (4.3) | 43.4 (5.5) | |
| Sensitivity to Infant Cues | 14.2 (1.4) | 14.3 (1.6) | |
| Response to Distress | 9.9 (1.4) | 9.8 (1.4) | |
| Social-Emotional Growth Fostering* | 12.4 (1.5) | 11.8 (2.0) | |
| Cognitive Growth Fostering | 7.9 (1.7) | 7.5 (2.0) | |
| Infant Score** | 19.9 (2.0) | 19.1 (2.5) | |
| Clarity of Cues ** | 12.7 (1.1) | 12.0 (1.5) | |
| Responsiveness to Caregiver | 7.3 (1.4) | 7.2 (1.6) | |
| H-HOPE (n = 68) % |
Control (n = 73) % |
||
| DMC* | |||
| Low responsiveness (6–8) | 23.5 | 34.2 | |
| Moderate responsiveness (9–10) | 32.4 | 37.0 | |
| High responsiveness (11–12) | 44.1 | 28.8 | |
NCAST = Nursing Child Assessment Satellite Training – Feeding Scale; DMC = Dyadic Mutuality Code
p < 0.10
p < 0.05
In multivariable models controlling for significant covariates, H-HOPE dyads had more positive mother-infant interaction during feeding than control dyads, as measured by the overall NCAST score, and differences were marginally significant (p = 0.06). For the infant subscale, H-HOPE dyads had significantly higher scores than control dyads (p = 0.05). For the maternal subscale, H-HOPE dyads had higher scores than control dyads, but this finding did not reach statistical significance. In general, dyads with mothers who were anxious performed worse on the NCAST than those with low trait anxiety and dyads with infants who had higher morbidity in the hospital performed better (Table 4).
Table 4.
Multivariable Normal Regression Models for the Relationship between the H-HOPE Intervention and the Quality of Mother-Infant Interaction During Feeding, as Measured by the NCAST Scales (n = 142)
| Outcome | Parameters | β | SE | p | |
|---|---|---|---|---|---|
| Overall NCAST Score (R2 = 0.10) | Intercept | 58.07 | 2.06 | ||
| (n = 134) | HHOPE vs. Control group | 2.03 | 1.05 | 0.06 | |
| High vs. Low Trait anxiety | −2.56 | 1.18 | 0.03 | ||
| Infant morbidity score (POPRAS) | 0.07 | 0.03 | 0.01 | ||
| NCAST Maternal Subscale (R2 = 0.11) | Intercept | 39.58 | 1.63 | ||
| (n = 134) | HHOPE vs. Control group | 1.34 | 0.83 | 0.11 | |
| High vs. Low Trait Anxiety | −2.33 | 0.94 | 0.01 | ||
| Infant morbidity score (POPRAS) | 0.06 | 0.02 | 0.006 | ||
| NCAST Infant Subscale (R2 = 0.05) | Intercept | 19.19 | 0.26 | ||
| (n = 142) | |||||
| HHOPE vs. Control group | 0.75 | 0.39 | 0.05 | ||
Note: Four mothers had missing data for the trait anxiety scale and four infants had missing data for the POPRAS score
3.3. Mother-Infant Interaction During Play by Intervention Group
Of the mothers and infants assigned to the H-HOPE group, 44.1% were identified as exhibiting high responsivity (11–12) on the Dyadic Mutuality Code (DMC) score, compared to only 28.8% of the control infants. In addition, fewer dyads in the H-HOPE versus control group exhibited low responsivity (score of 6–8), 23.5% vs. 34.2%, respectively (Table 3). The chi-square test for trend was marginally significant (p = 0.055).
After adjustment for significant covariates, H-HOPE dyads had 2.37 times (95% CI: 0.97–5.80) higher odds of high versus low responsivity compared to control dyads. The H-HOPE group also had slightly higher odds of having moderate versus low responsivity, but the 95% CI was wide and contained the null value of one (Table 5). Covariates significantly associated with high responsivity included higher infant birthweight, older infant chronological age at the 6 week CA visit, and maternal employment before delivery, although 95% CIs were wide around all OR estimates.
Table 5.
