Abstract
INTRODUCTION
Estrogen receptor alpha (ERα) has been identified in the vessel wall, offering vasoprotective effects when upregulated. Estrogens are known to mediate the inflammatory mileu, and inflammation has long been associated with abdominal aortic aneurysm (AAA) formation. Therefore it is theorized that increased estrogen receptor in females contributes to their relative resistance to AAAs. The study’s objective was determining gender differences in ERα levels during experimental AAA formation.
METHODS
Infrarenal aortas of male and female C57 mice (n=18 and n=16, respectively) were infused with 0.4% elastase. Diameters were measured at day 0 and day 14. Aortic mRNA expression of ERα was determined on day 3 by RTPCR, while ERα protein levels were measured via Western blot. Immunohistochemistry using rabbit antibody for ERα was performed on day 14 samples and quantified. Zymography was done for MMP2 and 9 activity levels. Samples of human AAAs were collected and Western blot performed. Data were compared for significance using a student t-test.
RESULTS
Infrarenal aortic diameter increased in elastase-perfused males (ME) by 80% at 14 days post perfusion, while females (FE) increased by only 35% (p=0.0012). FE had 10x greater ERα mRNA expression compared with ME at day three (p=0.003). Similarly, ERα protein levels were 100% higher in FE compared to ME on day 14 (p=0.035). ERα protein levels were 80% higher in female human patients with AAA than in their male counterparts (p=0.029). ERα visualized via immunohistochemistry was 1.5 fold higher in FE than ME (p=0.029). MMP2 and 9 activity levels were decreased in female as compared with male aortas.
CONCLUSION(S)
This study demonstrates an increase in aortic wall ERα in females compared with males that correlates inversely with MMP activity and AAA formation. These findings, coupled with observations that exogenous estrogen inhibits AAA formation in males, further suggest that estrogen supplementation may be important to prevent AAA formation and growth.
INTRODUCTION
Abdominal aortic aneurysm (AAA) formation is known to be an inflammatory process, involving infiltration of macrophages and lymphocytes, release of proinflammatory cytokines, and eventual activation of matrix metalloproteinases (MMPs) which degrade the extra cellular matrix (ECM). In humans, AAA disease affects men four times as often as women. Investigational studies from our labs and others1,2,3 suggest this is in part due to a protective role of estrogen.
The biochemistry of sex hormones and their role in AAA formation is made more complex by the multiple and varied hormone receptors throughout the vasculature. GPER, a g-protein related estrogen receptor, located in the endoplasmic reticulum, mediates rapid responses to changes in vascular tissues. In contrast, estrogen receptor alpha (ERα) and beta (ERβ) are classic nuclear receptors in the cardiovascular system. Specifically, ERα mediates endothelial responses after vascular injury, while ERβ mediates arterial tone and blood pressure. ERα has also been identified as offering vasoprotective effects when upregulated in the vessel wall, likely due to decreased inflammation suggesting a possible role during AAA formation and perhaps at least partially responsible for the gender differences in AAA formation. The objective of this study was to examine the role of ERα during experimental AAA formation in a murine model.
METHODS
Animal surgery
Mice were obtained from Jackson Laboratories. Infrarenal aortas of 8–12 week old male and female C57BL/6 mice (n=18 and n=16, respectively) were infused with 0.4% pancreatic porcine elastase. Animals were harvested at day 0, 1, 3, and 14. The 0 day were non-perfused animals for baseline control PCR. Day one and three samples were for PCR, and day three samples were also processed for zymography. Day 14 samples were prepared for western blot and immunohistochemistry. Aortic diameters were measured mid-aorta prior to perfusion and then at postoperative days 3, 7, and 14. This was done using a video micrometer and NIS Elements software on a computer attached to the microscope (Nikon, Melville, NY). The baseline (day 0) measurement was subtracted from subsequent measurements to determine the percent increase in diameter. All experiments were approved by the University of Michigan Universal Committee on the Use and Care of Animals (UCUCA No.09679).
Messenger RNA (mRNA) extraction, reverse transcription PCR, Real Time-PCR
Aortic mRNA expression of ERα was determined on day one and three by polymerase chain reaction (PCR). Later time points have not produced rigorous PCR data in our lab previously. Established techniques using TRIzol reagent (Invitrogen) were used to extract mRNA for reverse transcription PCR. In brief, fresh explanted aortic tissue was added to 1.5mL of TRIzol reagent and homogenized for 45 seconds. Samples were frozen at this point at −70° C. Chloroform (+99%) was then added to the homogenized tissue, vortexed, and centrifuged. The clear supernatant was pipetted into Eppendorf tubes while the RNA precipitation was performed with isopropanol (+99%) and 7.5ug of glycogen. The resulting solution was centrifuged and the supernatant was again poured off. The remaining mRNA pellet was then washed by adding 70% ethanol in DEPC water and centrifuged. The supernatant was aspirated off once more, and the pellet dried at room temperature. The pellet was redissolved in DEPC water at 58° C. RNA concentration was then measured on the Nanodrop 1000 Spectrophotometer (ThermoScientific, Pittsburgh,PA). Appropriate dilutions were made to produce 5ug/ul of RNA. Reverse transcription reaction with standard reagents in a GeneAmp 2400 PCR System (Perkin Elmer-Applied Biosystems, Norwalk, CT) was then performed. The concentration of the resulting cDNA was measured using the Nanodrop 1000 Spectrophotometer. Dilutions were made to calculate the amount needed to obtain 22ng/uL of cDNA for the Real Time-PCR reaction. The primers and SYBR Green Master Mix -PCR were obtained from SABiosciences (Qiagen, Frederick, MD). The RotorGene 6000 Series Software 1.7 (Corbett Research, Qiagen, Frederick, MD) was used with the following program: 95° C, 10 min; 40 cycles of (95° C, 15 sec; 60° C, 60 sec). Target mRNA was therefore amplified and the take-off values and melt curves were obtained for analysis. mRNA expression of Estrogen Receptor 1 (α) and 2 (β) was compared with that of β-actin, a housekeeping gene.
