Abstract
Background
Maternal smoking during pregnancy (MSDP) is an independent risk factor for offspring nicotine dependence (ND), but mechanisms remain unknown. We investigated prenatal glucocorticoid (cortisol) and androgen (testosterone) associations with offspring ND over 40 years, and the possibility that prenatal glucocorticoids and androgens would mediate links between MSDP and offspring ND.
Methods
Participants were 1,086 mother-adult offspring pairs (59% female) from the New England Family Study, a 40-year longitudinal follow up of the Collaborative Perinatal Project. MSDP was assessed prospectively at each prenatal visit. Maternal cortisol, testosterone, and cotinine (nicotine metabolite), were assayed from third trimester maternal sera. Offspring lifetime ND was assessed via structured interview.
Results
Significant bivariate associations emerged for: a) MSDP/cotinine and lifetime ND, and b) maternal cortisol and lifetime ND, for daughters only. In multivariate models, maternal cortisol and MSDP/cotinine remained significantly and independently associated with increased odds of daughters’ lifetime ND. However, cortisol did not mediate the MSDP-lifetime ND relation. No associations emerged between maternal testosterone and offspring ND.
Conclusions
Results provide the first evidence in support of prenatal glucocorticoid programming of adult ND over 40 years in daughters only. Our study highlights two independent prenatal pathways leading to increased risk for ND in daughters: elevated prenatal glucocorticoids and MSDP/nicotine exposure. Daughter-specific effects of glucocorticoid and MSDP programming over 40 years highlight the breadth and persistence of sexually dimorphic programming effects in humans. Results do not support androgen programming of offspring ND.
Keywords: Maternal smoking during pregnancy, nicotine dependence, cotinine, cortisol, testosterone, programming, glucocorticoid, androgen
INTRODUCTION
Maternal smoking during pregnancy (MSDP) remains a major public health problem. Despite pervasive medical and societal sanctions, thirteen to thirty percent of pregnant mothers continue to smoke in the United States, with highest rates in poor, less educated, underserved mothers (1–3). MSDP has been linked to numerous adverse medical and behavioral outcomes in offspring, including low birth weight and sudden infant death syndrome, and attention deficits/attention deficit hyperactivity disorder, disruptive behaviors/conduct disorder in older children and adults (4–8). MSDP has also been linked to a significantly increased risk for offspring smoking uptake and regular smoking (9–20). Most pronounced links have emerged between MSDP and progression to regular/heavy smoking and nicotine dependence (ND) (16, 17, 21, 22), phenotypes associated with resistance to quit attempts, nicotine craving, and alterations in neural processes and circuitry (23). In some studies, effects of MSDP were more pronounced for female offspring (12–14, 16, 17, 19, 24). Although findings supporting the MSDP-offspring smoking/ND links have been replicated across samples and measures, mechanisms remain largely unknown.
Over the last decade, a large body of human and preclinical research has highlighted the profound importance of the fetal environment in “programming” physiologic systems and structures leading to adult health and disease (25–27). Programming has been defined as permanent alterations in fetal tissues and physiological systems as a function of the prenatal environment (28). Programmed physiological changes are believed to lead to adjustments in developmental trajectories that may predispose offspring to either positive outcomes or impairments/disease depending on congruence with postnatal environmental demands (26, 29, 30). Steroid hormones including glucocorticoids and androgens have been proposed as prominent candidate mediators of prenatal programming (31, 32). Although necessary for fetal development, over-exposure to glucocorticoids during the sensitive prenatal period is proposed to alter or “program” numerous fetal physiological and neural systems leading to psychiatric, cardiovascular, and metabolic diseases in offspring (33–37). However, although several human studies have highlighted the impact of prenatal glucocorticoid over-exposure on physiological and behavioral outcomes in infancy and childhood (38–41), we know of no human studies investigating glucocorticoid programming of adult behavioral disorders including ND.
Several lines of research highlight the plausibility of glucocorticoid programming of ND. First, exposure to MSDP/nicotine has been associated with increased maternal glucocorticoids and alterations in offspring HPA stress response in animal and human studies (42–52). Second, preclinical studies have shown links between increased maternal glucocorticoids and alterations in reward pathways, including persistent effects on drug sensitivity, altered propensity for drug self-administration, and altered brain dopamine activity in offspring (53–56). Given relationships between brain dopamine activity and reinforcement from nicotine (57) and between prenatal glucocorticoid exposure and alterations in offspring dopamine activity (58), smoking-induced increases in maternal glucocorticoids may alter fetal dopamine activity leading to increased propensity for ND. Thus, we propose that maternal cortisol levels may mediate the relation between MSDP and offspring ND. Given evidence for more pronounced MSDP-offspring smoking links in daughters (12–14, 16, 17, 19, 24), and for sex differences in prenatal glucocorticoid levels (59) and offspring outcomes following prenatal glucocorticoid exposure (60), we also hypothesized that links between prenatal glucocorticoids and offspring ND would be stronger for daughters.
Prenatal androgens have been proposed as an additional candidate mediator of prenatal programming. Several studies support the plausibility of androgen programming of offspring ND. Lombardo et al. (32) showed associations between fetal testosterone exposure and alterations in responsiveness of neural reward regions and behavioral approach tendencies, both of which have been associated with ND (61). Further, Kandel and Udry (13) published the only human study, to our knowledge, investigating prenatal androgen (testosterone) programming of offspring smoking. They hypothesized that testosterone might program the developing brain, leading to increased offspring testosterone, corresponding to greater sensation-seeking behaviors, and increased likelihood of smoking. In their study of mother-daughter pairs, they found significant positive associations between MSDP and maternal prenatal testosterone and between prenatal testosterone adolescent and adult offspring smoking. Their results highlight the plausibility of prenatal androgen programming of offspring smoking, especially in daughters.
In sum, multiple converging lines of evidence support the plausibility of glucocorticoid and androgen programming of offspring smoking/ND. Yet, to our knowledge, only one study (13) has investigated one of these plausible mechanisms (testosterone) and in an all-female sample (n=240) focusing on offspring smoking but not ND. In the present study, we conduct the first large-scale (n=1,086) investigation of the plausibility of both prenatal glucocorticoid and androgen programming of offspring ND in both daughters and sons in relation to prospectively-assessed, biochemically validated MSDP. Specifically, we investigated maternal late third-trimester prenatal cortisol and testosterone as possible mediators between MSDP and offspring ND, capitalizing on mother-offspring pairs taking part in the New England Family Study, a 40-year longitudinal follow-up of the Collaborative Perinatal Project.