Multivariable Generalized Logit Models for the Relationship between the H-HOPE Intervention and Mother-Infant Responsiveness as Measured by the Dyadic Mutuality Code (DMC) (n = 140)
| Maternal/Infant Factor | High versus Low Responsiveness |
Moderate versus Low Responsiveness | ||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% Confidence Intervals | Odds Ratio | 95% Confidence Intervals | |||
| HHOPE vs. Control | 2.37 | 0.97 | 5.80 | 1.32 | 0.55 | 3.16 |
| Infant Birthweight (kg) | 3.87 | 0.99 | 15.10 | 2.02 | 0.54 | 7.58 |
| Infant Chronological Age at 6 week Follow-up (wks) | 1.39 | 1.04 | 1.85 | 1.08 | 0.82 | 1.44 |
| Mother worked vs did not work before delivery | 3.64 | 1.40 | 9.50 | 2.84 | 1.11 | 7.29 |
4. Discussion
The H-HOPE intervention offers a unique approach to help mother-preterm infant dyads establish positive interaction patterns. The infant-directed component of H-HOPE, the ATVV, helps the preterm infant achieve more mature behavioral organization, including clearer cues that are easier for mothers to interpret and increased capacity to maintain the quiet alert state that is optimal for social interaction (White-Traut et al., 2005; White-Traut & Nelson, 1988; White-Traut, Nelson, Silvestri, Vasan, Patel, et al., 2002; White-Traut & Pate, 1987). The mother-directed component of H-HOPE helps mothers understand and respond appropriately to their preterm infants’ behaviors. By building the capacities of both mother and infant for social interaction, H-HOPE was hypothesized to support the development of more positive mother-infant social interaction. Our results identified a trend toward more positive mother-infant interaction during both feeding and play for dyads who received the H-HOPE intervention compared to those in the attention control group, and these differences were significant or marginally significant when covariates were controlled. Moreover, the significantly higher score for H-HOPE infants on the infant clarity of cues subscale of the NCAST during feeding supports the effectiveness of the ATVV in helping the infant to organize his/her behaviors. The trend toward higher scores for the H-HOPE mothers on the social-emotional growth fostering subscale supports the importance of the H-HOPE mother-focused education component. Mothers who received this education responded to their infant with greater warmth and sensitivity, a key component for infant social development.
These results are consistent with findings from other interventional studies that aimed to improve parent-infant interactions. Kang and colleagues (1995) found that a hospital and home visit intervention teaching mothers to read their premature infant’s behavioral cues and modulate their behavioral states increased infant clarity of cues and responsiveness during feeding interactions compared to the control group. Additionally, mothers assigned to the intervention group demonstrated significantly more sensitivity and social-emotional and cognitive simulation during teaching interactions than mothers in the control group (Kang et al., 1995). Two other studies examined the effects of a different guided participation intervention for mothers of preterm infants (Pridham et al., 2005; Schroeder & Pridham, 2006). Infants in the guided participation group showed higher adaptiveness at 1 and 8 months and mothers showed higher adaptiveness at 4 months. In a subsequent study, Schroeder & Pridham (2006) found that the relationship competency scores for mothers in the guided participation group increased more rapidly to a higher level than the scores for mothers in the standard teaching group. Investigators in Norway also examined the effect of the Mother-Infant Transaction Program on mother-preterm infant dyads and reported that mothers scored higher on sensitivity and responsiveness at 12 months of age (Ravn et al., 2011).
Early mother-infant interaction is foundational, building the infant’s capacities to engage in social interaction and form and sustain relationships and promoting normal development. Previous research has reported positive associations between mother-infant interaction and longer term child development. For instance, Beckwith & Rodning (1996) found that maternal responsiveness to infant’s vocalizations were associated with both the child’s language competence at 3 years and social competence at 5 years (Beckwith & Rodning, 1996). Subsequent investigations have generated similar results, demonstrating that positive mother-infant interactions, characterized by responsiveness and sensitivity to an infant, are associated with a child’s social skills, more developed communication skills, higher cognitive scores, and early literacy skills (Dodici, Draper, & Peterson, 2003; Newnham, Milgrom, & Skouteris, 2009; Poehlmann & Fiese, 2001; Rauh, Nurcombe, Achenbach, & Howell, 1990; Steelman et al., 2002). In another study, Swiss researchers found that only 28% of mother-preterm infant dyads had a cooperative interaction pattern, characterized as a sensitive mother and cooperative-responsive infant, compared to 68% of full-term control dyads, and early cooperative interaction was associated with improved later development. Moreover, at 18 months, those preterm infants with a cooperative pattern did not differ from full-term controls in behavioral problems and overall development, while all other patterns (i.e., controlling) were associated with less optimal development (Forcada-Guex et al., 2006). This study provides further evidence of the need for intervention for mother-preterm infant dyads to ensure that developmental outcomes are met for these vulnerable infants. More recent developmental studies have shown that the impact of the quality of interaction between the mother-infant dyad extend into middle childhood and adulthood, affecting behavioral outcomes in school and adult mental health status (Easterbrooks, Bureau, & Lyons-Ruth, 2012; Schmid et al., 2011).