Western Blot
ERα protein levels were measured via Western blot from aortic tissue harvested on day 14 and human aortic tissue. For Western blot analysis the frozen tissues were thawed and then lysed by homogenization and sonication before overnight incubation in ice-cold RIPA buffer (Sigma, St. Louis, MO) containing protease and phosphatase inhibitors (Roche, Basel, Switzerland). Protein concentration in the lysate was determined with the BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were loaded into each well and resolved by SDS-PAGE with 10% gels (Novex, Invitrogen, Carlsbad, CA). They were then electroblotted onto polyvinylidene membranes (Immobilon-P, Millipore, Billerica, MA) by semidry transfer blot (Biorad, Hercules, CA) according to the manufacturer’s instructions. The membranes were incubated in StartBlock TBS (Pierce, Rockford, IL) for one hour and then with total-ERα or ERβ in StartBlock solution at 1:500 dilution overnight with gentle shaking. The membranes were washed in 25 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.4 (TBST) for one hour at room temperature. They were then incubated with HRP conjugated goat anti-rabbit secondary antibodies (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA) for one hour and again washed in TBST. For normalization of proteins on the western blots, the membranes were stripped and probed with anti-actin antibodies conjugated with HRP (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were developed with the West-Pico ECL kit (Pierce, Rockford, IL). Densitometry was performed using Image J program to quantify the levels of ER protein.
Immunohistochemistry
Animals were harvested at day 14 for histologic analysis. Fresh aortic tissue was fixed in 10% buffered formaldehyde for 2 h, transferred to 70% ethanol, and subsequently embedded in paraffin for immunohistochemistry. Sections were prepared with Hematoxylin and Eosin, Masson’s trichrome (total collagen), and Verhoeff-Van Gieson (elastin) stains. Blank sections were then stained for ERα using the following procedure. The aortic sections were deparaffinized in xylene and rehydrated in graded alcohols. The sections were subsequently incubated with 3% hydrogen peroxide to block endogenous peroxidase activity. Anti-Mouse ERα monoclonal antibody (1:200, ABCAM) was used in conjunction with M.O.M and Elite Vectastain ABC kit (Vector Laboratories, Burlingame, CA). The biotinylated-avidin complex was then visualized using Novared kit(Vector Laboratories, Burlingame, CA) following the instructions of these kits. The stained sections were visualized using confocal microscopy (Nikon Eclipse TiU). Percent stained was quantified using an Image J area fraction and thresholding technique. Data were compared for significance using student t test.
Substrate Gel Zymography
MMP2 and 9 activity was determined by Zymography on days 1, 3, and 14. Gelatin substrate zymograms were run in pre-cast 10% SDS-PAGE gels containing 1 mg/ml of gelatin (Invitrogen, Carlsbad, CA). Equal amounts of proteins were diluted into 2X tris-glycine SDS sample buffer and electrophoretically separated under non-reducing conditions. Proteins were renatured for 30 min in Renaturing Buffer (Invitrogen, Carlsbad, CA) and then the gels were incubated in the Developing Buffer (Invitrogen, Carlsbad, CA) for 30 min and again in the same buffer overnight shaking at 37°C. The gels were washed in water and stained in SimplyBlue SafeStain (Invitrogen, Carlsbad, CA) until the gelatinase activity was observed by clear bands against the blue background. Gels were scanned and densitometry was performed using Image J software (NIH) to quantify the levels of MMP activity.
Substrate gel zymography for determination of TIMP activity started with equal amounts of aortic proteins (0.9 ug) run in triplicate for each treatment group in a 15% SDS PAGE gel under non-reducing conditions. The gels were cast using serum free conditioned media from male rat aortic smooth muscle cells (SMC) as the source of MMPs. The completion of the protocol was the same as used above for MMP zymography.
Human Tissue
Aortic tissue (anterior wall of infra-renal aortas) was collected from patients undergoing AAA repair (n=6) and from cadavers (n=4) following the IRB guidelines of the Unviersity of Michigan (HUM1999-0413). Protein was isolated from the tissue using RIPA buffer (Thermo Scientific, Pittsburgh, PA) containing 1% SDS. Protein concentration was measured utilizing a BCA kit (Thermo Scientific, Pittsburgh, PA).
