Abstract
BANK1, an adaptor protein expressed in B-cells, plays a little understood role in B-cell signaling. Because BANK1 contains an N-terminal putative Toll-interleukin 1 receptor (TIR) domain, we used mouse Bank1−/− splenic B cells to test if BANK1 affects signaling induced by the TLR9 agonist CpG. Following CpG stimulation BANK1 deficiency reduced p38 phosphorylation without affecting that of ERK or JNK and reduced IL-6 secretion. Bank1−/− B cells showed reduced phosphorylation of MNK1/2 and eIF4E, suggesting an effect on translation initiation, while Bank1−/− had no effect on IL6 mRNA stability, thus suggesting that BANK1 has no effect on MK2 signaling. IL-6 secretion observed when CpG stimulation was combined with anti-CD40, was reduced in the absence of BANK1. While in the presence of anti-CD40 stimulation CpG induced a stronger phosphorylation of AKT, mTOR and 4E-BP1, Bank1−/− had no effect on phosphorylation of mTOR and 4E-BP1, and weakly on AKT, implying that BANK1 does not affect the release of eIF4E by phospho-4E-BP1. Together these data establish a previously unrecognized role for BANK1 in CpG-induced responses by splenic B cells on p38 signaling and control of translation initiation of IL-6 via MNK1/2 and eIF4E.
Keywords: endosomal toll-like receptors, mitogen activated protein kinases, p38, IL-6, CD40, autoimmunity, systemic lupus erythematosus, B lymphocytes
Introduction
Human and mouse Bank1 encode the B cell scaffold protein with ankyrin repeats 1. During B cell receptor (BCR) activation BANK1 becomes extensively tyrosine phosphorylated and is capable of binding the Src family kinases Lyn and Blk (1, 2). While apparently involved in BCR signaling, the function of BANK1 during signaling induced by CpG, an agonist of the major toll-like receptor, TLR9 expressed in B cells, is not known.
It has been proposed that BANK1 acts as an adaptor or scaffold protein in the same family as the B cell adapter for PI3K (BCAP) and the Drosophila homologue Dof (2). Consistent with this hypothesis, our recent studies have shown that exon 2 of human BANK1 encodes a highly hydrophobic domain, which renders the protein susceptible to aggregation (3); scaffold and adaptor proteins are known to form complex structures to facilitate intracellular signaling at the proper time and differentiation stage. Furthermore, exon 2 also encodes a predicted N-terminal toll/IL-1 receptor (TIR) domain that is shared by BCAP (4) and used in the interaction of BCAP with the adaptors MyD88 and TIRAP. TLR9 is the major endosomal TLR in B cells that recognizes viral nucleic acids, and TLR9 signaling is believed to have an important role in autoimmunity (5). TLR9 signaling is stimulated by hypomethylated DNA oligonucleotides or CpG (6), leading to a pro-inflammatory response (7).
CpG-induced signaling activates mitogen activated protein kinase (MAPK) pathways, including p38, JNK and ERK. Stimulation of p38 and ERK signaling by growth factors, stress or viral infections can induce transcriptional activation, but can also induce two pathways of post-transcriptional regulation of protein synthesis: control of mRNA stabilization (8, 9) by the mitogen activated protein kinase-activated protein kinase 2 (MAPKAP kinase) MK2, and the transient formation of the heterotrimeric eIF4E/eIF4F/eIF4G translation initiation complex through phosphorylation of eIF4E (10, 11). In mice, the only kinases known to phosphorylate eIF4E are MNK1 and MNK2. MNK2 is constitutively active, while MNK1 is regulated by the MAP kinases (12).
A second axis of control of eIF4E activation is through the AKT/mTORC1 pathway. This pathway regulates the phosphorylation of 4E-BP1, the eIF4E binding protein. Under non-phosphorylated conditions, 4E-BP1 retains eIF4E (13, 14). Once 4E-BP1 becomes phosphorylated by mTORC1, it releases eIF4E, which is in turn phosphorylated by MNK1/2 (15).
Because of the role of CpG-induced signaling in autoimmunity and the putative role of BANK1 as a TIR-containing adaptor, we hypothesized that BANK1 may participate in CpG-induced signaling. Our results establish a function for BANK1 in CpG-induced responses that could have important implications for the role of BANK1 in infections and autoimmunity, where BANK1 has been established as a susceptibility gene (16).
Materials and Methods
Mice
Bank1−/− mice were kindly provided by Dr T. Kurosaki (Riken Research Centre for Allergy and Immunology, Kyoto, Japan) and were backcrossed 9 generations onto the C57BL/6J background. C57BL/6J mice were purchased from Jackson Laboratory, Bar Harbor, Maine, USA. Mice were maintained under specific pathogen free (SPF) barrier conditions. This study was approved by the Oklahoma Medical Research Foundation Institutional Animal Care and Use Committee.
Antibodies and reagents
Phospho-specific antibodies to p38 (Thr180/Tyr182, clone12F8 #4631), JNK (Thr183/Tyr185, clone 81E11, #4668), ERK (Thr202/Tyr204, clone D13.14.4E, #4370), IκBα (Ser32, clone 14D4, #2859), eIF4E (Ser209, #9741), MNK1/2 (Thr197/202, #2111), 4E-BP1 (Thr37/46, clone 236B4, #2855), mTOR (Ser2448, clone D9C2, #5536), and AKT (Ser473, clone 193H12, #4058) and phosphorylation-state-independent antibodies to p38 (#9212), JNK, ERK, IκBα, eIF4E (clone C46H6, #2067), MNK1 (clone C4C1, #2195), 4E-BP1 (clone 53H11, #9644), mTOR (clone 7C10, #2983), and AKT (#9272), were purchased from Cell Signaling Technology (Danvers, MA). Phosphorylation-state-independent antibody for MNK2 (S-20) was purchased from Sigma-Aldrich (St. Louis, MI).
Purification of splenic B cells
Splenic B cells were purified by negative selection using magnetic bead separation. Briefly, spleen cells from Bank1+/+ and Bank1−/− littermates were labeled with a cocktail of biotin-conjugated antibodies for 15 minutes. Cells were incubated an additional 15 minutes with anti-biotin micro beads (B Cell isolation Kit, mouse; Miltenyi Biotech, Auburn, CA) at 4°C. The labeled non-B-cells were depleted by magnetic retention in a MACS column while unlabeled B cells were recovered. The purity of the resulting cell population was typically more than 95% B220+CD3− as assessed by flow cytometry analysis.
