Abstract
Objectives
To examine whether blood pressure reductions differ by estrogen use among overweight glucose-intolerant women.
Methods
We conducted a secondary analysis of postmenopausal Diabetes Prevention Program participants who used oral estrogen with or without progestogen at baseline and at 1-year follow-up (n=324) vs. those who did not use at either time point (n=382). Systolic (SBP) and diastolic blood pressure (DBP) changes were examined by randomization arm (intensive lifestyle change (ILS), metformin 850 mg twice daily, or placebo). Associations between changes in blood pressure with changes in sex hormone binding globulin, estradiol, testosterone, and dehydroepiandrosterone were also examined.
Results
Estrogen users and non-users had similar prevalences of baseline hypertension (33% vs. 34%, p=0.82) and use of blood pressure medications at baseline (p=0.25) and follow-up (p=0.10). Estrogen users and non-users randomized to ILS had similar decreases in SBP (-3.3 vs. -4.7 mmHg, p=0.45) and DBP (-3.1 vs. -4.7 mmHg, p=0.16). Among estrogen users, women randomized to ILS had significant declines in SBP (p=0.016) and DBP (p=0.009) vs. placebo. Among non-users, women randomized to ILS had significant declines in DBP (p=0.001) vs. placebo, but declines in SBP were not significant (p=0.11). Metformin was not associated with blood pressure reductions vs. placebo regardless of estrogen therapy. Blood pressure changes were not associated with changes in sex hormones regardless of estrogen therapy.
Conclusions
Among overweight women with dysglycemia, the magnitude of blood pressure reductions after ILS was unrelated to postmenopausal estrogen use.
Keywords: lifestyle change, hypertension, postmenopausal hormone therapy
Introduction
Numerous clinical trials have demonstrated that lifestyle interventions targeting weight reduction in overweight individuals also reduce blood pressure levels1–4. In the Diabetes Prevention Program (DPP), intensive lifestyle changes (ILS) led to reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels by several mmHg5 with persistent benefit observed up to 10 years later1.
It is not known whether blood pressure reductions differ between estrogen users and non-users. Randomized trials of exogenous estrogen therapy have variously reported that oral estrogen use increases6–8, decreases9, 10, or has no effect on blood pressure11–13 in postmenopausal women. These conflicting results could be due to the paradoxical effects of oral estrogen, which may increase activation of the renin-angiotensin system10 while simultaneously causing peripheral vasodilatation and endothelial remodeling14. Therefore, use of oral estrogen could potentiate or negate the effects of weight loss interventions upon blood pressure. Among postmenopausal women, a potential mechanism for the effects of weight loss upon blood pressure is concurrent alteration in endogenous sex hormone profiles, specifically decreases in endogenous estradiol (E2), testosterone (T), dehydroepiandrosterone (DHEA), and increases in the binding protein sex hormone binding globulin (SHBG). No randomized trials have reported whether endogenous sex hormone levels are associated with blood pressure changes independently of changes in body mass in postmenopausal women. Observational studies are inconsistent, with some studies reporting associations between higher E2 and T levels and lower SHBG levels with higher SBP and DBP15, 16, while other reports note no association17, 18.
Using DPP data, we have previously reported that postmenopausal participants randomized to ILS had the significant reductions in weight and waist circumference compared to placebo19. Therefore, in a secondary analysis of the DPP, we hypothesized that decreases in blood pressure among women randomized to ILS would be observed among both users and non-users of exogenous estrogen and the magnitude of such reductions in our overweight, dysglycemic sample would be similar by estrogen use. We also hypothesized that reductions in blood pressure would be associated with reductions in sex steroids and increases in SHBG.
