Abstract
Background:
Postpartum hemorrhage (PPH) and anemia thereafter can be a life threatening condition in parturients undergoing lower segment cesarean section (LSCS), especially when anemia is present.
Aim:
The objective of this study was to assess two doses of Tranexamic acid (TXA) in reducing intra operative blood loss and incidence of PPH, in terms of both efficacy and safety profiles, when used prophylactically.
Methods:
A monocenter prospective case control double blind randomized study was carried out on a consecutive series of 90 anemic patients undergoing LSCS, with hemoglobin between 7-10 g percent. Three random groups were formed. Group T1 (n=30) received 10mg/kg TXA in 20 ml of 5% dextrose intravenously, while T2 group (n=30) received 15mg/kg. Group C (n=30) received a placebo. Drug was administered prophylactically 20 min before skin incision. Blood loss was measured from placental delivery up to 24 hours by method of weight and volume.
Staistical Analysis:
To compare quantitative data between two groups, t-test, and for more than two groups ANOVA was used. To compare the mean for non- parametric data between two groups Mann – Whitney test was used, while in case of more than two groups Kruskal – Wallis test was employed. Probability (p) value was considered significant when it was 0.05 or less.
Results:
TXA significantly reduced blood loss in both the study groups. Mean total blood loss was 527.17±88.666 ml, 376.83±31.961ml and 261.17±56.777 ml in group C, T1, and T2 respectively. While reduction of blood loss in T1 group compared to control group was 146.34±56.32ml, it was 262±31.51ml in T2 group. Difference between T1 and T2 was 115.66±24.81ml, which was statistically significant (P<0.05). Postoperative blood loss was insignificant in all three groups. Pre- and post-operative hemoglobin levels differed significantly when compared to control group. Blood transfusion was needed in two patients in the control group, whereas no patient in groups T1 and T2 needed transfusion (P=0.02). No significant adverse effect was seen in all the three groups.
Conclusion:
Hence, TXA was found to be effective in reducing blood loss and transfusion in anemic parturients undergoing LSCS. 15mg/kg dose of TXA was more efficacious than the 10mg/kg dose and without any undue increase in adverse events. Postpartum anemia is a public health problem worldwide and TXA could prove to be a very useful drug to prevent blood loss and transfusions in patients undergoing LSCS, especially in the anemic subgroup.
Keywords: Anemia, lower segment cesarean section, postpartum haemorrhage, tranexamic acid, blood sparing
INTRODUCTION
One of the most common surgeries encountered in women of child bearing age is cesarean section. The incidence of cesarean section is as high as 25-30% in many areas of the world.[1] Delivery by cesarean section can cause more complications than normal vaginal delivery. The most common complications documented are primary and secondary postpartum hemorrhage (PPH).[2] These in turn increase maternal mortality, the incidence being as high as 20%.[3] It is a well-known fact that these complications as well as maternal mortality statistics are quite higher in the developing world. Maternal mortality rates in India are estimated at 560/100,000 live births and PPH accounts for 35-56% of these deaths.[4] Anemia, with its very high prevalence, adds to this mortality. In India, anemia is directly or indirectly responsible for 40% of maternal deaths.[5] PPH also contributes to morbidity because patients may require blood transfusion with its inherent hazards. Approximately, 1% of women with spontaneous vaginal deliveries receive a blood transfusion, but the rate increases to about 5% for women undergoing lower segment cesarean section (LSCS).[6] A loss of up to 800-1000 ml is seen on an average during LSCS.[7] A major contributing factor to this loss is placental delivery, which is known to cause a hyperfibrinolytic state.[8,9,10] In developing countries like India where prevalence of anemia is as high as 70%, this loss could lead to serious morbidity and mortality.
Tranexamic acid (TXA) is an antifibrinolytic agent, which causes reversible and competitive blockade of the lysine binding sites in plasminogen molecules. It is a synthetic analog of the amino acid lysine[11] and its action is to reduce blood loss. TXA is widely in use in the field of obstetrics. Both antepartum and PPH are being treated by TXA extensively. In their study, Ducloy-Bouthors et al. demonstrated for the first time that TXA administered to women with overt PPH decreases blood loss and maternal morbidity.[12] Prevention of PPH is another indication where TXA has been used.[3,8,13,14] Varied doses of TXA ranging from 1 mg/kg to more than 100 mg/kg have been used in various surgeries.[14,15,16,17] Even in studies involving LSCS, the doses used were either a bolus of 1 gm[3,8,13,14] or 10 mg/kg[15] intravenously.
