Abstract
NF-κB activation within the epithelium has been implicated in the pathogenesis of asthma, yet the exact role of epithelial NF-κB in allergen-induced inflammation and airway remodeling remains unclear. In the present study, we utilized an intranasal House Dust Mite (HDM) extract exposure regimen time course in BALB/c mice to evaluate inflammation, NF-κB activation, airway hyperresponsiveness (AHR), and airway remodeling. We utilized CC10-IκBαSR transgenic mice to evaluate the functional importance of epithelial NF-κB in response to HDM. After a single exposure of HDM, mRNA expression of pro-inflammatory mediators was significantly elevated in lung tissue of WT mice, in association with increases in nuclear RelA and RelB, components of the classical and alternative NF-κB pathway, respectively, in the bronchiolar epithelium. In contrast, CC10-IκBαSR mice displayed marked decreases in nuclear RelA and RelB and mRNA expression of pro-inflammatory mediators compared to WT mice. After 15 challenges with HDM, WT mice exhibited increases in inflammation, airway hyperresponsiveness, mucus metaplasia and peri-bronchiolar fibrosis. CC10-IκBαSR transgenic mice displayed marked decreases in neutrophilic infiltration, tissue damping, and elastance parameters, in association will less peri-bronchiolar fibrosis and decreases in nuclear RelB in lung tissue. However, central airway resistance and mucus metaplasia remained elevated in CC10-IκBαSR transgenic mice, in association with continued presence of lymphocytes, and partial decreases in eosinophils and IL-13. The current study demonstrates that following airway exposure with an asthma-relevant allergen, activation of classical and alternative NF-κB pathways occur within the airway epithelium and may coordinately contribute to allergic inflammation, AHR and fibrotic airway remodeling.
INTRODUCTION
The NF-κB pathway is a critical regulator of both innate and adaptive immune responses in a wide variety of cell types. Upon stimulation, the I kappa B kinase (IKK) signalsome, consisting of IKKβ, IKKα, and IKKγ, is activated, leading to IKKβ-mediated phosphorylation of IκBα. Phosphorylation of IκBα in turn leads to its subsequent ubiquitination and degradation by the 26S proteasome, thus allowing for transcription factor, RelA, to translocate to the nucleus. This event results in RelA-dependent transcription of genes important in cell survival, proliferation, and inflammation (1, 2). A wide variety of agonists can activate the classical NF-κB pathway in lung epithelial cells and the resultant release of pro-inflammatory mediators crucial in the recruitment and activation of dendritic cells, lymphocytes, neutrophils, and many other cells in the lung (3). Additionally, an alternative NF-κB pathway exists, which requires activation of NF-κB inducing kinase (NIK) and subsequent phosphorylation of IKKα. IKKα in turn phosphorylates p100, leading to its partial processing to p52. This allows subsequent nuclear translocation of RelB/p52 and transcriptional activation of a subset of NF-κB dependent genes (4, 5). It was originally thought that the alternative NF-κB pathway played a predominant role in lymphocyte activation and lymphoid organ development (6). However, recent work from our laboratory demonstrated that both classical and alternative NF-κB pathways are activated in lung epithelial cells in response to diverse pro-inflammatory stimuli and that both pathways coordinately regulate pro- inflammatory responses (7).
Activation of the classical NF-κB pathway within the airway epithelium has been demonstrated to play a critical role in acute inflammation and allergic airways disease. CC10-IκBαSR transgenic mice, which are refractory to IκBα degradation and NF-κB activation in the lung epithelium, were demonstrated to be strongly protected from airway inflammation induced by lipopolysaccharide (8). Following intraperitoneal sensitization and challenge with ovalbumin (Ova), CC10-IκBαSR transgenic mice showed a marked diminution of airway inflammation compared to WT littermate controls, although Ova-induced airways hyperresponsiveness (AHR) was unaffected in CC10-IκBαSR transgenic mice compared to controls (9). A similar protection against Ova-induced allergic inflammation and peri-bronchiolar fibrosis has been observed in mice following epithelial-specific ablation of IKKβ (10).
It remains unclear to date whether activation of NF-κB within epithelial cells plays a role in the orchestration of inflammatory responses in vivo to an asthma-relevant allergen following sensitization via the airways. It also remains unknown whether both NF-κB pathways are activated following exposure to an antigen. House dust mite (HDM) is a multifaceted allergen to which up to 85% of asthmatics are allergic (11). HDM has been shown to signal through the classical NF-κB pathway in human bronchial epithelial cells in vitro (12). Therefore, the goal of the present study was to determine the activation of classical and alternative NF-κB in epithelial cells in vivo in response to HDM, and to address its effect on HDM-triggered airway inflammation, remodeling, mucus, and AHR. Our results demonstrate the functional importance of epithelial NF-κB in HDM-induced acute inflammatory responses, AHR, and airway remodeling. We also demonstrate activation of both classical and alternative NF-κB pathways in response to HDM. These findings illustrate the complexity of activation of the NF-κB pathways in settings of allergic airways disease and suggest a broader role for epithelial NF-κB in lung disease pathogenesis.
MATERIALS AND METHODS
Animal Studies
CC10-IκBαSR mice were generated as previously described (8) and backcrossed onto the BALB/cJ (N=10) (The Jackson Laboratories, Bar Harbor, ME) background. Transgene-negative littermates were used as a control. All experiments were approved by the University of Vermont Institutional Animal Care and Use Committee.
Cell Culture
A human bronchial epithelial cell line (HBE) was kindly provided by Dr. Albert van der Vliet and cultured as described previously (13, 14) and primary human nasal epithelial cells were cultured as described previously (15). Human cell lines were exposed to either PBS or 25μg HDM (Greer, Lenoir, NC). All protocols that utilize primary human nasal epithelial cells were approved by the Institutional Review Board.
HDM exposure
BALB/cJ mice (Jackson Laboratories, Bar Harbor, ME) were subjected to daily intranasal instillation with 50μg HDM (35 endotoxin units/mg) extract resuspended in PBS, or PBS alone as a vehicle control. Briefly, mice were instilled with either 1 dose of HDM and euthanized: 2 h, 6 h, or 24 h later or instilled with 3 doses of HDM on 3 consecutive days and euthanized 24 h thereafter. In addition, mice were exposed to HDM 5 days/week for 1, 2 or 3 weeks, (5, 10 or 15 instillations, respectively), and harvested 72 h after the final exposure (Fig. 1A).