Methods
Characteristics of DPP participants have been published20–22, as well as characteristics of participants in this secondary analysis23. Briefly, the DPP was a randomized controlled trial conducted between 1996 and 2001. The DPP was originally developed to prevent or delay the onset of diabetes among persons at high-risk using interventions designed to improve abnormal glucose metabolism. The DPP had 26 clinical centers located throughout the United States which are listed in the study Appendix (Supplemental Digital Content 1, http://links.lww.com/MENO/A61). Eligible participants were randomly assigned to one of three groups: 850 mg metformin twice daily, placebo twice daily, or ILS. The goals of ILS were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a low-calorie, low-fat diet, plus moderate physical activity for at least 150 minutes per week 24. Inclusion criteria included age > 25 years, fasting plasma glucose (FPG) of 95–125 mg/dl and 2-hour plasma glucose of 140–200 mg/dl following a 75-gram glucose load, and body mass index (BMI) ≥24 kg/m2 (≥22 kg/m2 for Asian Americans). Written informed consent was obtained from all participants before screening, consistent with the guidelines of each participating center's institutional review board.
At the time of randomization, all women completed a questionnaire about their menses, gynecological history including surgeries, and about estrogen use (contraceptive and postmenopausal therapy). Medication use was reassessed every 6 months. For this report, we included women who were postmenopausal at randomization, who had an available stored blood sample, and who consented for participation in ancillary studies. We did not include women enrolled at the Native American/American Indian centers, as these women were not originally consented to participate in this ancillary study. Women were classified as postmenopausal if they met any of the following criteria: bilateral oophorectomy, lack of menses for at least one year while retaining uterus and at least one ovary, cessation of menses prior to hysterectomy, cessation of menses within the past year and age > 55 years, and cessation of menses with hysterectomy and age > 55 years. Women could be categorized as oral estrogen users both at randomization as well as at 1 year follow-up (n=324), or as non-users both at randomization and 1 year follow-up (n=382). Women who used injection, implant, transdermal, or transvaginal estrogen were excluded, as were women who used any estrogen at baseline but not at follow-up and vice-versa. We have previously reported the characteristics of estrogen use19; of the 324 women who reported using oral estrogen at baseline and at follow-up, 266 women were estrogen-only users at baseline and 58 women used estrogen-progestogen; at year 1 follow-up, 258 women were estrogen-only users and 66 women used estrogen-progestestogen, and the most common estrogen used was conjugated equine estrogen.
Weight and waist circumference were measured semiannually, and participants had an annual blood pressure measurement. Blood pressure measurements were performed with a standard mercury manometer by certified staff at baseline and quarterly visits using a standard protocol, with the participant seated for at least 5 minutes before the measurement. A second pressure measurement was obtained 30 seconds later after complete cuff deflation. The mean of the 2 measurements was used for analysis. Hypertension was defined as blood pressure > 140/90 mmHg or the use of antihypertensive medication. Changes in blood pressure medication dosages were not recorded. Blood was drawn after a 12-hour fast at baseline and annually thereafter.
We have previously reported sex hormone measurement procedures 25. Briefly, SHBG, follicle stimulating hormone (FSH), total E2, total T, and DHEA were measured on heparinized plasma collected at baseline and year 1. SHBG was measured at Endoceutics (Quebec City, Canada) using ELISA (Bioline) with interassay coefficients of variation of 7.8 and 5.0 at 18.2 and 63.1 nmol/l, respectively. FSH was measured at Endoceutics using ELISA (Bioline) with interassay coefficients of variation of 3.6 and 4.4 at 27.1 and 72.9 mIU/ml, respectively. E2, T, and DHEA were analyzed using gas chromatography/mass spectrometry at Endoceutics. The limits of detection were 3.0 pg/ml for total E2; 8.0 ng/dl for total T, and 0.30 ng/ml for DHEA. Interassay coefficients of variation for E2 were 17.5% at 4.7 pg/ml, for T were 13.0% at 14 ng/dl, and for DHEA were 24.0% at 0.77 ng/ml.