Cell Culture
Cell culture was performed with RPMI 1640 medium supplemented with 10% FBS, L-glutamine (2 mM), 2-ME (50 μM) and antibiotics for all experiments, except for p38 analyses and the Akt axis where cells were serum-starved two hours prior to stimulation. Purified splenic B cells (106 cells/mL) were treated with 10 μg/ml F(ab′)2 fragment of anti-mouse IgM (Jackson Immunoresearch, West Grove, PA), 2 μM CpG (ODN Type B;1668, Invivogen, San Diego, CA) or combination of anti-mouse IgM and CpG, 20μg/mL TLR4 agonist LPS or 1μg/mL of the TLR7 agonist R848 (Invivogen, San Diego, CA), or were left unstimulated. Supernatants were collected at 0, 12, 24 and 48 hr, and 72 and 96 hours for anti-CD40 stimulation (low-endotoxin, azide-free anti-mouse CD40, Biolegend, San Diego, CA) was included (10μg/ml final concentration), with or without CpG.
Western Blot
Stimulated splenic B cells were lysed in buffer containing 1% TritonX-100, 50 mM Tris pH 7.4, 50mM NaCl, 1mM EDTA, 2 mM Na3VO4, and a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). LDS sample buffer and reducing agent (Life Technologies, Carlsbad, CA) were added. Samples were then boiled, and protein was separated with NuPAGE 4–12% Bis-Tris Gels (Life Technologies, Carlsbad, CA). Proteins were blotted onto polyvinylidene difluoride membranes, blocked with 5% skimmed milk and the immunoblots were processed with specific antibodies diluted with 5% BSA or 5% skimmed milk solution, and detected with Novex ECL substrate (Invitrogen, Carlsbad CA).
ELISA Assays
Cytokine secretion by in vitro stimulated B cells was measured using the ELISA kit for IL-6 and IL-10 purchased from BD Bioscience (San Jose, CA). For this, 96-well plates were coated with antibody against mouse IL-6 or mouse IL-10 overnight at 4°C. Plates were then blocked for 1 h at room temperature with 10% FBS in PBS. Supernatants were then added to the plate and left for 2 h at room temperature. Following washing, biotinylated anti-IL-6 or anti-IL-10 was added and plates incubated for further an hour at room temperature. Streptavidine alkaline phosphatase was then added following incubation at room temperature and washing with tetramethylbenzidine liquid substrate. Absorbance was read at 450 nm, from which absorbance was subtracted at 570 nm within 30 min of stopping reaction. Cytokine concentration was determined by extrapolation from the standard curve. Standard curve was generated using recombinant mouse IL-6 or recombinant mouse IL-10 (BD Bioscience, San Jose, CA).
Splenic B cells (0.5×106 cells/well) were stimulated for 6 days and supernatants were analyzed for immunoglobulin isotype antibodies as per the instructions from BD Pharmingen™ mouse immunoglobulin isotype ELISA kit (BD Biosciences, San Diego, CA, US.). We quantified IgG2a and IgG2c isotype antibodies using mouse IgG2a Ready-Set-Go ELISA kit (eBioscience Inc. San Diego CA, US.) and mouse IgG2c ELISA Quantification Set (Bethyl Laboratories, Inc. TX, US.) respectively and data are presented as ng/ml. For other antibodies, only O.D.s are shown.
Determination of Cell Viability and Proliferation Assay
Purified splenic B cells were resuspended in pre-warmed PBS/ 0.1% BSA and labeled with 2μM CFSE (Life Technologies, Carlsbad, CA), and incubated at 37°C in a water bath for 10 min. Five volumes of culture medium were added for quenching. After incubating 5 min on ice, the cell pellet was collected by centrifugation, washed 3 times with fresh complete RPMI medium and resuspended to the appropriate density. Cells were then stimulated with 10μg/ml F(ab′)2 fragment of anti-IgM, 2μM CpG, 10μg/ml anti-CD40, 20μg/ml LPS, and 1μg/ml R848, respectively, or the shown combinations and cultured for 48, 72 and 96 hr. After harvest, cells were stained with propidium iodide (PI, final concentration of 1μg/ml; eBioscience Inc. San Diego CA, US.) and acquired by LSR II or FACScalibur (BD bioscience, San Jose, CA) 36,000 events in total. For flow cytometry, debris and doublet cells were discriminated and viable cells (PI- negative) were gated and % of cell viability was determined. The histograms shown with FL1 channel represent overlaid data from un-stimulated CFSE-labeled B cells, stimulated Bank1+/+ and Bank1−/− B cells, and the y-axis is presented as % viability of maximum. The analysis was conducted using FlowJo software (Tree Star Inc., Ashland, OR).
Inhibition of IL-6 production from splenic B cells
The MNK1 specific inhibitor, CGP57380 [4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d] pyrimidine] was obtained from Calbiochem®-Millipore (Billerica, MA), and MNKs inhibitor cercosporamide and MK2 inhibitor, PF-3644022 [(10R)-9,10,11,12-tetrahydro-10-methyl-3-(6-methyl-3-pyridinyl)-8H-[1,4]diazepino[5′,6′:4,5]thieno[3,2-f]quinolin-8-one hydrate] were purchased from Sigma-Aldrich (St. Louis, MI). The purified splenic B cells (5×105 cells/ well) were seeded in complete RPMI1640 medium in a 48 well tissue culture plate. Then the cells were pretreated with or without inhibitor for 30 min or 1h, respectively, before CpG stimulation for 24 hr time at which cells were harvested and subjected to determine % of viability by using propidium iodide staining (final concentration 1μg/ml) and flow cytometry analysis. Supernatants were collected and stored at −80°C until use and IL-6 was measured using a capture ELISA. The control well contained CpG and cell culture grade DMSO (Pierce, Rockford, IL) only (17).
