Abstract
Background:
Continous spinal anesthesia (CSA) and frequently unilateral spinal anesthesia (USpA) are usually preferred for lower extremity surgeries. In this study, we aimed to compare the effects of these anesthetic techniques, on hemodynamic parameters, quality of anesthesia and complications in elderly patients undergoing hip surgeries.
Methods:
Forty patients aged 65 years and older, assigned to receive either CSA or USpA with 7.5 mg (1.5 cc) 0.5% hyperbaric bupivacaine initially. In CSA group, additional doses of 2.5 mg bupivacaine were applied until sensory block reach to T10. Maximum sensorial block level, time to reach the level of T10 (defined as onset time) and to regress to T12, hemodynamic parameters and ephedrine requirements were recorded peroperatively and during 2 h postoperatively.
Results:
Hemodynamic parameters, ephedrine requirements and regression of sensory block by two levels were similar in two groups. The onset time of anesthesia was significantly longer in USpA group than CSA group. Neuraxial anesthesia had to be converted to general anesthesia in 5 patients (25%) in CSA group and 1 patient (5%) in USpA group.
Conclusions:
We conclude that both USpA and CSA techniques have similar effects in elderly high risk patients. On the other hand, USpA is more preferable for surgeries with shorter durations due to its low cost and high success rate.
Keywords: Anesthesia, continous, elderly, spinal, unilateral
INTRODUCTION
Spinal anesthesia is often used for orthopedic surgery in the elderly. However, because of the high prevalence of medical problems and a reduction in physiologic compensatory mechanisms in these patients, spinal anesthesia is associated with a risk of severe and prolonged hypotension.
The technique of continous spinal anesthesia (CSA) is thought to have the advantage of providing greater control over anesthetic management than the conventional single-bolus needle injection technique.[1,2,3] CSA allows titration of small amounts of local anesthetic to achieve the appropriate level and provide adequate duration of anesthesia with only minimal hemodynamic changes;[4] thus, minimizing the risks of cardiovascular and respiratory disturbances.[5,6] Primary indications for CSA in elderly and high-risk patients are lower abdominal and lower limb surgery.[1,7,8,9]
Low dose local anesthetic solutions by using a pencil-point needle and slow intrathecal injection have been reported to obtain satisfactory unilateral spinal anesthesia (USpA), which should also minimize the cardiovascular effects of spinal block.[6,10]
Both CSA and USpA techniques allow the administration of small doses of local anesthetic and thus provide a more controllable sensory and sympathetic level of anesthesia.
The purpose of this study was to compare the hemodynamic consequences and the effectiveness of CSA versus USpA.
METHODS
After approval by the Medical Ethics Committee of the Sisli Etfal Training and Research Hospital and informed patient consent, 40 patients aged 65 years or over in the American Society of Anesthesiologists (ASA) physical status III undergoing elective hip surgery were included in this prospective, randomized study. Exclusion criteria were contraindications to spinal anesthesia, having peripheral neuropathy, neurological disturbances or disorder, comorbidities pre-dispose severe hypotension and/or severely altered mental status. Patients who had a history of having controlled hypertension with medical treatment (6 of the patients) were included the study.
Midazolam 1 mg and 50 μg fentanyl intravenous (IV) was used in all patients for pre-medication in pre-anesthetic care room, 45 min before the anesthetic procedure was performed. Heart rate, invasive arterial blood pressure (BP), peripheral oxygen saturation and electrocardiography were continously monitored with PETAS KMA-175 Monitor (PETAS, Istanbul, Turkey). An observer who were unaware the study groups recorded these parameters with 1-min intervals for the first 10 min, then at 5-min intervals. Furthermore, pre-anesthetic hydration consisted of 7 mL/kg crystalloid solution infused 30 min before the patient's arrival in the operating room.