Statistical Analysis
Women who did not use any estrogen at baseline or follow-up were compared to women who used oral estrogen at both baseline and follow-up. For each sample or estrogen group, baseline characteristics were described using percentages for categorical variables and means (SD) for quantitative variables. Baseline sex hormone distributions were skewed, and therefore log-transformed values and median values were used for comparison of these baseline distributions. We used t-tests or Wilcoxon rank-sum tests to compare change in SBP and DBP between randomized treatment arms. In the DPP, maximal reductions in weight loss were noted at 1 year post randomization; therefore, we examined blood pressure changes between baseline and 1-year.24 Change in blood pressure was calculated as year 1 level - baseline level. Next, the magnitude of blood pressure changes among women randomized to ILS; metformin; and placebo were compared between estrogen users and non-users. We examined the association between sex hormone changes and blood pressure changes separately for estrogen-users and nonusers. Due to differences in baseline FSH by randomization arm, as well as previously reported effects of menopausal stage upon sex steroid-adipose tissue relationships26, baseline FSH levels were also examined as a covariate. A series of linear regression models additionally adjusted for age, race/ethnicity, and use of blood pressure medications.
In sensitivity analyses, we also evaluated models that adjusted for changes in waist circumference and changes in fasting insulin. Waist circumference and weight are highly correlated, and we chose to use waist circumference due to its stronger associations with reductions in adiposity among postmenopausal women enrolled in weight loss interventions27. Characteristics of estrogen-only and estrogen-progestogen users did not differ (results not shown), and therefore these women were grouped together. Finally, in models that contained estrogen users and non-estrogen users, we evaluated the significance of an interaction term between estrogen use and intervention arm for the outcomes of changes in weight, waist circumference, and sex hormones. We also evaluated the significance of an interaction term between hormone use and sex hormone level among women randomized to ILS for the outcomes of changes in SBP and DBP. The SAS analysis system was used for all analyses (SAS Institute, Cary, NC).
Results
Baseline characteristics of postmenopausal women by estrogen use are shown in Table 1. The baseline prevalence of hypertension, defined by blood pressure levels or use of blood pressure medications, was similar by estrogen use. There were no significant differences in DBP by estrogen use, while SBP was slightly but significantly lower among women using oral estrogen. Use of blood pressure medications at baseline was also similar between estrogen users and non-users. Reflecting DPP recruitment criteria, all women were overweight or obese at baseline and glucose-intolerant. Women who did not use estrogen were slightly older and more often non-white than estrogen users. Among non-users, the most common cause of menopause was natural or non-surgical cessation of menses, whereas among estrogen users, the most common cause of menopause was oophorectomy. Non-users weighed more and had greater waist circumferences and higher BMIs than estrogen users. Non-users had higher baseline levels of fasting insulin and FSH but lower baseline levels of SHBG and E2 than estrogen users. Differences in T between estrogen users and non-users were not observed; DHEA was slightly higher in non-users. Among women who did not use estrogen, there were no significant baseline differences between women randomized to ILS (n=133), metformin (n=122), or placebo (n=129), with the exception that women randomized to metformin had slightly lower FSH levels than women randomized to placebo (51.5 IU/l vs. 59.3 IU/l, p=0.02). Among estrogen users, there were no significant baseline differences between women randomized to ILS (n=107), metformin (n=122), and placebo (n=95), with the exception that there were slightly more African-American women in the metformin arm than the placebo arm (9% vs. 5%, p=0.02).
Table 1.
Baseline characteristics of participants by oral estrogen use.*
| Characteristic | No Estrogen Use (N=382) |
Oral Estrogen Use at Baseline and 1-year (N=324) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 59 (9) | 57 (8) | 0.0003 |
| Race/ethnicity (%) | |||
| Non-Hispanic white | 53 | 66 | 0.001 |
| African-American | 28 | 16 | |
| Hispanic | 16 | 13 | |
| Asian | 3 | 4 | |
| Type of menopause (%) | |||
| Bilateral oophorectomy | 19 | 42 | <0.0001 |
| Natural menopause | 67 | 39 | |
| Age ≥55 years and hysterectomy | 13 | 19 | |
| Anthropometrics and sex hormones | |||
| Baseline weight (kg) | 91 (19.7) | 87.6 (17.8) | 0.038 |
| Baseline waist circumference (cm) | 104 (14) | 101 (14) | 0.003 |
| Baseline follicle stimulating hormone (IU/l)† | 55.3 (26.6) | 34.8 (22.5) | <0.0001 |
| Baseline sex hormone binding globulin (nM/l)† | 33.2 (18.3) | 85.3 (77.0) | <0.0001 |
| Baseline dehydroepiandrosterone (ng/ml)† | 1.6 (1.3) | 1.3 (1.2) | 0.01 |
| Baseline total estradiol (pg/ml)† | 8.5 (8.0) | 17.6 (14.8) | <0.0001 |
| Baseline total testosterone (pg/ml)† | 14.0 (13.0) | 15.0 (11.0) | 0.44 |
| Blood pressure | |||
| Baseline hypertension (%)‡ | 34 | 33 | 0.82 |
| Baseline blood pressure medication use (%) | 18 | 21 | 0.25 |
| Baseline systolic blood pressure (mmHg) | 128 (16) | 126 (15) | 0.04 |
| Baseline diastolic blood pressure (mmHg) | 77 (9) | 77 (9) | 0.33 |
Means (standard deviation) or percentages shown unless otherwise indicated.