In multivariable analyses we identified infant and mother factors, in addition to the intervention, that also related to the quality of mother infant interaction. However, the infant health factors related to mother-infant interaction differ for feeding and play. During play, our findings showed that heavier birth weight and older chronological age at the 6 week CA follow-up visit were associated with higher mother-infant dyad responsiveness. These findings are consistent with prior research which reports that mothers display more positive interactions towards healthier premature infants (Feingold, 1994; Landry, Smith, Miller-Loncar, & Swank, 1997; Muller-Nix et al., 2004). However, during feeding at 6 weeks corrected age, mothers whose infants had poorer health during hospitalization (POPRAS Score) demonstrated more positive maternal behaviors. These findings were unexpected and contradict the previous research discussed above. One other study by Holditch-Davis and colleagues (2007) similarly found that infant illness was related to more positive maternal involvement in a sample of mother-preterm infant dyads. One possible interpretation of these findings is that mothers with severely ill premature infants may provide more positive interactions to compensate for the severity of their infant’s illness (Holditch-Davis, Bartlett, Blickman, & Miles, 2003; Holditch-Davis et al., 2007; McGrath, Sullivan, & Seifer, 1998).
Maternal background factors were also related to the quality of mother-preterm infant interaction. During feeding, mother-infant interaction scores were lower for mothers with high trait anxiety. Mothers with high trait anxiety may be more concerned about the feeding itself because they are more anxious about the infant’s nutrient intake and growth (Crockenberg, 2000; Pridham et al., 2005). This may make them less able to integrate social interaction with the feeding experience. This finding is consistent with previous research, which has shown that maternal anxiety is related to decreased responsiveness to their premature infants (Feeley, Gottlieb, & Zelkowitz, 2005; Schmucker et al., 2005).
During play, mothers who reported that they worked during pregnancy had more responsive mother-infant interaction scores. Past maternal employment may represent greater financial stability that allows mothers to focus more on playing with their infants. Having been employed may also reflect greater social capital and regular socializing outside the family that promote the mother’s capacity for synchrony and reciprocity during play.
The findings of this study highlight the importance of examining mother-preterm infant interaction during both feeding and play to obtain a full picture of the overall quality of their developing interaction patterns. Feeding takes priority during the early days of life, especially for more fragile preterm infants. At 6 weeks CA, mothers and preterm infants are beginning to engage in play more regularly. Thus, at our 6 week CA evaluation, the dyads had more experience in interaction during feeding, and are just beginning to develop their mutual responsiveness during play. It is noteworthy that the H-HOPE intervention, which builds capacities of both mother and preterm infant, had a positive impact on mother-infant interaction during both feeding and play.
There were a number of limitations to this study. The number of participants with complete data for mother-infant interaction at six weeks CA was diminished because some mothers moved out of the area or discontinued their telephone services and some mother-infant dyads were not able to complete both videos for a variety of reasons. We also failed to include a measure of maternal post-traumatic stress symptomology, which is increasingly recognized as a factor that may add to distress and anxiety for mothers of preterm infants (Forcada-Guex et al., 2011; Holditch-Davis et al., 2003; Pierrehumbert, Nicole, Muller-Nix, Forcada-Guex, & Ansermet, 2003). Finally, mothers in the study were all African American or Latina and predominately low income, limiting generalizability to other groups. A major strength of the study is the use of two different ways of examining mother infant interaction, during feeding and play. Videotaping interactions enabled us to verify the reliability of coding of mother-infant interactions, another strength of the study.
4.1. Future research
One major question that warrants future research is the long-term impact of H-HOPE on parent-child interaction and the child’s social, language cognitive and motor development. While the long-term benefits of positive mother-infant interaction are well established, more work is needed to establish the role of intervention in fostering positive interactions and long-term benefits. Another area where more research is needed is the development of reliable measures of the quality of mother-infant interaction across feeding and play situations that are easy to administer and predictive of future development. Current measures require training and time, and they are not yet appropriate for use by busy clinicians.
4.2. Implications
There is a growing national consensus that sensory interventions should be incorporated into the standard of care for preterm infants. The Physical Environment Exploratory Group (Liu et al., 2007) has endorsed the incorporation of sensory interventions, including massage, as the standard of care to support newborn brain development and long-term health and development. Moreover, they recommend that these interventions should begin early, after 30–31 weeks GA. Because mothers of preterm infants also have unmet needs for early support and education, interventions that support both mothers and their preterm infants would be especially valuable. H-HOPE offers a model for early intervention that addresses the needs of both mother and infant to improve their mutual responsiveness and social interaction. The infant-directed component of H-HOPE, the ATVV, supported the preterm infant’s capacity to exhibit clearer cues during interaction with their mothers, facilitating the mother’s ability to read and interpret the infant’s social cues. The mother-directed component of H-HOPE helped mothers understand and respond appropriately to their preterm infants’ behaviors. By building the capacities of both the infant and the mother, mother-infant social interaction was improved. H-HOPE should be considered as part of the standard of care for mothers and preterm infants.
