Abstract
Acetaminophen (APAP) hepatotoxicity is the leading cause of acute liver failure in the US. Although many aspects of the mechanism are known, recent publications suggest that gap junctions composed of connexin32 function as critical intercellular communication channels which transfer cytotoxic mediators into neighboring hepatocytes and aggravate liver injury. However, these studies did not consider off-target effects of reagents used in these experiments, especially the gap junction inhibitor 2-aminoethoxy-diphenyl-borate (2-APB). In order to assess the mechanisms of protection of 2-APB in vivo, male C56Bl/6 mice were treated with 400 mg/kg APAP to cause extensive liver injury. This injury was prevented when animals were co-treated with 20 mg/kg 2-APB and was attenuated when 2-APB was administered 1.5h after APAP. However, the protection was completely lost when 2-APB was given 4–6h after APAP. Measurement of protein adducts and c-jun-N-terminal kinase (JNK) activation indicated that 2-APB reduced both protein binding and JNK activation, which correlated with hepatoprotection. Although some of the protection was due to the solvent dimethyl sulfoxide (DMSO), in vitro experiments clearly demonstrated that 2-APB directly inhibits cytochrome P450 activities. In addition, JNK activation induced by phorone and tert-butylhydroperoxide in vivo was inhibited by 2-APB. The effects against APAP toxicity in vivo were reproduced in primary cultured hepatocytes without use of DMSO and in the absence of functional gap junctions. We conclude that the protective effect of 2-APB was caused by inhibition of metabolic activation of APAP and inhibition of the JNK signaling pathway and not by blocking connexin32-based gap junctions.
Keywords: acetaminophen hepatotoxicity, gap junctions, connexin32, c-jun-N-terminal kinase, protein adducts, 2-aminoethoxy-diphenyl-borate, oxidative stress
INTRODUCTION
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug, which is safe at therapeutic doses. However, intentional or accidental overdosing can induce severe liver injury and in some patients even acute liver failure (Larson 2007, McGill et al., 2012). Early animal studies identified reactive metabolite formation, glutathione (GSH) depletion and protein adduct formation as critical events in the toxicity (Mitchell et al., 1973; Nelson, 1990; McGill and Jaeschke, 2013). This mechanistic insight led to the introduction of N-acetylcysteine (NAC) as a clinical antidote against APAP poisoning (Prescott et al., 1977). NAC promotes GSH synthesis and thus protects by enhancing the detoxification capacity for the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Corcoran et al, 1985; Corcoran and Wong, 1986). NAC-supported GSH synthesis also replenishes the mitochondrial GSH content, which allows detoxification of reactive oxygen species and peroxynitrite in the mitochondria (Knight et al., 2002; Cover et al., 2005). In addition, surplus NAC not needed to synthesize GSH will be degraded, and the resulting Krebs cycle intermediates support mitochondrial energy metabolism (Saito et al., 2010b). Despite these multiple protective mechanisms of NAC, it is very obvious that NAC is most effective when given as early as possible after the APAP overdose. However, the clinical reality is that many patients only seek medical attention when liver injury is already present (Larson, 2007). Therefore, there is clearly a need to develop drugs that are effective after the metabolism phase.
A recent paper by Patel et al. (2012) identified gap junctions containing connexin32 (Cx32) as critical intercellular communication channels responsible for the progression of liver injury after thioacetamide (TAA) and APAP overdose. The authors suggested that gap junctions allow the transfer of a lethal dose of reactive oxygen species (ROS) into neighboring hepatocytes. The most remarkable feature of this study was the identification of a small molecule Cx32-gap junction inhibitor, 2-aminoethoxy-diphenyl-borate (2-APB) (Tao and Harris, 2007), which was not only >99% effective in preventing APAP- or TAA-induced liver injury when given 1 h before drug overdose, but also reduced liver injury by 60% when administered as late as 6 h after the toxicants (Patel et al., 2012). This remarkable protection of 2-APB, especially with delayed administration, garnered significant attention (Fromenty, 2013; Maurel and Rosenbaum, 2012). The editorial commentaries expressed the hope that this might be a novel and promising treatment option for drug-induced liver injury. However, the virtually perfect protection with 2-APB raises some concerns regarding the mechanisms involved. Although Patel et al. (2012) evaluated the formation of TAA metabolites, this was not done with APAP. In addition, 2-APB is only soluble in diluted dimethyl sulfoxide (DMSO), which is known to effectively block APAP toxicity through inhibition of drug metabolism even at very low doses (Jaeschke et al., 2006). Given these serious concerns and the potential benefits of 2-APB treatment, we evaluated the mechanism of protection of 2-APB in a murine model of acetaminophen hepatotoxicity in vivo and in cultured mouse hepatocytes.
MATERIALS AND METHODS
Animals
Male C57Bl/6 mice (8–12 weeks old) purchased from Jackson Laboratories (Bar Harbor, ME) were used in our experiments. The mice were kept in an environmentally controlled room with a 12 h light/dark cycle and free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals.
Experiment design
After overnight fasting, mice were treated with 400 mg/kg APAP (Sigma-Aldrich, St. Louis, MO) (i.p.) dissolved in warm saline. A dose of 20 mg/kg of the Cx32 gap junction inhibitor 2-aminoethoxy-diphenyl-borate (2-APB) (Sigma-Aldrich) dissolved in DMSO was administered with APAP, or at 1.5 h, 4.5 h and 6 h after APAP treatment. All vehicle control mice received the same volume of DMSO (0.2 ml/kg) and saline (20 ml/kg). Mice were euthanized at 2 h, 6 h or 24 h after APAP injection and blood and livers were harvested. In addition, some animals were treated with 20 mg/kg 2-APB followed 1 h later by 100 mg/kg phorone (Sigma-Aldrich) dissolved in corn oil and then 1 h later with 1 mmol/kg tert-butylhydroperoxide (tBHP) (Sigma-Aldrich) and sacrificed 1 h after tBHP (Xie et al., 2013). Blood was drawn into a heparinized syringe to determine alanine aminotransferase (ALT) activity with a kit from Pointe Scientific (Canton, MI). The liver was removed and pieces were fixed in phosphate-buffered formalin or used for mitochondrial isolation. The rest of the liver was snap-frozen in liquid nitrogen and subsequently stored at −80°C.