Patients were randomly divided into two groups. Patients in group USpA were placed in the lateral position with the operative side in the dependent position. Dural puncture was performed using a 25-gauge Quincke point needle (Spinocan, Braun Melsungen, Germany) inserted in the midline at the L2-3 or L3-4 interspace under aseptic conditions. After dural puncture, the needle hole was turned toward the dependent side and 0.5% hyperbaric bupivacaine (Marcaine amp, 4 mL, Astra-Zeneca, Turkey) 7.5 mg was injected over 80 s. The lateral position was maintained for 15 min and then the patients were turned to the supine position.
Group CSA patients were placed in the lateral position. Lumbar puncture was performed by the midline approach at the L2-3 or L3-4 interspace with an 18-gauge Tuohy needle; a 22-gauge spinal cathether (Spinocath, Braun Melsungen, Germany) was then introduced 2-3 cm in a cephalad direction and patients were turned back to the horizontal supine position for the rest of the study. An initial dose of 7.5 mg 0.5% hyperbaric bupivacaine was injected. Ten minute later, if the level of sensory block was lower than T10, intermittent doses of 2.5 mg 0.5% hyperbaric bupivacaine were injected at 5-min intervals until a T10 or higher level of the block was achieved. The total dose of bupivacaine administered was limited to 15 mg.
The sensory block level was assessed by pinprick test and motor block was evaluated with a modified Bromage scale (0 = no motor block, 1 = hip flexion with extended leg blocked, 2 = knee flexion blocked, 3 = complete motor block) by a blinded observer at 2-min intervals until sensorial block level reached T10 and at 5 min intervals for 60 min. Time to reach the level of T10 and to maximum sensorial block level and regress to T12 was also noted.
General anesthesia was planned in the patients that we failed to perform both neuraxial anesthetic techniques (three unsuccessful attempts to reach to spinal space or insert the spinal catheter was defined as procedure failure) or had insufficient anesthesia (unable to reach T10 dermatome in both techniques was defined as insufficient anesthesia). The failure rate was defined as the patients’ percentage who had general anesthesia. Surgery was performed in lateral position. During the surgical procedure, all patients received oxygen by face mask at 2 “L/min,” lactated Ringer solution” 5 mL/kg/h,” and perioperative bleeding was immediately treated with either colloid infusion or paced red cells, depending on the hematocrit level (monitoring 30-32% hematocrit level). Any decrease in mean arterial pressure (MAP) below 30% of preoperative value was defined as hypotension and treated with a 5-mg ephedrine bolus. Bradycardia (defined as a heart rate under 40 beats/min) was treated with atropine 0.5 mg IV.
Catheters were removed from group CSA in the operating room at the end of surgery, then the patients were observed in the post-operative care unit for 1 h and side-effects (nausea, vomiting, bradicardia and hypotension) were recorded. Postdural puncture headache (PDPH) and late complications such as back pain and neurologic sequelae were investigated.
Statistical analysis
Sample size was calculated as minimum 15 patients, based on our preliminary results, to provide 90% power and α =0.05 to detect a mean difference in the MAP of 15 mmHg between two groups. We decided to study 40 patients to account for possible dropouts. The results are expressed as mean±standard deviation or as a median range for ordinal data. For statistical analysis, the t-test was used for comparison of normally distributed data and the Mann-Whitney U test was used for non-parametric values. The Chi-square test was used to compare the frequencies, with P<0.05 considered significant.
RESULTS
A total of 40 patients were studied. They were randomly divided into two groups of 20 patients each. Six patients had general anesthesia because of failure in USpA or CSA. In group USpA 1 patient and in group CSA 2 patients (10%) were not sufficiently anesthetized; in group CSA in 3 patients (15%), we failed to insert the catheter.
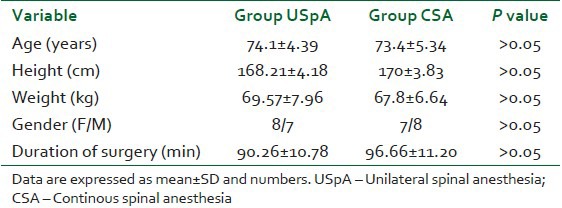
Both groups were comparable with regard to age, duration of surgery, height, ASA, physical status and gender ratio [Table 1].