Taqman RT-PCR and Il6 mRNA Stability Assay
Total RNA was isolated with Trizol (Invitrogen, Carlsbad, CA) from triplicate cultures of 5×105 splenic B cells in the presence or absence of CpG treatment for the indicated times. After quantification and quality control of the RNA with a Nanodrop spectrophotometer (Thermo Scientific, West Palm Beach, FL), 400ng RNA was subjected to first-strand cDNA synthesis for qRT-PCR (Origene, Rockvile MD). 5ng total cDNA/RNA was used per reaction in TaqMan Fast Universal PCR Master Mix (Life Technologies, Carlsbad, CA). The qRT-PCR reactions were performed on a 7900 HT Fast Real-Time PCR system. The primers and probes for mouse Tlr9 (Assay ID: Mm00446193_m1), Prdm1 (Mm00476128_m1) and Il6 (Assay ID: Mm00446190_m1) and internal control gene 18S rRNA (4319413E) were purchased from Taqman Gene expression Assays (Life Technologies, Carlsbad, CA).
For the Il6 mRNA stability assay, 5×105 splenic B cells were cultured in triplicate and treated with 2μM CpG for 20hr followed by addition of 1μg/ml actinomycin D (Sigma-Aldrich, St. Louis, MI) for 0, 50, 100, 200 min. RNA was isolated and Il6 transcription was determined with Taqman qRT-PCR using the primers as described above.
Results
BANK1 Deficiency Reduces CpG-Induced p38 Activation in Splenic B Cells
To discern the signaling cascades affected by BANK1, we tested if BANK1 deficiency altered B cell proliferation after stimulation with CpG. Using purified splenic B-cells from Bank1−/− and littermate control (+/+) mice, we did not observe any differences at any of the time points tested in proliferation with CpG or LPS, a TLR4 agonist (Figure 1A). We also did not observe differences in cell proliferation when using anti-IgM, anti-IgM+CpG, anti-CD40, CpG + anti-CD40, R848, R848+anti-CD40, LPS, LPS + anti-CD40, or anti-IgM+LPS (Supplementary Figure S1). We then tested the effects of BANK1 deficiency on IgM-induced activation of NFκB by analyzing phosphorylation and degradation of IκBα, and activation of the MAPK pathways by studying phosphorylation of ERK and p38. BANK1 deficiency did not affect phosphorylation of MAPKs, or IκBα following stimulation with anti-IgM alone (Figure 1B).
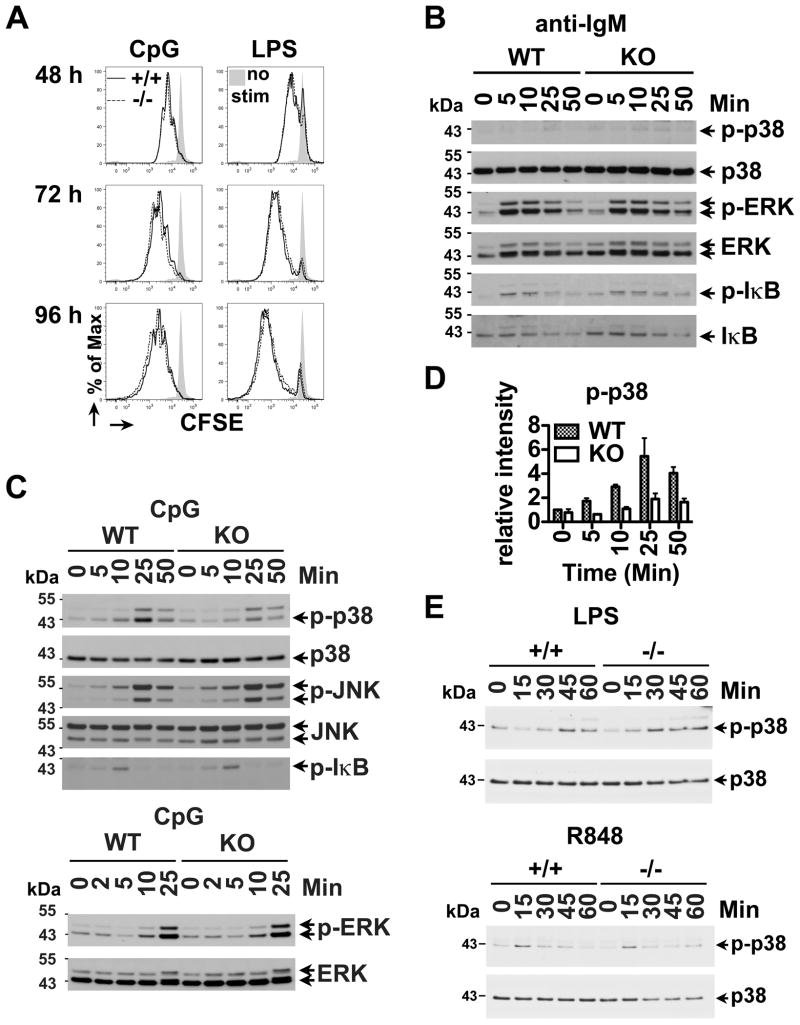
Figure 1. CpG-induced p38 MAPK activation is down-regulated in Bank1−/− B cells.
(A) Proliferation assay of CFSE-labeled splenic B cells from Bank1−/− mice and its littermate control (+/+) mice following 2μM CpG or 20μg/ml LPS stimulation for 48, 72 and 96h. Propidium iodide (PI)-negative viable cells were gated and analyzed by flow cytometry. Data shown is representative of 3 independent experiments. (B–C) Cell extracts from WT and Bank1 KO splenic B cells stimulated with 10μg/mL anti-mouse IgM F(ab′)2 (anti-IgM) (B) or 2μM CpG (C) and analyzed by Western blot with specific antibodies for ERK, JNK, p38 and IκB and the corresponding phospho-specific antibodies. (D) Quantification of densitometry analysis of phospho-p38 Western blot data is shown as mean ± SEM. (E) Cell extracts from splenic B cells stimulated with 20μg/mL LPS and 1μg/mL of the TLR7 agonist (R848) were analyzed by Western blot with specific antibodies to p38. WB data shown is representative of 2 independent experiments.
After CpG stimulation, p38 phosphorylation was importantly reduced (Figure 1C and D), but ERK, JNK and IκBα phosphorylation were unchanged, in Bank1−/− cells compared to cells from WT mice (Figure 1C) or from Bank1+/+ littermates (data not shown). For all pathways, stimulation with anti-IgM+CpG (data not shown) yielded results identical to CpG alone (Figure 1C). BANK1 deficiency had no effect on p38 signaling induced by LPS or R848 (Figure 1E). Therefore, BANK1 specifically modulates CpG-induced p38 signaling in vitro.