Highlights.
H-HOPE offers a model for early intervention that addresses the needs of both mother and infant to improve their mutual responsiveness and social interaction.
The H-HOPE intervention had a positive impact on mother-infant interaction during both feeding and play.
H-HOPE provides a model that should be considered as part of the standard of care for mothers and preterm infants because improving mother-infant interaction enhances infant overall development.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Child Health and Human Development, the National Institute of Nursing Research (1 R01 HD050738-01A2), and the Harris Foundation. We also wish to acknowledge the infants and mothers who participated in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, Eichenwald EC. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH, Juffer F. Less is more: Meta-analyses of sensitivity and attachment interventions in early childhood. Psychological Bulletin. 2003;129(2):195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Barnard K. Nursing child assessment sleep/activity record. Seattle: University of Washington Press; 1979. [Google Scholar]
- Barnard KE. Influencing parent-child interactions for children at-risk. In: Guralnick MJ, editor. The effectiveness of early intervention. Baltimore: PH: Brookes; 1997. pp. 249–268. [Google Scholar]
- Barnard KE, Hammond MA, Booth CL, Mitchell SK, Spieker SJ. Measurement and meaning of parent-child interaction. In: Morrison FJ, editor. Applied Developmental Psychology. Vol. Vol. 3. New York: Academic Press; 1989. pp. 39–80. (Reprinted from: NOT IN FILE). [Google Scholar]
- Barnard KE, Hammond MA, Booth CL, Mitchell SK, Spieker SJ. Measurement and meaning of parent -child interaction. In: Morrison FJ, editor. Applied Developmental Psychology. New York, NY: Academic Press; 1989. pp. 39–80. [Google Scholar]
- Barnard KE, Kelly . Assessment of parent-child interaction. In: Meisels SJ, Shonkoff JP, editors. Handbook of early childhood intervention. New York: Cambridge University Press; 1990. (Reprinted from: NOT IN FILE). [Google Scholar]
- Barnett B, Parker G. Professional and non-professional intervention for highly anxious primiparous mothers. The British Journal of Psychiatry. 1985;146:287–293. doi: 10.1192/bjp.146.3.287. [DOI] [PubMed] [Google Scholar]
- Beck CT, Gable RK. Further validation of the Postpartum Depression Screening Scale. Nursing Research. 2001;50(4):155–164. doi: 10.1097/00006199-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Beckwith L, Rodning C. Dyadic processes between mothers and preterm infants: Development at ages 2 to 5 years. Infant Mental Health Journal. 1996;17(4):322–333. [Google Scholar]
- Bendersky M, Lewis M. Environmental Risk, Biological Risk, and Developmental Outcome. Developmental Psychology. 1994;30(4):484–494. [Google Scholar]
- Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, Quigley MA. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. British Medical Journal. 2012;344 doi: 10.1136/bmj.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Whiteside L, Mundfrom DJ, Casey PH, Kelleher KJ, Pope SK. Early indications of resilience and their relation to experiences in the home environments of low birthweight, premature children living in poverty. Child Development. 1994;65(2 Spec No):346–360. [PubMed] [Google Scholar]
- Brandon DH, Tully KP, Silva SG, Malcolm WF, Turner BS, Holditch-Davis D. Emotional responses of mothers of late pre-term and term infants. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2011;40(6):719–731. doi: 10.1111/j.1552-6909.2011.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JR. Maternal anxiety: course and antecedents during the early postpartum period. Depression & Anxiety (1091–4269) 2008;25(9):793–800. doi: 10.1002/da.20325. [DOI] [PubMed] [Google Scholar]
- Burchinal M, Roberts JE, Zeisel SA, Hennon EA, Hooper S. Social risk and protective child, parenting, and child care factors in early elementary school years. Parenting-Science and Practice. 2006;6(1):79–113. [Google Scholar]
- Burns K, Cunningham N, White-Traut R, Silvestri J, Nelson MN. Infant stimulation: modification of an intervention based on physiologic and behavioral cues. J Obstet Gynecol Neonatal Nurs. 1994;23(7):581–589. doi: 10.1111/j.1552-6909.1994.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Censullo M, Bowler R, Lester B, Brazelton TB. An instrument for the measurement of infant-adult synchrony. Nursing Research. 1987;36(4):244–248. [PubMed] [Google Scholar]