METHODS AND MATERIALS
Participants and Sample Selection
The Collaborative Perinatal Project (CPP) was a multi-site, prospective investigation of the prenatal and familial antecedents of pediatric, neurological, and behavioral disorders of childhood. The CPP enrolled more than 50,000 pregnancies between 1959 and 1966 from 12 university-affiliated medical centers, and followed offspring through 7 years of age (62–64). The New England Family Study (NEFS) was established to locate and interview adult offspring of mothers enrolled in the Boston and Providence cohorts of the CPP. Selection and sampling for 1,674 NEFS participants has previously been described (65–67). All NEFS participants provided written informed consent, and followed procedures reviewed and approved by Human Subjects Committees at The Miriam Hospital, Brown University and the Harvard School of Public Health. Participants in the present study were mother-offspring pairs enrolled in the NEFS from live, singleton births who had available maternal prenatal serum sampled between 31 and 36 weeks gestation (1086 of 1674; 65%).
Procedures
Following enrollment, CPP mothers completed numerous measures, including socio-demographic and pregnancy characteristics, and cigarette smoking during pregnancy. Non-fasting maternal blood was collected at each prenatal visit. Serum was extracted, and samples were frozen and shipped to a CPP repository in Bethesda, MD. Offspring birth characteristics were recorded by study examiners. Adult offspring enrolled in the NEFS follow up study (1999–2004) completed a number of interview and self-report measures including ND (67).
We obtained late third trimester maternal serum samples from the CPP central storage repository to assay for cotinine (nicotine metabolite), testosterone, and cortisol. Sex hormone binding globulin (SHBG), and cortisol binding globulin (CBG) were also assayed to determine concentrations of free cortisol (free cortisol index; FCI) and testosterone (free androgen index; FAI) for a more accurate estimate of fetal exposure (68, 69). We selected NEFS mother-offspring pairs with a serum sample drawn between 31 and 36 weeks following the last menstrual period and at least 14 days prior to the infant’s birth date given known effects of labor/delivery on steroid hormone levels (70–72). Weeks 31–36 were selected because: a) they provided a relatively tight window within third trimester to examine hormone levels; b) they included greatest number of participants with available serum samples; and c) prior literature showed links between third trimester stress and hormone levels and offspring neurobehavioral outcomes (73–76). Mean gestational age at sampling was 31 weeks (SD =1.5) after last menstrual period. Time of day of sampling was not recorded in the CPP. Validity of cotinine, testosterone, and cortisol values from the CPP has been demonstrated previously (77, 78).
Measures
Maternal Smoking during Pregnancy (MSDP)
Pregnant mothers were queried regarding cigarette smoking by study physicians at each prenatal visit. Mothers were asked whether they were currently smoking, and, if so, the number of cigarettes smoked per day. Validity of CPP maternal smoking reports through comparison with serum cotinine levels has been shown to be excellent (kappas=83–87%) (78). Following Kandel and Udry (13), bivariate analyses utilized an ordered categorical MSDP variable: none (did not smoke), low (smoked <15 cigarettes per day), and high (smoked ≥15 cigarettes per day). For multivariate analyses, this 3-level ordinal MSDP measure was recoded using 2 dummy variables contrasting low and high MSDP with no MSDP.
Adult Lifetime Nicotine Dependence (ND)
Adult history of ND was based on Diagnostic and Statistical Manual IVth Edition (DSM IV) criteria (79) and assessed using a modified (80) version of the Composite International Diagnostic Interview (81). Lifetime ND was summarized as a binary variable (non-dependent, nicotine dependent) covering all ages through the adult follow-up interview (M=39 years, SD=2).
Biological Variables
Cotinine was assayed using liquid chromatography – tandem mass spectrometry (LC-MS/MS) (82, 83), laboratory of Neal Benowitz, M.D., University of California, San Francisco. Limit of quantitation was 1 ng/ml. Cortisol, testosterone, and SHBG were assayed using enzyme-linked immunosorbent assay (ELISA) kits; CBG was measured using radioimmunoassay (laboratory of C. Kirschbaum, University of Duesseldorf; assays described at www.ibl-hamburg.com).. Inter/intra-assay coefficients of variability ranged from 3–12%. Free Androgen Index (FAI) and Free Cortisol Index (FCI) were calculated from testosterone and SHBG, and cortisol and CBG, respectively (84, 85). (85) For further details of sample collection, storage and analysis, see Stroud et al. (77).
Potential Confounding Variables
Maternal age, race/ethnicity, education, occupation, income, gravida, and parity (number of prior live births) were assessed during the first prenatal visit. A composite index of socio-economic status (SES; range: 1= lowest through 100=highest) was derived from education (years), occupation (manual, non-manual, unemployed) of the head of household, and household income (based on US poverty threshold at the time) using methods developed by the US Census Bureau (86). Maternal psychiatric conditions and excessive alcohol and drug use during pregnancy were recorded by study personnel as part of an obstetric diagnostic summary. Maternal history of treatment for mental illness prior to pregnancy was assessed by maternal report. Gestational age was calculated based on maternal report of last menstrual period. Birth weight was recorded by a nurse observer at delivery.
Statistical Analysis
Bivariate associations between MSDP, cotinine, cortisol (FCI), testosterone (FAI), and offspring ND were estimated using polychoric, polyserial (φ), and Pearson correlations for the full sample (n=1086), stratified by gender (649 daughters; 437 sons). Following LeWinn et al. (87), given potential for confounding by low birthweight and prematurity (4, 88), the sample for mediation modeling was restricted to 986 mother-offspring pairs (584 daughters) with gestational age ≥37 weeks, and birthweight ≥2500 grams. A causal steps approach was utilized to test cortisol and testosterone as mediators of the MSDP/offspring ND link (89). This requires significant associations between: a) predictor and outcome, b) predictor and mediator; and c) mediator and outcome adjusted for predictor. Further, it requires attenuation of the predictor-outcome association when the putative mediator is included in the model. Causal steps were tested using multivariate logistic regression analyses in Splus 8.2 (90), using ordinal MSDP followed by maternal cotinine as predictors. Potential confounders were then tested for inclusion in regression models based on significant associations with both MSDP and offspring ND. These included: gravida/parity, maternal age at delivery (centered at 35 years, scaled by 5 years), maternal race/ethnicity (Caucasian, other), SES, maternal other drug use (use, no use), history of maternal treatment for mental illness (yes, no). Continuous predictors/confounders were centered at the median, and scaled by the median to 3rd quartile.