Table 1.
Demographic characteristics of the patients, duration of surgery
The maximum sensory block level was not significantly different between the two groups, but the time to reach the T10 level was significantly longer in group USpA [Table 2]. Motor block was not significantly different between groups; no patients had a grade 0 Bromage score [Table 2].
Table 2.
Block characteristics and total bupivacaine consumption of the groups
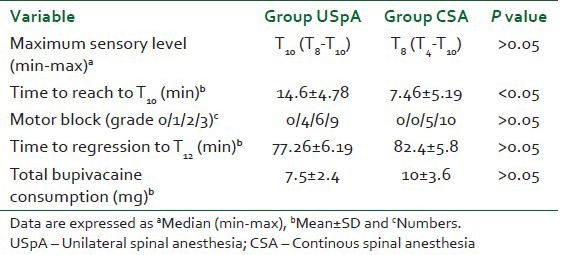
Failure rate was significantly higher in CSA group (25%) than USpA group (5%) (P<0.05).
The variation in MAP did not significantly differ between the two study groups. The significant decrease in the MAP was determined at 30, 45 and 60 min in the CSA group and at 90 min in the USpA group compared with baseline values [Figure 1]. Heart rate was similar in the two groups [Figure 2].
Figure 1.
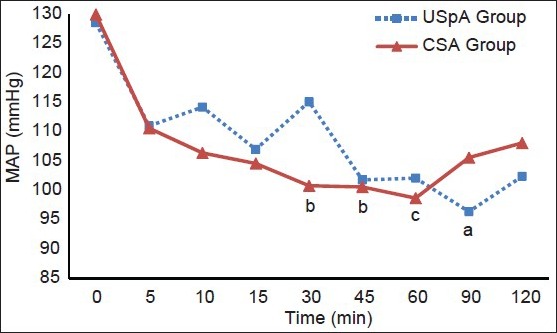
Mean arterial pressure values of the groups. USpA – Unilateral spinal anesthesia, CSA – Continous spinal anesthesia. aP<0.01, bP<0.05, cP<0.01 compared with 0 min
Figure 2.
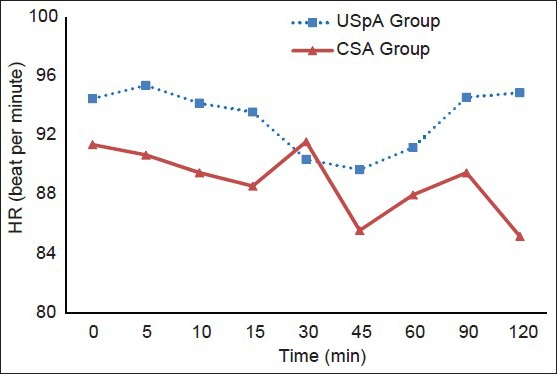
HR values of the groups. USpA – Unilateral spinal anesthesia, CSA – Continous spinal anesthesia
Four patients (20%) in group USpA and 3 (15%) in group CSA experienced at least one episode of severe hypotension. The mean dose of ephedrine was similar in both groups (8.3±2.8 mg in group USpA and 7.5±3.5 mg in group CSA). In the peroperative period, one CSA patient and one USpA patient experienced bradycardia and was treated with 0.5 mg atropine. In the post-operative period, no cardiovascular complications were observed in either group.
Nausea and vomiting was observed in 1 patient in group CSA.
The amount of perioperative crystalloid and colloid solutions did not differ among the groups.
No patients suffered from headache in the days following spinal anesthesia and no late complications were observed in either group.