BANK1 Deficiency Reduces CpG-Stimulated IL-6 Secretion
It is known that the MAPK p38-signaling pathway regulates the production of several cytokines including IL-6, TNFα, IFNγ, and IL-10 (18–20). Having shown that BANK1 deficiency reduced p38 phosphorylation after CpG stimulation, we asked whether reduction in p38 phosphorylation leads to modulation of IL-6 or IL-10 secretion by Bank1−/− B cells. As expected (21–23), anti-IgM alone did not induce detectable IL-6 or IL-10 in splenic B-cells from WT, Bank1+/+ or Bank1−/− littermates (data not shown), while CpG induced detectable levels of IL-10 and IL-6 production. Bank1−/− B-cells showed consistently reduced IL-6 but normal IL-10 secretion in response to CpG alone or the combination of CpG and BCR ligation (Figure 2A).
Figure 2. CpG-induced IL-6 production is downregulated in Bank1−/− B cells, but Tlr9 gene expression is not affected.
(A) IL-6 and IL-10 were measured by ELISA of WT (+/+) and Bank1 KO (−/−) B-cell supernatants collected at the indicated times following CpG, anti-IgM + CpG or CpG + anti-mouse CD40 (10μg/ml). Data shown are mean ± SD of 3 replicates, and is representative of 4 independent experiments. (B) Splenic B cells from Bank1+/+ and Bank1−/− mice were stimulated with 2μM CpG for the indicated times. Relative expression of the Tlr9 gene was analyzed by Taqman real-time PCR. Data shown is the mean ± SEM of 3 replicates from a representative experiment out of 3 performed. (C) Bank1+/+ and Bank1−/− splenic B cells were stimulated with LPS (20 μg/ml) or R848 (1 μg/mL), or left unstimulated for the times shown. Data are the representative of two independent experiments with 3 replicates each. Bars represent mean ± SD.
In general, it has been observed that the combination of anti-CD40 and CpG leads to the most optimal secretion of IL-6 by B cells (24, 25), and BANK1 deficiency has been shown to lead to activation of Akt following stimulation through CD40. When we investigated production of IL-6 by combining anti-CD40 and CpG, we clearly observed that the amount of IL-6 secreted was importantly increased when adding anti-CD40 to CpG as compared to CpG alone in Bank1+/+ B cells. Deficiency of BANK1 reduced the levels of secreted IL-6 (Figure 2A). We tested if stimulation with CpG would lead to a reduction in cell viability by BANK1 deficient cells and hence to reduced IL-6 secretion. This was not the case (Supplementary Figure S1A). We did observe slightly fluctuated cell viability when combining CpG with anti-CD40 in BANK1 deficient cells, but proliferation was normal. Hence we conclude that reduced secretion of IL-6 is not due to changes in cell viability in BANK1 deficient B cells.
CpG is a known agonist of Tlr9. To test if the reduced expression of IL-6 and reduced phosphorylation of p38 could be due to reduced expression of Tlr9, we tested mRNA expression. Tlr9 gene expression was no different between Bank1+/+ and Bank1−/− mice (Figure 2B).
Next, we examined whether BANK1 deficiency had an effect on the production of IL-6 induced by TLR7 and TLR4. However, LPS and the TLR7 agonist R848 induced subtle amounts of IL-6 (21, 22) as compared to CpG stimulation and BANK1 deficiency had no effect (Figure 2C). Taken together, these results show that BANK1 deficiency reduces secretion of IL-6 in response to CpG stimulation, overcomes the effect of anti-CD40 and is not due to changes in cell viability or to reduced Tlr9 expression.
Bank1 affects translation initiation via the MNK1/2 and eIF4E pathway but not mRNA stability, which is controlled by MK2
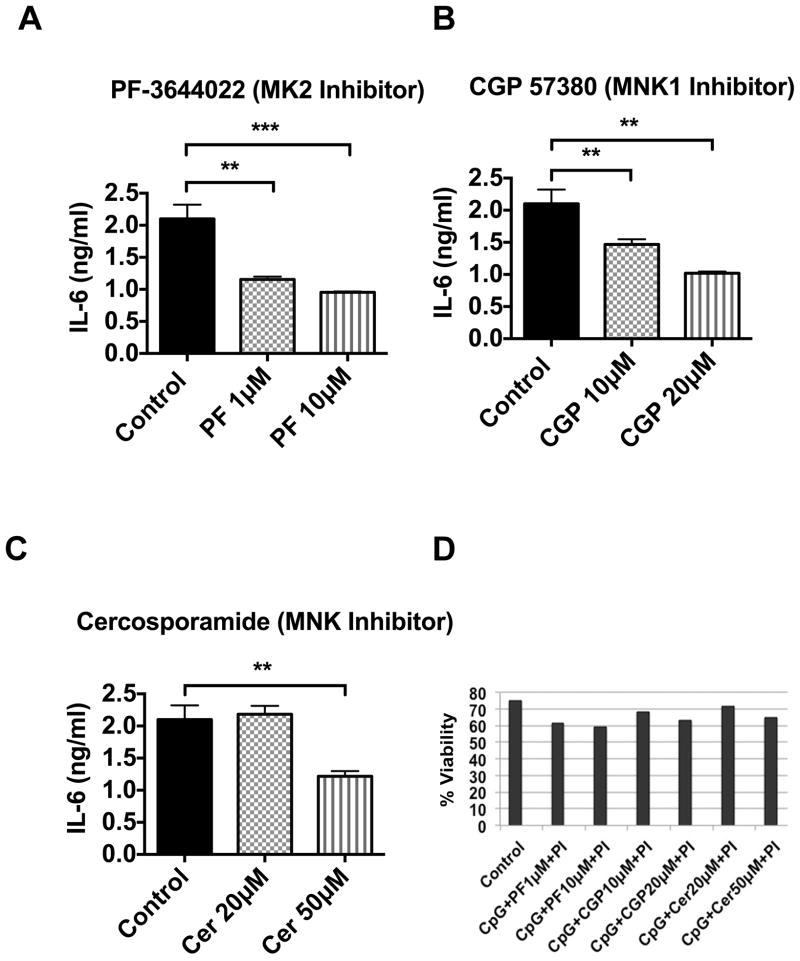
We suspected that BANK1 deficiency could decrease IL-6 production by reducing Il6 translation initiation and/or mRNA stability, since p38 directly controls these via the kinases MNK1/2 and MK2, respectively (26, 27). MK2 inhibitors are known to reduce IL-6 secretion by human peripheral blood mononuclear cells (28). Consistent with this, we observed reduced IL-6 production in CpG-stimulated WT mouse B cells treated with the MK2 inhibitor PF3644022 (Figure 3A). In addition, there was a significant and reproducible, though less marked reduction of IL-6 secretion after treatment with the MNK1/2/eIF4E inhibitors CGP57380 and cercosporamide using doses that minimally affect cell viability (Figure 3B, 3C and 3D). These results show that MNK1/2 and MK2 inhibitors can reduce IL-6 secretion in mouse B cells and confirm that both MNK1/2 and MK2 are important for CpG-stimulated IL-6 secretion from mouse splenic B cells.