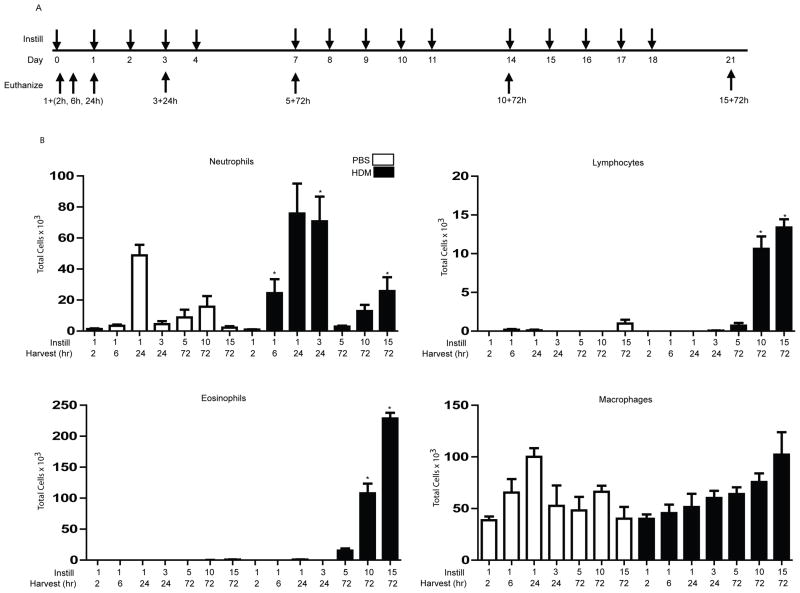
Figure 1. Analysis of cell totals and differentials in BAL of mice exposed to HDM.
(A) Schematic depicting the time course of HDM exposure and euthanization. Instill: 50μg HDM, or PBS as the vehicle control were administered intranasally once at the days indicated via downward arrows. Mice were euthanized 2 h, 6 h or 24 h post a single challenge, 24 h after 3 challenges or 72 h after 5, 10, or 15 challenges, indicated by the upward arrows. (B) Mice (n=5/group/time point) were exposed to PBS or HDM according to the schematic in A, and BAL was assessed for neutrophils, eosinophils, lymphocytes, and macrophages. * p< 0.05 (ANOVA) compared to the PBS group at the same time point.
Assessment of AHR
Mice subjected to 5, 10, or 15 administrations of HDM were anesthetized with an intraperitoneal injection of pentobarbital sodium (90mg/kg), tracheotomised, and mechanically ventilated at 200 breaths/minute and assessed in response to increasing doses of methacholine (0, 3.125mg, 12.5mg, and 25mg). Respiratory mechanics were assessed with a forced oscillation technique on a computer controlled small animal ventilator (Flexi-vent™, SCIREQ, QC, Canada), as previously described (16, 17), and the parameters Newtonian resistance (Rn), tissue damping (G), and elastance (H) were calculated.
Serum IgG1 and IgE
Following euthanization, blood was collected by heart puncture and immediately spun through a microtainer and serum was separated. Analysis of serum IgG1 and IgE was performed via enzyme-linked immunosorbent assay (ELISA) methods, using 1μg/ml HDM to coat the 96 well plates.
Bronchoalveolar lavage
Following euthanization, bronchoalveolar lavage (BAL) was collected using 1ml of PBS. Cell counts were determined (Advia 120 automated hematology analyser), and differential cells were analysed by the Hema3 kit (Fisher Scientific, Kalamazoo, MI) by counting a minimum of 300 cells per mouse as previously described (17).
mRNA/protein analysis
Right lung lobes were flash frozen and pulverized for protein and mRNA. Total mRNA was isolated using the RNeasy kit (Qiagen, Valencia, CA). 1 μg of mRNA was used to generate cDNA, followed by quantitative polymerase chain reaction (qPCR) using SYBR green (BioRad, Hercules, CA) via in order to assess expression of: Muc5ac, KC, MIP-2, GM-CSF, IL-6, CCL20, and IL-33. Primers were: KC (Fwd:5′-GCTGGATTCACCTCAAGAA-3′, Rv:5′-TGGGGACACCTTTTAGCATC-3′), IL-6 (Fwd:5′-CTGATGCTGGTGACAACCAC-3′, Rv:5′-CAGAATTGCCATTGCACAAC-3′), CCL20 (Fwd:5′-AAGACAGATGGCCGATGAAG-3′, Rv:5′-AGCCCTTTTCACCCAGTTCT-3′), Muc5ac (Fwd:5′-CAGTGAATTCTGGAGGCCAACAAGGTAGAG-3′, Rv:5′-AGCTAAGCTTAGATCTGGTTGGGACAGCAGC-3′), MIP-2 (Fwd:5′-AGTGAACTGCGCTGTCAATG-3′, Rv:5′-TTCAGGGTCAAGGCAAACTT-3′), GM-CSF (Fwd:5′-GGCCTTGGAAGCATGTAGAGG-3′, Rv:5′-GGAGAACTCGTTAGAGACGACTT-3′), and IL-33 (Fwd:5′-GCTGCGTCTGTTGACACATT-3′, Rv:5′-GACTTGCAGGACAGGGAGAC-3′). The expression of all genes was normalized to the housekeeping gene, cyclophilin, (Fwd:5′-TTCCTCCTTTCACAGAATTATTCCA-3′, Rv:5′-CCGCCAGTGCCATTATGG-3′) and calculated via the ddCt method. Antibodies for Western blot analysis were: RelB and NIK (Santa Cruz Biotchnologies, Santa Cruz, CA), Histone H3, p52, and phospho RelA536 (Cell Signaling Technology, Danvers, MA) and β-Actin (Sigma-Aldrich, St. Louis, MO). Enzyme-linked Immunosorbent assays (ELISA) were performed according to manufacturer’s instructions (R & D Systems, Minneapolis, MN) on either BAL or homogenized lung extracts. Protein was equalized before analysis.