DISCUSSION
In this study, we used CSA and USpA techniques with low dose hyperbaric bupivacaine for lower limb surgery in elderly patients. The primary end-point was the difference in hemodynamic parameters between the two groups. Other end-points were differences between the two groups with respect to the characteristics of anesthesia and complications in elderly patients. Both techniques produced suitable and comfortable anesthesia without severe hemodynamic changes, with comparable anesthesia characteristics and side effects. But USpA has the advantage of high success rate because it is technically simpler to perform than CSA.
In 6 patients, we had to perform general anesthesia. In 3 patients (15%) in group CSA, we failed to insert the catheter. All three were aged above 65 years and one of them had mild scoliosis. Similar with our study in Surange and Mohan study,[11] epidural catheter couldn’t be cited in patients with mild scoliosis, aged over 70 years. In another study by Lux[8] of a retrospective analyses of the cases had CSA for lower limb surgery, in 7,4% of the patients were not included the study due to failure of micro-catheter placement or lack of analgesia. In our study in group USpA, 1 patient (5%) and in group CSA 2 patients (10%) had insufficient anesthesia. Both patients had a history of a disk hernia surgery. These 6 patients were operated under general anesthesia. We concluded that old age, history of surgery or anatomical abnormalities of spine can be pre-disposing factors in failure of catheter insertion in neuraxial techniques in elderly patients.
The many theoretical advantages of CSA include good-quality blockade at low doses; good hemodynamic stability; rapid onset; better control of anesthesia level, intensity and duration; and no or lower risk of toxicity.[5,12,13] Clinical studies have shown that hemodynamic stability is greater with CSA than with other neuraxial anesthesia techniques,[1,2,3,8,14] but limited studies have been published on this technique for elderly patients. Few studies or case reports[9,10,15,16] have evaluated the efficacy and safety of CSA in this population, probably because of concerns about potential adverse effects-principally neurologic complications and PDPH. We also think that; the high failure rate of CSA compared with USpA as we had in our study can be evaluated as a disadvantage. The pre-disposing factors that we concluded in the previous paragraph should be taken into account in the high failure rate of CSA in elderly patients.
USpA aims to limit the distribution of spinal block to the operated side, because all operations involved only one lower limb. USpA is the use of small doses of local anesthetic solution injected by a directional, pencil-point needle with the patient in the lateral decubitus position for 15-20 min and the use of a hyperbaric solution. Compared with the conventional technique, it requires a bit longer preparation time, but it causes fewer hemodynamic side-effects and has higher cardiovascular stability, increased autonomy after surgery and better patient acceptance. It also reduces the incidence of clinically relevant hypotension. Finally, USpA has more stable cardiovascular parameters compared with conventional bilateral spinal block.[6,10,17]
We failed to find any randomized studies in the literature comparing CSA with USpA. In our study, we aimed to compare the effects of these two techniques on hemodynamic parameters, quality of anesthesia and complications in elderly patients.
In Imbelloni et al. study,[13] arterial hypotension was observed significantly more often in the combined spinal epidural anesthesia group compared with the CSA group. In Reisli et al. study,[18] there was a significant decrease in the MAP in the continuous epidural anesthesia group compared with the CSA group. In some other studies comparing CSA with other neuraxial techniques, the authors found less frequent and less pronounced decreases in MAP in the CSA groups.[2,4] In our study, MAP values were decreased in both groups, with the significant decrease occurring first at 30 min and at 90 min in the CSA and USA groups, respectively. In Bai et al. study,[19] a significant initial decrease was determined at 25 min in the CSA group, corroborating nearly all our data. Casati et al.[6] compared USpA and single-dose spinal anesthesia and found a significant difference in hypotension frequency. They also determined that there were minimal hemostatic changes when 0.5% hyperbaric bupivacaine was administered with USpA.[10] In our study, hemodynamic changes were similar in both groups. MAP, which decreased in tolerable ranges, was related to baseline in both groups. The relative hemodynamic stability of CSA and USpA in our study was considered to be a result of slow development of the sympathetic blockade.