RESULTS
Sample Characteristics
Pregnant mothers
Mean maternal age at delivery was 25 years (SD=6). Racial/ethnic characteristics of mothers included 87.3% Non-Hispanic White, 12.0% Black, 0.2% Hispanic, and 0.5% other. Average gravida was 2 (SD=2). Mean composite maternal SES was 56 (SD=19), on a 100-point scale. 58% of mothers endorsed smoking during pregnancy; 43% reported smoking during third trimester. Among smokers, average maximum cigarettes per day during pregnancy was 18 (SD=11), highly similar to third trimester levels (M=18, SD=10); mean cotinine levels were 95 ng/ml (SD=78, range=1–526).
Adult Offspring
59% were female. Mean age at adult follow up was 39 years (SD=2; range 34–44). Average gestational age at birth was 40 weeks (SD=2); 5% of infants were born premature (<37 weeks). Mean birthweight was 3310 grams (SD=506); 6% of infants were born low birthweight (<2500 grams). 39% of offspring (42% of daughters) met criteria for lifetime ND.
Bivariate associations
Bivariate associations between MSDP, maternal cotinine, cortisol (FCI), testosterone (FAI), and offspring ND for daughters (n=649) and sons (n =437) are shown in Table 1. As in prior CPP samples (78), MSDP showed strong associations with maternal cotinine for daughters and sons (φ’s>0.825, p’s<.0001). Significant associations between maternal cortisol and testosterone also emerged for both daughters and sons (r’s>.222, p’s<.0001). For daughters only, increasing MSDP exposure (did not smoke, <15 cigarettes/day, 15+ cigarettes/day; n’s=254, 132, 258) and maternal cotinine were associated with increased likelihood of offspring lifetime ND (p’s<.01). Increased MSDP was associated with increased maternal cortisol (p<.05); increased cortisol was also associated with increased likelihood of lifetime ND (p<.05) in daughters only. No associations emerged between maternal cotinine and cortisol or between testosterone and either MSDP/cotinine or offspring ND for daughters. For sons, no significant associations emerged between either MSDP (did not smoke, <15 cigarettes/day, 15+ cigarettes/day, n’s=195, 78, 163) or maternal cotinine with offspring ND, or between either cortisol or testosterone with MSDP, cotinine, or offspring ND.
Table 1.
Bivariate associations between maternal smoking, maternal prenatal cotinine, and maternal prenatal testosterone and cortisol levels and with offspring daughters’ (N= 649) and sons’ (N= 437) lifetime nicotine dependence.
| Maternal Smokinga | Maternal Cotinineb | Maternal Cortisolb | Maternal Testosteroneb | |
|---|---|---|---|---|
| Daughters (N=649) | ||||
| Maternal Cotinineb | 0.856*** | _ | ||
| Maternal Cortisolb | 0.110* | 0.062 | _ | |
| Maternal Testosteroneb | −0.017 | −0.032 | 0.222*** | _ |
| Adult Offspring | 0.161** | 0.136** | 0.145* | −0.079 |
| Lifetime NDc | ||||
|
| ||||
| Sons (N=437) | ||||
| Maternal Cotinineb | 0.825*** | -- | ||
| Maternal Cortisolb | 0.059 | 0.080 | _ | |
| Maternal Testosteroneb | 0.023 | −0.030 | 0.264*** | -- |
| Adult Offspring | 0.010 | 0.010 | 0.053 | −0.020 |
| Lifetime NDc | ||||
NOTES. Polychoric correlations were estimated between categorical variables, polyserial correlations between categorical and continuous variables, and Pearson correlations between continuous variables.
Maternal smoking included 3 categories: none (did not smoke), low (<15 cigarettes per day), high (15+ cigarettes per day). N’s =254, 132, and 258 for daughters; n’s=195, 78, and 163 for sons.
Maternal cotinine is the logarithm of maternal cotinine levels. Maternal cortisol is the logarithm of the free cortisol index (FCI). Maternal testosterone is the logarithm of the free androgen index (FAI).
Adult lifetime nicotine dependence included: nicotine dependent, non-nicotine dependent.
p<.0001;
p<.001;
p<.05.
Thus, cortisol was the only putative mediator associated with both MSDP and lifetime ND for daughters only. Accounting for missingness, final mediation sample for daughters was n=544. Because cortisol was not associated with maternal cotinine in bivariate analyses (r=.062, p=.09), mediation models were not pursued with cotinine. Instead, interest centered on whether bivariate associations were robust to control for potential confounders. Maternal testosterone did not qualify as a possible mediator, because it was not significantly related to MSDP/maternal cotinine in either daughters or sons.
Multivariate model: maternal cotinine as a predictor of lifetime ND in adult daughters (n=544)
Maternal cotinine remained associated with an increased likelihood of daughters’ lifetime ND (β=.114, SE=.055, p=.039) in the restricted sample after controlling for gravida, advanced maternal age at delivery, race, and SES. Specifically, increases from the median to the 3rd quartile of cotinine raised odds of lifetime ND by 12% (OR=1.12, 95% CI=1.01–1.25).
Multivariate model: maternal cortisol as a mediator of associations between MSDP and lifetime ND in adult daughters (n=544)
MSDP, dummy coded as low vs. none (n’s=110, 218) and high vs. none; (n’s=216, 218), was entered into the model along with significant covariates (Step 1), followed by cortisol (FCI) as the mediator (Step 2). Logistic regression coefficients (log odds β) for both steps are presented in Table 2. In Step 1, high MSDP (≥15 cigarettes per day) was associated with an increased likelihood of lifetime ND (β=0.419, SE=.207, p=.043). In Step 2, maternal cortisol was significantly associated with daughters’ lifetime ND (β=0.121, SE=.055, p=.029), with increases from the median to the 3rd quartile raising odds of lifetime ND by 13% (OR=1.13, 95% CI=1.01–1.26). The association between high MSDP and lifetime ND showed little change when cortisol was added to the model (β=0.409, SE=.207, p=.048). High MDSP increased the odds of lifetime ND by approximately 50% both before (OR=1.52, 95% CI=1.01- 2.28) and after (OR=1.51, 95% CI=1.00–2.26) adjustment for maternal cortisol. Low MSDP was not significantly related to offspring daughters’ ND before or after adjustment for cortisol (p’s>.30).
Table 2.