Histopathology/immunofluorescence/αSMA immunohistochemistry
Following euthanization, the left lobe was inflated with 4% paraformaldehyde and mounted in paraffin embedded 5μm sections. Hematoxylin and Eosin (H&E) and Periodic Acid Schiff (PAS) imaging were all performed with 3 small bronchioles (x20 magnification) per animal. Mucus metaplasia was assessed with a blinded scoring system by two independent investigators with the following scale: 0, no reactivity; 1, minimal staining; 2, moderate staining; and 3, prominent staining. Scores were averaged according to treatment group. Immunofluorescence was performed as previously described to detect nuclear RelA and RelB in situ (18). RelA and RelB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Staining for α-SMA was performed on lung sections following incubation of slides for 20 minutes in 0.01M sodium citrate, pH 6.0 at 95°C. Slides were then blocked with 2% normal goat serum for 30 minutes, incubated with monoclonal mouse antibody against α-SMA (1:5000 dilution; Sigma Aldrich, St. Louis, MO) overnight at 4°C, and then incubated in biotinylated anti-mouse IgG for 30 minutes at room temperature. Subsequently, the slides were incubated in avidin-biotin- complex-alkaline phosphatase (Vectastain ABC-AP, Vector Laboratories, Burlingame, CA) for another 30 minutes at room temperature. After rinsing the sections in PBS, the substrate, Vector Red (Vector Laboratories), was added for 20 minutes, which reacts with the bound alkaline phosphatase, thus producing an intense red color. The slides were then counterstained in hematoxylin and imaged (x20 magnification) for analysis.
Assessment of collagen content
Collagen was assessed from the upper right lobe of the lung after an overnight digestion with 10mg pepsin in 0.5M acetic acid. Quantification was performed by the Sircol Assay according to manufacturer’s instructions (Accurate Chemical and Scientific corp, Westbury, NY). Masson’s Trichrome reactivity was evaluated in 3 bronchioles (x20 magnification) per animal, and analyzed by two blinded investigators with the following scale: 0, no reactivity; 1, minimal staining; 2, moderate staining; and 3, prominent staining.
Statistical Analysis
All data were evaluated using Graphpad Prism 6 Software (Graphpad, Inc, San Diego, CA). A one-way ANOVA was used with Bonferoni corrections to adjust for multiple comparisons and all p-values <0.05 were considered statistically significant. Histopathological/αSMA IHC scores were analyzed using the Kruskal-Wallis test and Dunn’s multiple comparison post hoc tests.
RESULTS
Inflammatory response in BALB/c mice exposed to HDM
Because of the well-known role of NF-κB in the orchestration of inflammation, we first sought to assess the extent and kinetics of the HDM-induced inflammatory response through a time-course analysis illustrated schematically in Fig. 1A. Neutrophils in bronchoalveolar lavage fluid (BAL) were significantly increased 6 h following a single intranasal challenge with HDM, and remained elevated until 24 h post 3 challenges, as compared to PBS controls (Fig. 1B). It is important to note that neutrophils were elevated 24 h following exposure to the vehicle PBS, potentially indicative of a non-specific response to the intranasal instillation. Neutrophils were also detectable in BAL 72 h post 5, 10, or 15 challenges, with statistically significant increases occurring in response to HDM 72 h post 15 challenges. Although macrophages in BAL tended to increase in response to HDM, these trends were not statistically significant. No eosinophils or lymphocytes were detected in BAL up to 24 h post 3 challenges. In contrast, robust increases in eosinophils and lymphocytes occurred in BAL 72 h post 10 and 15 challenges with HDM compared to PBS controls (Fig. 1B). Although some fluctuations in the total number of macrophages in BAL were observed throughout these time points, these were not statistically significant (Fig. 1B).
Increases in nuclear localization of RelA and RelB in airway epithelium in response to HDM
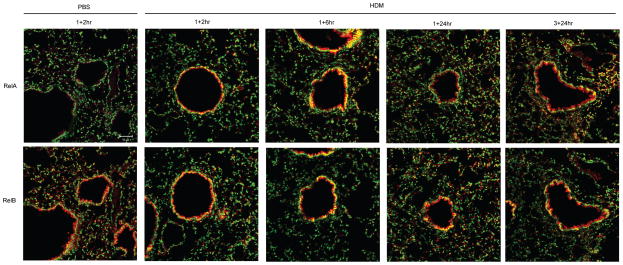
We next evaluated a potential role for epithelial NF-κB in response to HDM. We investigated kinetics of activation of both classical and alternative pathways in the bronchiolar epithelium via the assessment of nuclear presence of RelA and RelB. Nuclear presence of RelA and RelB (indicated by yellow staining) within the bronchiolar epithelium was increased 2 and 6 h following a single challenge with HDM, compared to PBS controls in the nuclei (Fig. 2). Nuclear RelA decreased to control reactivity by 24 h, but increased again 24 h post-3 challenges with HDM, indicative of a bi-modal activation pattern (Fig. 2). In contrast, increases in nuclear RelB were apparent throughout the time course evaluated here (Fig. 2). No clear evidence for nuclear localization of RelA or RelB within the airway epithelium was apparent 72 h after 5, 10 or 15 challenges with HDM compared to PBS (data not shown).
Figure 2. Assessment of nuclear localization of RelA and RelB in airway epithelium in response to HDM.
BALB/c mice were exposed to PBS and HDM and harvested as indicated. De-paraffinized lung sections were incubated with antibodies directed against RelA or RelB, followed by fluorophore-conjugated secondary antibodies, and the nuclei were counterstained with Sytox Green. Images were captured by laser scanning confocal microscopy using identical instrument settings in all groups. Images are representative results of two independent experiments, n=5/group/time. RelA/RelB: red, DNA: green, Nuclear RelA/RelB: yellow. scale bar=50μm.
Pro-inflammatory mediator expression following acute HDM exposures
In order to investigate the early inflammatory response after HDM exposure, we evaluated mRNA expression of Interleukin-33 (IL-33), Keratinocyte Derived Chemokine (KC), Granulocyte Macrophage Colony Stimulating-Factor (GM-CSF), Macrophage Inflammatory Protein-2 (MIP-2), Chemokine (C-C motif) ligand 20 (CCL20), Interleukin-6 (IL-6), Interleukin-25 (IL-25) and Thymic Stromal Lymphopoietin (TSLP), pro-inflammatory mediators shown to be produced by epithelial cells. Expression levels of all genes analyzed were significantly increased following 2 h exposure HDM, with the exception of IL-25 and TSLP, whose expression did not change (data not shown). mRNA expression of CCL20, GM-CSF, MIP-2, KC, and IL-6 remained elevated 6 h after a single administration of HDM, and tended to decrease towards control levels thereafter (Fig S1).