Mouse hepatocyte isolation and cell viability assessment
Primary hepatocytes were isolated from mice by means of a 2-step collagenase perfusion technique as described previously (Bajt et al., 2004). Cell viability was generally more than 90% based on trypan blue exclusion, and cell purity of hepatocytes was more than 95%. The cells were plated in a density of 6 ×105 cells/well in six-well plates (BioCoat collagen I cellware plates; Becton Dickinson, Franklin Lakes, NJ). Cells were grown in Williams E medium (Life Technologies, Grand Island, NY) containing 100 U/mL penicillin/streptomycin, 1×10−7 M insulin, and 10% fetal bovine serum. After 3.5 h of attachment to the bottom of the plate, cells were treated with 5 mM APAP and 2-APB at the same time or delayed for 1.5 h at a concentration of 1, 5, 10, or 25 µM. Both APAP and 2-APB were dissolved in 37°C Williams E medium. Cells were harvested for lactate dehydrogenase (LDH) activity, GSH and APAP-protein adducts determination.
Measurement of LDH and GSH
The measurement of LDH activities was performed as described in detail (Bajt et al., 2004). In short, after removing the medium, cells were scraped off and lysed in cell lysis buffer (25 mM HEPES, 5 mM EDTA, 0.1% CHAPS, and 1 mg/ml each of pepstatin, leupeptin, and aprotinin). The lysates were sonicated and centrifuged for 10 min at 20,000 g at 4°C. Both the resulting supernatant and previous cell medium were incubated with potassium phosphate buffer containing pyruvate and NADH, and LDH activities were determined by the decline of absorbance at 340 nm. GSH levels in primary mouse hepatocyte were determined using a modified Tietze assay (Yan et al., 2010). In brief, primary cells were lysed and homogenized in 3% sulfosalicylic acid and centrifuged to remove precipitated proteins. The supernatant was further diluted with 0.1 M potassium phosphate buffer, pH 7.4. The samples were then assayed with dithionitrobenzoic acid as described (Jaeschke and Mitchell, 1990). A 10% sodium dodecyl sulfate solution was applied to the pellet to solubilize the protein. Protein concentrations were determined using the bicinchoninic acid kit (Pierce, Rockford, IL). The GSH levels were expressed per mg protein.
PAP protein adducts and cytochrome P450 activity
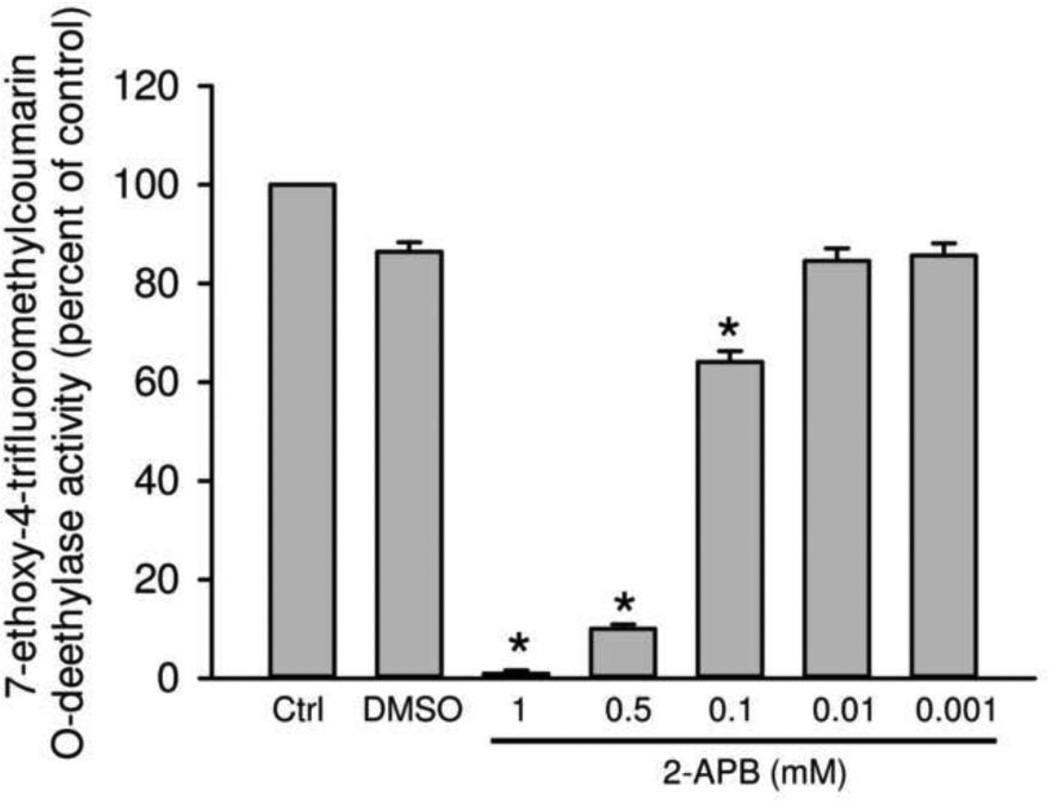
APAP-protein adducts in liver tissues were measured by high-pressure liquid chromatography with electrochemical detection (HPLC-ECD) according to the method of Muldrew et al. (2002) with some modifications (Ni et al., 2012). APAP-protein adducts concentration in primary mouse hepatocytes was determined as described (McGill et al., 2011). The cytochrome P450 activity was determined using the 14,000 g supernatant from a total liver homogenates in the 7-ethoxy-4-trifluoromethylcoumarin (7EFC) deethylase assay (Buters et al., 1993), as described in detail (Ramachandran et al., 2011). The substrate 7EFC is known to be metabolized by Cyp1A2 and 2E1 (Buters et al., 1993).