Figure 3. MK2 and MNK1/2 inhibitors suppress IL-6 cytokine secretion in mouse splenic B cells.
Mouse C57BL/6J (WT) splenic B cells (1×106 cells/ml) were seeded in triplicate in RPMI 1640 complete medium in a 48 well tissue culture plate. The cells were pretreated with and without (A) MK2 specific inhibitor, PF3644022 (1 and 10μM) for an hour, (B) MNK1 inhibitor, CGP57380 (10 and 20μM) for 30 min, and (C) MNKs inhibitor cercosporamide (20 and 50μM) for an hour before stimulation of CpG (2μM) for 24h. The control well contains CpG and cell culture grade DMSO (<0.2% v/v) only. The culture supernatants were collected after 24h of stimulation and the capture ELISA for IL-6 was performed. The culture supernatants were placed in triplicate in a 96 well ELISA plate and IL-6 ELISA was performed. Bars represent mean ± SD from two independent experiments. Unpaired t-test was performed and all the treated groups were compared with control, **p<0.001 and ***p<0.0001. (D) Viability of cells after above treatment was tested by propidium iodide (PI) labeling, the whole cells were gated and analyzed by flow cytometry, the figure shows (PI)-negative viable cells.
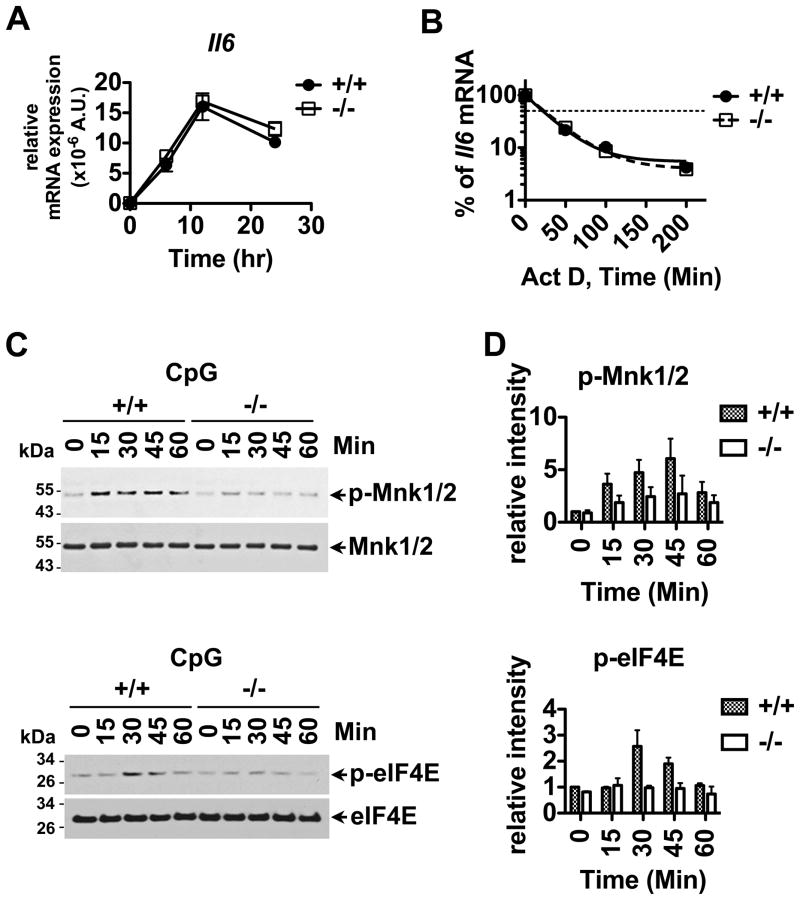
Since CpG-induced IL-6 production was reduced in Bank1−/− cells, we tested if Il6 gene expression was altered by the deficiency of BANK1. As shown in Figure 4A, Il6 gene expression showed no differences between BANK1 sufficient or BANK1 deficient B cells following CpG stimulation.
Figure 4. Reduced IL-6 secretion can be attributed to downregulation of the phosphorylation of the p38-MNK1/2-eIF4E cascade in Bank1−/− B cells following CpG stimulation and not to Il6 gene transcription and Il6 mRNA stability.
(A) Splenic B cells from Bank1+/+ and Bank1−/− mice were stimulated with 2 μM CpG for indicated time shown on the plot. Relative expression of the Il6 gene was determined using Taqman RT-PCR. Data shown is the mean ± SEM of 3 replicates from 1 out of 3 independent experiments. (B) Splenic B cells from Bank1+/+ and Bank1−/− littermate mice were stimulated with 2μM CpG for 20h followed by the addition of 1μg/mL of actinomycin D (Act D). Relative expression of the Il6 gene was analyzed with RT-PCR, and % of Il6 mRNA was determined. Data shown is the mean of 3 replicates from 1 representative experiment out of 3 performed. The thin dotted line indicates 50% degradation of Il6 mRNA. (C) Bank1+/+ or −/− splenic B cells were stimulated with 2μM CpG for the indicated times and tested by Western blot using p-MNK1/2, MNK1/2, p-eIF4E, and eIF4E specific antibodies. The data are representative of 3 independent experiments. (D) Quantification analysis of phospho-MNK1/2 and phospho-eIF4E Western blot data by densitometry. The relative intensities of phospho-MNK1/2 and phospho-eIF4E were quantified using ImageJ software (freely available through the National Institutes of Health). The plots are from 3 independent experiments and data are expressed as mean ± SD.