Role of epithelial NF-κB in pro-inflammatory cytokine expression induced by HDM
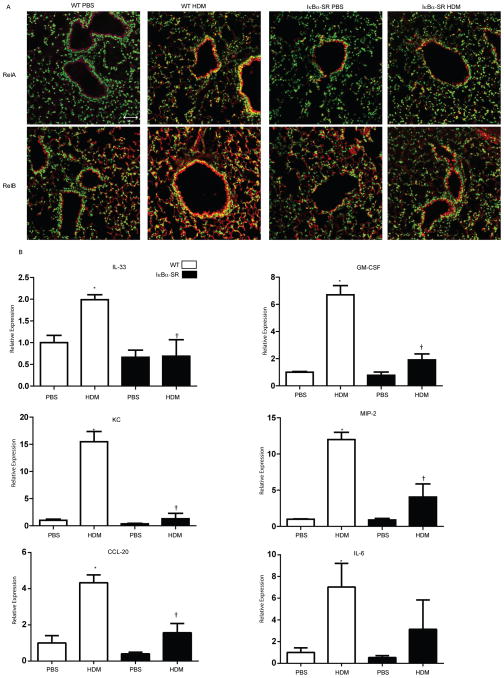
We next sought to address the role of epithelial NF-κB in HDM-induced pro-inflammatory responses utilizing CC10-IκBαSR transgenic mice. While nuclear RelA and RelB content increased in the bronchiolar epithelium in WT mice in response to HDM, these increases were not observed in CC10-IκBαSR mice exposed to HDM (Fig 3A). HDM- mediated increases in mRNA expression of CCL20, GM-CSF, MIP-2, KC, IL-33, and IL-6 in WT mice were strongly attenuated in CC10-IκBαSR mice (Fig. 3B), demonstrating the importance of NF-κB activation in the bronchiolar epithelium in the orchestration of the acute pro-inflammatory responses to HDM.
Figure 3. Nuclear RelA and RelB, and pro-inflammatory cytokine mRNA expression in CC10-NF-κBSR mice following exposure to HDM.
CC10-NF-κBSR mice, or WT littermate controls were exposed to PBS or HDM for 2 h. (A) Nuclear localization of RelA and RelB was assessed in lung tissues as described in Fig. 2. Images are representative results of two independent experiments with 5 mice/group, RelA/RelB, red; DNA, green; Nuclear RelA/RelB, yellow, scale bar=50μm. (B) Assessment of mRNA expression for IL-33, GM-CSF, CCL20, KC, MIP-2, and IL-6 in homogenized lung tissue. Data were normalized to cyclophilin and are presented as relative expression. Values reflect 6 mice/group/time point. * p< 0.05 (ANOVA) compared to the PBS group at the same time point. † p<0.05 (ANOVA) compared to WT mice exposed to HDM
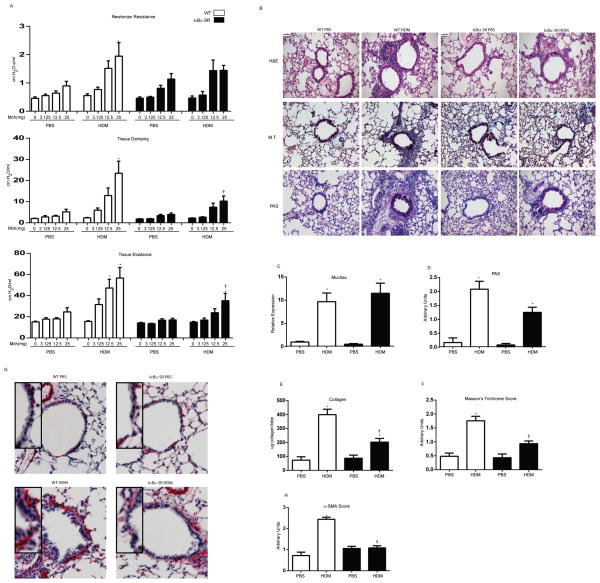
Effects of repeated HDM exposure on AHR, mucus metaplasia, and remodeling
We next subjected mice to 5, 10, and 15 challenges of HDM. Serum content of HDM-specific IgG1 and IgE showed no apparent increases 72 h following 5 days challenge. Marked increases in HDM-specific IgG1 and IgE were apparent following 10 and 15 challenges with HDM, compared to PBS controls, indicative of activation of adaptive immune responses (Fig. S2A). AHR, a feature of allergic airways disease, was assessed via forced oscillation mechanics using ascending doses of methacholine. Newtonian resistance (Rn) increased significantly over PBS controls after 10 and 15 challenges with HDM, while no changes were apparent in mice subjected to 5 challenges with HDM (Fig S2B). Tissue damping (G), which is indicative of tissue resistance and small airway dysfunction, was increased following 5 challenges HDM, and remained increased throughout 10 and 15 challenges. Elastance (H) was also increased following 5, 10, and 15 challenges of HDM. Increases in elastance were most prominent after 10 challenges of HDM and tended to decrease after 15 challenges (Fig. S2B). Histo-pathological evaluation revealed a robust inflammatory response to HDM, with prominent peri-bronchiolar and perivascular cellular infiltrates being apparent following 10 and 15 challenges with HDM (Fig. S2C). Additionally, mucus metaplasia was apparent following 5, 10, and 15 challenges HDM, based upon staining with Periodic Acid Shiff reagent and Muc5ac expression (Fig. S2C and S2D). Another hallmark of severe allergic airways disease is peri-bronchiolar fibrotic remodeling. Following 15 challenges with HDM, overall collagen content increased in the lung tissue (Fig S2E), consistent with increases in peri-bronchiolar collagen deposition evaluated via Masson’s Trichrome staining (Fig S2C). These results collectively demonstrate that the HDM exposure regimen used herein induces a number of the hallmark features of allergic airway disease.