Histology and Immunoprobing
Formalin-fixed tissue samples were embedded in paraffin and 5 µm thickness sections were cut and stained with hematoxylin and eosin (H&E) for blinded evaluation of tissue necrosis as described (Gujral et al., 2002). Western blotting was performed as described in detail previously (Bajt et al., 2000). Briefly, a 20% liver homogenate was prepared for each liver sample in homogenizing buffer (25 mM HEPES, 5 mM EDTA, 2 mM DTT, 0.1% CHAPS supplemented with 1 µg/mL leupeptin, aprotinin and pepstatin) with 10 stroke of a tight fitting Teflon pestle in a mortar. The homogenate was centrifuged at 14,000 g for 20 min and the supernatant was collected. After determination of protein concentration using the bicinchoninic acid kit (Pierce, Rockford, IL), 30 µg of protein was loaded per lane. Western blotting for JNK was performed using rabbit anti-JNK and anti-phospho-JNK antibodies (Cell Signaling Technology, Danvers, MA) with horseradish peroxidase-coupled donkey anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) (Bajt et al., 2000). Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech., Inc., Piscataway, NJ).
Statistics
All data were expressed as mean ± SEM. For normally distributed data, statistical significance was evaluated by one-way analysis of variance (ANOVA), followed by Student Newman-Keul’s test for multiple comparisons. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn’s multiple comparisons. P < 0.05 was considered significant.
RESULTS
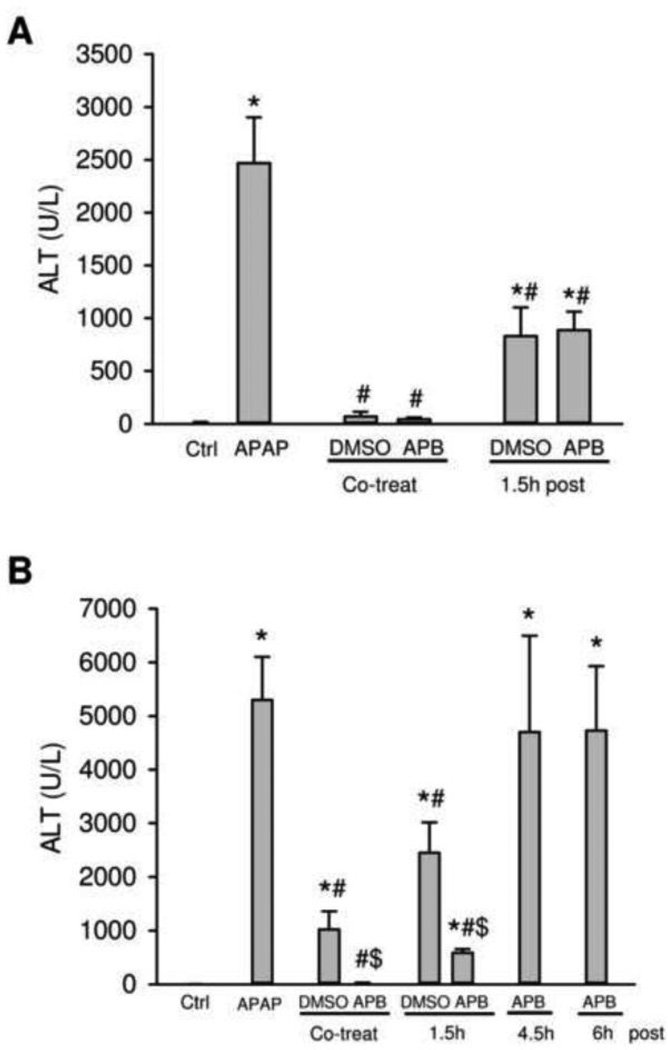
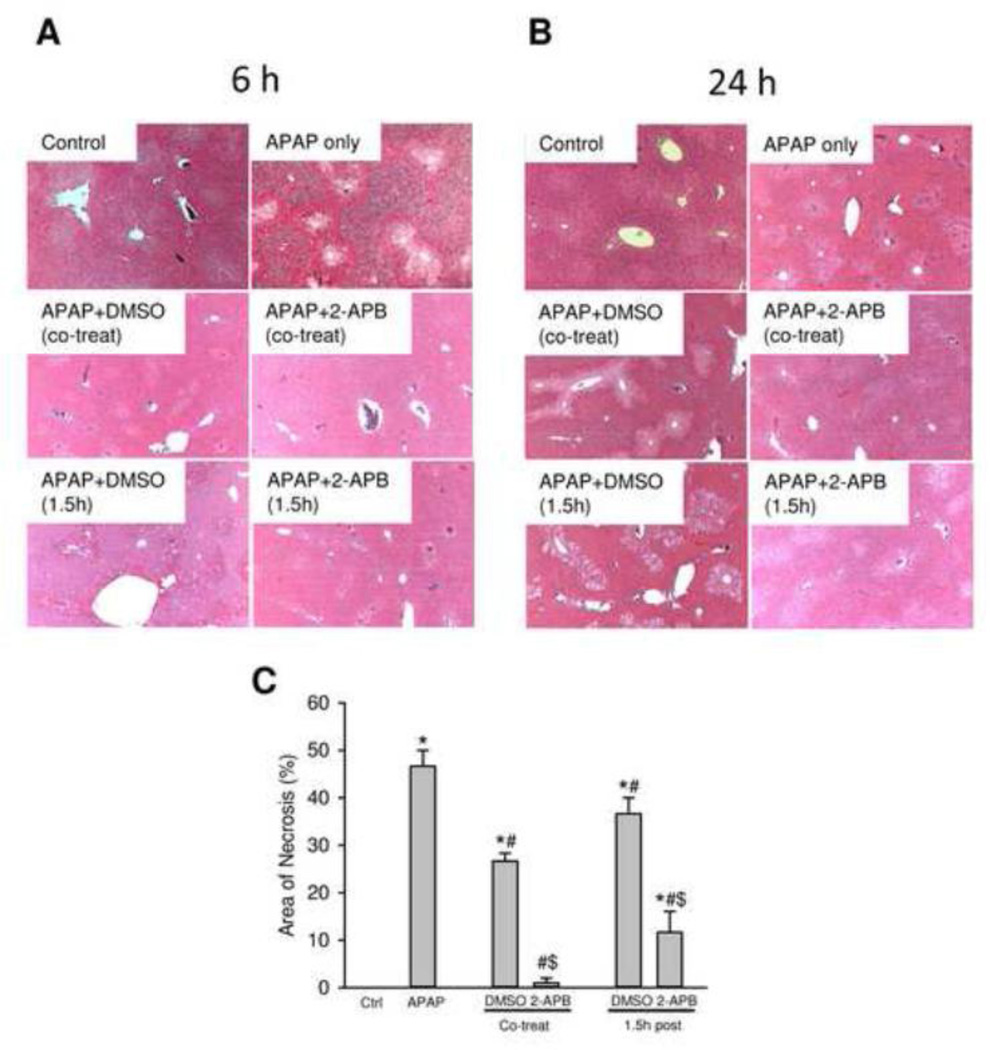
Treatment of fasted mice with 400 mg/kg APAP resulted in severe liver injury as indicated by the massive increase in plasma ALT activities at 6 h (Figure 1A) and 24 h (Figure 1B). The liver injury was further confirmed by the extensive centrilobular necrosis observed at both time points (Figure 2). Treatment with the gap junction inhibitor 2-APB (20 mg/kg) at the same time as APAP resulted in almost complete protection at 6 h and at 24 h (Figure 1A,B; 2). However, the solvent of 2-APB, diluted DMSO (0.2 ml/kg), reduced the ALT increase by 100% at 6 h and 85% at 24 h (Figure 1A,B; 2). Treatment with 2-APB or DMSO 1.5 h after APAP attenuated serum ALT activities by 70% at 6 h (Figure 1A) and by 90% or 55%, respectively, at 24 h (Figure 1B). These results were confirmed by assessing necrosis by histology (Figure 2). Blinded