Parameter estimates for regression analyses to test mediation models linking MSDP, maternal prenatal cortisol, and offspring nicotine dependence in daughters (n=544a).
| Maternal smoking to daughter’s lifetime nicotine dependence (n=544) b | ||||
|---|---|---|---|---|
| Step 1 | ||||
| β | SE β | z | p | |
| Constant | −1.554 | 0.382 | −4.068 | <0.001 |
| Gravidac | 0.199 | 0.049 | 4.044 | <0.001 |
| Maternal age at deliveryd | 0.080 | 0.032 | 2.490 | 0.013 |
| Maternal race (White vs. Other) | 0.798 | 0.343 | 2.328 | 0.020 |
| Maternal SES | −0.135 | 0.067 | −2.022 | 0.043 |
| Maternal smoking (low vs. none)e | 0.239 | 0.250 | 0.954 | 0.340 |
| Maternal smoking (high vs. none)f | 0.419 | 0.207 | 2.028 | 0.043 |
|
| ||||
| Step 2 | ||||
| β | SE β | z | p | |
| Constant | −1.512 | 0.385 | −3.926 | <0.001 |
| Gravidac | 0.200 | 0.050 | 4.048 | <0.001 |
| Maternal age at deliveryd | 0.076 | 0.033 | 2.334 | 0.020 |
| Maternal race (White vs. Other) | 0.795 | 0.345 | 2.307 | 0.021 |
| Maternal SES | −0.110 | 0.068 | −1.627 | 0.104 |
| Maternal smoking (low vs. none)e | 0.217 | 0.252 | 0.863 | 0.388 |
| Maternal smoking (high vs. none)f | 0.409 | 0.207 | 1.974 | 0.048 |
| Maternal Cortisol (FCI)g | 0.121 | 0.055 | 2.177 | 0.029 |
NOTES. In Step 1, significant covariates followed by maternal cigarettes smoked per day (low vs. none, and high vs. none) were entered. In Step 2, significant covariates followed by both level of maternal cigarettes per day and maternal cortisol were entered into the model.
Sample is restricted to gestational age ≥ 37 weeks and birthweight ≥ 2500 grams.
Adult lifetime nicotine dependence: (1=nicotine dependent, 0=non-nicotine dependent).
Number of prior pregnancies (square root)
Maternal age in years centered at 35 and scaled by 5 (pure quadratic term)
Sample sizes for the dummy low versus no cigarettes smoked per day during pregnancy variable (dummy coded): low, n=110; none, n=218.
Sample sizes for high versus no cigarettes smoked per day during pregnancy variable (dummy coded): high, n=216; none n=218.
Maternal cortisol (logarithm of the free cortisol index (FCI)).
Although MSDP was positively correlated with cortisol in bivariate analyses (Table 3, φ=.11), it was no longer significant at either low (β=.191, SE= .119, p=.337) or high MSDP (β=.138, SE=.165, p=.403) after adjusting for potential confounders.
Table 3.
Parameter estimates for normal regression analysis predicting maternal prenatal cortisol (n=544a).
| Maternal Smoking to Maternal Cortisol (FCI; n=544)b | ||||
|---|---|---|---|---|
| β | SE β | z | p | |
| Constant | −0.586 | 0.283 | −2.073 | 0.039 |
| Gravidac | 0.016 | 0.037 | 0.436 | 0.663 |
| Maternal age at deliveryd | 0.051 | 0.025 | 2.034 | 0.042 |
| Maternal race (White vs. Other) | 0.140 | 0.256 | 0.546 | 0.585 |
| Maternal SES | −0.214 | 0.053 | −4.048 | <0.001 |
| Maternal smoking (low vs. none)e | 0.191 | 0.199 | 0.962 | 0.337 |
| Maternal smoking (high vs. none)f | 0.138 | 0.165 | 0.837 | 0.403 |
Sample is restricted to gestational age ≥ 37 weeks and birthweight ≥ 2500 grams.
Maternal cortisol (logarithm of the free cortisol index (FCI)).
Number of prior pregnancies (square root)
Maternal age in years centered at 35 and scaled by 5 (pure quadratic term)
Sample sizes for the dummy low versus no cigarettes smoked per day during pregnancy variable (dummy coded): low, n=110; none, n=218.
Sample sizes for high versus no cigarettes smoked per day during pregnancy variable (dummy coded): high, n=216; none n=218.
Results do not support maternal cortisol as a mediator of links between MSDP and offspring ND, but suggest that high MSDP and cortisol are independently associated with daughters’ lifetime ND. Figure 1 presents path coefficients (log ORs, significance levels) for high MSDP and maternal cortisol, with covariate paths omitted for simplicity. Path coefficients were identical when Structural Equation Modeling (SEM) (91) was utilized to simultaneously estimate all paths.
Figure 1.
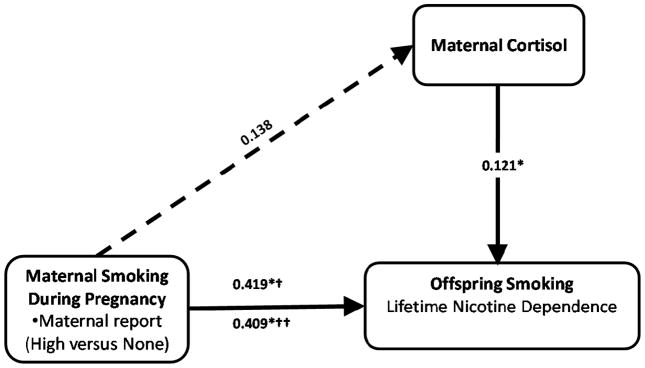
Prenatal glucocorticoids and maternal smoking independently predict nicotine dependence in adult daughters (n =544).
NOTES. Separate regression models were analyzed to derive path coefficients (standard errors) for each endogenous variable (normal linear regression for maternal cortisol, logistic regression for offspring ND). Results were identical using Structural Equation Modeling to simultaneously estimate all path coefficients. Each regression model controlled for maternal race (Caucasian vs. Other), maternal age at delivery (pure quadratic term), gravida (square root transformed), and socioeconomic status (continuous). Maternal smoking included 3 categories: none (did not smoke; n=218), low (<15 cigarettes per day; n=110), high (15+ cigarettes per day; n=216). Dashed line from MSDP to maternal cortisol highlights the non-significance of this path coefficient.
*p<.05
† before including maternal cortisol in the model;
†† after including maternal cortisol in the model.