Role of epithelial NF-κB in HDM-induced inflammation
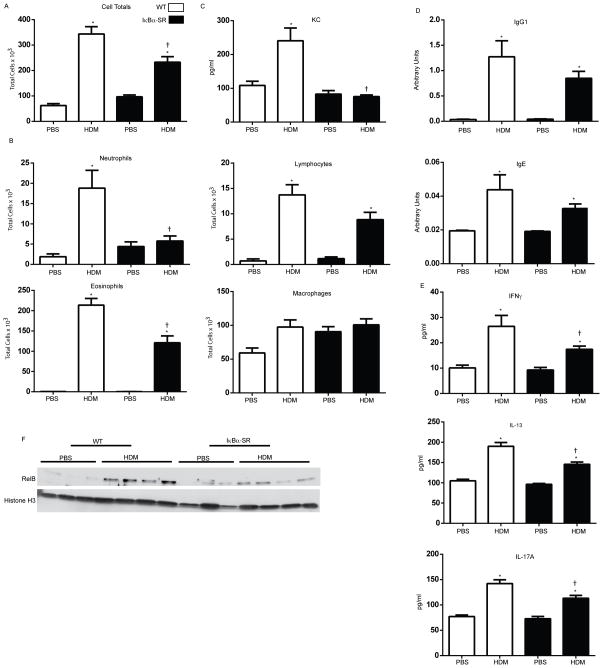
We next determined the role of epithelial NF-κB on the inflammatory response induced following 15 challenges of HDM. HDM-mediated increases in total cell counts in BAL observed in WT mice were significantly decreased in CC10-IκBαSR mice (Fig. 4A). BAL eosinophils were significantly decreased in CC10-IκBαSR transgenic mice exposed to HDM, while BAL lymphocytes remained elevated in HDM exposed CC10-IκBαSR transgenic mice compared to WT littermates (Fig. 4B). Total macrophage number in BAL remained unchanged in CC10-IκBαSR mice (Fig. 4B). In contrast HDM-mediated increases in BAL neutrophils in WT mice were completely attenuated in CC10-IκBαSR mice. Consistent with these observations, increases in BAL content of the neutrophil chemoattractant, KC, in WT mice exposed to HDM were absent in CC10-IκBαSR mice (Fig. 4C). HDM-specific IgG1 and IgE levels in serum were decreased slightly in CC10-IκBαSR HDM-exposed mice, although not significantly, compared to littermate controls, but remained elevated over PBS controls (Fig. 4D). Levels of IFN-γ, IL-13, and IL-17A in homogenized lung tissue were significantly decreased in CC10-NF-κBSR mice exposed to HDM, compared to WT animals (Fig. 4E), collectively suggesting an attenuation of Th-mediated responses to HDM following epithelial-specific inhibition of NF-κB. We next sought to assess inhibition of nuclear RelA and RelB in lung tissue in CC10-NF-κBSR mice exposed to HDM. After 15 challenges with HDM, no consistent increases in nuclear RelA or RelB were detected in the bronchiolar epithelium (data not shown), in contrast to earlier time points at which increases in nuclear RelA and RelB were readily observed (Fig 3). Assessment of homogenized lung tissue also showed variable and inconsistent patterns of nuclear RelA (data not shown). However, clear increases in nuclear RelB content were observed in WT mice, which were absent in the CC10-IκBαSR transgenics (Fig. 4F).
Figure 4. Assessment of HDM-induced inflamation, immunoglobins, T-cell cytokines, and nuclear RelB in WT or CC10-NF-κBSR mice following 15 challenges with HDM.
Assessment of (A) total cells and differential cell counts (B) and KC levels(C) in BAL. (D) Total IgG1 and IgE in serum. (E) IFNγ, IL-13, and IL-17A in homogenized tissue by ELISA. (F) Nuclear content of RelB in homogenized lung tissue. Histone H3 is shown as a loading control. Data represents 8 mice/group/time point. * p< 0.05 (ANOVA) compared to the PBS group at the same time point. † p<0.05 (ANOVA) compared to WT mice exposed to HDM.
Role of epithelial NF-κB in HDM-induced AHR and remodeling
WT and CC10-IκBαSR mice subjected to 15 challenges with HDM demonstrated equivalent increases in Newtonian resistance (Rn). In contrast, HDM-mediated increases in tissue damping (G) and elastance (H) were significantly decreased in CC10-IκBαSR mice compared to littermate controls (Fig. 5A). Consistent with attenuated inflammatory cell profiles in BAL, peri-bronchiolar infiltrates were attenuated CC10-IκBαSR mice compared to controls (Fig. 5B). In contrast, mucus metaplasia was not observed to be decreased in HDM-exposed CC10-IκBαSR mice, compared to WT animals (Fig. 5B+D). These findings are consistent with a lack of attenuation of HDM-induced Muc5AC mRNA (Fig. 5C) and modest decreases in IL-13 content (Fig. 4E). Biochemical analysis of collagen demonstrated that HDM-mediated increases in WT mice were significantly decreased in CC10-IκBαSR transgenic mice (Fig.5E), consistent with less peri-bronchiolar collagen detected via histopathology (Fig. 5B+F). Furthermore, α-Smooth Muscle Actin (α-SMA), a known marker of airway thickening and remodeling, was assessed via immunohistochemistry. Peri-bronchiolar α-SMA staining was significantly increased following HDM administration in WT mice, as compared to PBS controls. In contrast, no increases in peri-bronchiolar α-SMA reactivity were detected in CC10-IκBαSR mice exposed to HDM, in comparison to PBS controls (Fig. 5G+H).
Figure 5. Assessment of HDM-induced alterations in respiratory mechanics and airway remodeling in WT or CC10-NF-κBSR mice following 15 challenges with HDM.
(A) Evaluation of airway hyperresponsiveness via forced oscillation mechanics in response to 3.125, 12.5, and 25 mg methacholine. Shown are the parameters Newtonian resistance (Rn), Tissue damping (G) and Elastance (H) assessed via forced oscillation (B) Histopathological evaluation of tissue inflammation (H&E), mucus metaplasia (PAS) and peri-bronchiolar collagen deposition (M.T.). scale bar=50μm. (C) Analysis of Muc5ac mRNA expression in homogenized lung tissue. Data were normalized to cyclophilin and are presented as relative expression. (D) Quantification of mucus metaplasia. Bronchioles of similar size (n=3) were analyzed/mouse by two blinded scorers, and average scores presented as average units. (E) Assessment of collagen from the upper right lobe. (F) Quantification of peri-bronchiolar collagen deposition. Bronchioles of similar size (n=3) were analyzed/mouse by two blinded scorers, and average scores presented as average units. (G) Assessment of α-SMA immunohistochemistry, red=α-SMA, scale bar=1μm. (F) Quantification of α-SMA immunoreactivity. Bronchioles of similar size (n=3/mouse) were analyzed by two independent blinded scorers, and average scores presented as average units. Data represent 8 mice/group/time point. * p< 0.05 (ANOVA) compared to the PBS group at the same time point. † p<0.05 (ANOVA, Kruskal Wallis) compared to WT mice exposed to HDM.