Since the p38-MK2 signal pathway is responsible in maintaining mRNA stability, we tested the effect of BANK1 deficiency on MK2-mediated IL-6 secretion by testing the stability of Il6 mRNA. CpG-stimulated Bank1+/+ and Bank1−/− splenic B-cells showed comparable Il6 mRNA stability (Figure 4B). Next, we tested the effects of BANK1 on the activation of the p38-MNK1/2-mediated signaling pathway. MNK1/2 regulates the translation initiation factor eIF4E through phosphorylation (26, 29, 30). We therefore analyzed phosphorylation of MNK1/2 and eIF4E. CpG-induced MNK1/2 and eIF4E phosphorylation were consistently reduced in Bank1−/− cells (Figure 4C and D).
These results show that BANK1 influences IL-6 secretion by its effects on the p38-regulated MNK1/2/eIF4E/eIF4G pathway of translation initiation while BANK1 does not affect IL-6 secretion via the MK2 pathway.
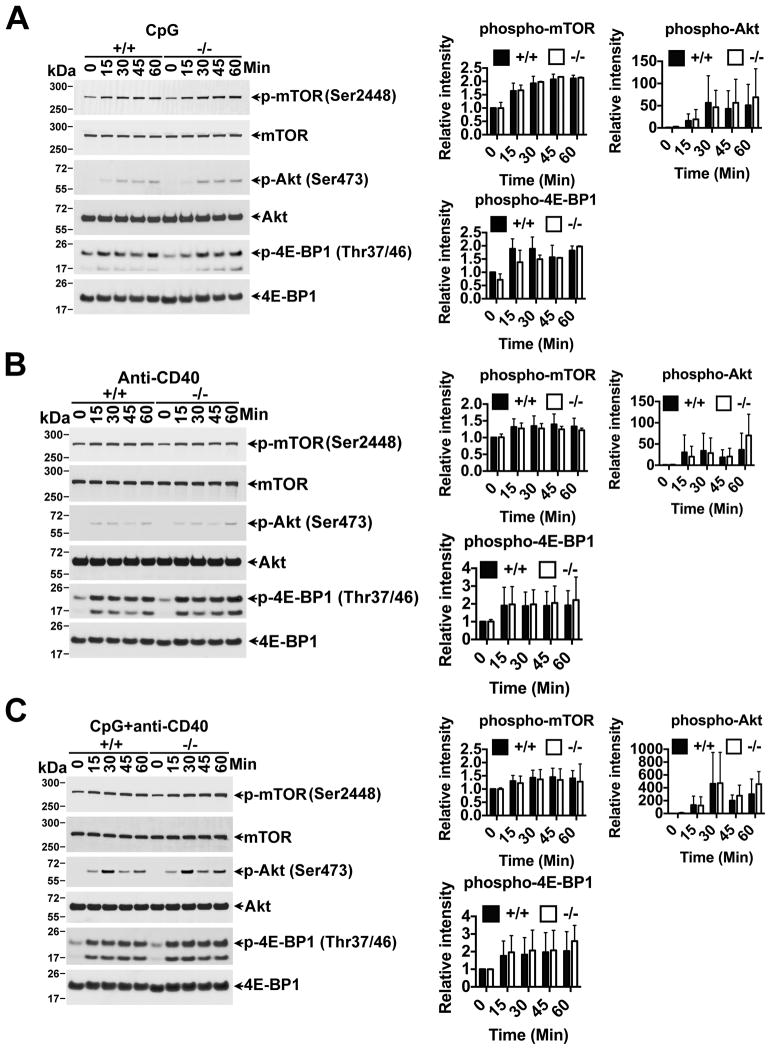
BANK1 has no effect on the AKT-mTORC1–4E-BP1 Signaling Cascade
Activation of eIF4E is also controlled via the AKT-mTORC1–4E-BP1 signaling cascade. Activation of this pathway leads to phosphorylation of 4E-BP1, which in turn releases eIF4E for it to be phosphorylated by MNK1/2 and initiate translation. In addition, stimulation through CD40 has been reported to lead to increased AKT phosphorylation in Bank1−/− purified B cells (31). The cascade was induced with CpG alone (Figure 5A), and the phosphorylation of AKT, mTOR and 4E-BP1 was strongly induced when anti-CD40 was included alone or in combination with CpG (Figure 5B and C). We did observe an increase, albeit weak, in phospho-AKT following anti-CD40 and anti-CD40+CpG treatment on Bank1−/− B cells (Figure 5B and C) (31). However, we did not observe any differences in the downstream mTORC1 to 4E-BP1, when we used anti-CD40 alone or combined with CpG between Bank1−/− and Bank1+/+ B cells or on phospho-AKT when using CpG alone (Figure 5A, B and C). Overall our results show that BANK1 acts only in the MNK1/2 and eIF4E arm of p38 signaling to induce IL-6 secretion following CpG stimulation, and that absence of BANK1 does not affect activation of AKT, mTORC1 or 4E-BP1 of induction of translation initiation.
Figure 5. Bank1 deficiency does not affect 4E-BP1 phosphorylation controlled through the AKT-mTORC1 signaling cascade.
Splenic B cells from Bank1+/+ and Bank1−/− littermate mice were stimulated with (A) CpG (2μM), (B) anti-mouse CD40 (10μg/ml), and (C) CpG (2μM) in combination with anti-mouse CD40 (10μg/ml) at 37°C in a water bath for the indicated time points. Cytoplasmic cell extracts were prepared by using B cell lysis buffer containing appropriate amount of protease inhibitor and sodium vanadate. Equal amount of cell lysate protein (approx. 2×106cell) was loaded in each lane and western blot was performed as written in the material and methods. The data are representative of two independent experiments. The relative intensities of the phospho-bands were quantified as described in Figure 4.
BANK1 deficiency leads to a tendency towards increased production of IgG2a/c subclass antibodies but does not affect expression of the Blimp1 gene (Prdm1)
Finally, we tested if BANK1 had an effect on the in vitro secretion of antibodies following CpG stimulation. Our results show a non-significant tendency towards increased, rather than decreased secretion of IgG2a and IgG2c antibodies by Bank1−/− B cells. Further, we did not observe any difference in the expression of the Prdm1 gene that codifies for the transcription factor Blimp1 (Supplementary Figure S2).
Our results suggest that secretion of IgG2a/2c antibodies is not significantly affected by reduced secretion of IL-6 by Bank1−/−B cells.