Statistical Analysis
Data points were collected and entered into a database (Microsoft Excel 2007, Microsoft Corp., Redmond, WA). Comparison statistics between groups were determined using unpaired student’s t-test in PRISM software (GraphPad Software Inc., La Jolla, CA). Data are presented as means ± standard deviation, along with significance values. Differences were considered statistically significant at p<0.05.
RESULTS
Murine Tissue
Phenotype
An AAA was defined as an increase in aortic diameter greater than 50% from baseline (preperfusion) to harvest. The aortic diameters of male mice at baseline were not significantly different from females. There were no aneurysms by day three, but there was a trend of greater aortic diameter in elastase-perfused males (ME) compared with females (FE). By day seven, the aortas of ME were significantly larger than FE (p=0.01). At day 14 post perfusion, the infrarenal aortic diameter increased in ME by 80%, while FE increased by only 35% (p=0.0012). In terms of the incidence of AAA, 90% of ME developed AAAs at this time point, while only 14% of FE did.
RT-PCR
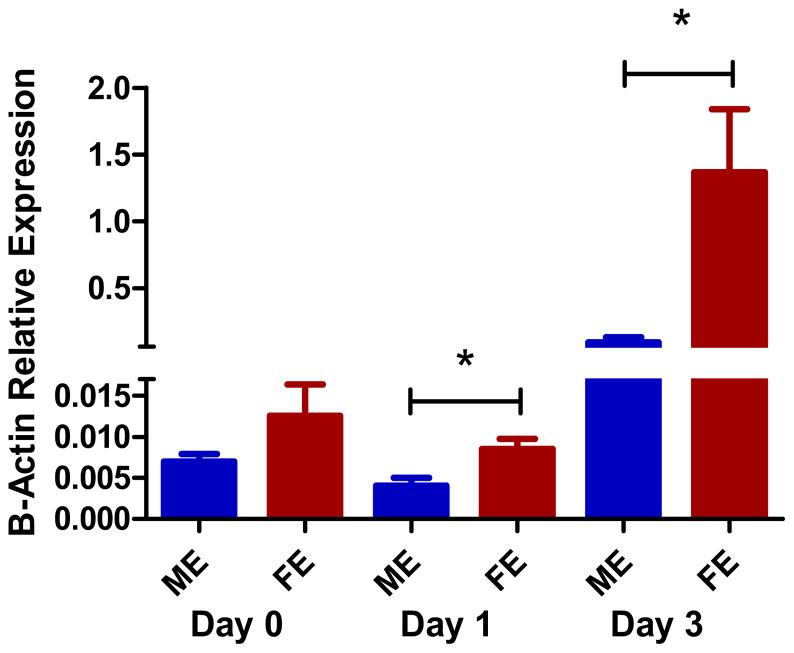
At baseline, non-perfused male and female aortas had equivalent ERα levels of mRNA by RT-PCR (p=0.15). The elastase perfused mice however, showed that FE had 10x greater ERα mRNA expression compared with ME at day one (p=0.013). Day three RT-PCR also showed the FE have significantly more ERα mRNA expression than ME (p=0.003) (Figure 1). There were no data obtained for ERβ; as multiple primers for ERβ attempted were not successful, suggesting that no ERβ was present in the mouse aortic samples.
Figure 1.
Increased estrogen receptor α mRNA expression in female compared with male aortas by rt-PCR at day 1 and 3 (p=0.013, p=0.003). Females and males had equivalent amounts at day 0 baseline (n=3 per group).