- Censullo MNK. Synchronous patterns in appropriate and small-for-gestational age infant-mother dyads. (no date). [Google Scholar]
- Coffman S, Levitt MJ, Deets C, Quigley KL. Close relationships in mothers of distressed and normal newborns: Support, expectancy confirmation, and maternal well-being. Journal of Family Psychology. 1991;5:93–107. [Google Scholar]
- Conley D, Bennett NG. Is biology destiny? Birth weight and life chances. American Sociological Review. 2000;65:458–467. [Google Scholar]
- Coyl DD, Roggman LA, Newland LA. Stress, maternal depression, and negative mother–infant interactions in relation to infant attachment. Infant Mental Health Journal. 2002;23(1/2):145–163. [Google Scholar]
- Crockenberg SLE. Infant social and emotional development in family context. In: Zeanah CH, editor. Handbook of infant mental health. 2nd edition ed. New York: Guilford Press; 2000. pp. 60–90. [Google Scholar]
- Cserjesi R, Van Braeckel K, Butcher PR, Kerstjens JM, Reijneveld SA, Bouma A, Bos AF. Functioning of 7-Year-Old Children Born at 32 to 35 Weeks' Gestational Age. Pediatrics. 2012;130(4):E838–E846. doi: 10.1542/peds.2011-2079. [DOI] [PubMed] [Google Scholar]
- Cusson RA. Factors influencing language development in preterm infants. Jognn-Journal of Obstetric Gynecologic and Neonatal Nursing. 2003;32(3):402–409. doi: 10.1177/0884217503253530. [DOI] [PubMed] [Google Scholar]
- Davidson EC, Hobel CJ. POPRAS: A guide to using the prenatal, intrapartum, postpartum record. Torrence, CA: South Bay Regional Perinatal Project Professional Staff Association; 1978. [Google Scholar]
- Davis L, Edwards H, Mohay H, Wollin J. The impact of very premature birth on the psychological health of mothers. Early Human Development. 2003;73(1):61–70. doi: 10.1016/s0378-3782(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Dodici BJ, Draper DC, Peterson CA. Early parent-child interactions and early literacy development. Topics in Early Childhood Special Education. 2003;23(3):124–136. [Google Scholar]
- Easterbrooks MA, Bureau JF, Lyons-Ruth K. Developmental correlates and predictors of emotional availability in mother-child interaction: A longitudinal study from infancy to middle childhood. Development and Psychopathology. 2012;24(1):65–78. doi: 10.1017/S0954579411000666. [DOI] [PubMed] [Google Scholar]
- Feeley N, Gottlieb L, Zelkowitz P. Infant, mother, and contextual predictors of mother-very low birth weight infant interaction at 9 months of age. Journal of Developmental and Behavioral Pediatrics. 2005;26(1):24–33. [PubMed] [Google Scholar]
- Feeley N, Zelkowitz P, Westreich R, Dunkley D. The evidence base for the cues program for mothers of very low birth weight infants: an innovative approach to reduce anxiety and support sensitive interaction. The Journal of perinatal education. 2011;20(3):142–153. doi: 10.1891/1058-1243.20.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold C. Correlates of cognitive development in low-birth-weight infants from low-income families. Journal of Pediatric Nursing. 1994;9(2):91–97. [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. Pediatrics. 2006;118(3):E869–E878. doi: 10.1542/peds.2005-2040. [DOI] [PubMed] [Google Scholar]
- Feldman R, Keren M, Gross-Rozval O, Tyano S. Mother-child touch patterns in infant feeding disorders: Relation to maternal, child, and environmental factors. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(9):1089–1097. doi: 10.1097/01.chi.0000132810.98922.83. [DOI] [PubMed] [Google Scholar]
- Forcada-Guex M, Borghini A, Pierrehumbert B, Ansermet F, Muller-Nix C. Prematurity, maternal posttraumatic stress and consequences on the mother–infant relationship. Early Human Development. 2011;87(1):21–26. doi: 10.1016/j.earlhumdev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. pediatrics. 2006;118(1):e107–e114. doi: 10.1542/peds.2005-1145. [DOI] [PubMed] [Google Scholar]
- Glascoe FP, Leew S. Parenting Behaviors, Perceptions, and Psychosocial Risk: Impacts on Young Children's Development. Pediatrics. 2010;125(2):313–319. doi: 10.1542/peds.2008-3129. [DOI] [PubMed] [Google Scholar]
- Grant K-A, McMahon C, Austin M-P. Maternal anxiety during the transition to parenthood: A prospective study. Journal of Affective Disorders. 2008;108(1/2):101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gravener JA, Rogosch FA, Oshri A, Narayan AJ, Cicchetti D, Toth SL. The Relations among Maternal Depressive Disorder, Maternal Expressed Emotion, and Toddler Behavior Problems and Attachment. Journal of Abnormal Child Psychology. 2012;40(5):803–813. doi: 10.1007/s10802-011-9598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelmann KKO. Design and analysis of experiments. New York, NY: J. Wiley; 1994. [Google Scholar]