Van Gessel et al.[20] reported that hyperbaric bupivacaine produced major hemodynamic consequences with high cephalad spread compared with both isobaric or hypobaric bupivacaine in CSA. The decrease in MAP was significantly more severe wýth the hyperbaric bupivacaine (30%). We used hyperbaric bupivacaine in both groups and in the CSA group the maximum decrease in MAP was 24%.
Because Lundorf et al.[3] monitored arterial BP invasively, they were able to treat arterial BP drops immediately. We also used invasive arterial BP monitoring; therefore, we could instantly interfere with the BP drops by using the ephedrine. For both groups, the ephedrine usage was similar. In our study, the mean dose of ephedrine was similar for both groups. Similar to our results, of the 19 high-risk patients administered CSA in the Vijayan et al. study, only 2 required ephedrine to treat hypotension.[21]
In our study, only one patient in group USpA had inadequate anesthesia during the peroperative period. However in group CSA, we failed to insert the catheter in 3 patients (15%), so surgery was completed under general anesthesia. As in our study, Lundorff et al.[3] had technical problems with the spinal catheter in 4 of 30 patients (13.3%). In CSA, orientation of the catheter tip appears to be a major factor in the distribution of isobaric and hyperbaric bupivacaine.[22] There seems to be a connection between the caudal direction taken by the catheter and the restricted diffusion of the dye solution.[20,23] A caudally orientated tip of an end-holed spinal catheter is a major factor in restricted block.[24]
The time to reach the level of maximum sensorial block was significantly longer in group USpA, being related to the time needed for subarachnoid distribution of the local anesthetic. In the CSA group, the catheter was introduced 3-4 cm in a cephalad direction and local anesthetic was injected, whereas in the unilateral group, the local anesthetic was injected toward the dependent side with the Quincke spinal needle. This probably reduced the mixing of local anesthetic molecules with the CSF, leading to a significant delay in the cephalad spread of the spinal block in the unilateral group compared with group CSA. In various studies, incremental injections of bupivacaine through a subarachnoid catheter produced an equally effective block with fewer cardiovascular changes than a single injection of the same dose of local anesthetic solution.[3]
CSA was initially described in 1907[25] for anesthesia practice and is now used in Europe when cardiovascular stability is desired in poor-risk patients undergoing lower limb and lower abdominal surgery,[1,8,21] but it is still an underutilized technique in modern anesthesia practice. The use of CSA is limited by concerns about the risk profile and absence of approved devices for continuous intrathecal infusion.[9] Major concerns about CSA are Cauda Equina syndrome and PDPH. After case reports of Cauda Equina syndrome were reported with the use of spinal micro-catheters for CSA, these micro-catheters were withdrawn from clinical practice in the United States and Australia,[21] but continued to be used in Europe with no further neurological sequelae. Current opinion however is that the reported Cauda Equina syndrome was due to the neurotoxic effects of lignocaine 5% that was used and not the micro-catheter per se.[21,25] Poor distribution of local anesthetic through the micro-catheter has also been blamed for Cauda Equina syndrome in CSA. With the advent of intermediate (over-the-needle) catheters and the low incidence of headaches and neurological symptoms, this technique has been gaining credibility. The Imbelloni et al. study[12] reported the possible safety of the new catheter with a large dose of hyperbaric 0.5% bupivacaine and hyperbaric 2% lidocaine in a physical status ASA III, citing a 78-year-old patient who underwent CSA for surgery for huge bilateral inguinal and umbilical hernias. In this case, the authors concluded that, with the administration of high doses of hyperbaric anesthetics through the new catheter, poor distribution or risk of Cauda Equina syndrome were not observed. In two recent studies, CSA was used in elderly patients without any meaningful adverse effects.[1,9] Nonetheless, authors concluded that potential complications would always be taken into account especially in this fragile population. In Lux study[8] also, 1,212 patients had a median age 61 (56-76) years no. case of Cauda Equina syndrome or other major neurologic complications were reported. In our study, we used an over-the-needle catheter and observed no PDPH or Cauda Equina syndrome in any patient.