quantitation of APAP-induced necrosis by the pathologist indicated that co-treatment of APAP with DMSO alone or 2-APB reduced necrosis by 42% and 98%, respectively (Figure 2C). Even the delayed treatment with DMSO or 2-APB resulted in 21% and 75%, respectively, less cell death (Figure 2C). Later post-treatments with 2-APB (4.5 h or 6 h) after APAP showed no protection based on ALT activities (Figure 1B) or necrosis (data not shown). Together our data confirmed the highly effective protection of 2-APB against APAP hepatotoxicity when treated around the time of APAP administration, but not when the drug treatment was more delayed (Patel et al., 2012).
Figure 1.
Plasma ALT activities in mice treated with APAP, 2-APB, or both. Mice were treated with 400 mg/kg APAP or saline (blank control), and 2-APB was co-treated with APAP, or post-treated at 1.5 h, 4.5 h or 6 h after APAP administration with DMSO (0.2 ml/kg, vehicle control) as the solvent for 2-APB. Mice were sacrificed at 6 h (A) or 24 h (B) after APAP administration. Data are expressed as means ± SE, n=3–5 mice per group. *P<0.05 (compared to blank control). #P<0.05 (compared to APAP). $P<0.05 (compared to vehicle control).
Figure 2.
Representative H&E-stained liver sections (×50 magnification) of mice at 6 h (A) and 24 h (B) after APAP treatment with or without 2-APB. Mice were treated with 400 mg APAP/kg body weight or saline, and 2-APB (20 mg/kg) was co-treated with APAP or 1.5 h post-treated after APAP administration with DMSO (0.2 ml/kg) as the vehicle control. (C) Blinded quantitation of the area of necrosis for the 24 h samples. Data represent mean ± SE of n = 3–5 mice per group. *P<0.05 (compared to blank control). #P<0.05 (compared to APAP). $P<0.05 (compared to vehicle control).
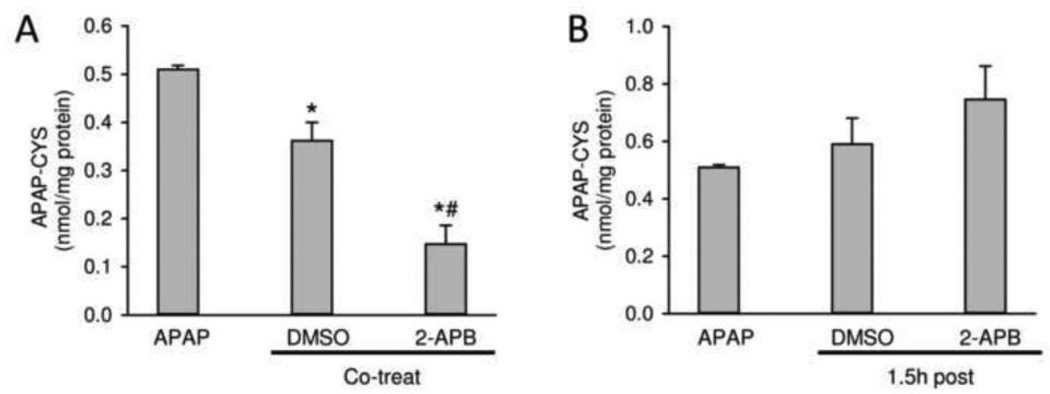
In order to evaluate the potential mechanism(s) of protection by 2-APB and its solvent, protein adducts were determined at 6 h after APAP (Figure 3A,B). Co-treatment of the solvent DMSO with APAP resulted in a 36% reduction of protein adducts compared to APAP alone. However, 2-APB co-treatment reduced protein adducts even further (−70%) (Figure 3A). Because the peak of adduct formation occurs around 1.5–2 h after 300 mg/kg APAP (McGill et al., 2013), it is not surprising that the delayed treatment (1.5 h) with 2-APB or solvent did not significantly reduce protein adduct formation (Figure 3B).