- Holditch-Davis D, Bartlett TR, Blickman AL, Miles MS. Posttraumatic stress symptoms in mothers of premature infants. Jognn-Journal of Obstetric Gynecologic and Neonatal Nursing. 2003;32(2):161–171. doi: 10.1177/0884217503252035. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Miles MS, Belyea M. Feeding and nonfeeding interactions of mothers and prematures. Western Journal of Nursing Research. 2000;22(3):320–334. doi: 10.1177/01939450022044449. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Schwartz T, Black B, Scher M. Correlates of mother-premature infant interactions. Research in Nursing & Health. 2007;30(3):333–346. doi: 10.1002/nur.20190. [DOI] [PubMed] [Google Scholar]
- Howland LC, Pickler RH, McCain NL, Glaser D, Lewis M. Exploring Biobehavioral Outcomes in Mothers of Preterm Infants. Mcn-the American Journal of Maternal-Child Nursing. 2011;36(2):91–97. doi: 10.1097/NMC.0b013e318205587e. [DOI] [PubMed] [Google Scholar]
- Kaaresen PI, Ronning JA, Ulvund SE, Dahl LB. A randomized, controlled trial of the effectiveness of an early-intervention program in reducing parenting stress after preterm birth. Pediatrics. 2006;118(1):E9–E19. doi: 10.1542/peds.2005-1491. [DOI] [PubMed] [Google Scholar]
- Kang R, Barnard K, Hammond M, Oshio S, Spencer C, Thibodeaux B, Williams J. Preterm infant follow-up project - a multisite field experiment of hopsital and home intervention programs for mothers and preterm infants. Public Health Nursing. 1995;12(3):171–180. doi: 10.1111/j.1525-1446.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Morisset CE, Barnard KE, Hammond MA, Booth CL. The influence of early mother-child interaction on preschool cognitive/linguistic outcomes in a high-social-risk group. Infant Mental Health Journal. 1996;17(4):310–321. [Google Scholar]
- Kelly MM. Comparison of Functional Status of 8-to 12-Year-Old Children Born Prematurely: An Integrative Review of Literature. Journal of Pediatric Nursing-Nursing Care of Children & Families. 2012;27(4):299–309. doi: 10.1016/j.pedn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher's handbook. 2nd edition ed. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, ten Vergert EMJ, Reijneveld SA, Bos AF. Developmental Delay in Moderately Preterm-Born Children at School Entry. Journal of Pediatrics. 2011;159(1):92–98. doi: 10.1016/j.jpeds.2010.12.041. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar CL, Swank PR. Responsiveness and initiative: Two aspects of social competence. Infant Behavior & Development. 1997;20(2):259–262. [Google Scholar]
- Lee TY, Holditch-Davis D, Miles MS. The influence of maternal and child characteristics and paternal support on interactions of mothers and their medically fragile infants. Research in Nursing & Health. 2007;30(1):17–30. doi: 10.1002/nur.20184. [DOI] [PubMed] [Google Scholar]
- Lekskulchai R, Cole J. Effect of a developmental program on motor performance in infants born preterm. Australian Journal of Physiotherapy. 2001;47(3):169–176. doi: 10.1016/s0004-9514(14)60264-6. [DOI] [PubMed] [Google Scholar]
- Lester BM, Hoffman J, Brazelton TB. The rhythmic structure of mother-infant interaction in term and preterm infants. Child Development. 1985;56(1):15–27. [PubMed] [Google Scholar]
- Liu WF, Laudert S, Perkins B, MacMillan-York E, Martin S, Graven S, Explorato NQPE. The development of potentially better practices to support the neurodevelopment of infants in the NICU. Journal of Perinatology. 2007;27:S48–S74. doi: 10.1038/sj.jp.7211844. [DOI] [PubMed] [Google Scholar]
- Magill-Evans J, Harrison MJ. Parent-child interactions, parenting stress, and developmental outcomes at 4 years. Childrens Health Care. 2001;30(2):135–150. [Google Scholar]
- Martin J, Hamilton B, Ventura S, Osterman M, Wilson E, Mathews T. Births: Final data for 2010. US Department of Health and Human Services, Centers for Disease Control and Prevention. National Center for Health Statistics. 2012 [PubMed] [Google Scholar]
- McGauhey PJ, Starfield B, Alexander C, Ensminger ME. Social environment and vulnerability of low birth weight children: A social epidemiological perspective. Pediatrics. 1991;88(5):943. [PubMed] [Google Scholar]
- McGrath MM, Sullivan MC, Seifer R. Maternal interaction patterns and preschool competence in high-risk children. Nursing Research. 1998;47(6):309–317. doi: 10.1097/00006199-199811000-00004. [DOI] [PubMed] [Google Scholar]