DISCUSSION
The present study provides the first evidence of prenatal glucocorticoid programming of an adult psychiatric disorder, namely, nicotine dependence (ND), among daughters over a 40 year prospective study. Uniquely, this study also highlights two independent and additive prenatal pathways to daughters’ ND: elevated prenatal glucocorticoid exposure and elevated maternal smoking during pregnancy (MSDP) exposure. Our findings fail to confirm prior evidence for androgen programming of offspring ND (13). Strengths of the present study include the large sample size (n=1086), prospective assessment of MSDP and maternal and perinatal characteristics, unique distribution of MSDP (58% smokers), 40-year longitudinal follow up, interview measure of DSM-IV ND, and availability of third trimester serum samples for neuroendocrine assays. Available serum samples allowed measurement of free cortisol and testosterone, indicating bioavailable cortisol and testosterone, as well as cotinine, a biomarker of MSDP/nicotine exposure (92). Further, roughly equal numbers of female and male offspring allowed us to conduct all analyses stratified by gender.
Increased exposure to maternal prenatal glucocorticoids was associated with a 13% increased odds of daughters’ lifetime ND over 40 year follow-up. To our knowledge, this is the first study to reveal effects of prenatal glucocorticoid exposure on risk for nicotine addiction and the first to reveal effects of endogenous glucocorticoid exposure enduring to adulthood in daughters. Evidence for prenatal glucocorticoid programming in the present study complements a number of animal studies highlighting the causal role of over-exposure to maternal glucocorticoids in programming CNS dysfunction and disease in adult offspring (33, 93). Results also complement an emerging human literature suggesting that over-exposure to prenatal glucocorticoids may program early behavioral, physiologic, and neurocognitive outcomes (38–41, 87). The present study extends evidence in humans for associations between endogenous prenatal glucocorticoids and offspring outcomes to include adult ND.
Smoking 15 cigarettes per day or more was associated with a 52% increased odds of ND in daughters. Our finding of an independent pathway between MSDP and elevated risk for offspring ND is consistent with numerous prior studies (16, 17, 21), including a prior study from the Collaborative Perinatal Project (CPP) (21). Previous studies have shown links between MSDP and all stages of offspring smoking progression (smoking uptake, regular smoking, ND) (10, 18, 19, 21), but with most pronounced effects for progression to regular/heavy smoking and ND (17, 21, 22). ND specifies a maladaptive pattern of tobacco use involving withdrawal, tolerance, and/or inability to quit smoking that is more closely linked to alterations in neural, affective, and hedonic processes as well as smoking-related diseases and health care burden relative to regular smoking (94–97). Results highlight the influence of MSDP on the phenotype of nicotine addiction in female offspring.
Maternal cotinine also predicted ND in adult daughters, highlighting the importance of nicotine in the long-term behavioral consequences of MSDP. Our results contrast with Kandel and Udry (13), who found no effects of maternal cotinine on adolescent daughters’ smoking. Because smoking in adolescence may be a better representation of smoking initiation versus persistent smoking or ND, it is possible that exposure to maternal nicotine is associated with alterations in fetal neuroteratogenesis increasing propensity to ND in adulthood (97, 98), whereas other aspects of MSDP may impact smoking initiation in adolescence (13).
Associations between both prenatal glucocorticoids and MSDP/nicotine and offspring ND emerged only for daughters. Results are unlikely to be due to sex differences in ND in the population as men have shown slightly increased ND prevalence (99), or sex differences in fetal metabolism as the fetus shows little ability to independently metabolize drugs or glucocorticoids (100). Results are consistent with numerous preclinical and recent human studies revealing sex differences in effects of prenatal insults and in neuroendocrine programming pathways (60, 101). Daughter-specific effects of MSDP in the present study complement prior studies revealing more pronounced effects of MSDP on offspring smoking in daughters (9, 10, 12–16, 18–20, see also 21, 24). Daughter-specific effects of prenatal glucocorticoid exposure are consistent with a recent study revealing increased late-gestational cortisol levels in mothers carrying daughters vs. sons (59) and daughter-specific effects of maternal prenatal cortisol on child amygdalar volume, a neural marker of affective disorders (102). Likely mechanisms are sex differences in placental glucocorticoid regulation and adaptations to environmental insults, as well as differential effects of cortisol and nicotine on a sexually-differentiating fetal brain (103). That daughter-specific effects emerged for both MSDP and prenatal glucocorticoids in the present and prior studies highlights consistent sexual dimorphism in programming outcomes across a broad range of prenatal exposures. Future research is needed to further elucidate sexually dimorphic prenatal programming pathways in relation to a broad range of prenatal insults.
Initially, we hypothesized that prenatal glucocorticoid programming would mediate links between MSDP and offspring ND. Instead, our findings suggest that exposure to maternal elevated glucocorticoids and high MSDP were associated with independent and additive increased risk for female offspring ND, together increasing odds of ND by 72%. Results complement emerging epidemiologic theories highlighting the interplay of allostatic load and environmental toxicants on maternal and child health disparities (104). Additive effects of elevated prenatal glucocorticoids and nicotine also complement an intriguing series of preclinical studies of offspring exposed to prenatal nicotine and dexamethasone, a synthetic glucocorticoid (105–107). Dually exposed offspring showed synergistic effects on brain cholinergic, serotonergic, and dopaminergic circuitry, with effects persisting to adulthood and evidence for sex differences in developmental trajectories.
In contrast to Kandel and Udry (13), our results fail to confirm prenatal testosterone as a mechanism linking MSDP and offspring ND despite a large sample size and prospective assessment of MSDP. We believe it is unlikely that our failure to replicate Kandel and Udry’s (13) findings, is due to lack of power. Our sample size was large (n=1086), even when stratifying by gender with 80% power to detect small effects: r’s=.11 and .13 for daughters and sons; however, effect sizes for cortisol were statistically significant, and approximately two times greater than those for testosterone. Although prenatal androgens did not show associations with the ND phenotype, they may be more closely linked to other points in offspring smoking trajectories, such as smoking initiation (13).
We acknowledge several key limitations of our study. First, time of day of serum collection and additional factors related to variability in cortisol and testosterone levels (food intake, caffeine) were not recorded in the CPP (108–110). However, if these factors are assumed to be random across prenatal visits (87), variation in hormone levels due to time of day/nutritional status would be included as error variance, and would serve to attenuate rather than strengthen links between maternal glucocorticoids and offspring ND. That findings emerged despite likely high error variance suggests that effects would be stronger with more precise measures of maternal glucocorticoids. Second, measures of postnatal environment are lacking, particularly exposure to secondhand smoke, which was not assessed in CPP. Although CPP mothers who smoked during pregnancy likely continued to smoke postpartum, several prior studies of MSDP and offspring smoking/ND have shown associations to be robust to control for postnatal secondhand smoke exposure (14–17) and parental lifetime smoking status (111). Additional postnatal environmental factors (e.g., maternal care, parental sensitivity) have also been shown to mitigate effects of prenatal adversity (112–114). Future studies are needed to investigate postnatal environmental moderators of links between gestational glucocorticoids and MSDP and offspring ND (e.g., parental sensitivity, early life stressors, behavioral modeling).