Western blot and Immunochemistry
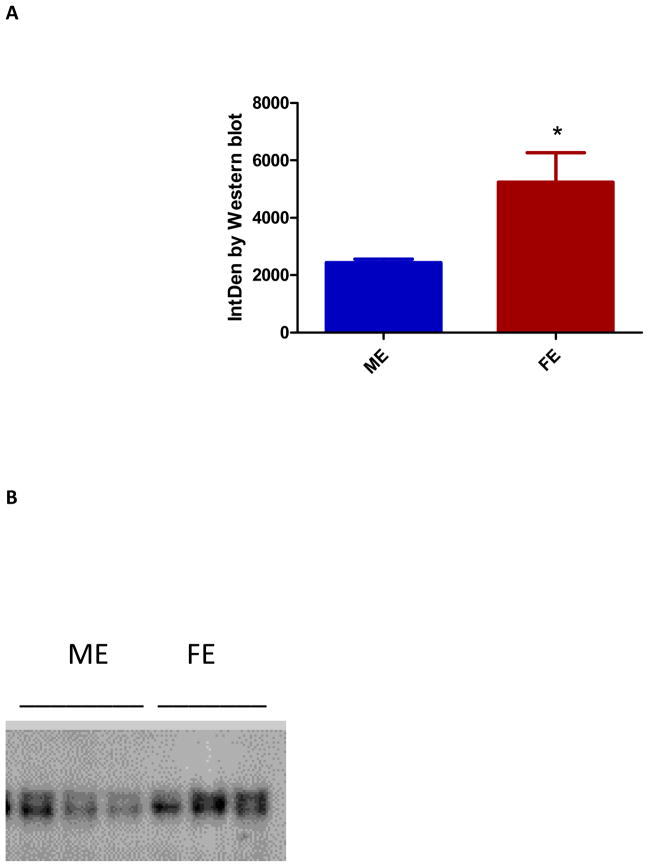
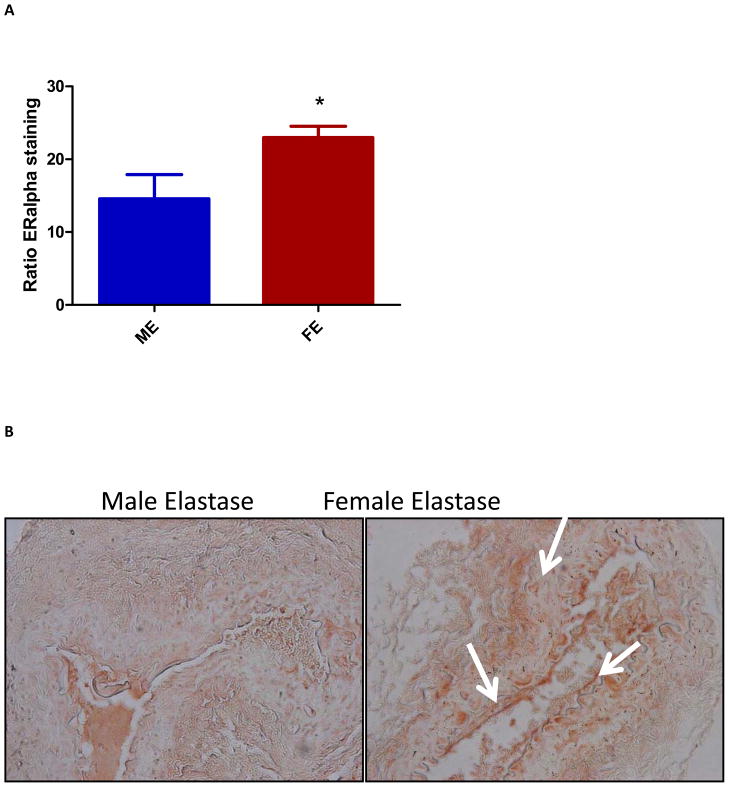
Western blot performed on day three did not document statistically significant differences in ERα protein levels between ME and FE. However, by day 14 ERα protein levels were 100% higher in FE compared with ME (p=0.035) (Figure 2). Similar to the results of ERβ PCR, none of the antibodies for ERβ used were successful in producing quantifiable blots. In keeping with the results from Western blotting, ERα visualized in the luminal side of the aortic wall via immunohistochemistry staining was 1.5 fold higher in FE than ME (p=0.029) (Figure 3). Hematoxylin and eosin, Masson’s trichrome, and Verhoeff-Van Gieson staining of ME aortas confirmed increased inflammatory infiltrate and disrupted elastic lamellae at day 14, as compared with FE aortas.
Figure 2.
(A) Increased estrogen receptor (ER) α protein levels by western blot in female as compared to male aortas. This was significant at day 14 (p=0.035) (n=3). (B) Western blot for ERα.
Figure 3.
(A) Increased estrogen receptor (ER) α staining in female compared with male aortas at day 14 with significance (p=0.029) (n=3). (B) Increased ERα staining seen in female aortas as compared with males at 40x.