In this study, two doses of TXA were compared with placebo to compare their efficacy and safety profile in anemic parturients undergoing LSCS. The prophylactic blood sparing effect of TXA in both the intra-operative and post-operative period (PPH) was evaluated.
METHODS
After approval from the hospital ethical committee, this monocentre double-blind randomized, case controlled prospective trial was conducted from the year of 2009 to 2011. Inclusion criteria were ASA grade I and II patients, age more than 18 years and anemic patients with hemoglobin 7-10 g%. Any patient belonging to ASA physical status III and IV; patients having a history of coagulopathy or thrombo-embolism, or both; patients who had received Acenocoumerol or platelet antiaggregant such as Aspirin in the week before surgery or NonSteroidal Anti Inflammatory Drugs (NSAIDS) 2 days before surgery; patients having pre-operative plasma creatinine greater than 130 μmol/L; patients having a history of Myocardial Infarction (MI) or chronic arteriopathy or unstable angina in the previous 12 months; patients with the mental status preventing them from understanding the study prospectus; patients having a history of renal or hepatic impairment or any hypersensitivity to TXA and any patient refusing to be a participant were excluded from the study. No support from any pharmaceutical company was taken for this study.
After a detailed pre-anesthetic evaluation of selected patients, laboratory tests including hemoglobin level, hematocrit, blood sugar (fasting), electrocardiogram, blood urea, serum creatinine, prothrombin time, and International Normalised Ratio (INR) were carried out. Hemoglobin and hematocrit levels were ascertained for every patient on the day before surgery in the hospital laboratory. LSCS was carried out under subarachnoid block using 2-2.5 ml of 0.5% hyperbaric bupivacaine after an informed written consent. Blockade up to T4-T6 level was considered adequate level of anesthesia. After delivery of the neonate, 20 unit of oxytocin in 500 ml normal saline were given at the rate of 8 mU/min intravenously.
All consenting patients were recruited as a consecutive series to one of the three study groups of 30 patients each, on the basis of block random allocation protocol. Neither the patient nor the investigator was aware of the group assignment. An anesthesiologist not related to the study prepared the drug for every patient.
Groups were labeled as follows:
Group C (30) – 5 ml of distilled water in 20 ml of 5% dextrose
Group T1 (30) – TXA in the dose of 10 mg/kg in 20 ml of 5% dextrose
Group T2 (30) – TXA in the dose of 15 mg/kg in 20 ml of 5% dextrose.
The drug in all the groups was given intravenously over 20 min before skin incision.
Monitoring of the pulse rate, blood pressure, Pulse Oximetry (SpO2) and Electrocardiograph (ECG) was carried out every 2 min up to 10 min of starting the study drug; then every 5 min until the delivery of baby and thereafter every 15 min until the end of the surgery. Blood loss was measured intra-operatively and post-operatively up to 24 h. All material such as sponges, mops, pads, and drapes were weighed with an electronic weighing scale before and at the end of surgery. Volume of blood in the suction bottle was considered only after the placental delivery, to exclude any amniotic fluid volume. The quantity of intra-operative blood loss (ml) = (weight of the abdominal swabs and drapes after LSCS – weight of materials prior to LSCS) + (the volume in the suction bottle after placental delivery in ml). Post-operative blood loss was measured by weighing and numbering the vaginal pads used by the patient after completion of LSCS 2 hourly up to 6 h and then 6 hourly up to 24 h.
Uterine contractility, placental separation, neonatal condition and any side effect caused by TXA were noted. Intramuscular methylergometrine would be used as a rescue uterotonic treatment when required. Post-operative hemoglobin, hematocrit, serum creatinine, prothrombin time, and INR values were recorded at 24 h. All the parturients were encouraged to start early leg exercises and ambulation in the post-operative period.
Sample size was calculated based on a pilot study so as to keep the power of study more than 0.80. Demographic data were compared using the Chi-square test. To compare quantitative data between two groups t-test was used although ANOVA was used for more than two groups. To compare the mean for non-parametric data between two groups Mann-Whitney test was used, although Kruskal-Wallis test was used in case of more than two groups. Probability values were considered significant when values were 0.05 or less.
RESULTS
Excepted sample size was 30 patients each in all the three groups. There was no statistically significant difference among the groups in terms of the demographic profile. This has been shown in Table 1. Hemodynamic parameters such as pulse rate, blood pressure, respiratory rate and SpO2 were seen to be comparable after statistical analysis using the student t-test (P>0.05).