Third, our study design did not allow assessment of familial confounding factors. Several recent studies revealed evidence for familial confounding of links between MSDP and offspring behavioral outcomes, although offspring ND was not measured and the majority did not include biochemical verification of MSDP (115–117). Nonetheless, future studies with genetically informative designs (e.g., sibling pairs differing in MSDP exposure levels), which also include intermediate phenotypes and biological mediators, are needed (118, 119). Finally, maternal glucocorticoids in the present study are presumed to indicate fetal glucocorticoid exposure. Future studies of both maternal prenatal and offspring cortisol regulation in relation to risk for adult ND would be a major contribution.
Conclusions
This 40-year longitudinal study reveals the first evidence, to our knowledge, that prenatal exposure to glucocorticoids predicts ND in adult daughters. Specifically, two independent and additive pathways to daughters’ ND were identified: exposure to elevated prenatal glucocorticoids and exposure to high MSDP. Results highlight the enduring influence of gestational glucocorticoid exposure and also support differential vulnerability of daughters to long-term adverse outcomes following gestational exposure to glucocorticoids and MSDP.
Acknowledgments
Preparation of this manuscript was supported by the National Institutes of Health (R01 HD043844 to L.R.S./R.N. and P50 CA84719 to R.N.) and the Flight Attendant Medical Research Institute Clinical Innovator Award to L.R.S. There are no conflicts of interest. We are grateful to mothers and children who contributed to the CPP and NEFS studies. We are indebted to John Lewis for his expertise in the biochemistry of binding globulins. We also acknowledge the role of the Biospecimen Repository Access and Data Sharing Committee (BRADSC) in the Division of Epidemiology, Statistics and Prevention Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in providing access to CPP serum samples. We also thank Stephanie Paton and Kathy McGaffigan for administrative and programming assistance, respectively. Finally, we are grateful for the excellent suggestions of three anonymous reviewers.
Footnotes
FINANCIAL DISCLOSURES
The authors have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura R. Stroud, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University
George Papandonatos, Center for Statistical Sciences, Brown University
Edmond Shenassa, Program in Maternal-Child Health, School of Public Health, University of Maryland
Daniel Rodriguez, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University
Raymond Niaura, Schroeder Institute for Tobacco Research and Policy Studies, American Legacy Foundation
Kaja LeWinn, Department of Psychiatry, University of California, San Francisco
Lewis P. Lipsitt, Department of Psychology, Brown University
Stephen L. Buka, Department of Epidemiology, School of Public Health, Brown University
References
- 1.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. MMWR. Atlanta, GA: Centers for Disease Control; 2009. Trends in Smoking Before, During, and After Pregnancy --- Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 Sites, 2000–2005; pp. 1–29. [PubMed] [Google Scholar]
- 2.Mathews TJ. Smoking during pregnancy in the 1990s. Natl Vital Stat Rep. 2001;49:1–14. [PubMed] [Google Scholar]
- 3.Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med. 2003;24:29–35. doi: 10.1016/s0749-3797(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 4.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant Morbidity and Mortality Attributable to Prenatal Smoking in the US. American Journal of Preventive Medicine. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: the case of prenatal smoking and disruptive behavior. Child Dev. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimasu K, Kiyohara C, Minami T, Yoshikawa N, Kihira S, Toyonaga K, et al. Maternal smoking during pregnancy and offspring attention-deficit/hyperactivity disorder: a case-control study in Japan. Attention deficit and hyperactivity disorders. 2009;1:223–231. doi: 10.1007/s12402-009-0015-1. [DOI] [PubMed] [Google Scholar]
- 7.Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius MD, Goldschmidt L, De Genna NM, Larkby C. Long-term Effects of Prenatal Cigarette Smoke Exposure on Behavior Dysregulation Among 14-Year-Old Offspring of Teenage Mothers. Matern Child Health J. 2012;16:694–705. doi: 10.1007/s10995-011-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, et al. The effects of maternal smoking during pregnancy on offspring outcomes. Preventive Medicine. 2010;50:13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Mamun A, O’Callaghan FV, Alati R, O’Callaghan M, Najman JM, Williams GM, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15:452–457. doi: 10.1136/tc.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol Teratol. 2005;27:667–676. doi: 10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Griesler PC, Kandel DB, Davies M. Maternal smoking in pregnancy, child behavior problems, and adolescent smoking. Journal of Research on Adolescence. 1998;8:159–185. [Google Scholar]
- 13.Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters’ smoking: nicotine or testosterone exposure? Am J Public Health. 1999;89:1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieb R, Schreier A, Pfister H, Wittchen HU. Maternal smoking and smoking in adolescents: A prospective community study of adolescents and their mothers. European Addiction Research. 2003;9:120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- 16.Oncken C, McKee S, Krishnan-Sarin S, O’Malley S, Mazure C. Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res. 2004;6:829–833. doi: 10.1080/1462220042000282555. [DOI] [PubMed] [Google Scholar]
- 17.Rydell M, Cnattingius S, Granath F, Magnusson C, Galanti MR. Prenatal exposure to tobacco and future nicotine dependence: population-based cohort study. British Journal of Psychiatry. 2012;200:202–209. doi: 10.1192/bjp.bp.111.100123. [DOI] [PubMed] [Google Scholar]
- 18.Weden MM, Miles JNV. Intergenerational Relationships Between the Smoking Patterns of a Population-Representative Sample of US Mothers and the Smoking Trajectories of Their Children. American Journal of Public Health. 2012;102:723–731. doi: 10.2105/AJPH.2011.300214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts KH, Munafo MR, Rodriguez D, Drury M, Murphy MFG, Neale RE, et al. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behavior of offspring. Nicotine & Tobacco Research. 2005;7:801–808. doi: 10.1080/14622200500262840. [DOI] [PubMed] [Google Scholar]
- 20.Tehranifar P, Liao YY, Ferris JS, Terry MB. Life course socioeconomic conditions, passive tobacco exposures and cigarette smoking in a multiethnic birth cohort of US women. Cancer Causes & Control. 2009;20:867–876. doi: 10.1007/s10552-009-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. Am J Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 22.O’Callaghan FV, Al Mamun A, O’Callaghan M, Alati R, Najman JM, Williams GM, et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults - a birth cohort study. Australian and New Zealand Journal of Public Health. 2009;33:371–377. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 23.Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan KM, Bottorff J, Reid C. Does Mother’s Smoking Influence Girls’ Smoking More Than Boys’ Smoking? A 20-Year Review of the Literature Using a Sex- and Gender-Based Analysis. Substance Use & Misuse. 2011;46:656–668. doi: 10.3109/10826084.2010.528122. [DOI] [PubMed] [Google Scholar]
