Abstract
The participation of extranuclear steroid receptor signaling in organ physiology and the impact for pathobiology has increasingly been demonstrated. Important functions of membrane estrogen receptors in the cardiovascular system demonstrate new mechanisms of rapid steroid signaling to gene regulation, preventing cardiovascular disease and maintaining healthy arterial function. In cancer cells, kinase signaling initiated by extranuclear estrogen, progesterone, and androgen receptors modulates transcriptional events in the nucleus, which in turn regulate proliferation, migration, and invasion. Important mediators of cross talk between cytoplasmic and nuclear steroid receptor signaling are the proline-, glutamic acid-, and leucine-rich protein-1 and paxillin proteins, both of which modulate membrane and nuclear receptor pool signaling to promote a variety of cell biological functions.
Signaling by extranuclear steroid receptors has increasingly been shown to alter existing protein location and function, resulting in both transcription-independent (nongenomic) and transcription-dependent (genomic) actions (1). In this rapidly moving field, we highlight some recent developments and new concepts for the impact of these receptor pools in contributing to overall steroid hormone action.
Steroid Signaling to Functions in the Absence of Transcription
A major difficulty in studying the biological effects of nongenomic steroid signaling is that most physiologic processes (e.g. proliferation, differentiation, or growth) involve both extranuclear and intranuclear signaling. Separating these two processes can be problematic, especially because both pools of receptors frequently contribute to the genomic effects of steroid hormones. The simplest means of gaining insights for extranuclear receptor functions is to study biologically relevant systems that do not involve transcription. Two such systems are oocyte maturation and the sperm acrosomal response.
Oocyte maturation refers to a reentry into the meiotic cycle that occurs just before ovulation. In Xenopus laevis, oocyte maturation is triggered by steroids independent transcription. Therefore, Xenopus oocyte maturation serves as a valuable physiologically relevant model for studying nongenomic steroid signaling (2–4). Androgen signaling through classical androgen receptors (AR) located at or near the plasma membrane serves as the physiologic mediator of Xenopus oocyte maturation (4, 5). In vitro, progestins can also promote Xenopus oocyte maturation, likely through classical progesterone receptors (PR) signaling outside the nucleus (6, 7). Notably, progestins and androgens can also promote mammalian oocyte maturation via classical steroid receptors independent of transcription (8–13), although the absolute requirement of nongenomic steroid-mediated signaling for mammalian oocyte maturation has not been demonstrated. Fish oocytes also mature in response to progesterone, but evidence suggests that the nonclassical PR family termed membrane progesterone receptor (mPR) mediates this process (14, 15). It is not established that mPR have a similar function in mammalian oocytes.
Importantly, many concepts and pathways described in this transcription-independent steroid signaling system apply to more complex systems, where genomic and nongenomic steroid signaling occur simultaneously. For example, AR signaling at the oocyte plasma membrane suppresses constitutive Gαs and Gβγ signaling to decrease intracellular cAMP. This decrease results in activation of MOS (the germ cell equivalent of Raf) and subsequent Erk signaling (4, 5). As described elsewhere (1) and below, G protein and kinase signals are also triggered by steroids activating extranuclear classical steroid receptors in mammalian somatic cells.
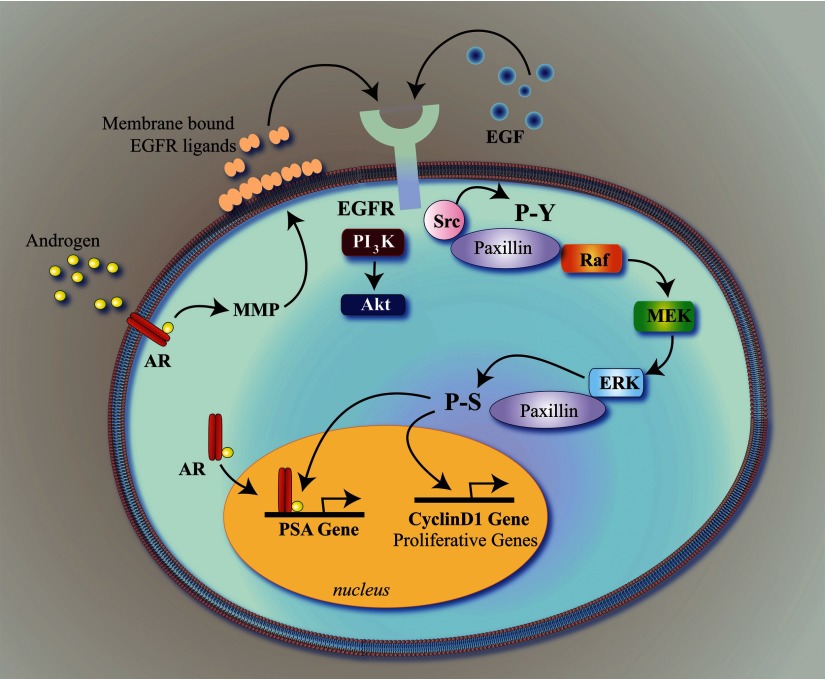
Notably, an important regulator of androgen-triggered Erk signaling and subsequent oocyte maturation is the protein paxillin. Paxillin is required for steroid-triggered expression and activation of MOS and downstream Erk signaling (Fig. 1). After activation, Erk phosphorylates paxillin on serine residues, and this alteration is necessary for cyclin activation and meiotic resumption (16). Thus, paxillin serves as both an affector and effector of Erk signaling.