Zymography
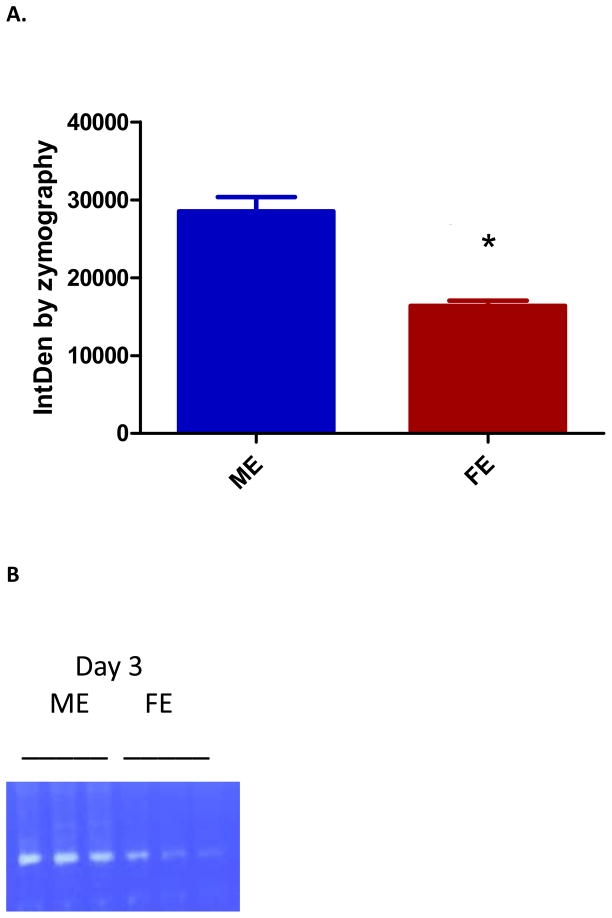
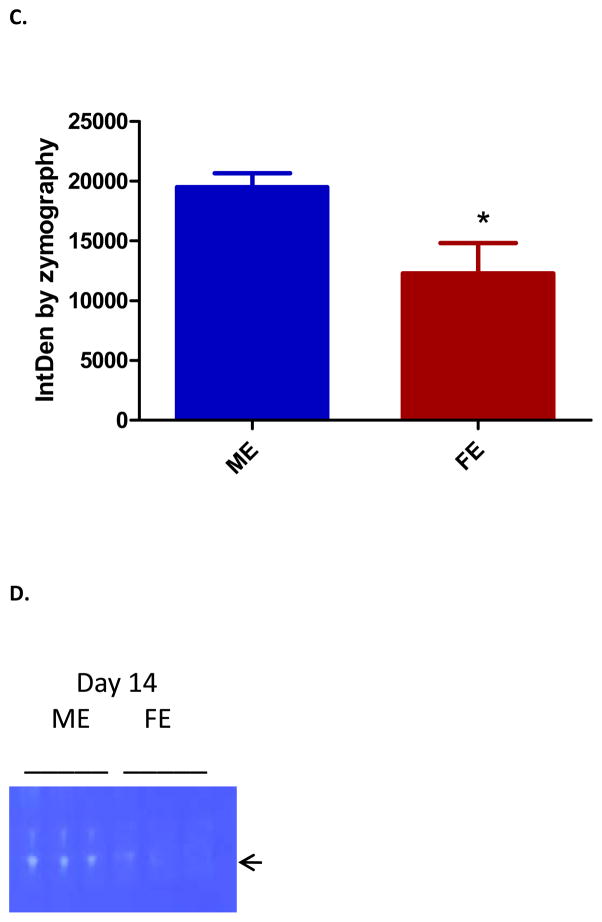
MMP activity, known to be elevated in aneurysm tissue, was analyzed by gel zymography. Day one showed equal levels of MMP 2 and 9 activity between ME and FE. However, by day three there was significantly less MMP9 activity in FE as compared with ME (p=0.003)(Figure 4). Although our day 14 data did not show significance, previous studies from our lab have shown that this difference persists at day 141. Active MMP2 was also significantly less in FE compared to ME at day three (p=0.022) and day 14 (p=0.011). There was no significant difference in TIMP activity found at day 3.
Figure 4.
(A) Decreased matrix metalloproteinase (MMP) 9 in female aortas at day 3 as compared with males (p=0.003) (n=3). (B) Zymogram for MMP9. (C) Decreased MMP2 in female aortas at day 14 as compared with males (p=0.011) (n=3). (D) Zymogram for active MMP2.
Human aorta tissue
Aortic tissue samples were collected from the operating room from patients undergoing open AAA repair. Western blot showed that female patients with AAA had over 80% more ERα protein than did their male counterparts (p=0.029).
DISCUSSION
This study, using the elastase perfusion model of AAA formation, confirmed a phenotypic difference between male and female mice. After documenting that females formed smaller aneurysms and did so less frequently, we documented that this occurred in conjunction with increased early expression of ERα mRNA and later ERα protein and staining in females compared to males. This correlated with changes in MMP activity. We were also able to show an increase in ERα protein in female compared to male human AAA samples.
Males form AAAs four to five times more often than females2, although the risk for females after menopause approaches the risk in males. This, along with evidence that estrogen decreases inflammation in other cardiovascular diseases, suggests a protective role for circulating estrogens during AAA formation in females. We have previously shown that aneurysms are formed in part by elastin degradation by protease activity of MMP9 and that estrogen mediates a reduction in macrophage production of this crucial enzyme1. Male experimental AAAs have also been shown to have more MMP13(collagenase3) than females, which results in more collagen degradation, loss of wall tensile strength, and AAA formation3. Although the situation may be complicated by the point in menstruation cycle, this study showed females and males have no significant differences in ERα mRNA expression levels at baseline. Therefore and importantly differences in ERα seen occur after AAA induction. Aside from time course, we have also shown that the protection in females is due to systemic as compared with local resident factors, since female aortas transplanted into male rats lost their resistance to AAA and exogenous estrogen administered to male rats before AAA induction decreased AAA formation by 300%1.
Estrogens, nitric oxide (NO), and reactive oxygen species are a few possible circulating factors that are responsible4 for gender differences in AAA formation. Tamoxifen, a SERM (Selective Estrogen Receptor Modulator), had the same effect of attenuating experimental AAA formation, as did estrogen; this was associated with an increase in catalase leading to less neutrophil infiltration and decreased reactive oxygen species formation5. Phytoestrogens (ex. red wine polyphenols) have also been shown to have similar protective effects, increasing vasorelaxation via NO in females as compared with males6.