Figure 3.
Effects of 2-APB on APAP-protein adduct formation at 6 h after APAP administration. 2-APB (20 mg/kg) was co-treated or 1.5 h post-treated with APAP (400 mg/kg), and DMSO (0.2 ml/kg) was used as the vehicle control. APAP-protein adducts were quantified by HPLC-ECD using total liver homogenate. (A) APAP-protein adducts in co-treatment mice. (B) APAP-protein adducts in post-treatment mice. Data are expressed as means ± SE, n = 4–5 mice per group. *P<0.05 (compared to APAP). #P<0.05 (compared to vehicle control).
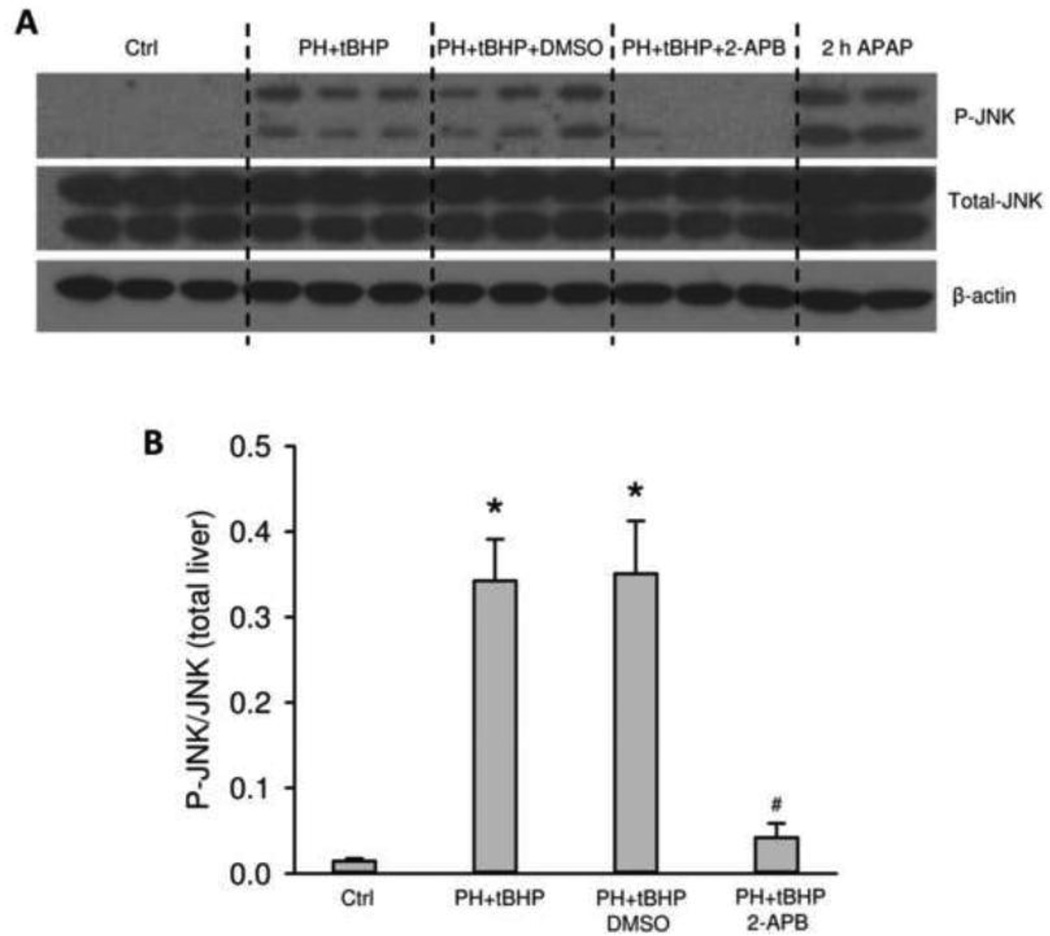
It is well-established that the early and sustained activation of the MAP kinase c-jun-N-terminal kinase (JNK) is a critical feature of the intracellular signaling mechanism of APAP toxicity (Gunawan et al., 2006; Henderson et al., 2007; Latchoumycandane et al., 2007). To test if 2-APB affects JNK activation after APAP overdose, total JNK and P-JNK were evaluated in liver homogenates by Western blotting (Figure 4A). The results clearly indicate that 2-APB treatment attenuates JNK activation while not significantly affecting total JNK expression levels (Figure 4A,B). There is evidence that the early APAP-protein adduct formation and mitochondrial oxidant stress may initiate JNK activation (Hanawa et al., 2008; Saito et al., 2010a), so the reduced protein adduct formation caused by 2-APB co-treatment may be at least partially responsible for the reduced JNK activation. However, in the 1.5 h post-treatment group, the APAP-protein adduct levels were the same (Figure 3B), but P-JNK was still reduced (Figure 4). Based on these data, we hypothesized that 2-APB may also serve as a direct inhibitor of JNK activation. To test this, animals were treated with the GSH-depleting agent phorone and oxidant stress was induced with tBHP in order to mimic the conditions of APAP without dependence on cytochrome P450-mediated metabolic activation (Saito et al., 2010a). Phorone/tBHP induced P-JNK formation similar to APAP (Figure 5A,B). Importantly, however, pretreatment with 2-APB, but not the solvent DMSO, almost completely eliminated the JNK activation induced by GSH depletion/oxidant stress (Figure 5A,B). Together, these data show that 2-APB and its solvent affect reactive metabolite formation after APAP treatment, and that 2-APB can act as a direct inhibitor of JNK signaling.
Figure 4.