Median (interquartile range).
Hypertension is defined as systolic blood pressure ≥ 140 mmHg at baseline OR diastolic blood pressure ≥ 90 mmHg at baseline OR use of blood pressure medication at baseline.
Table 2 shows reductions in weight and waist circumference by intervention arm among women who used and did not use estrogen. Among both estrogen users and non-users, women randomized to ILS or metformin lost weight and reduced waist circumference compared to placebo. The magnitude of weight loss and reductions of waist circumference did not differ significantly by estrogen use, within or across randomization arms. Table 2 also shows changes in SHBG and sex steroid levels by estrogen use. Among women who did not use estrogen, randomization to ILS was associated with increases in SHBG and decreases in DHEA compared to placebo; randomization to ILS was not associated with significant sex hormone changes among women randomized to metformin compared to placebo. However, the interaction term between randomization arm and estrogen use was not significant. The exception was that the interaction between hormone use and intervention arm for change in E2 level was significant, suggesting that estrogen use modified metformin effects upon estradiol levels; however, in stratified analysis, metformin did not decrease E2 relative to placebo among estrogen users or non-users.
Table 2.
Changes in weight, waist circumference, and sex hormone levels among hormone users and non-users by lifestyle change (ILS), metformin, and placebo arm.*
| Characteristic | No Estrogen Use N=382 |
Oral Estrogen Use at Baseline and at 1-year (N=324) |
p-value for interaction between estrogen use and intervention arm |
|---|---|---|---|
| Change in weight (kg) | |||
| ILS | −6.5 (−7.5, −5.5) | −7.0 (−8.1, −5.9) | 0.22 |
| Metformin | −3.2 (−4.2, −2.3) | −2.8 (−3.5, −2.0) | 0.81 |
| Placebo | −0.9 (−1.6, −0.3) | −0.3 (−1.3, 0.8) | |
| p-value for ILS vs. placebo | <0.0001 | <0.0001 | |
| p-value for metformin vs. placebo | =0.0001 | =0.0001 | |
| Change in waist circumference (cm) | |||
| ILS | −6.5 (−7.5, −5.4) | −6.0 (−7.3, −4.8) | 0.70 |
| Metformin | −3.0 (−3.9, −2.0) | −2.1 (−3.2, −1.0) | 0.97 |