Gender differences are seen often in the cardiovascular system, from cell signaling to the immune response. Previous work in our lab has shown that in the experimental AAA model female rats exhibited a five-fold decrease in expression of BMP and TNF superfamily ligands compared to males. Females had less than half of the TGFβ and VEGF expression males had and resultant lower macrophage counts7. More evidence that gonadal hormones differentially regulate AAA growth in concert with macrophage levels came from a study in which orchiectomies or estrogen treatment decreased male rat AAA size, while testosterone treatment increased them. Estrogen also decreased female AAA size, but no changes resulted from testosterone or oophorectomies8. Although most studies have suggested estrogen is protective instead of testosterone harmful, some preliminary studies using a different model of rodent AAA formation have seen that testosterone instead of estrogen was responsible. Angiotensin II chronic infusion induced AAAs in orchiectomized males which were smaller than normal females9.
ERs are steroid hormone receptors mostly on SMCs that act as dimers to mediate the vasculoprotective effects of estrogen via both genomic, and rapid, non-genomic mechanisms. Genomic regulation occurs via the estrogen-ER complex causing migration from cytosol to nucleus, dimerization, and binding to Estrogen-Response-Elements in transcriptional control regions of target genes to start transcriptional activation10. The non-genomic membrane-initiated steroid signaling (MISS) causes vasodilation through an ER, c-Src, NO-dependent mechanism which is thought to occur in the caveolar membranes (specialized plasma membrane lipid rafts) of endothelial cells(ECs)11. NO is well known to be vasodilatory, atheroprotective, angiogenic, anti-thrombotic, anti-leukocyte adhesive, and anti-smooth muscle cell proliferative. Some of these properties, along with the inhibition of aortic collagen accumulation and elastic loss, are clearly beneficial in protection from AAA development11,12.
Further complexity to estrogen signaling is added because there are three known estrogen receptor subtypes: GPER, ERα, and ERβ. GPR30/GPER is already primarily localized to the endoplasmic reticulum found in high levels in human vascular smooth muscle cells (VSMCs)13. ERα and ERβ are classic nuclear receptors encoded by genes on different chromosomes. Some tissues have a predominance of one subtype (ERα in uterus and ERβ in ovarian and testes) while the cardiovascular tissues express both14. Most physiologic ligands (E2, androgens, antiestrogens) bind with similar affinity to α and β, while estrone preferentially binds to ERα and estriol to ERβ15. However, ERα was shown to be more potent than ERβ at low concentrations in response to E216. ER alpha −/− mice male and female mice underwent elastase perfusion and there were no differences in AAA formation between gender with and without gonads (personal communication Dr. Guanyi Lu). Importantly, the background on the available mice was not fully C57B.
It is now generally accepted that within the cardiovascular system ERα and ERβ have distinct roles. ERα protects endothelium after vascular injury and against atherosclerosis, while ERβ mediates arterial tone and blood pressure. This distinction wasn’t always known. Some early 1990s studies were referred to as indicating that ERα gene deletion had little or no effect on the cardiovascular system14,17, 18,19. This posed the question: is either receptor sufficient to protect against vascular injury or is another receptor responsible? A double knockout ERα,β was subjected to the same vascular injury and given estrogen to answer this question20. The answer was ‘it depends’ as E2 inhibited increases in medial area but not VMSC proliferation in the knockout mice. Now three theories were posed: there was a third estrogen receptor yet unidentified, certain functions were receptor-independent, or there was still a splice variant exerting activity. A new fully null ERα KO mouse was created that did not contain the ERα splice variant (ER46) found in all prior ERα knockout mice from Chapel Hill. E2 treatment in the new ERα knockout mice had no protective effect on any measure of vascular injury finally showing that ERα, as opposed to ERβ, is necessary for E2 to mediate the vascular injury response21. This was paralleled in vitro by showing estrogens can inhibit ERα positive VSMCs, but not ERβ positive VSMCs22. ERα and ERβ signaling may be redundant in some functions in females, but ERα appears necessary for many involved with atherosclerosis and vascular wall injury repair23.
In the present study, ERα was chosen to focus on due to the recent evidence of its predominance in mediating the cardiovascular protective effects attributed to estrogen. However, there is not a clear cut distinction. Both ERα and ERβ are found in arterial walls, yet ERα was shown to be the predominant ER in vascular ECs by level of expression24,25. Although a 2009 review concluded that there is no definite evidence for predominant expression of one ER subtype over the other in human arteries, the pattern depends on gender, hormonal environment, disease state, vascular bed, level of wall layer, and cell type (EC, VSMC) 22,26. Selective activation of ERα on ECs in rat aortas increased ERK and cell proliferation(ERK1/2), while ERβ on VSMCs lead to ERK inhibition, reversal of cell proliferation, and apoptosis via p38/MAPK activation27. The two ERs can also interact with each other and can influence the effects of one other, such as ERβ2 variant inhibiting ERα transcriptional activity and inducing proteasome-dependent degradation of ERα10. In 2007, Traupe et al suggested that E2 activation of ERα lead to NO and vasodilation while E2 activation of ERβ just dysregulated the ERα triggered effects on endothelial function28. Related, ERα was shown to be essential for E2-related increases in gene expression, while ERβ for decreases (ex. Subunits for mitochondrial respiratory chain)29. Despite complex interactions and regulation, ERα has emerged as the primary receptor for vasoprotection by numerous studies28,30,31.