HDM-induced activation of the classical and alternative NF-κB pathways in human nasal and bronchial epithelial cells
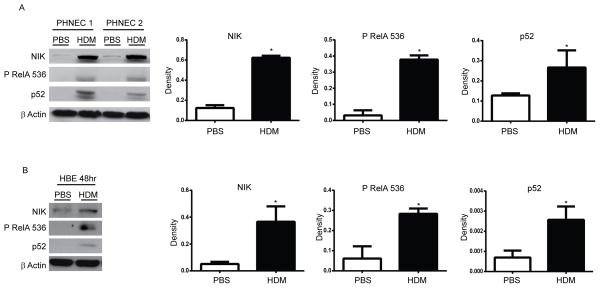
Since we demonstrated increases in nuclear RelB in the bronchial epithelium in response to HDM administration, we sought to determine whether the alternative NF-κB pathway could be activated directly by HDM in human epithelial cells. Results in Fig. 6A demonstrate increased levels of NIK and p52 in response to HDM in Primary Human Nasal Epithelial Cells (PHNEC) obtained from two independent donors, and similar increases were observed (Fig. 6B) in human bronchial epithelial cells (HBEC), albeit less robust, demonstrating activation of the alternative NF-κB pathway. In addition, phosphorylation of RelA at serine 536, a separate parameter of NF-κB activation, was also increased in response to HDM (Fig. 6A+B), demonstrating that in addition to the known ability of HDM to activate the classical NF-κB pathway, it also induces activation of the alternative NF-κB pathway in lung epithelial cells.
Figure 6. Assessment of activation of classical and alternative NF-κB pathways in human lung epithelial cells exposed to HDM.
(A) Primary human nasal epithelial (PHNE) cells or (B) Human bronchial epithelial (HBE) cells were exposed to PBS or 25 μg HDM once a day for consecutive days and harvested thereafter at either 72 h (PHNE) or 48 h (HBE). Cells were lysed for evaluation of NIK, p52, phospho RelA 536, by Western blot analyses. β-Actin (loading control). Right panels: Densitometric evaluation of Western blots shown in (A) PHNE (n=3 patients) or (B) HBE (n=3 experimental repeats). Results are expressed as arbitrary density and were normalized to corresponding β-Actin bands.
DISCUSSION
NF-κB is a regulator of inflammation and immunity, and its role in the pathogenesis in asthma has been suggested based upon evidence of its activation in the bronchiolar epithelium from asthmatics (19) and from studies in mouse models of allergic airways disease (18). Notably, studies previously performed in our laboratory have revealed a crucial role for lung epithelial NF-κB in the Alum/Ova model of allergic airways disease (9). Moreover, transgenic expression of constitutively active IKKβ in lung epithelial cells was sufficient to cause neutrophilic inflammation and airways hyperresponsiveness, and enhanced sensitization to an inhaled antigen (17, 20). Despite these prior observations, the more generalized importance of epithelial NF-κB in allergic airways disease following sensitization/exposure to a relevant allergen via the airways has yet to be determined. Results presented herein describe a critical role for non-ciliated airway epithelial NF-κB in promoting inflammation, AHR, and fibrotic remodeling following extended challenges of HDM, while mucus metaplasia differences did not appear affected.
Results of the present study somewhat contrast our previous work using the Alum/Ova model of intraperitoneal sensitization followed by challenges of aerosolized Ova. Notably, CC10-IκBαSR mice subjected to the Alum/Ova protocol were strongly protected against the development of eosinophilic inflammation and mucus metaplasia, but were not protected against Ova-induced AHR (9). The reasons for these discrepant findings remain unclear. It is likely that the route of sensitization of an allergen dictates the nature of the subsequent immunological and pathophysiological response. In support of the latter is a previous report demonstrating that the IP sensitization regimen with Alum/Ova triggers eosinophilic, Th2-driven inflammation (21). In contrast, sensitization with Ova via the airways, along lipopolysaccharide as the adjuvant, led to neutrophilic-dependent AHR and Th17-associated inflammation (22).
In the present study we demonstrated that CC10-IκBαSR mice did not display increases in airway neutrophils following 15 challenges with HDM compared to PBS controls, while neutrophils were robustly increased in BAL of WT mice. In contrast, HDM-induced eosinophilia was only partially attenuated in CC10-IκBαSR mice, while BAL lymphocytes were not significantly affected. IL-5, a potent eosinophil chemoattractant in mice and humans, was not detectable at any experimental time points (data not shown); however, we did demonstrate a strong decrease in KC (human IL-8) in CC10-IκBαSR mice in comparison to WT mice exposed to HDM. These findings demonstrate that following inhibition of epithelial NF-κB in the setting of HDM-induced disease, the inflammatory process is not uniformly inhibited, but preferentially affects neutrophils. This potential differential effect of NF-κB inhibition on the inflammatory process could explain the impact of airway remodeling and AHR. Consistent with the continued presence of eosinophils, HDM-specific IgG1 and IgE, and IL-13 in CC10-IκBαSR mice exposed to HDM, mucus metaplasia and Muc5AC expression remained elevated in these animals. Previous studies demonstrated that IL-13 can activate epithelial cells to produce Muc5ac in an NF-κB independent mechanism (23). In light of those observations, the lack of an impact of CC10-I-κBαSR mice in HDM-induced mucus metaplasia is therefore, perhaps, not surprising.
The role of neutrophils in the pathogenesis of asthma remains unclear. HDM exposure in mice has been associated with mixed neutrophilic/eosinophilic, Th2/Th17-linked inflammation, and production of IL-13 and IL-17A (24). In this study, the preferential diminution of neutrophils following inhibition of epithelial NF-κB was associated with normalization of tissue damping and elastance parameters towards values observed in controls, while in contrast, Newtonian resistance, reflective of the central airways, was unaffected. The disparate effects of the CC10-IκBαSR transgene on tissue damping and elastance, as compared to Newtonian resistance, are puzzling. Previous studies have suggested that inflammation contributed to enhanced leakage of fibrin to the airway surface, leading to decreased stability of surfactant proteins and increased surface tension, and facilitated contractility of smooth muscle. These mechanisms have been linked to increases in the closure of distal airways (25–27). It is plausible that decreases in inflammation, along with decreased α-smooth muscle actin in bronchioles, account for decreases in tissue damping and elastance, which were observed in CC10-IκBαSR transgenic mice. This putative explanation would need to be addressed with additional studies. Our data also demonstrate that KC, a potent neutrophil chemokine, was not increased in CC10-IκBαSR HDM-exposed mice in comparison to PBS controls, indicating a potential mechanism whereby neutrophils are decreased in CC10-IκBαSR mice. All together, these findings are suggestive of a role for neutrophils in promoting increases in peri-bronchiolar collagen deposition and associated changes in tissue damping and elastance. Alternatively, it is also plausible that other mediators control neutrophil trafficking to the airways, promote peri-bronchiolar remodeling, and changes in AHR. In this regard, IL-17A, which we also demonstrate to be decreased in the tissue of CC10-IκBαSR mice, has been shown to stimulate production of chemokines important in neutrophil recruitment in a KC-dependent manner (28) and has been implicated in the pathogenesis of pulmonary fibrosis (29), yet its functional contribution here remains to be determined. Additionally, eosinophils expressing TGF-β1 have been shown to be important in allergen-induced peribronchial fibrosis (30), and hence it is possible that TGF-β1 also plays a role in peri-bronchiolar fibrosis downstream of activation of NF-κB. Additional studies will be required to formally address these putative scenarios.