Effects of 2-APB on JNK phosphorylation at 6 h after APAP administration. Mice were treated with 400 mg/kg APAP or saline, and 2-APB (20 mg/kg) was co-treated or 1.5 h post-treated with APAP using DMSO (0.2 ml/kg) as the vehicle control. (A) P-JNK, total JNK and β-actin were measured by Western blotting in total liver homogenate. (B) Densitometric analysis of P-JNK and total JNK. Data are expressed as means ± SE, n=3 mice per group. *P<0.05 (compared to vehicle control).
Figure 5.
JNK phosphorylation in mice treated with APAP, or phorone (PH)/tBHP after 2-APB administration. Mice were pre-treated with saline or 2-APB (20 mg/kg) with DMSO (0.2 ml/kg) as the vehicle control. One hour later, 100 mg/kg phorone was injected, and 1 mmol/kg tBHP was given one hour after phorone. These mice were sacrificed 1 h after tBHP injection. One group of mice were challenged with 400 mg/kg APAP for 2 h (A) Western blotting for P-JNK, total JNK and β-actin. (B) Densitometric analysis of P-JNK/total JNK ratio. Data are expressed as means ± SE, n=3 mice per group. *P<0.05 (compared to saline control). #P<0.05 (compared to DMSO control).
APAP is metabolically activated mainly by Cyp2e1 and to a lesser degree by Cyp1a2 in mice (Zaher et al., 1998). To confirm that 2-APB can directly inhibit these cytochrome P450 enzymes, the 7EFC deethylase assay was used (Buters et al., 1993). As indicated in Figure 6, the solvent (0.5 % DMSO) had only a minimal effect on Cyp activity. However, 2-APB dose-dependently inhibited cytochrome P450 enzymes, with >30% inhibition at 100 µM and >90% inhibition at 500 µM and above (Figure 6). These data clearly demonstrate that 2-APB is an inhibitor of cytochrome P450 enzymes.
Figure 6.
Effect of 2-APB on P450 activities. Cytochrome P450 activities affected by various concentrations of 2-APB (dissolved in 0.5% DMSO) were measured by the 7EFC deethylase assay using the 14,000 ×g supernatant of mouse liver homogenate. n=4 mice per group. *P<0.05 (compared to vehicle control).
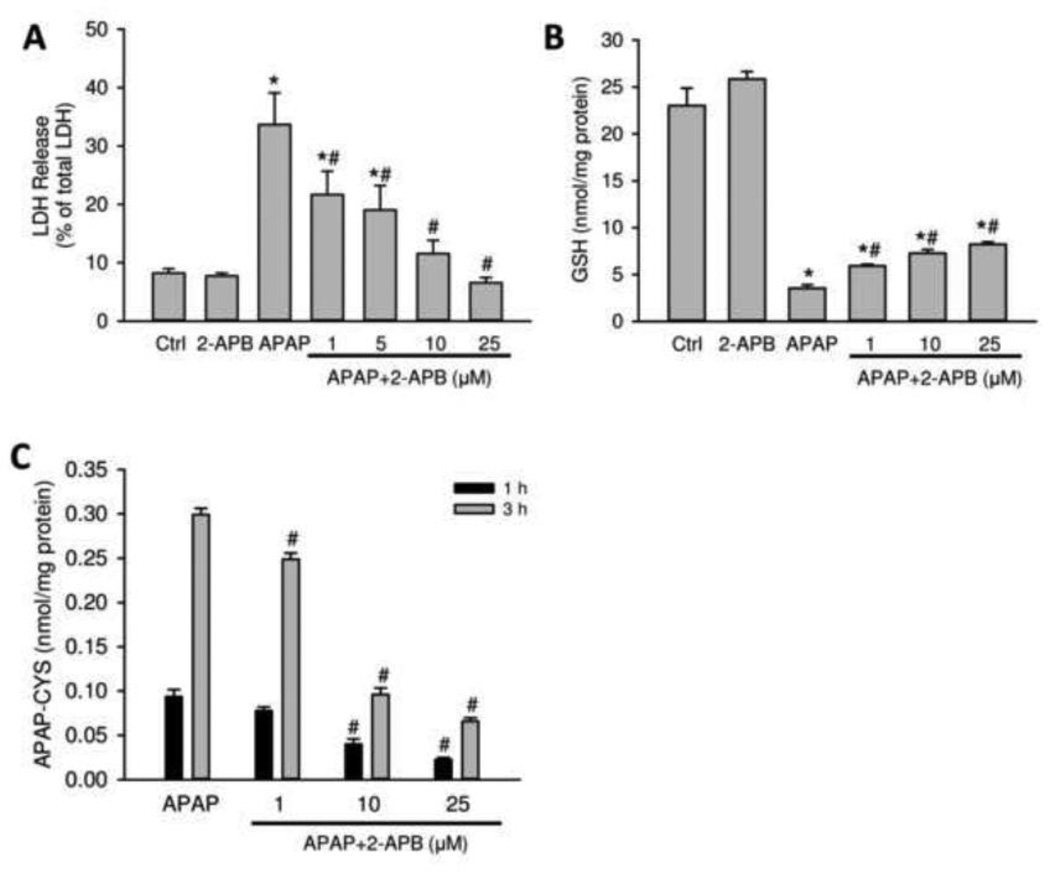
It has been demonstrated that isolation of hepatocytes from freshly removed rodent liver tissue results in the acute loss of gap junctions at the plasma membrane surface and that it requires at least 24 h in culture to restore their functionality (Vinken et al., 2006a,b). Thus, freshly isolated primary hepatocytes can be a model system to test the effects of the gap junction inhibitor in the absence of functional channels. Therefore, mouse hepatocytes were isolated, allowed to adhere for 3.5 h and then treated with 5 mM APAP and various concentrations of 2-APB (0–25 µM) directly dissolved in warm Williams E medium. Exposure to APAP alone resulted in significant cell death indicated by LDH release at 9 h (Figure 7A), GSH depletion at 1 h (Figure 7B) and time-dependent increase in protein adduct formation at 1 and 3 h (Figure 7C). 2-APB, without the use of DMSO and in the absence of functional gap junctions, dose-dependently attenuated APAP-induced cell death, which correlated with reduced GSH depletion and in particular with reduced protein adduct formation (Figure 7).