| Placebo | −1.3 (−2.2, −0.3) | −0.4 (−1.5, 0.8) | |
| p-value for ILS vs. placebo | <0.0001 | <0.0001 | |
| p-value for metformin vs. placebo | 0.01 | 0.03 | |
| Change in sex hormone binding globulin (nmol/l) | |||
| ILS | 10.4 (7.3, 13.5) | 16.8 (8.8, 24.8) | 0.94 |
| Metformin | 1.4 (−1.7, 4.4) | 15.1 (8.0, 22.1) | 0.21 |
| Placebo | 0.1 (−3.1, 3.4) | 6.1 (−4.8, 17.0) | |
| p-value for ILS vs. placebo | <0.0001 | 0.12 | |
| p-value for metformin vs. placebo | 0.58 | 0.17 | |
| Change dehydroepiandrosterone (ng/ml) | |||
| ILS | −0.3 (−0.5, −0.1) | −0.3 (−0.5, −0.1) | 0.22 |
| Metformin | −0.2 (−0.4, 0.01) | −0.2 (−0.5, −0.02) | 0.23 |
| Placebo | 0.0 (−0.2, 0.2) | −0.2 (−0.5, −0.1) | |
| p-value for ILS vs. placebo | 0.03 | 0.67 | |
| p-value for metformin vs. placebo | 0.18 | 0.62 | |
| Change in total estradiol (pg/ml) | |||
| ILS | −5.4 (−8.9, −1.9) | −3.1 (−9.1, 2.8) | 0.22 |
| Metformin | −1.9 (−6.0, 2.3) | −5.1 (−9.7, −0.4) | 0.02 |
| Placebo | −9.1 (−16.7, −1.5) | −0.3 (−4.7, 4.2) | |
| p-value for ILS vs. placebo | 0.38 | 0.45 | |
| p-value for metformin vs. placebo | 0.10 | 0.15 | |
| Change in total testosterone (pg/ml) | |||
| ILS | −3.3 (−12.1, 5.3) | −7.6 (−20.9, 5.6) | 0.76 |
| Metformin | −1.1 (−4.3, 2.0) | −1.6 (−13.3, 10.2) | 0.91 |
| Placebo | −2.0 (−8.8, 4.8) | −3.5(−9.6, 2.6) | |
| p-value for ILS vs. placebo | 0.81 | 0.57 | |
| p-value for metformin vs. placebo | 0.82 | 0.77 |
Means (95% confidence intervals) or percentages shown unless otherwise indicated.
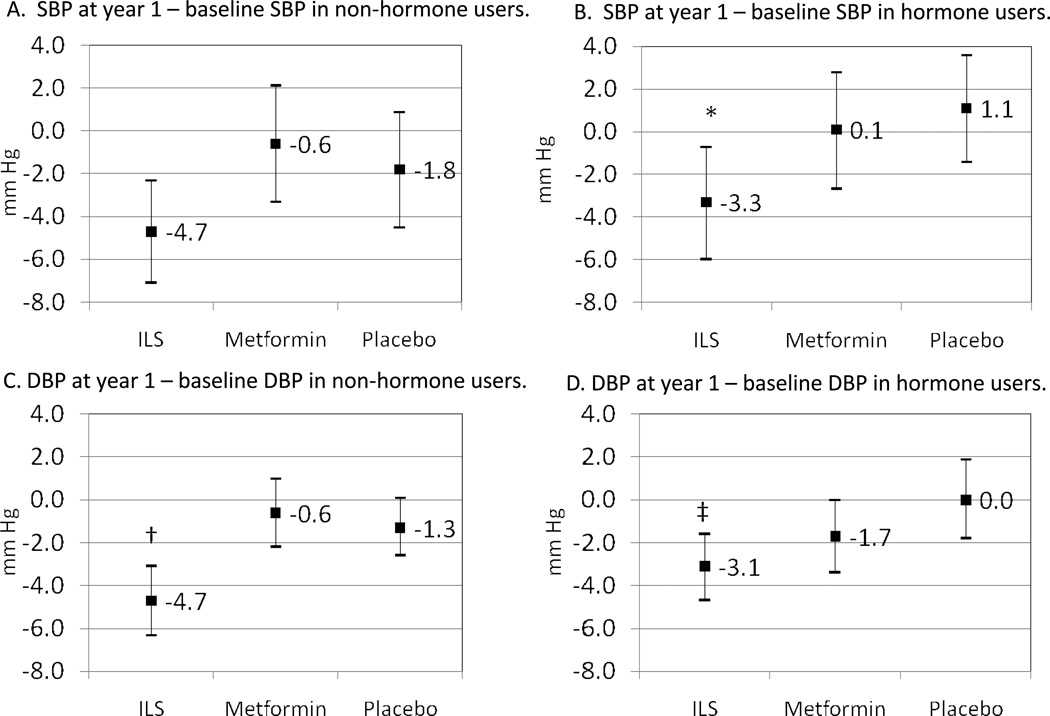
Figure 1 illustrates corresponding changes in blood pressure by randomization arm and by estrogen use. Among estrogen users, women randomized to ILS had reductions in SBP (p=0.016) and DBP (p=0.009) compared to placebo. Women randomized to metformin had non-significant changes in SBP (p=0.59) and DBP (p=0.19) compared to placebo. Among women who did not use estrogen, women randomized to ILS had significant reductions in DBP (p=0.001) compared to placebo, although reductions in SBP did not meet criteria for significance (p=0.11) compared to placebo. Women randomized to metformin had non-significant reductions in SBP (p=0.53) and DBP (p=0.53).