- McGroder SM. Parenting among low-income, African American single mothers with preschool-age children: Patterns, predictors, and developmental correlates. Child Development. 2000;71(3):752–771. doi: 10.1111/1467-8624.00183. [DOI] [PubMed] [Google Scholar]
- McManus BM, Poehlmann J. Parent-child interaction, maternal depressive symptoms and preterm infant cognitive function. Infant Behavior & Development. 2012;35(3):489–498. doi: 10.1016/j.infbeh.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D, Schwartz TA. Depressive symptoms in mothers of prematurely born infants. Journal of Developmental and Behavioral Pediatrics. 2007;28(1):36–44. doi: 10.1097/01.DBP.0000257517.52459.7a. [DOI] [PubMed] [Google Scholar]
- Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother-child interactions. Early Human Development. 2004;79(2):145–158. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Halverson CF. Threats to optimal development: Integrating biological psychological and social risk factors: The Minnesota Symposium on Child Psychology (Vol. 27) Merrill-Palmer Quarterly. 1996;42(2):331–336. [Google Scholar]
- Newnham CA, Milgrom J, Skouteris H. Effectiveness of a Modified Mother-Infant Transaction Program on Outcomes for Preterm Infants from 3 to 24 months of age. Infant Behavior & Development. 2009;32(1):17–26. doi: 10.1016/j.infbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Nicolaou M, Rosewell R, Marlow N, Glazebrook C. Mothers' experiences of interacting with their premature infants. Journal of Reproductive and Infant Psychology. 2009;27(2):182–194. [Google Scholar]
- Parker SJ, Zahr LK, Cole JG, Brecht ML. Outcome after developmental intervention in the neonatal intensive-care unit for mothers of preterm infants with low socioeconomic status. Journal of Pediatrics. 1992;120(5):780–785. doi: 10.1016/s0022-3476(05)80248-3. [DOI] [PubMed] [Google Scholar]
- Pickler RH, McGrath JM, Reyna BA, McCain N, Lewis M, Cone S, Best A. A Model of Neurodevelopmental Risk and Protection for Preterm Infants. Journal of Perinatal & Neonatal Nursing. 2010;24(4):356–365. doi: 10.1097/JPN.0b013e3181fb1e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrehumbert B, Nicole A, Muller-Nix C, Forcada-Guex M, Ansermet F. Parental post-traumatic reactions after premature birth: implications for sleeping and eating problems in the infant. Arch Dis Child. 2003;88(5):F400–F404. doi: 10.1136/fn.88.5.F400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Fiese BH. Parent-infant interaction as a mediator of the relation between neonatal risk status and 12-month cognitive development. Infant Behavior & Development. 2001;24(2):171–188. [Google Scholar]
- Pridham K, Brown R, Clark R, Limbo RK, Schroeder M, Henriques J, Bohne E. Effect of guided participation on feeding competencies of mothers and their premature infants. Research in Nursing & Health. 2005;28(3):252–267. doi: 10.1002/nur.20073. [DOI] [PubMed] [Google Scholar]
- Pridham K, Lin CY, Brown R. Mothers' evaluation of their caregiving for premature and full-term infants through the first year: Contributing factors. Research in Nursing & Health. 2001;24(3):157–169. doi: 10.1002/nur.1019. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rauh VA, Nurcombe B, Achenbach T, Howell C. The mother-infant transaction program - the content and implications of an intervention for the mothers of low-birth-weight infants. Clinics in Perinatology. 1990;17(1):31–45. [PubMed] [Google Scholar]
- Ravn IH, Smith L, Lindemann R, Smeby NA, Kyno NM, Bunch EH, Sandvik L. Effect of early intervention on social interaction between mothers and preterm infants at 12 months of age: A randomized controlled trial. Infant Behavior & Development. 2011;34(2):215–225. doi: 10.1016/j.infbeh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. Journal of Health and Social Behavior. 2001;42(3):258–276. [PubMed] [Google Scholar]