Figure 7.
Effects of 2-APB on APAP toxicity in primary mouse hepatocytes. Cells were treated with APAP (5 mM), or 2-APB (25 µM), or both at various concentration of 2-APB (1–25 µM). (A) LDH release at 9 h after treatment. (B) GSH depletion at 1 h after treatment (C) APAP protein adducts at 1 and 3 h after treatment. Data represent means ± SE of n=3 separate experiments. *P<0.05 (compared to control). #P<0.05 (compared to APAP).
DISCUSSION
The main objective of this study was to evaluate the hepatoprotective effect of the gap junction inhibitor 2-APB in a mouse model of APAP-induced liver injury. Similar to the report by Patel et al. (2012), our data showed that 2-APB is highly effective against APAP hepatotoxicity, especially when the inhibitor was administered around the time of APAP treatment. However, in contrast to the findings by Patel et al. (2012), we were unable to demonstrate a beneficial effect of 2-APB when the drug was injected 4.5 – 6 h after APAP. The reason for the lack of efficacy at these later time points can be explained by the mechanism of protection of this drug.
2-APB and the solvent used in vivo can inhibit the metabolic activation of APAP
It is well established that APAP toxicity depends on the metabolic activation of APAP, i.e. formation of a reactive metabolite which depletes GSH and binds to cellular proteins (Mitchell et al., 1973; Cohen et al., 1997; McGill and Jaeschke, 2013). Although the signaling mechanisms of APAP-induced cell death are highly complex (Jaeschke et al., 2012; Ramachandran et al., 2013; Hinson et al., 2010), it is still assumed that the cytochrome P450-dependent reactive metabolite formation and protein binding are critical early events in the toxicity. Thus, any interference with this early step can result in profound protection against the toxicity. Most importantly, if the potential effect of a drug intervention on the metabolic activation of APAP is not carefully investigated, this may lead to misinterpretation of the mechanisms of protection (Jaeschke et al., 2011). Our data clearly demonstrated in an in vitro assay of cytochrome P450 enzyme activity that 2-APB can inhibit these enzymes. In addition, the fact that 2-APB attenuated protein adduct formation after APAP in vivo and in vitro supports the conclusion that the co-treatment of 2-APB with APAP caused the inhibition of NAPQI formation and protein binding. The presence of DMSO in vivo, a known inhibitor of cytochrome P450 enzymes (Park et al., 1988) even at the low concentrations used here (Jaeschke et al. 2006), further supports this mechanism. Nevertheless, the fact that 2-APB is still highly effective in reducing protein binding and preventing cell death in the absence of DMSO in vitro suggests that much of the inhibition of metabolic activation was caused by the drug itself. The impact of the solvent DMSO was highest with co-treatment of the drug and APAP and diminished with more delayed treatment. A contribution of the solvent may also be the reason why the use of ethanol as solvent instead of DMSO did not change the results (Patel et al., 2012) because ethanol is also metabolized by Cyp2e1 and is thus a competitive inhibitor of APAP metabolism (Sato and Lieber, 1981). Taken together, our data suggest that a substantial part of the protection of 2-APB was caused by inhibition of cytochrome P450 enzymes and protein binding by the drug itself and by the solvent DMSO.
2-APB as direct inhibitor of JNK activation
Although it is quite obvious that the inhibition of metabolic activation and protein binding plays a role in the mechanism of protection by 2-APB, there is evidence that other mechanisms are also involved. For example, the protection at 24 h with the delayed treatment of 2-APB (Figure 1B) without an effect on protein adducts (Figure 3B) suggests additional mechanisms of protection. It is known that the early mitochondrial dysfunction and ROS formation in APAP hepatotoxicity leads to JNK activation, which amplifies the oxidant stress and causes the mitochondrial membrane permeability transition pore opening and necrotic cell death (Kon et al., 2004; Hanawa et al., 2008; Saito et al., 2010a). When the oxidant stress-mediated JNK activation was reproduced by GSH depletion and tBHP, 2-APB was able to inhibit this effect. This suggests that 2-APB is an inhibitor of JNK activation independent of its effect on metabolic activation. It has been suggested that JNK can be activated by oxidant stress through both the apoptosis signal-regulating kinase-1 (Nagakawa et al., 2008) and mixed-lineage kinase 3 (Sharma et al., 2012). Thus, the effect of 2-APB on JNK activation could have been caused by a direct inhibition of JNK or one of the upstream enzymes.
Lack of protection after delayed treatment with 2-ABP
Although we obtained similar protection with pretreatment of 2-ABP as previously reported (Patel et al., 2012), we were unable to reproduce protection when administering the drug 4.5–6 h after APAP. There were several minor differences between the experiments of Patel et al. (2012) and our study. The dose of APAP used by Patel et al. was 500 mg/kg and mice were sacrificed 16 h post-APAP. In our study we used a dose of 400 mg/kg APAP and sacrificed mice at 6 and 24 h post-APAP. Additionally, the 2-APB vehicle used by Patel et al. was 0.1ml/kg DMSO. In our hands, 2-APB was not completely soluble in 0.1 ml/kg DMSO, so the vehicle used in our study was 0.2 ml/kg DMSO. However, it is highly unlikely that these minor differences in the experimental design would lead to fundamentally different results. Nevertheless, when detailed time course studies with APAP are performed, the results are variable when one compares ALT values at 6 h versus 24 h (Jaeschke, 1990; Cover et al., 2006). Thus, it is possible that under certain conditions an intervention administered 4–6 h after APAP may still exert a limited protective effect. Because this time point is beyond the drug metabolism phase, it is feasible that 2-APB acted as JNK inhibitor. Indeed in some studies, JNK inhibitors were shown to be partially protective when administered 4–6 h after APAP (Latchoumycandane et al., 2007; Henderson et al., 2007). However, such an extensive delay in treatment is clearly not reproducibly protective under all circumstances.