Figure 1.
Change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) between year 1 and baseline, mean (95% confidence interval). *Indicates that p=0.016 for ILS vs. placebo; † indicates that p=0.001 vs. placebo; ‡ p=0.009 compared to placebo Other comparisons not statistically significant.
When we compared the magnitude of blood pressure reduction by estrogen use, particularly in the ILS arm where significant decreases in BP were observed, we found no significant differences by estrogen use (Figure 1). That is, both estrogen users and non-users randomized to ILS had a similar magnitude of reductions in SBP (p=0.45) and DBP (p=0.16). Use of blood pressure medication at 1-year follow-up was also similar by estrogen use (21% among non-users and 27% among users, p=0.10).
We did not find statistically significant associations between changes in blood pressure with changes in sex hormones. Specifically, among women randomized to ILS, where the largest blood pressure reductions were observed, sex hormone changes were not associated with changes in SBP or DBP in unadjusted models or models that adjusted for age, race/ethnicity, blood pressure medication use, and FSH; models that also adjusted for waist circumference and 1/fasting insulin; models stratified by estrogen use that included the entire sample of women and adjusted for randomization arm; and models that included all women and evaluated the interaction between sex hormone level and estrogen use.
Discussion
In a secondary analysis of a randomized trial of weight loss interventions in overweight postmenopausal women, we found that elective estrogen users and non-users had similar patterns of blood pressure reductions. Among both estrogen users and non-users, women randomized to ILS had the greater decreases in blood pressure, whereas women randomized to metformin had non-significant decreases in blood pressure despite significant decreases in weight and waist circumference compared to placebo. The magnitude of blood pressure reductions among women in the ILS arms and the proportion of women using blood pressure medications at 1-year follow-up were also similar by estrogen use. To our knowledge, no other randomized studies have examined whether the use of oral estrogen reduces the effects of lifestyle upon blood pressure, particularly for postmenopausal women who are at risk for adverse cardiovascular events, i.e. overweight and glucose-intolerant.
Our results are reassuring in that estrogen use does not completely negate the beneficial effects of lifestyle change upon weight and blood pressure. In observational studies, estrogen use has been reported to be associated with increased blood pressure levels28, 29. Reports of randomized trials conflict, with reports of increased blood pressure vs. placebo6–8, no effect11–13 or even lowered blood pressure9, 10. Previous studies have reported that in postmenopausal women, randomized trials of lifestyle modification can improve metabolic syndrome components, specifically blood pressure30 31. These reductions are highly correlated with reductions in fat mass30. Weight loss was associated with concurrent reductions in adipokines such as C-reactive protein and tumor necrosis factor-alpha levels that might have contributed to decreases in blood pressure30. Our results suggest that the magnitude of weight loss necessary for blood pressure reduction needs to be fairly large among overweight, glucose intolerant women, as these women randomized to ILS lost more weight than those randomized to metformin, and only women randomized to ILS had concomitant reductions in blood pressure. While women randomized to ILS lost approximately 7 kg of weight regardless of estrogen use, accompanying reductions in SBP and DBP were only 3–5 mm Hg. This suggests that smaller amounts of weight loss i.e. aimed at targets less than the DPP ILS target of loss of 7% of total weight might not lead to significant blood pressure declines.