Several examples of vascular events found to be mediated by ERα as opposed to ERβ are: critical post-translational modifications (such as S-palmitoylation at cysteine-447) of ERα causing membrane targeting11, estrogen induced proliferative signaling via the ERK/MAPK pathway, and g-protein Gα13 induced rapid remodeling of actin cytoskeleton27.
The main endogenous estrogen, E2, exists as free, albumin-bound, and sex-hormone bound. Because bound estrogen can dissociate in capillaries and cells can make their own estrogen via DHEA, target tissues can be exposed to much higher concentration than plasma levels. This makes responses to higher levels of estrogens used in studies physiologically relevant not simply pharmacologically interesting32. Estrogens are overall beneficial to the circulatory system by direct and indirect means. Direct includes anti-inflammatory processes, regulating vascular cell growth, proliferation, and migration, promoting recovery of intimal injury, and vasodilatory actions via endothelial independent (via cyclooxygenase-2 and prostacyclin or VSM Ca and K channels) or dependent (stimulating release of NO by activating eNOs) methods30,32,33. Aside from these direct mechanisms on the cardiovascular system, indirect effects of estrogen can be vascular protective by decreasing LDL, increasing HDL, decreasing renin, ACE, and endothelin-1, increasing insulin sensitivity, and increasing collagen and elastin cross-linking via steady state regulation of lysyl oxidase38.
There are several limitations to this study. The elastase perfusion model of AAA formation is an artificial model utilizing mechanical pressure along with enzymatic degradation that has the inherent problems in translation to humans as seen in much of animal model research. Although we explained the rationale behind choosing ERα, we were unable to quantify ERβ and did not evaluate the samples for GPER levels. We also did not measure other elements of the proposed pathways or outcomes involved in AAA formation, such as eNOS, NO, ERK, Akt, or MAP-kinases. Therefore, we cannot completely describe the inflammatory mileu or the causal relationship between estrogen, its receptor expression, and MMP activity.
While this study did not utilize cell culture, in vitro and in vivo work have led to some inconsistencies in estrogen-CVD studies, underlining the danger in relying on one to make conclusions of the other. The example of E2 inhibiting production of proinflammatory chemokines preventing leukocyte adhesion in vitro but increasing production of them in chronically treated mice33 highlights that acute and chronic effects of E2 may not be equivalent, and the difficulties in differentiating local vessel wall resident versus circulating immune cells. This warns of the applicability of applying conclusions made from in vitro systems to in vivo organisms.
Future work to be pursued includes repeating experiments with different ERβ primers or antibodies, uing ERα46 splic variant, and creating ERβKO strains. Aortic transplants between the sexes could help identify local versus systemic sources of vasoprotection. A murine rupture model could further elaborate on gender differences in AAA disease as females are more likely to rupture. Enhanced sample collection is imperative in order to measure ERs in human female aortic controls to compare with female AAA samples. Treatment strategies are needed for timing and delivery of estrogen and possible receptor agonist or antagonists. Treatments could be delivered locally (ex. dedrimer nanoparticles designed to colocalize with specific macrophage populations to target inflammation) to decrease the side effects of systemic delivery.Finally, manipulation of peripheral and central estrogen though gonadectomy and aortic transplantation and their subsequent effects on AAA phenotype, however elucidating, are being undertaken in another study.
More specific hormonal replacement therapy in the form of ERα or ER46 selective compounds, a SERM minimally stimulating ERα AF1 specifically, or phytoestrogens may offer the cardiovascular protective effects without unwanted side effects, such as breast cancer. Tamoxifen is an example of a ligand being an agonist in tissues where coactivators predominate (via GPER), but antagonistic where corepressors dominate (via ERα and β). Phytoestrogens (genistein, resveratrol) act as ER agonist in vasculature, but antagonist in breast and uterine tissue35, and have been shown to cause acute relaxation in arteries of post-menopausal women with CHD versus healthy controls, whereas in healthy controls PPT (selective ERα agonist) causes more relaxation than E235. This suggests selectively targeting GPER or perhaps ERα46 could therefore uncouple the vascular beneficial and undesirable effects of estrogen.
CONCLUSION
This study demonstrates an increase in aortic wall ERα in females compared with males that correlates inversely with MMP activity and AAA formation. These findings, coupled with our earlier observation that exogenous estrogen inhibits AAA formation in males, further suggest an important protective role for estrogen via ERα during AAA formation.
Acknowledgments
This work was supported by NIH grant # NIH R01 HL081629-01, NIH R01 supplemental HL081629-03S1, and the University of Michigan Cardiovascular Center Aortic Program Research Fellowship 2009–2010.