- Ruth CA, Roos N, Hildes-Ripstein E, Brownell M. The influence of gestational age and socioeconomic status on neonatal outcomes in late preterm and early term gestation: a population based study. Bmc Pregnancy and Childbirth. 2012;12 doi: 10.1186/1471-2393-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Szatmari P, Rosenbaum P, Campbell D, King S. Cognitive abilities and school performance of extremely low birth weight children and matched term control children at age 8 years: A regional study. Journal of Pediatrics. 1991;118(5):751–760. doi: 10.1016/s0022-3476(05)80043-5. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, MacKenzie MJ. Research strategies for capturing transactional models of development: The limits of the possible. Development and Psychopathology. 2003;15(3):613–640. doi: 10.1017/s0954579403000312. [DOI] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Buchmann AF, Trautmann-Villalba P, Zimmermann US, Schmidt MH, Laucht M. Quality of early mother-child interaction associated with depressive psychopathology in the offspring: A prospective study from infancy to adulthood. Journal of Psychiatric Research. 2011;45(10):1387–1394. doi: 10.1016/j.jpsychires.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Schmucker G, Brisch KH, Kohntop B, Betzler S, Osterle M, Pohlandt F, Buchheim A. The influence of prematurity, maternal anxiety, and infants' neurobiological risk on mother-infant interactions. Infant Mental Health Journal. 2005;26(5):423–441. doi: 10.1002/imhj.20066. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Pridham K. Development of relationship competencies through guided participation for mothers of preterm infants. Jognn-Journal of Obstetric Gynecologic and Neonatal Nursing. 2006;35(3):358–368. doi: 10.1111/j.1552-6909.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Smith KE, Landry SH, Swank PR. The influence of early patterns of positive parenting on children's preschool outcomes. Early Education and Development. 2000;11(2):147–169. [Google Scholar]
- Spielberger CD, Gorsuch RL. State-trait anxiety inventory for adults: sampler set: manual, test, scoring key. Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- Steelman LM, Assel MA, Swank PR, Smith KE, Landry SH. Early maternal warm responsiveness as a predictor of child social skills: Direct and indirect paths of influence over time. Journal of Applied Developmental Psychology. 2002;23(2):135–156. [Google Scholar]
- Stroemple R. Medical Foster Parent Handbook. National Center for Clinical Infant Programs. 1992 Zero to Three. [Google Scholar]
- Sumner G, Spietz A. NCAST Caregiver/parent-child interaction teaching manual. Seattle, WA: NCAST Publications, University of Washington, School of Nursing; 1994. [Google Scholar]
- Sumner GSA. NCAST-Caregiver/parent-child interaction feeding manual. Seattle, WA: 1994. [Google Scholar]
- Treyvaud K, Doyle LW, Lee KJ, Roberts G, Cheong JLY, Inder TE, Anderson PJ. Family functioning, burden and parenting stress 2 years after very preterm birth. Early Human Development. 2011;87(6):427–431. doi: 10.1016/j.earlhumdev.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Brazelton TB. Monadic phases - structural descriptive analysis of infant-mother face to face interaction. Merrill-Palmer Quarterly-Journal of Developmental Psychology. 1980;26(1):3–24. [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depression symptoms in the psychiatric population: a validation study. American Journal of Epidemiology. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants: The relationship between preterm behavioral state and feeding readiness behaviors and efficiency during transition from gavage to oral feeding. MCN. 2005;30(1):52–59. [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation: Relationship to later interactions between mothers and premature infants. Research in Nursing & Health. 1988;11(1):31–39. doi: 10.1002/nur.4770110106. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatric Nursing. 1997;23(2):169–175. 193. [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy-Rey P, Patel M. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Developmental medicine and child neurology. 2002;44(2):91–97. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Patel M, Cardenas L. Feeding readiness behaviors and feeding efficiency in response to ATVV intervention. Newborn and Infant Nursing Reviews. 2002;2(3):166–173. [Google Scholar]
- White-Traut RC, Norr KF. An ecological model for premature infant feeding. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2009;38(4):478–490. doi: 10.1111/j.1552-6909.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut RC, Pate CM. Modulating infant state in premature infants. Journal of Pediatric Nursing. 1987;2(2):96–101. [PubMed] [Google Scholar]
- Williams BL, Dunlop AL, Kramer M, Dever BV, Hogue C, Jain L. Perinatal Origins of First-Grade Academic Failure: Role of Prematurity and Maternal Factors. Pediatrics. 2013;131(4):693–700. doi: 10.1542/peds.2012-1408. [DOI] [PubMed] [Google Scholar]