In addition to demonstrating a role for epithelial NF-κB in promoting inflammation, AHR, and fibrosis following 15 challenges of HDM, we also established a contributing role for epithelial NF-κB in regulating pro-inflammatory gene expression following a single exposure of HDM. Numerous pro-inflammatory mediators have been implicated in the development of allergic airways disease (3), and our studies demonstrate a putative role for epithelial NF-κB in regulating expression of several of these molecules in response to HDM. GM-CSF and CCL20 have been shown to be important in the recruitment/activation of dendritic cells, which are required for T cell activation and recognition of antigens. Notably, exposure of human asthmatic bronchial epithelial cells to the HDM component, Derp1, was shown to be important in dendritic cell recruitment in a CCL20 dependent manner (31). Additionally, IL-33 has been shown to be important in the activation of a variety of cell types crucial to the development of asthma, such as T helper type 2 cells, eosinophils, dendritic cells, and mast cells (32). As previously mentioned, the activation and infiltration of neutrophils is emerging as a potential phenotype in severe, steroid resistant asthma. Cytokines KC and MIP-2 have both been shown to be important in the recruitment of neutrophils and production of HDM-specific IgE (33). Altogether, our data indicate a likely role for epithelial NF-κB in the recruitment/activation of several cell types important for the development of HDM-induced asthma. This suggests a mechanism whereby epithelial NF-κB activation is the crucial step between contact with an allergen and downstream manifestations of asthma.
In addition to the role of NF-κB in airway epithelium demonstrated herein, it is plausible that activation NF-κB in other cell types contributes to the pathophysiology of allergic airways disease. Notably our findings demonstrate increased immunofluorence of nuclear RelA and RelB in parenchymal regions following exposure to HDM (Fig. 3). Unraveling the cell types in which NF-κB is activated and their functional contribution to allergic airways disease would require additional cell specific labeling and targeting strategies, which were beyond the scope of the present study. For example, neutrophil elastase-induced secretion of Transforming Growth Factor β-1 from smooth muscle cells was shown to be dependent on NF-κB activation (34), and the smooth muscle contractile force in response to IL-17A was dependent on NF-κB activation (24), suggesting a putative role of NF-κB activation in smooth muscle cells in airways hyperresponsiveness and remodeling. Secretion of eotaxin, a potent eosinophil activating factor, by fibroblasts was also shown to be dependent on NF-κB activation (35). One notable finding of the present study is that HDM activates both the classical and alternative NF-κB pathway within the bronchiolar epithelium, evidenced by the increases in nuclear presence of both RelA as well as RelB in mice exposed to HDM. Similar to observations herein, we recently demonstrated increases in nuclear RelA and RelB in the parenchymal regions following administration of lipopolysaccharide, a component of HDM (7), suggesting that TLR4 activation by HDM may be contributing to the observed increases in RelA and RelB observed. Increases in nuclear RelA and RelB within the epithelium occurred rapidly and were sustained at least for 24 h after 3 challenges. However, 72 h post 5, 10 or 15 challenges, there was no clear evidence of increased RelA or RelB in the bronchiolar epithelium, possibly due to the timing of tissue analysis post the last administration of HDM, which was 72 h, a time when NF-κB activation may have resolved. However, we did observe sustained increases in nuclear RelB in lung tissue homogenates 72 hr post 15 challenges with HDM in WT mice, suggestive of NF-κB activation in other cell types. Increases in nuclear RelB were attenuated in CC10-IκBαSR mice, suggesting a putative role for RelB in the orchestration of HDM-mediated AHR and fibrotic remodeling. In addition to the demonstration that RelB was increased in the bronchiolar epithelium following HDM exposure, we also demonstrated that HDM directly activated both NF-κB pathways in both human bronchial and nasal epithelial cells. Although extensive studies have been conducted to unravel the molecular regulation and pathophysiological relevance of the classical NF-κB pathway, far less information is available for the alternative pathway. The latter pathway was originally thought to play a role in adaptive immune responses, development of lymphocytes and lymphoid organs. However, emerging studies have pointed to a coordinate function of both classical and alternative NF-κB in the orchestration of pro-inflammatory responses. For example, exposure of lung epithelial cells with Tumor Necrosis Factor-α, Polyinosinic acid, LPS, IL-17A, lipoteichoic acid, and CD40L, agonists that signal through distinct families of receptors, led to a coordinate activation of classical and alternative NF-κB pathways and subsequent pro-inflammatory responses (7). In contrast, adenovirus-mediated delivery of RelB afforded protection against cigarette smoke-induced neutrophilic inflammation (36), suggesting potentially complex roles of the alternative NF-κB pathway in the regulation of pro-inflammatory and immune responses. The classical NF-κB pathway previously has been shown to be important in HDM-mediated pro-inflammatory responses in human bronchial epithelial cells in vitro (12), and Derp1, a component of HDM, was shown to be important in the activation of NF-κB in human asthmatic bronchial epithelial cells (37). Patients with allergic asthma demonstrate increased classical NF-κB activation in nasal epithelial cells in response to HDM in comparison to healthy controls (38). Intriguingly, the HDM component, β-glucan, was shown to be crucial for activation of allergic rhinitis in nasal epithelial cells via TLR2, in contrast to another HDM component, LPS, which is important in promoting allergic airways disease via activation of TLR4 in bronchial epithelial cells (39). Despite these previous studies and data presented herein, additional studies are needed to better understand the timing and locale of activation of classical and alternative NF-κB pathways, the components of HDM that are required to activate either pathway, as well as the relative contributions of the classical and alternative NF-κB pathways in eliciting HDM- triggered allergic airways disease.