Table 1.
Demographic data
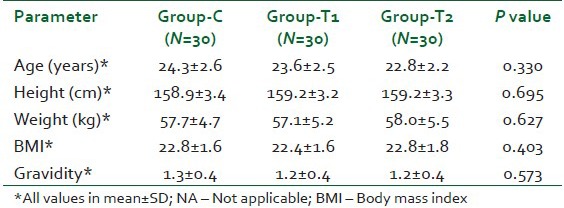
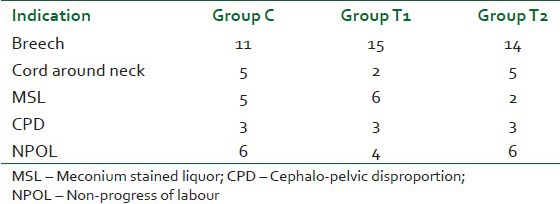
Indications of surgery were comparable in all the three groups. This is shown in Table 2. Breech presentation had the highest number in all three groups. There was a significant difference between the durations of surgery among the three groups, the longest being 67.80±9.704 min in group T2.
Table 2.
Indications of surgery
The total intra-operative blood loss seen in the three groups are shown in Table 3. The mean blood loss reduction when both groups were compared to the control group were 146.34±56.32 ml and 262±31.51 ml for group T1 and T2 respectively [Table 4]. Blood loss reduction in the T2 group when compared to the T1 group came to be 115.66±24.81 ml. F value of Analysis of Variance (ANOVA) for estimated blood loss was found to be 128.876 and the significance was 0.000.
Table 3.
Mean blood loss during surgery in all three groups
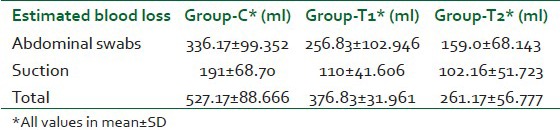
Table 4.
Difference in intra-operative blood loss between groups

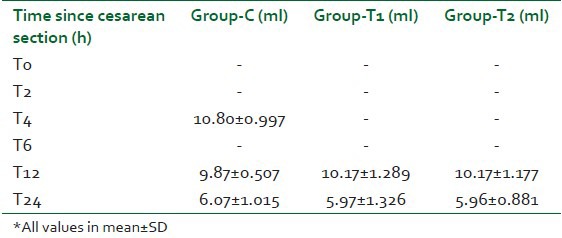
Post-operative blood loss was negligible in all the three groups with no statistically significant difference [Table 5]. Hence, there was no incidence of PPH in any of the patients. Blood transfusion was needed in two patients in the control group, there being a significant difference in comparison to groups T1 and T2 where no patient needed transfusion (P=0.02). Intramuscular methylergometrine had to be given in eight, three, and two patients in the control, T1 and T2 groups respectively.
Table 5.
Mean post-operative blood loss in all three groups
After statistical analysis of difference in hemoglobin values in the pre- and post-operative periods, P value came to be 0.00 when control and study groups were compared [Figure 1]. Same was the case with hematocrit. But when T1 and T2 groups were compared, the difference was significant for hematocrit (P=0.00) and not for hemoglobin.
Figure 1.
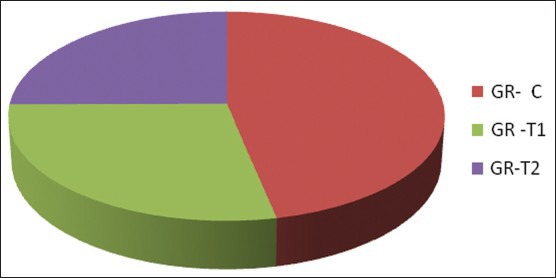
% Change in Hemoglobin from pre- to post-operative period. Gr-C – Group C, Gr-T1 – Group T1, Gr-T2 – Group T2
As far as adverse events are concerned, two patients each in group C and T2 and one patient in group T1 complained of nausea. There was no other significant adverse event such as deep vein thrombosis or other thrombotic events up to 24 h post-operatively. Serum creatinine and INR values were carried out post-operatively at 24 h and their values were comparable to the pre-operative values. Urine output was within normal limits in all patients.