Footnotes
Presented at the American College of Surgeons, Washington DC, October 6th, 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ailawadi G, Eliason JL, Roelofs KJ, et al. Gender differences in experimental aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24:2116–22. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 2.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25:561–8. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 3.Cho BS, Roelofs KJ, Ford JW, et al. Decreased collagen and increased matrix metalloproteinase-13 in experimental abdominal aortic aneurysms in males compared with females. Surgery. 2010;147:258–67. doi: 10.1016/j.surg.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci. 1992;89:11259–63. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigoryants V, Hannawa KK, Pearce CG, et al. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41:108–14. doi: 10.1016/j.jvs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Kane MO, Anselm E, Rattman YD, Auger C, Schini-Kerth VB. Role of gender and estrogen receptors in the rat aorta endothelium-dependent relaxation to red wine polyphenols. Vascul Pharmacol. 2009;51(2–3):140–6. doi: 10.1016/j.vph.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR. Female gender attenuates cytokine and chemokine expression and leukocyte recruitment in experimental rodent abdominal aortic aneurysms. Ann NY Acad Sci. 2006;1085:367–79. doi: 10.1196/annals.1383.027. [DOI] [PubMed] [Google Scholar]
- 8.Cho BS, Woodrum DT, Roelofs KJ, et al. Differential regulation of aortic growth in male and female rodents is associated with AAA Development. J Surg Res. 2009;155:330–38. doi: 10.1016/j.jss.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriques TA, Huang J, D’Souza SS. Orchidectomy, But not ovariectomy, Regulates Angiotensin II-induced vascular diseases in apolipoprotein E-deficient Mice. Endocrinology. 2004;145(8):3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Dahlman-Wright K, Gustafsson J. Estrogen receptor β: an overview and update. Nucl Recept Signal. 2008;6:e003. doi: 10.1621/nrs.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–9. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massart F, Marini F, Menegato A, et al. Allelic genes involved in artery compliance and susceptibility to sporadic abdominal aortic aneurysm. J Ster Biochem Mol Bio. 2004;92:413–18. doi: 10.1016/j.jsbmb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–91. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enmark E, Pelto-Huikko M, Grandien K, et al. Human estrogen Receptor β-Gene Structure, Chromosomal Localization, and Expression Pattern. J Clin Endocrinol Metab. 1997;82:4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper GJM, Carlsson B, Grandien K, et al. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 16.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors α and β prevalence of estrogen receptor βmRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101:1792–98. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 17.Lubahn DB, Moyer JS, Golding TS, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci. 1993;90:11162–66. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iafrati MD, Karas RH, Aronovitz M, et al. Estrogen inhibits the vascular injury response in estrogen receptor α-deficient mice. Nature Medicine. 1997;3:545–48. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 19.Karas RH, Hodgin JB, Kwoun M, et al. Estrogen inhibits the vascular injury response in estrogen receptor β-deficient female mice. PNAS. 1999;96:15133–36. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karas RH, Schulten H, Pare G, et al. Effects of Estrogen on the Vascular Injury response in Estrogen Receptor α,β(Double ) Knockout Mice. Cir Res. 2001;89:534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 21.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–92. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Suzuki T, Miki Y, Tazawa C, Senzaki K, Moriya T, et al. Estrogen receptors in atherosclerotic human aorta: inhibition of human vascular smooth muscle cell proliferation by estrogens. Mol Cell Endrocrinol. 2004;219:17–26. doi: 10.1016/j.mce.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Hogg ME, Jiang Q, Banerjee M. Estrogen receptors Knockout the efficacy of Nitric Oxide. J Sur Res. 2010;158:299–300. [Google Scholar]
- 24.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74:337–43. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 26.Luksha L, Kublickiene K. The role of estrogen receptor subtypes for vascular maintenance. Gynecological Endocrinology. 2009;25:82–95. doi: 10.1080/09513590802485038. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MR, Haas E, Prossnitz ER, et al. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traupe T, Stettler CD, Li H, et al. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–70. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- 29.O’Lone R, Knorr K, Jaffe IZ, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–96. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 30.Simoncini T. Mechanisms of action of estrogen receptors in vascular cells: relevance for menopause and aging. Climacteric. 2009;12(Supp 1):6–11. doi: 10.1080/13697130902986385. [DOI] [PubMed] [Google Scholar]
- 31.Chambliss KL, Yuhanna IS, Mineo C, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87(11):E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 32.White RE. Estrogen and vascular function. Vascular Pharmacology. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- 33.Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen Receptors and Endothelium. Aterioscler THromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez Schulman I, Raij L. Salt sensitivity and hypertension after menopause: role of nitric oxide and angiotensin II. Am J Nephrol. 2006;26:170–180. doi: 10.1159/000092984. [DOI] [PubMed] [Google Scholar]
- 35.Cruz MN, Agewall S, Schenck-Gustafsson K, Kublickiene K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis. 2008;196:49–58. doi: 10.1016/j.atherosclerosis.2007.01.038. [DOI] [PubMed] [Google Scholar]