Discussion
We show for the first time that the B cell adaptor with ankyrin repeats BANK1 influences signaling leading to the formation of the translation initiation eIF4E complex following stimulation with CpG, and that BANK1 deficiency results in reduction of the translation of the proinflammatory cytokine IL-6. We have shown that mRNA stability of IL6 is not affected, nor the second axis of regulation of translation initiation via AKT, which is also induced by CpG and strongly amplified with anti-CD40 treatment. Clearly, the combination of CpG and anti-CD40 treatment leads to stronger signaling of the AKT axis followed by strong activation of mTOR and 4E-BP1. However absence of BANK1 has no effect on the signaling pathway through this axis, except weak activation of AKT. While the secretion of IL-6 is optimal (22, 23, 32) with the combination of CpG and anti-CD40, BANK1 deficiency leads to its reduction, and anti-CD40 cannot overcome this effect. Our results clearly show that overall BANK1 controls the production of IL-6 via the p38-MNK1/2-eIF4E pathway. At the same time, activation of the CD40-induced pathway leads to strong phosphorylation of the AKT axis, which would eventually promote translation initiation of IL-6 through phosphorylation of 4E-BP1 and the release of eIF4E for it to become phosphorylated by MNK1/2. However, we clearly observe that this is not the case.
The involvement of BANK1 in modulating the IL-6 response after CpG stimulation has important implications in the pathogenesis of autoimmunity and viral infection.
Sera from autoimmunity-prone animals and cerebrospinal fluid from SLE patients have elevated levels of IL-6 (33, 34) and peripheral blood cells from SLE patients spontaneously secrete increased levels of IL-6 (35). Autoimmunity is ameliorated by IL-6 ablation (36). It is also important to note that IL-6, coordinated with IL-21, has been reported to control the differentiation towards plasma cells (37). More recently, B cell derived IL-6 was shown to function in an autocrine manner and trigger receptor revision through re-expression of RAG in the post germinal center response (38).
IL-6 has also important roles in infectious diseases. It has been reported that B cells release IL-6 to promote a follicular helper T cell (TFH) response to viral infection. B cell–derived IL-6 was necessary and sufficient to induce IL-21 from CD4+ T cells in vitro and to support TFH cell development in vivo upon acute influenza virus infection (39). IL-6 is also involved in the induction but not maintenance of plasma cells (40). BANK1 deficient mice have normal T cell-dependent humoral responses following immunization with NP-CGG (31). One explanation for the unchanged primary humoral response reported by Aiba et al. in Bank1−/− mice is likely to be the lack of TLR agonist challenge and IL-6 production by B cells in their in vivo system. Therefore, it will be worth to address if infection with DNA- or RNA-viruses, instead of NP-CGG immunization can initiate TLR9 or TLR7 activation that would probably result in reduced IL-6 production by Bank1−/− B cells, and suppressed germinal center responses. Regarding humoral responses, at this point we are unable to explain the tendency towards increased IgG2a/2c production induced by CpG and we do not know if this bears any relationship with the increased serum IgG2a observed by Aiba et al (31).
BANK1 has been genetically associated with SLE and other autoimmune diseases, and here we observe that BANK1 deficiency decreased IL-6 secretion by altering the translation initiation pathway upon CpG stimulation. Our results therefore suggest that BANK1 could be involved in controlling disease development through the control of IL-6 secretion. While there are several mechanisms through which IL-6 production is regulated, it is clear that the production of IL-6 is controlled through a multitude of pathways and through different genes.
BANK1 was found to contain a conformational modular TIR domain at the N-terminus, similar to its relative, the molecule BCAP. BCAP is required for TLR-mediated activation of PI3K and AKT in macrophages (4). In contrast, CpG-induced AKT activation is normal in Bank1−/− B cells (Figure 5A). The data suggest that BCAP, rather than BANK1, may play a critical role in TLR-mediated activation of AKT, and further, BCAP and CD19 have complementary roles in BCR-mediated-PI3K activation (41). While BANK1 appears to mediate AKT activation upon CD40 ligation in B cells ((31) and Figure 5B), our results support a specific role for BANK1 in transducing CpG-induced signals via p38-MNK1/2 and the translation initiation factor eIF4E in B cells. Thus, BANK1 and BCAP rather than playing redundant roles, appear to have very different ones.
BANK1, with a putative TIR domain, would be prone to bind molecules containing TIR domains, and explain the very specific role of BANK1 in p38 signaling and translation initiation. BANK1 is an adaptor molecule with a modular structure. It has up to 23 tyrosines susceptible of phosphorylation, and their substrate could alternate among a variety of molecules forming specific complexes during CpG-induced activation, different from those occurring following BCR-induced ligation, for instance. One of those complexes induced by CpG could include p38. How BANK1 controls the p38-MNK1/2 pathway downstream of TLR9 is at present not known, but is a subject of study in our laboratory.
We have described that BANK1 shows an interaction with the Src tyrosine kinase BLK (1), and that BLK serves to promote the interaction between BANK1 and phospholipase C gamma 2, a key molecule in signal transduction during BCR ligation (42). This phenomenon is apparently not linked to CpG-induced signaling, as we do not observe phosphorylation of BANK1 or changes in binding of BANK1 to BLK following CpG stimulation (data not shown). Nevertheless, we provide here the first hints of a role for BANK1 in CpG-induced signaling in the production of IL-6, which could be highly relevant in the study of autoimmunity and inflammation.
Supplementary Material
Acknowledgments
The authors would like to thank Huining Da and Farideh Movafagh for technical assistance.
Footnotes
The authors acknowledge the financial support from NIH (GM103456), Alliance for Lupus Research and OCAST.