- 25.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2012 doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochemical Journal. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 27.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Bergh BR. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev Med Child Neurol. 2011;53(Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- 29.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 30.Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychol Sci. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Lai M-C, Taylor K, et al. Fetal Programming Effects of Testosterone on the Reward System and Behavioral Approach Tendencies in Humans. Biological Psychiatry. 2012;72:839–847. doi: 10.1016/j.biopsych.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis--2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 38.de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 39.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 40.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander N, Rosenlocher F, Stalder T, Linke J, Distler W, Morgner J, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97:3538–3544. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 42.Chen M, Ting W, Zhang-xiu L, Xiao-liang P, Ying-Hong F, Hui W. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Experimental and Toxicological Pathology. 2007;59:245–251. doi: 10.1016/j.etp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Xu D, Liang G, Yan YE, He WW, Liu YS, Chen LB, et al. Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicology Letters. 2012;209:282–290. doi: 10.1016/j.toxlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Poland RE, Lutchmansingh P, Au D, Edelstein M, Lydecker S, Hsieh C, et al. Exposure to threshold doses of nicotine in utero: I. Neuroendocrine response to restraint stress in adult male offspring. Life Sciences. 1994;55:1567–1575. doi: 10.1016/0024-3205(94)00318-1. [DOI] [PubMed] [Google Scholar]
- 45.Poland RE, Lutchmansingh P, Au D, Hsieh C, Acosta S, Lydecker S, et al. Exposure to threshold doses of nicotine in utero: II. Neuroendocrine response to nicotine in adult male offspring. Brain Research and Developmental Brain Research. 1994;83:278–284. doi: 10.1016/0165-3806(94)00143-x. [DOI] [PubMed] [Google Scholar]
- 46.Poland RE, Lutchmansingh P, Au D, McGeoy S, Que M, Acosta S, et al. Exposure to threshold doses of nicotine in utero: III. Augmentation of the prolactin and ACTH response to 8-OH DPAT by desipramine treatment is compromised in adult male offspring. Neurotoxicology. 1996;17:351–358. [PubMed] [Google Scholar]
- 47.Lieberman E, Torday J, Barbieri R, Cohen A, Vanvunakis H, Weiss ST. Association of intrauterine cigarette-smoke exposure with indexes of fetal lung maturation. Obstet Gynecol. 1992;79:564–570. [PubMed] [Google Scholar]
- 48.McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, et al. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. Bjog. 2006;113:1289–1295. doi: 10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 49.Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. J Perinat Med. 2006;34:466–470. doi: 10.1515/JPM.2006.091. [DOI] [PubMed] [Google Scholar]
- 50.Varvarigou AA, Liatsis SG, Vassilakos P, Decavalas G, Beratis NG. Effect of maternal smoking on cord blood estriol, placental lactogen, chorionic gonadotropin, FSH, LH, and cortisol. J Perinat Med. 2009;37:364–369. doi: 10.1515/JPM.2009.028. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Dev Psychobiol. 2008;50:819–834. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fameli M, Kitraki E, Stylianopoulou F. Effects of hyperactivity of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy on the development of the HPA axis and brain monoamines of the offspring. Int J Dev Neurosci. 1994;12:651–659. doi: 10.1016/0736-5748(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 54.Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, et al. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res. 1995;685:179–186. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- 55.Hermans RH, Longo LD. Altered catecholaminergic behavioral and hormonal responses in rats following early postnatal hypoxia. Physiol Behav. 1994;55:469–475. doi: 10.1016/0031-9384(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574:131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- 57.Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nature Reviews Neuroscience. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 58.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neuroscience and Biobehavioral Reviews. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 59.DiPietro JA, Costigan KA, Kivlighan KT, Chen P, Laudenslager ML. Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology. 2011;36:588–591. doi: 10.1016/j.psyneuen.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiol Behav. 2012;106:736–740. doi: 10.1016/j.physbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Di Matteo V, Pierucci M, Di Giovanni G, Benigno A, Esposito E. The neurobiological bases for the pharmacotherapy of nicotine addiction. Current Pharmaceutical Design. 2007;13:1269–1284. doi: 10.2174/138161207780618920. [DOI] [PubMed] [Google Scholar]
- 62.Broman S, Bien E, Shaughnessy P. Low Achieving Children: The First Seven Years. Hillsdale, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- 63.Broman S, Nichols PL, Shaughnessy P, Wallace K. Retardation in Young Children: A Developmental Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]
- 64.Niswander KR, Gordon M. DHEW Publication NIH-73-379. Washington, D.C: U.S. Government Printing Office; 1972. The Women and Their Pregnancies. [Google Scholar]
- 65.Gilman SE, Martin LT, Abrams DB, Kawachi I, Kubzansky L, Loucks EB, et al. Educational attainment and cigarette smoking: a causal association? International Journal of Epidemiology. 2008;37:615–624. doi: 10.1093/ije/dym250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graham AL, Papandonatos GD, Depue JD, Pinto BM, Borrelli B, Neighbors CJ, et al. Lifetime characteristics of participants and non-participants in a smoking cessation trial: Implications for external validity and public health impact. Annals of Behavioral Medicine. 2008;35:295–307. doi: 10.1007/s12160-008-9031-1. [DOI] [PubMed] [Google Scholar]
- 67.Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Clark MA, et al. Parental Smoking and Adolescent Smoking Initiation: An Intergenerational Perspective on Tobacco Control. Pediatrics. 2009;123:E274–E281. doi: 10.1542/peds.2008-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slaunwhite WR, Lockie GN, Back N, Sandberg AA. Inactivity in vivo of transcortin-bound cortisol. Science. 1962;135:1062–1063. doi: 10.1126/science.135.3508.1062. [DOI] [PubMed] [Google Scholar]
- 69.Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90:43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 70.Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, et al. Mode of delivery is associated with maternal and fetal endocrine stress response. Bjog. 2006 doi: 10.1111/j.1471-0528.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 71.Tuimala R, Kauppila A, Ronnberg L, Jouppila R, Haapalahti J. The effect of labour on ACTH and cortisol levels in amniotic fluid and maternal blood. Br J Obstet Gynaecol. 1976;83:707–710. doi: 10.1111/j.1471-0528.1976.tb00917.x. [DOI] [PubMed] [Google Scholar]