While we observed changes in sex hormone profiles, particularly increases in SHBG levels among women randomized to ILS, these changes were not significantly associated with declines in SBP or DBP among estrogen users and non-users. While no randomized trials have examined the role of sex hormones in altering blood pressure levels, observational studies have suggested that endogenous sex steroid levels might also contribute to blood pressure levels, although reports have conflicted. In the Multi-Ethnic Study of Atherosclerosis, higher baseline DHEA, E2, and T and lower baseline SHBG predicted incident hypertension and increases in blood pressure levels15, with associations between blood pressure and SHBG and DHEA persisting after adjustment for body mass index. Similar results were observed in a Swedish cohort of middle-aged women16. Among women in the Beaver Dam Eye Study, SHBG was strongly and inversely associated with DBP, while greater T was associated with greater SBP and DBP; relationships between estrone and blood pressure and DHEA and blood pressure were not observed32. In contrast, a cross-sectional report from the Study of Women’s Health Across the Nation (SWAN) did not find significant associations between SHBG with SBP or DBP after adjustment for BMI17. Similar lack of association was reported among women enrolled in the San Antonio Heart Study18. Our results may conflict with studies that found an association between sex hormones and blood pressure because the role of sex hormones in elevating blood pressure may differ among women who are already insulin-resistant and significantly overweight, or because the contribution of sex hormones to blood pressure, independent of weight loss and waist circumference reductions, is minimal.
Strengths of our report include its randomized study of weight loss interventions which led to significant changes in body mass and blood pressure. Although we used mass spectrometric assays which may be more sensitive for the low sex steroid levels typically observed in postmenopausal women, variance was still high at lower levels and may have biased estimates to the null33. Finally, this study was a secondary analysis of a randomized trial not designed a priori to assess the interaction between estrogen use, sex hormones, and blood pressure; such a study which randomized women to estrogen therapy as well as to weight loss would be unlikely to be performed today due to logistics and cost. Of note, the DPP was conducted before the publication of the Women’s Health Initiative Trial results that led to decreases in estrogen prescription.
Conclusion
We conclude that lifestyle changes targeting weight loss can reduce blood pressure in overweight, glucose-intolerant women. The benefit of lifestyle change was observed in both estrogen users and non-users and the magnitude of blood pressure reduction did not differ by estrogen use. Therefore, successful attempts at weight loss and physical activity could be expected to lead to lowered blood pressure regardless of estrogen use. Additional investigation is needed upon the potential impact of hormone therapy upon blood pressure among normal weight, glucose tolerant populations, as well as the potential mediating role of sex hormones in such populations.
Supplementary Material
Acknowledgments
The Investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The opinions expressed are those of the investigators and do not reflect the views of the Indian Health Service or other funding agencies.
Financial support: The project described was supported by Award Numbers U01DK048489, R01DK083297, and K23DK071552 from The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIDDK provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; collection, management, analysis, and interpretation of the Diabetes Prevention Program. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A list of centers, investigators, and staff can be found in the Appendix (Supplemental Digital Content 1, http://links.lww.com/MENO/A61)
Conflict of interest/financial disclosures: none
References
- 1.Diabetes Prevention Program Outcomes Study Research Group. Orchard T, Temprosa M, et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30:46–55. doi: 10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodpaster B, Delany J, Otto A, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel L, Champagne C, Harsha D, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 4.Mulrow C, chiquette E, Angel L, et al. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev. 2000;2:CD000484. doi: 10.1002/14651858.CD000484. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso C, Jr, Rosas F, Oneda B, et al. Aerobic training abolishes ambulatory blood pressure increase induced by estrogen therapy: a double-blind randomized clinical trial. Maturitas. 2011;69:189–194. doi: 10.1016/j.maturitas.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Kaya C, Dincer Cengiz S, Cengiz B, Akgun G. The long-term effects of low-dose 17 beta-estradiol and dydrogesterone hormone replacement therapy on 24-hour ambulatory blood pressure in hypertensive postmenopausal women: a 1-year randomized, prospective study. Climacteric. 2006;9:437–445. doi: 10.1080/13697130601003094. [DOI] [PubMed] [Google Scholar]