References
- 1.Castillejo-Lopez C, Delgado-Vega AM, Wojcik J, Kozyrev SV, Thavathiru E, Wu YY, Sanchez E, Pollmann D, Lopez-Egido JR, Fineschi S, Dominguez N, Lu R, James JA, Merrill JT, Kelly JA, Kaufman KM, Moser KL, Gilkeson G, Frostegard J, Pons-Estel BA, D’Alfonso S, Witte T, Callejas JL, Harley JB, Gaffney PM, Martin J, Guthridge JM, Alarcon-Riquelme ME. Genetic and physical interaction of the B-cell systemic lupus erythematosus-associated genes BANK1 and BLK. Ann Rheum Dis. 2012;71:136–142. doi: 10.1136/annrheumdis-2011-200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokoyama K, Su Ih IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. Embo J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozyrev SV, Bernal-Quiros M, Alarcon-Riquelme ME, Castillejo-Lopez C. The dual effect of the lupus-associated polymorphism rs10516487 on BANK1 gene expression and protein localization. Genes Immun. 2012;13:129–138. doi: 10.1038/gene.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, Pasare C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Nat Acad Sci (USA) 2012;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 7.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targ. 2007;6:223–235. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 8.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem. 2002;277:3065–3068. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- 9.Bollig F, Winzen R, Gaestel M, Kostka S, Resch K, Holtmann H. Affinity purification of ARE-binding proteins identifies polyA-binding protein 1 as a potential substrate in MK2-induced mRNA stabilization. Biochem Biophys Res Com. 2003;301:665–670. doi: 10.1016/s0006-291x(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Narayanan K, Mizutani T, Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J Virol. 2002;76:5937–5948. doi: 10.1128/JVI.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morley SJ. Intracellular signalling pathways regulating initiation factor eIF4E phosphorylation during the activation of cell growth. Biochem Soc Trans. 1997;25:503–509. doi: 10.1042/bst0250503. [DOI] [PubMed] [Google Scholar]
- 12.Sonenberg N. eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem Cell Biol. 2008;86:178–183. doi: 10.1139/O08-034. [DOI] [PubMed] [Google Scholar]
- 13.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 15.Livingstone M, Larsson O, Sukarieh R, Pelletier J, Sonenberg N. A chemical genetic screen for mTOR pathway inhibitors based on 4E-BP-dependent nuclear accumulation of eIF4E. Chem Biol. 2009;16:1240–1249. doi: 10.1016/j.chembiol.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Kozyrev SV, Abelson AK, Wojcik J, Zaghlool A, Linga Reddy MV, Sanchez E, Gunnarsson I, Svenungsson E, Sturfelt G, Jonsen A, Truedsson L, Pons-Estel BA, Witte T, D’Alfonso S, Barizzone N, Danieli MG, Gutierrez C, Suarez A, Junker P, Laustrup H, Gonzalez-Escribano MF, Martin J, Abderrahim H, Alarcon-Riquelme ME. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet. 2008;40:211–216. doi: 10.1038/ng.79. [DOI] [PubMed] [Google Scholar]
- 17.Rowlett RM, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, Ernst PB, Pizarro TT, Sturgill TW, Worthington MT. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am J Physiol Gastro Liver Physiol. 2008;294:G452–G459. doi: 10.1152/ajpgi.00077.2007. [DOI] [PubMed] [Google Scholar]
- 18.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 19.Nagaleekar VK, Sabio G, Aktan I, Chant A, Howe IW, Thornton TM, Benoit PJ, Davis RJ, Rincon M, Boyson JE. Translational control of NKT cell cytokine production by p38 MAPK. J Immunol. 2011;186:4140–4146. doi: 10.4049/jimmunol.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 21.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38:400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poudrier J, van Essen D, Morales-Alcelay S, Leanderson T, Bergthorsdottir S, Gray D. CD40 ligand signals optimize T helper cell cytokine production: role in Th2 development and induction of germinal centers. Eur J Immunol. 1998;28:3371–3383. doi: 10.1002/(SICI)1521-4141(199810)28:10<3371::AID-IMMU3371>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito E, Fujimoto M, Hasegawa M, Komura K, Hamaguchi Y, Kaburagi Y, Nagaoka T, Takehara K, Tedder TF, Sato S. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002;109:1453–1462. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haxhinasto SA, Bishop GA. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J Biol Chem. 2004;279:2575–2582. doi: 10.1074/jbc.M310628200. [DOI] [PubMed] [Google Scholar]
- 26.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 27.Ronkina N, Kotlyarov A, Dittrich-Breiholz O, Kracht M, Hitti E, Milarski K, Askew R, Marusic S, Lin LL, Gaestel M, Telliez JB. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol Cell Biol. 2007;27:170–181. doi: 10.1128/MCB.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourey RJ, Burnette BL, Brustkern SJ, Daniels JS, Hirsch JL, Hood WF, Meyers MJ, Mnich SJ, Pierce BS, Saabye MJ, Schindler JF, South SA, Webb EG, Zhang J, Anderson DR. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor alpha production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J Pharmacol Exp Ther. 2010;333:797–807. doi: 10.1124/jpet.110.166173. [DOI] [PubMed] [Google Scholar]
- 29.Andersson K, Sundler R. Posttranscriptional regulation of TNFalpha expression via eukaryotic initiation factor 4E (eIF4E) phosphorylation in mouse macrophages. Cytokine. 2006;33:52–57. doi: 10.1016/j.cyto.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Shveygert M, Kaiser C, Bradrick SS, Gromeier M. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol Cell Biol. 2010;30:5160–5167. doi: 10.1128/MCB.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiba Y, Yamazaki T, Okada T, Gotoh K, Sanjo H, Ogata M, Kurosaki T. BANK negatively regulates Akt activation and subsequent B cell responses. Immunity. 2006;24:259–268. doi: 10.1016/j.immuni.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185:2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcocer-Varela J, Aleman-Hoey D, Alarcon-Segovia D. Interleukin-1 and interleukin-6 activities are increased in the cerebrospinal fluid of patients with CNS lupus erythematosus and correlate with local late T-cell activation markers. Lupus. 1992;1:111–7. doi: 10.1177/096120339200100209. [DOI] [PubMed] [Google Scholar]
- 34.Stuart RA, Littlewood AJ, Maddison PJ, Hall ND. Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol. 1995;13:17–22. [PubMed] [Google Scholar]
- 35.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 36.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoSONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Y, Wang YH, Diamond B. IL-6 contributes to an immune tolerance checkpoint in post germinal center B cells. J Autoimmun. 2012;38:1–9. doi: 10.1016/j.jaut.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, Tarlinton DM, Kallies A, Corcoran LM. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 41.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 42.Bernal-Quiros M, Wu YY, Alarcon-Riquelme ME, Castillejo-Lopez C. BANK1 and BLK Act through Phospholipase C Gamma 2 in B-Cell Signaling. PLoSONE. 2013;8:e59842. doi: 10.1371/journal.pone.0059842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.