The limitation of our study is the small size of the groups. We studied with the minimum number of the calculated sample size. CSA was a new technique for our daily practice. The high failure rate in CSA group was our concern. However, we think more randomized studies with the higher number of patients needed for definite conclusions in this subject.
In conclusion, we found that in elderly patients with a potentially high-risk for anesthesia, CSA and USpA are produced comparable hemodynamic changes with similar block characteristics and side-effects. Nonetheless the potential failure risk of CSA should be taken into account compared with USpA, which can be assumed by that reason an easier technique to perform in elderly patients. USpA can be offered as a more appropriate technique than CSA for this group of patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Goyal M, Taxak S, Kshetrapal KK, Goel MK. Continuous spinal anesthesia in a high risk elderly patient using epidural set. J Anaesthesiol Clin Pharmacol. 2011;27:139–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Favarel-Garrigues JF, Sztark F, Petitjean ME, Thicoïpé M, Lassié P, Dabadie P. Hemodynamic effects of spinal anesthesia in the elderly: Single dose versus titration through a catheter. Anesth Analg. 1996;82:312–6. doi: 10.1097/00000539-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Lundorff L, Dich-Nielsen JO, Laugesen H, Jensen MM. Single-dose spinal anaesthesia versus incremental dosing for lower limb vascular surgery. Acta Anaesthesiol Scand. 1999;43:405–10. doi: 10.1034/j.1399-6576.1999.430407.x. [DOI] [PubMed] [Google Scholar]
- 4.Klimscha W, Weinstabl C, Ilias W, Mayer N, Kashanipour A, Schneider B, et al. Continuous spinal anesthesia with a microcatheter and low-dose bupivacaine decreases the hemodynamic effects of centroneuraxis blocks in elderly patients. Anesth Analg. 1993;77:275–80. doi: 10.1213/00000539-199377020-00011. [DOI] [PubMed] [Google Scholar]
- 5.Maurer K, Bonvini JM, Ekatodramis G, Serena S, Borgeat A. Continuous spinal anesthesia/analgesia vs. single-shot spinal anesthesia with patient-controlled analgesia for elective hip arthroplasty. Acta Anaesthesiol Scand. 2003;47:878–83. doi: 10.1034/j.1399-6576.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- 6.Casati A, Fanelli G, Aldegheri G, Colnaghi E, Casaletti E, Cedrati V, et al. Frequency of hypotension during conventional or asymmetric hyperbaric spinal block. Reg Anesth Pain Med. 1999;24:214–9. doi: 10.1016/s1098-7339(99)90130-x. [DOI] [PubMed] [Google Scholar]
- 7.Ketelaars R, Wolff AP. Unexpected High Sensory Blockade during Continuous Spinal Anesthesiology (CSA) in an Elderly Patient. Case Rep Anesthesiol 2012. 2012 doi: 10.1155/2012/648921. 648921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lux EA. Continuous spinal anesthesia for lower limb surgery: A retrospective analysis of 1212 cases. Local Reg Anesth. 2012;5:63–7. doi: 10.2147/LRA.S35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krämer S, Wenk M, Fischer G, Möllmann M, Pöpping DM. Continuous spinal anesthesia versus continuous femoral nerve block for elective total knee replacement. Minerva Anestesiol. 2011;77:394–400. [PubMed] [Google Scholar]
- 10.Casati A, Fanelli G, Beccaria P, Aldegheri G, Berti M, Senatore R, et al. Block distribution and cardiovascular effects of unilateral spinal anaesthesia by 0.5% hyperbaric bupivacaine. A clinical comparison with bilateral spinal block. Minerva Anestesiol. 1998;64:307–12. [PubMed] [Google Scholar]