- 9.Cacciatore B, Paakkari I, Hasselblatt R, et al. Randomized comparison between orally and transdermally administered hormone replacement therapy regimens of long-term effects on 24-hour ambulatory blood pressure in postmenopausal women. Am J Obstet Gynecol. 2001;184:904–909. doi: 10.1067/mob.2001.111246. [DOI] [PubMed] [Google Scholar]
- 10.Mirza F, Ong P, Collins P, et al. Effects of estradiol and the angiotensin II receptor blocker irbesartan on vascular function in postmenopausal women. Menopause. 2008;15:44–50. doi: 10.1097/gme.0b013e318150d13e. [DOI] [PubMed] [Google Scholar]
- 11.Manson J. The Kronos Early Estrogen Prevention Study. Womens Health (Lond Engl) 2013;9:9–11. doi: 10.2217/whe.12.69. [DOI] [PubMed] [Google Scholar]
- 12.The Writing Group for the PEPI Trial, Miller V, LaRosa J, et al. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 13.Steiner A, Hodis H, Lobo R, Shoupe D, Xiang M, Mack W. Postmenopausal oral estrogen therapy and blood pressure in normotensive and hypertensive subjects: the Estrogen in the Prevention of Atherosclerosis Trial. Menopause. 2005;12:728–733. doi: 10.1097/01.gme.0000184426.81190.01. [DOI] [PubMed] [Google Scholar]
- 14.Kublickiene K, Fu X, Svedas E, Landgren B, Genazzini A, Simoncini T. Effects in postmenopausal women of estradiol and medroxyprogesterone alone and combined on resistance artery function and endothelial morphology and movement. J Clin Endocrinol Metab. 2008;93:1875–1883. doi: 10.1210/jc.2007-2651. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Szklo M, Folsom A, Cook N, Gapstur S, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:228–234. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakir Y, Samsioe G, Nyberg P, Lidfeldt J, Nerbrand C, Agardh C. Do sex hormones influence features of the metabolic syndrome in middle-aged women? A population-based study of Swedish women: the Women's Health in the Lund Area (WHILA) Study. Fertil Steril. 2007;88:163–171. doi: 10.1016/j.fertnstert.2006.11.111. [DOI] [PubMed] [Google Scholar]
- 17.Sutton-Tyrrell K, Wildman R, Matthews K, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 18.Haffner S, Katz M, Stern M, Dunn J. Association of decreased sex hormone binding globulin and cardiovascular risk factors. Arteriosclerosis. 1989;9:136–143. doi: 10.1161/01.atv.9.1.136. [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Kong S, Laughlin G, et al. Reductions in glucose among postmenopausal women who use and do not use estrogen therapy. Menopause. 2012;20:393–400. doi: 10.1097/gme.0b013e3182703b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: recruitment methods and results. Controlled Clinical Trials. 2001;23:157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowler W, Hamman R, Edelstein S, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Kong S, Laughlin G, et al. Reductions in glucose among postmenopausalw omen who use and do not use estrogen therapy. Menopause. 2013;20:393–400. doi: 10.1097/gme.0b013e3182703b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C, Nan B, Laughlin G, et al. Endogenous sex hormone changes in postmenopausal women in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2012;97:2853–2861. doi: 10.1210/jc.2012-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildman R, Tepper P, Crawford S, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1614. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster-Schubert K, Alfano C, Duggan C, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu C, Lujic S, Thornton C, et al. Menopausal hormone therapy is associated with having high blood pressure in postmenopausal women: observational cohort study. PLoS One. 2012;7:40260. doi: 10.1371/journal.pone.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassertheil-Smoller S, Anderson G, Psaty B, et al. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension. 2000;36:780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 30.Joseph L, Prigeon R, Blumenthal J, Ryan A, Goldberg A. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerntol A Biol Sci Med Sci. 2011;66:1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villareal D, Miller 3rd B, Banks M, Fontana L, Sinacore D, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 32.Haffner S, Newcomb P, Marcus P, Klein B, Klein R. Relation of sex hormones and dehydroepiandrosterone sulfate to cardiovascular risk factors in postmenopausal women. Am J Epidemiol. 1995;142:925–934. doi: 10.1093/oxfordjournals.aje.a117740. [DOI] [PubMed] [Google Scholar]
- 33.Rosner W, Hankinson S, Sluss P, Vesper H, Wierman M. Challenges to the measurement of estradiol: an Endocrine Society Position Statement. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2012-3780. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.