DISCUSSION
TXA has been used to reduce the bleeding in a wide variety of surgeries. On searching for similar literature, we found a few studies where TXA has been used in LSCS patients. It has been found to be useful in PPH also, a Cochrane review (2011) regarding this being ample evidence.[18] Various doses ranging from 1 mg/kg to more than 100 mg/kg have been used in various surgeries as we have already discussed.[14,15,16,17] A double-blind randomized clinical trial by Karski et al. used TXA pre-operatively for prevention of bleeding after cardiopulmonary bypass in a very high dose of 10 g intravenously over 20 min before sternotomy followed by another 10 g infused intravenously over 5 h.[17] Even in studies involving LSCS cases, the doses used were either a bolus of 1gm[3,8,13,14] or 10 mg/kg[15] intravenously. 15 mg/kg doses were used in other surgeries like arthroplasties.[16] The time of administration of the drug in relation to the incision also varied in the different studies. Horrow et al. used prophylactic TXA in the dose range of 2.5-40 mg/kg in patients undergoing cardiac surgeries and concluded that the dose of 10 mg/kg followed by 1 mg/kg/h decreased bleeding after extracorporeal circulation. Larger doses did not provide additional hemostatic benefit in the study.[19] In the study by Karski et al. too, a high dose of infusion at the rate of 10 g intravenously over 5 h after a bolus of 10 g showed no additional benefit over placebo infusion.[17]
Placental delivery being a hyperfibrinolytic state, we decided to conduct a study using two doses of TXA in anemic parturients undergoing LSCS. In this study, there was a significant reduction in blood loss in both the groups using TXA when compared to the control group (P=0.00). When the two groups using the TXA, that is group T1 (10 mg/kg) and T2 (15 mg/kg), were compared the difference in reduction of blood loss was again significant, the mean reduction being 115±24 ml. T-test was used to analyze this and the P value was 0.00. Hence, the inference can be made that 15 mg/kg dose was more efficacious in reducing blood loss during LSCS. Previous studies attempting to find the reduction in blood loss after use of TXA in LSCS and vaginal delivery cases found it to be in the range of 32.5 ml to 92 ml.[3,8,11,18,20]
Changes in the hemoglobin and hematocrit values in the post-operative period were significantly less in the groups using TXA. Similar finding was shown in a randomized control trial by Sekhavat et al.[13] in patients undergoing LSCS in 2009. Blood transfusion requirements were also significantly reduced by both doses of the drug. Postpartum anemia is a public health problem with massive proportions in the world over – more so in developing countries like India. This study was conducted in the anemic subgroup of parturients and proved to be a very useful drug to prevent blood loss in these particular patients undergoing LSCS. These blood sparing effects of TXA would definitely be useful in reducing transfusion requirements in the anemic subgroup in which the incidence of transfusion generally tends to be high.
A study by Movafegh et al.[15] in 2011, found that the total oxytocin administration after the delivery of the fetus was less in the TXA group when compared to the control group. This finding was not measured in the present study. Like a similar study by Gungorduk et al.,[14] more additional uterotonic agents were needed in the control group in this study also. On cost analysis, TXA use was found to be much more cost-effective in comparison to blood transfusion.
There were a few limitations in this study. The blood loss that we calculated in our study did not include the amount collected in suction bottle before the placental delivery and hence the volume calculated would definitely be slightly less than the actual loss. Almost all similar studies that we came across have excluded the loss in suction bottle before placental delivery to exclude the amniotic fluid volume. Furthermore, the duration of surgeries in T2 group was significantly higher. The reason for this could be attributed to the fact that the skill and experience of the surgeons varied immensely. Blood loss, on the other hand, was significantly lower in this group in comparison to bothT1 and control group. This could mean that even though the chances of blood loss were increased with the longer duration of surgery, the 15 mg/kg dose was capable of reducing it with no significant increase in adverse events. The third problem that we saw in our study was that anemic patients are more prone to be encountered in unsupervised emergency LSCS cases. However, the timing of administration of the drug that was followed in our study would not be appropriate in cases like fetal distress where emergency LSCS has to be carried out.
CONCLUSION
In this study, TXA in doses of 10 mg/kg and 15 mg/kg, was found to be effective in significantly reducing blood loss and transfusion requirements in anemic parturients undergoing LSCS. A good safety profile was seen for both the doses. 15 mg/kg dose was more efficacious and without any undue increase in adverse events. Postpartum anemia is a public health problem with massive proportions in the world over – more so in developing countries like India. Hence, TXA could prove to be a very useful drug to prevent blood loss in patients undergoing LSCS, especially in the anemic subgroup where transfusion requirements can be brought down significantly. Further studies would however be useful and required to corroborate these findings with different doses of TXA and time of prophylactic administration of this drug in cases of LSCS.