- 11.Surange PN, Mohan CV. Comparative evaluation of continuous lumbar paravertebral versus continuous epidural block for post-operative pain relief in hip surgeries. Anesth Pain. 2012;1:178–83. doi: 10.5812/kowsar.22287523.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imbelloni LE, Neto SG, Ganem EM. Continuous spinal anesthesia with high dose of local anesthetics. Rev Bras Anestesiol. 2010;60:537–43. doi: 10.1016/S0034-7094(10)70065-9. [DOI] [PubMed] [Google Scholar]
- 13.Imbelloni LE, Gouveia MA, Cordeiro JA. Continuous spinal anesthesia versus combined spinal epidural block for major orthopedic surgery: Prospective randomized study. Sao Paulo Med J. 2009;127:7–11. doi: 10.1590/S1516-31802009000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilhelm S, Standl T, Burmeister M, Kessler G, Schulte am Esch J. Comparison of continuous spinal with combined spinal-epidural anesthesia using plain bupivacaine 0.5% in trauma patients. Anesth Analg. 1997;85:69–74. doi: 10.1097/00000539-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kumar CM, Corbett WA, Wilson RG. Spinal anaesthesia with a micro-catheter in high-risk patients undergoing colorectal cancer and other major abdominal surgery. Surg Oncol. 2008;17:73–9. doi: 10.1016/j.suronc.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Döhler S, Klippel A, Richter S. Continuous spinal anesthesia in very elderly patients with high anesthesia risk in traumatologic-orthopedic and general surgery interventions. Anaesthesiol Reanim. 1999;24:157–63. [PubMed] [Google Scholar]
- 17.Casati A, Fanelli G, Cappelleri G, Leoni A, Berti M, Aldegheri G, et al. Does speed of intrathecal injection affect the distribution of 0.5% hyperbaric bupivacaine? Br J Anaesth. 1998;81:355–7. doi: 10.1093/bja/81.3.355. [DOI] [PubMed] [Google Scholar]
- 18.Reisli R, Celik J, Tuncer S, Yosunkaya A, Otelcioglu S. Anaesthetic and haemodynamic effects of continuous spinal versus continuous epidural anaesthesia with prilocaine. Eur J Anaesthesiol. 2003;20:26–30. doi: 10.1017/s026502150300005x. [DOI] [PubMed] [Google Scholar]
- 19.Bai NY, Guo QL, Liu Y. Comparison of continuous spinal and combined spinal-epidural anesthesia in patients for uterectomy. Hunan Yi Ke Da Xue Xue Bao. 2002;27:539–41. [PubMed] [Google Scholar]
- 20.Van Gessel E, Forster A, Gamulin Z. A prospective study of the feasibility of continuous spinal anesthesia in a university hospital. Anesth Analg. 1995;80:880–5. doi: 10.1097/00000539-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Vijayan R, Chan L, Raveenthiran R. Continuous spinal anaesthesia – Early experience in University Hospital, Kuala Lumpur. Med J Malaysia. 1995;50:401–10. [PubMed] [Google Scholar]
- 22.Puolakka R, Pitkänen MT, Rosenberg PH. Comparison of three catheter sets for continuous spinal anesthesia in patients undergoing total hip or knee arthroplasty. Reg Anesth Pain Med. 2000;25:584–90. doi: 10.1053/rapm.2000.16157. [DOI] [PubMed] [Google Scholar]
- 23.Chan VW, Smyth RJ. Radiographic examination of catheter position in restricted sacral block after continuous spinal anesthesia. Anesth Analg. 1992;75:449–52. doi: 10.1213/00000539-199209000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Biboulet P, Capdevila X, Aubas P, Rubenovitch J, Deschodt J, d’Athis F. Causes and prediction of maldistribution during continuous spinal anesthesia with isobaric or hyperbaric bupivacaine. Anesthesiology. 1998;88:1487–94. doi: 10.1097/00000542-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Moore JM. Continuous spinal anesthesia. Am J Ther. 2009;16:289–94. doi: 10.1097/MJT.0b013e3181729d2a. [DOI] [PubMed] [Google Scholar]