2-APB and Cx32 KO mice
Although we have unequivocally demonstrated that 2-APB is a direct inhibitor of cytochrome P450 enzymes and of JNK activation and that these effects appear to be mainly responsible for the protection, we cannot exclude that Cx32-based gap junctions may also play a role in the pathophysiology of APAP-induced liver injury. This hypothesis is supported by the protection against APAP hepatotoxicity in Cx32-deficient mice (Patel et al., 2012) and rats ((Naiki-Ito et al., 2010). However, there are substantial differences between these studies. Whereas the rat study showed a highly variable protective effect with substantial overlap between the groups (Naiki-Ito et al., 2010), the mouse study demonstrated virtually 100% protection (Patel et al., 2012). Neither study included an evaluation of metabolic activation of APAP or of any other off-target effects. The latter phenomenon is becoming increasingly recognized as a problem for the use of gene KO mice. If the deletion (whole body or cell type-specific) of an important gene creates a substantial stress within the cell, a compensatory response leads to upregulation of defense mechanisms rendering the KO animal less susceptible to APAP. Recent examples include the conditional deletion of the autophagy-related protein Atg5 (Ni et al., 2012) or the antioxidant gene thioredoxin-1 (Patterson et al., 2013). In both cases, i.e. elimination of autophagy or removal of a vital antioxidant defense gene, the resulting cellular stress caused activation of Nrf2-dependent genes including glutamate-cysteine ligase leading to enhanced GSH synthesis with higher capacity to detoxify NAPQI and reactive oxygen (Ni et al., 2012; Patterson et al., 2013). In addition, the stress in the absence of autophagy caused apoptotic cell death and triggered continuous regenerative activity in the liver (Ni et al., 2012). These compensatory effects caused by deletion of important genes dramatically reduced the susceptibility to APAP. Thus, because Cx32-based gap junctions are important for intercellular communication between hepatocytes (Vinken et al., 2008), deletion of these proteins may result in stress responses that alter the susceptibility to APAP overdose. Therefore, the experiments with the Cx32 KO mice require further verification and validation before reliable conclusions can be drawn regarding the role of Cx32-based gap junctions in the pathophysiology of APAP-induced liver injury.
In summary, we conclusively demonstrated that 2-APB is an inhibitor of both cytochrome P450 enzymes and JNK activation. In addition, the solvent for 2-APB, DMSO, is an effective inhibitor of cytochrome P450 isoenzymes. These experiments clearly demonstrate that most, if not all, of the protection by 2-APB was caused by off-target effects rather than inhibition of Cx32-based gap junctions. Although we cannot exclude that Cx32-based gap junctions can play a role in the pathophysiology, more carefully designed experiments and consideration of off-target effects are required to decide whether or not Cx32-based gap junctions may play a relevant role in the mechanisms of APAP-induced liver injury.
Highlights.
2-APB protected against APAP-induced liver injury in mice in vivo and in primary hepatocytes
2-APB protected by the inhibition of APAP metabolic activation and JNK signaling pathway
DMSO inhibited APAP metabolic activation as the solvent of 2-APB
Off-target effects of connexin32 gene knock-out mice need to be considered
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
REFERENCES
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: Evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Buters JT, Schiller CD, Chou RC. A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethyl-coumarin. Biochem. Pharmacol. 1993;46:1577–1584. doi: 10.1016/0006-2952(93)90326-r. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J. Pharmacol. Exp. Ther. 1985;232:864–872. [PubMed] [Google Scholar]
- Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Fromenty B. Bridging the gap between old and new concepts in drug-induced liver injury. Clin. Res. Hepatol. Gastroenterol. 2013;37:6–9. doi: 10.1016/j.clinre.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Maurel M, Rosenbaum J. Closing the gap on drug-induced liver injury. Hepatology. 2012;56:781–783. doi: 10.1002/hep.25779. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Naiki-Ito A, Asamoto M, Naiki T, Ogawa K, Takahashi S, Sato S, Shirai T. Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol. Pathol. 2010;38:280–286. doi: 10.1177/0192623309357951. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology. 1988;52:165–175. doi: 10.1016/0300-483x(88)90202-8. [DOI] [PubMed] [Google Scholar]
- Patel SJ, Milwid JM, King KR, Bohr S, Iracheta-Velle A, Li M, Vitalo A, Parekkadan B, Jindal R, Yarmush ML. Gap junction inhibition prevents drug-induced liver toxicity and fulminant hepatic failure. Nat. Biotechnol. 2012;30:179–183. doi: 10.1038/nbt.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Carlson BA, Li F, Bonzo JA, Yoo MH, Krausz KW, Conrad M, Chen C, Gonzalez FJ, Hatfield DL. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem. Res. Toxicol. 2013 Jun 7; doi: 10.1021/tx4001013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;27:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. The receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013 Jun 6; doi: 10.1002/hep.26547. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Lieber CS. Mechanism of the preventive effect of ethanol on acetaminophen-induced hepatoxicity. J. Pharmacol. Exp. Ther. 1981;218:811–815. [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol. Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Harris AL. 2-aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol. Pharmacol. 2007;71:570–579. doi: 10.1124/mol.106.027508. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47:1077–1088. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, Vanhaecke T, Papeleu P, Geerts A, Van Rossen E, Chipman JK, Meda P, Rogiers V. Trichostatin a enhances gap junctional intercellular communication in primary cultures of adult rat hepatocytes. Toxicol. Sci. 2006a;91:484–492. doi: 10.1093/toxsci/kfj152. [DOI] [PubMed] [Google Scholar]
- Vinken M, Papeleu P, Snykers S, De Rop E, Henkens T, Chipman JK, Rogiers V, Vanhaecke T. Involvement of cell junctions in hepatocyte culture functionality. Crit. Rev. Toxicol. 2006b;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen -induced liver injury by inhibiting P450 isoenzymes not inflammasome activation. Toxicol. Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol. Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol. Appl. Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]