In summary, we demonstrate in the present study the importance of NF-κB activation within the bronchiolar epithelium in HDM-induced inflammation, AHR and fibrotic airway remodeling. We also demonstrate that both classical and alternative NF-κB pathways are activated by HDM. Data presented herein showed that in the setting of HDM-induced allergic airway disease, inhibition of epithelial NF-κB plays a more prominent role in attenuating neutrophilia, AHR, and remodeling, in comparison to eosinophilia, IgE, and mucus metaplasia. Therefore, it is plausible that therapeutic approaches which target NF-κB via interference with degradation of IκBα may have a stronger impact on asthmatic patients with predominant neutrophila in contrast to patients with predominant eosinophilia. Corticosteroids, the most common therapy for asthma, inhibit NF-κB, but have many off target effects. Current therapies that are being developed for asthma are aimed at inhibiting the pro-inflammatory effects of NF-κB signalling in the lung (40), and are focused on inhibition of IKKβ, the dominant kinase in the classical NF-κB pathway (40). The small molecule IKKβ inhibitor, IMD-0354, attenuated HDM- induced eosinophilia, globlet cell hyperplasia, subepithelial fibrosis, smooth muscle cell hypertrophy, and lung resistance using an intraperitoneal senstization model (41). The disparate findings of the latter study with the present findings may relate to the different sensitization route, mechanism of inhibition of NF-κB, and the cell types wherein NF-κB inhibition occurred. Furthermore, intravenous administration of RelA antisense oligonucleotides prior to ovalbumin challenge resulted in dampened responses of inflammation, AHR, and TH2 responses in mice (42). Based upon these collective findings, therapeutics designed to inhibit both facets of the NF-κB pathway may hold larger therapeutic potential for allergic asthma and other allergic diseases of the lung.
Supplementary Material
Acknowledgments
We would like to thank the University of Vermont Microscopy Imaging Center and the Vermont Lung Center for their assistance with these studies.
This work was supported by grants: T32 HL076122, T32 ES07122, NIGMS P30 GM103532, and R01 HL060014 from the National Institutes of Health; ATS unrestricted grant and Parker B. Francis Fellowship to VA.
References
- 1.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 2.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 3.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 5.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 6.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 2006;17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Tully JE, Nolin JD, Guala AS, Hoffman SM, Roberson EC, Lahue KG, van der Velden J, Anathy V, Blackwell TS, Janssen-Heininger YM. Cooperation between classical and alternative NF-kappaB pathways regulates proinflammatory responses in epithelial cells. Am J Respir Cell Mol Biol. 2012;47:497–508. doi: 10.1165/rcmb.2012-0014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 9.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol. 2004;173:7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterlund C, Gronlund H, Polovic N, Sundstrom S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen Der p 2 induce NF-kappaB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009;39:1199–1208. doi: 10.1111/j.1365-2222.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 13.Olson N, Greul AK, Hristova M, Bove PF, Kasahara DI, van der Vliet A. Nitric oxide and airway epithelial barrier function: regulation of tight junction proteins and epithelial permeability. Archives of biochemistry and biophysics. 2009;484:205–213. doi: 10.1016/j.abb.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J. 1997;10:2398–2403. doi: 10.1183/09031936.97.10102398. [DOI] [PubMed] [Google Scholar]
- 15.Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, Madden MC. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85:990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 16.Riesenfeld E, Allen GB, Bates JH, Poynter ME, Wu M, Aimiand S, Lundblad LK. The Temporal Evolution of Airways Hyperresponsiveness and Inflammation. J Allergy Ther. 2012;1:1–7. doi: 10.4172/2155-6121.S1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, Irvin CG, Janssen-Heininger YM. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Qi Y, Liu X, Jiang Q, Liu S, Jiang Y, Jiang Z. Activation of NF-kappa B in bronchial epithelial cells from children with asthma. Chinese medical journal. 2001;114:909–911. [PubMed] [Google Scholar]
- 20.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–638. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kung TT, Jones H, Adams GK, 3rd, Umland SP, Kreutner W, Egan RW, Chapman RW, Watnick AS. Characterization of a murine model of allergic pulmonary inflammation. International archives of allergy and immunology. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, Cohn L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol. 2002;27:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 24.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nature medicine. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohlfeld JM. The role of surfactant in asthma. Respir Res. 2002;3:4. doi: 10.1186/rr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest. 2004;114:104–111. doi: 10.1172/JCI19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yager D, Kamm RD, Drazen JM. Airway wall liquid. Sources and role as an amplifier of bronchoconstriction. Chest. 1995;107:105S–110S. doi: 10.1378/chest.107.3_supplement.105s. [DOI] [PubMed] [Google Scholar]
- 28.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 29.Hasan SA, Eksteen B, Reid D, Paine HV, Alansary A, Johannson K, Gwozd C, Goring KA, Vo T, Proud D, Kelly MM. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, Gosset P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 32.Borish L, Steinke JW. Interleukin-33 in asthma: how big of a role does it play? Curr Allergy Asthma Rep. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. CXC chemokines modulate IgE secretion and pulmonary inflammation in a model of allergic asthma. Cytokine. 2005;32:178–185. doi: 10.1016/j.cyto.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, Wang CH, Chung KF, Kuo HP. Neutrophil-derived elastase induces TGF-beta1 secretion in human airway smooth muscle via NF-kappaB pathway. Am J Respir Cell Mol Biol. 2006;35:407–414. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- 35.Rokudai A, Terui Y, Kuniyoshi R, Mishima Y, Mishima Y, Aizu-Yokota E, Sonoda Y, Kasahara T, Hatake K. Differential regulation of eotaxin-1/CCL11 and eotaxin-3/CCL26 production by the TNF-alpha and IL-4 stimulated human lung fibroblast. Biological & pharmaceutical bulletin. 2006;29:1102–1109. doi: 10.1248/bpb.29.1102. [DOI] [PubMed] [Google Scholar]
- 36.McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP. Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation. Am J Pathol. 2011;179:125–133. doi: 10.1016/j.ajpath.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]
- 38.Vroling AB, Jonker MJ, Luiten S, Breit TM, Fokkens WJ, van Drunen CM. Primary nasal epithelium exposed to house dust mite extract shows activated expression in allergic individuals. Am J Respir Cell Mol Biol. 2008;38:293–299. doi: 10.1165/rcmb.2007-0278OC. [DOI] [PubMed] [Google Scholar]
- 39.Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, Choi MK, Joo JH, Kim CH, Lee SN, Lee WJ, Kim J, Shin DM, Kweon MN, Bae YS, Yoon JH. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–561. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 40.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa H, Azuma M, Muto S, Nishioka Y, Honjo A, Tezuka T, Uehara H, Izumi K, Itai A, Sone S. IkappaB kinase beta inhibitor IMD-0354 suppresses airway remodelling in a Dermatophagoides pteronyssinus-sensitized mouse model of chronic asthma. Clin Exp Allergy. 2011;41:104–115. doi: 10.1111/j.1365-2222.2010.03564.x. [DOI] [PubMed] [Google Scholar]
- 42.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. International immunopharmacology. 2004;4:1817–1828. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.