ACKNOWLEDGMENTS
Deen Dayal Upadhyay Hospital. Study was conducted in the Department of Anaesthesiology, Deen Dayal Upadhyay Hospital, New Delhi, India.
Footnotes
Source of Support: Deen Dayal Upadhyay Hospital
Conflict of Interest: None declared.
REFERENCES
- 1.Kambo I, Bedi N, Dhillon BS, Saxena NC. A critical appraisal of cesarean section rates at teaching hospitals in India. Int J Gynaecol Obstet. 2002;79:151–8. doi: 10.1016/s0020-7292(02)00226-6. [DOI] [PubMed] [Google Scholar]
- 2.Abou Zahr C. 1st ed. Ch 4. Vol. 4. Boston (United States of America), Geneva, (Switzerland): Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1998. Antepartum and Postpartum Hemorrhage. [Google Scholar]
- 3.Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after caesarean section: A randomized case controlled prospective study. Obstet Gynaecol India. 2007;57(3):227–30. [Google Scholar]
- 4.Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: A joint collaboration between the United States and India. Int J Fertil Womens Med. 2004;49:91–6. [PubMed] [Google Scholar]
- 5.Kalaivani K. Prevalence and consequences of anaemia in pregnancy. Available from: http://www.icmr.nic.in/ijmr/2009/november/1125 . [PubMed]
- 6.Ekeroma AJ, Ansari A, Stirrat GM. Blood transfusion in obstetrics and gynaecology. Br J Obstet Gynaecol. 1997;104:278–84. doi: 10.1111/j.1471-0528.1997.tb11454.x. [DOI] [PubMed] [Google Scholar]
- 7.Healy TEJ, Knight PR. 7th edition. Churchill-Davidson; 2003. Obstetric Anaesthesia. Wylie and Churchill-Davidson's A Practice of Anaesthesia; p. 934. [Google Scholar]
- 8.Gai MY, Wu LF, Su QF, Tatsumoto K. A clinical observation of blood loss reduced by tranexamic acid during and after caesarean section: A multi-central trial, randomized trial. Eur J Obstet Gynecol Reprod Biol. 2004;112:154–7. doi: 10.1016/s0301-2115(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 9.Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–73. doi: 10.1111/j.1538-7836.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 10.Lynn M, Jeroukhimov I, Klein Y, Martinowitz U. Updates in the management of severe coagulopathy in trauma patients. Intensive Care Med. 2002;28:S241–7. doi: 10.1007/s00134-002-1471-7. [DOI] [PubMed] [Google Scholar]
- 11.Thorsen S, Clemmensen I, Sottrup-Jensen L, Magnusson S. Adsorption to fibrin of native fragments of known primary structure from human plasminogen. Biochim Biophys Acta. 1981;668:377–87. doi: 10.1016/0005-2795(81)90171-9. [DOI] [PubMed] [Google Scholar]
- 12.Ducloy-Bouthors AS, Jude B, Duhamel A, Broisin F, Huissoud C, Keita-Meyer H, et al. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15:R117. doi: 10.1186/cc10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Matern Fetal Neonatal Med. 2009;22:72–5. doi: 10.1080/14767050802353580. [DOI] [PubMed] [Google Scholar]
- 14.Gungorduk K, Yıldırım G, Asıcıoğlu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: A prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28:233–40. doi: 10.1055/s-0030-1268238. [DOI] [PubMed] [Google Scholar]
- 15.Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet. 2011;115:224–6. doi: 10.1016/j.ijgo.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemelä HM, Mäntylä SK, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–44. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Karski JM, Teasdale SJ, Norman P, Carroll J, VanKessel K, Wong P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid. Double-blind, randomized clinical trial. J Thorac Cardiovasc Surg. 1995;110:835–42. doi: 10.1016/S0022-5223(95)70118-4. [DOI] [PubMed] [Google Scholar]
- 18.Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2010;7:CD007872. doi: 10.1002/14651858.CD007872.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82:383–92. doi: 10.1097/00000542-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opin Pharmacother. 2011;12:503–16. doi: 10.1517/14656566.2011.545818. [DOI] [PubMed] [Google Scholar]