- 72.Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139:416–422. doi: 10.1016/0002-9378(81)90318-5. [DOI] [PubMed] [Google Scholar]
- 73.Davis EP, Glynn LM, Hobel C, Dunkel-Schetter C, Chicz-Demet A, Sandman C. Prenatal Exposure to Maternal Cortisol Influences Infant Temperament. 2006. Manuscript Under Review. [DOI] [PubMed] [Google Scholar]
- 74.Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 75.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 76.Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Human Development. 2005;81:183–190. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Stroud LR, Solomon C, Shenassa E, Papandonatos G, Niaura R, Lipsitt LP, et al. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: still valid after all these years. Psychoneuroendocrinology. 2007;32:140–150. doi: 10.1016/j.psyneuen.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klebanoff MA, Levin RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. American Journal of Epidemiology. 1998;148:259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- 79.APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. TR ed. [Google Scholar]
- 80.Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug and Alcohol Dependence. 2007;86:106–114. doi: 10.1016/j.drugalcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 81.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 82.Eisner MD, Balmes J, Yelin EH, Katz PP, Hammond SK, Benowitz N, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulm Med. 2006;6:12. doi: 10.1186/1471-2466-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cottler LB, Robins LN, Grant BF, Blaine J, Towle LH, Wittchen HU, et al. The CIDI-core substance abuse and dependence questions: cross-cultural and nosological issues. The WHO/ADAMHA Field Trial. Br J Psychiatry. 1991;159:653–658. doi: 10.1192/bjp.159.5.653. [DOI] [PubMed] [Google Scholar]
- 84.Mathur RS, Moody LO, Landgrebbe S, Williamson HO. Plasma androgens and sex hormone binding globulin in the evaluation of hirsute patients. Fertil Steril. 1981;35:29–37. doi: 10.1016/s0015-0282(16)45254-4. [DOI] [PubMed] [Google Scholar]
- 85.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26:197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 86.Myrianthopoulos NC, French KS. An application of the U.S. bureau of the census socioeconomic index to a large diversified patient population. Social Science and Medicine. 1968;2:283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 87.LeWinn KZ, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. International Journal of Epidemiology. 2009;38:1700–1710. doi: 10.1093/ije/dyp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krentz H, Voigt M, Hesse V, Guthmann F, Wenzlaff P, Straube S. Influence of Smoking during Pregnancy Specified as Cigarettes Per Day on Neonatal Anthropometric Measurements - an Analysis of the German Perinatal Survey. Geburtshilfe Und Frauenheilkunde. 2011;71:663–668. [Google Scholar]
- 89.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 90.TIBCO Spotfire SPLUS 8.2 for Solaris/Linux User’s Guide. Seattle, WA: TIBCO Software, Inc; 2010. [Google Scholar]
- 91.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 92.Ray R, Tyndale RF, Lerman C. Nicotine Dependence Pharmacogenetics: Role of Genetic Variation in Nicotine-Metabolizing Enzymes. Journal of Neurogenetics. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 94.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNeill A, Munafò MR. Reducing harm from tobacco use. Journal of Psychopharmacology. 2013;27:13–18. doi: 10.1177/0269881112458731. [DOI] [PubMed] [Google Scholar]
- 96.DiFranza J, Ursprung WWS, Lauzon B, Bancej C, Wellman RJ, Ziedonis D, et al. A systematic review of the Diagnostic and Statistical Manual diagnostic criteria for nicotine dependence. Addictive Behaviors. 2010;35:373–382. doi: 10.1016/j.addbeh.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 97.Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 2012;123(Suppl 1):S59–71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 99.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 100.Juchau MR, Chao ST, Omiecinski CJ. Drug metabolism by the human fetus. Clin Pharmacokinet. 1980;5:320–339. doi: 10.2165/00003088-198005040-00002. [DOI] [PubMed] [Google Scholar]
- 101.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 102.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slotkin TA, Ryde IT, Seidler FJ. Additive and synergistic effects of fetal nicotine and dexamethasone exposure on cholinergic synaptic function in adolescence and adulthood: Implications for the adverse consequences of maternal smoking and pharmacotherapy of preterm delivery. Brain Res Bull. 2010;81:552–560. doi: 10.1016/j.brainresbull.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain Res Bull. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 107.Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: fetal nicotine exposure enhances the effect of late gestational dexamethasone treatment on noradrenergic circuits. Brain Res Bull. 2011;86:435–440. doi: 10.1016/j.brainresbull.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 109.Kirschbaum C, Wust S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 110.Lovallo WR, Whitsett TL, al’Absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom Med. 2005;67:734–739. doi: 10.1097/01.psy.0000181270.20036.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selya AS, Wakschlag LS, Dierker LC, Rose JS, Hedeker D, Mermelstein RJ. Exploring Alternate Processes Contributing to the Association Between Maternal Smoking and the Smoking Behavior Among Young Adult Offspring. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buss C, Entringer S, Swanson JM, Wadhwa PD. The Role of Stress in Brain Development: The Gestational Environment’s Long-Term Effects on the Brain. Cerebrum. 2012;2012:4. [PMC free article] [PubMed] [Google Scholar]
- 113.Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Sharp H, Pickles A, Meaney M, Marshall K, Tibu F, Hill J. Frequency of infant stroking reported by mothers moderates the effect of prenatal depression on infant behavioural and physiological outcomes. PLoS One. 2012;7:e45446. doi: 10.1371/journal.pone.0045446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.D’Onofrio BM, Rickert ME, Langstrom N, Donahue KL, Coyne CA, Larsson H, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring substance use and problems. Arch Gen Psychiatry. 2012;69:1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry. 2010;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.D’Onofrio BM, Van Hulle CA, Goodnight JA, Rathouz PJ, Lahey BB. Is maternal smoking during pregnancy a causal environmental risk factor for adolescent antisocial behavior? Testing etiological theories and assumptions. Psychol Med. 2012;42:1535–1545. doi: 10.1017/S0033291711002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bublitz MH, Stroud LR. Maternal smoking during pregnancy and offspring brain structure and function: review and agenda for future research. Nicotine Tob Res. 2012;14:388–397. doi: 10.1093/ntr/ntr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]