Fig. 1.
Paxillin regulates androgen- and growth factor-mediated proliferation in prostate cancer cells as a liaison between extranuclear and intranuclear steroid receptor and kinase signaling. The EGFR is activated either directly by EGF or indirectly by androgen/AR-induced, matrix metalloproteinase (MMP)-mediated release of EGFR ligands. In addition to activating PI3K and Akt, EGFR activation triggers Src-mediated phosphorylation of paxillin on tyrosine residues, which promotes Raf/MEK/Erk activation. Erk then phosphorylates paxillin on serine residues, after which paxillin assists in the regulation of both intranuclear AR-mediated transcription (e.g. the PSA promoter), as well as Erk-mediated transcription of proliferative genes, such as cyclin D1. Notably, homologous pathways are likely triggered estrogens and progestins, as well as growth factors, in breast cancer cells. P-S, Phosphoserine; P-Y, phosphotyrosine.
Similar to oocytes, the male germ cells, sperm, also lack transcription and have been used for many decades as a model for transcription-independent steroid signaling. In the 1990s, progesterone was shown to promote the acrosome reaction in sperm (17). The acrosome reaction occurs just before fertilization of the egg and involves fusion of the cap-like structure over the anterior half of the sperm's head to the plasma membrane of the oocyte. The theory is that progesterone made in and released by cumulus granulosa cells surrounding the egg enhances calcium influx within the sperm (18). This calcium flux then promotes hyperactivation (increased motility), chemotaxis, and the acrosome reaction just before fertilization. The PR mediating this response has been difficult to define. Initially, progesterone receptor membrane component 1 was identified in mammalian sperm as a potential progesterone-binding protein; however, its role in regulating sperm function remains controversial (19, 20). In fish, evidence suggests that progesterone might bind to mPRα, which then signals through the stimulatory G protein Golf to enhance sperm hypermotility (21). However, whether these receptors function similarly in mammalian sperm is currently not known.
Interestingly, two recent papers examined calcium flux in human spermatozoa and showed that physiologic concentrations of progesterone enhance calcium influx by activating a pH-dependent calcium channel in sperm flagellum called CatSper (22, 23). This in turn helps improve fertilization by enhancing sperm hypermotility and the acrosome reaction. Unfortunately, the PR mediating calcium influx is still not known. However, these studies suggest novel targets for regulating sperm actions during fertilization that could lead to new advances in male contraception and fertility.
Progesterone Effects in Breast Cancer Cells
Many of the progesterone- and androgen-triggered pathways described in transcription-free germ cell systems are also present in somatic cell systems that use transcription independent and dependent functions. As in oocytes, progesterone triggers Erk activation in breast cancer cells. Here, classical PR located at or near the plasma membrane mediate rapid kinase signaling through either direct interactions with Src or trans-activation of the epidermal growth factor receptor (EGFR) (likely via matrix metalloproteinase-mediated release of EGFR ligands) (24–26). Progesterone-triggered extranuclear kinase signals profoundly effect PR-mediated transcription through kinase-mediated phosphorylation of the PR that leads to decreased receptor sumoylation and subsequent alterations in PR-mediated chromatin remodeling (27, 28). In the absence of progesterone, growth factor receptor-induced phosphorylation of nuclear PR occurring via Erk or casein kinase 2 can markedly increase or decrease transcription (29).
Thus, from rapid signaling, posttranslational alterations of PR have dramatic effects on nuclear PR-mediated transcription. Such actions modulate proliferation, migration, and invasion, emphasizing the biological importance of cross talk between extranuclear kinase activation and intranuclear transcription. Notably, in addition to Erk, progesterone rapidly enhances focal adhesion kinase activation through Src signaling, which promotes breast cancer cell motility and invasion (30). Paxillin is a known enhancer of both focal adhesion kinase and Erk activation in response to growth factors and extranuclear androgen and estrogen signals (16, 31, 32). Therefore, as with androgens, paxillin might also mediate nongenomic progesterone actions.
Androgen Effects in Prostate Cancer Cells
Similar to progestins in breast cancer cells, androgens bind classical AR at or near the membrane to rapidly trans-activate the EGFR and promote Erk, as well as Akt, signaling (31, 33–35). Erk activity is required for AR-mediated transcription, which is essential for steroid-triggered proliferation, migration, and invasion by prostate cancer cells (31, 35). Notably, as in frog oocytes, paxillin mediates AR-triggered Erk signaling in prostate cancer cells by regulating Raf activation. Subsequently, Erk phosphorylates paxillin, allowing paxillin to regulate important AR- and Erk-mediated transcriptional processes in the prostate cancer cell nucleus (31). As with AR and PR, paxillin regulates estrogen receptor (ER)-mediated extranuclear signaling and downstream breast cancer cell proliferation (32). In fact, paxillin regulates Erk activation and downstream Erk-mediated transcription in response to multiple inputs arising from growth factors and their receptors (e.g. EGF or fibroblast growth factor) (31). Thus, paxillin is a general liaison between extranuclear and intranuclear Erk functions (Fig. 1).
Plasma Membrane ER and the Cardiovascular System
Estrogen signaling from membrane ERα and ERβ in endothelial or other vascular cells stimulates nitric oxide (NO) production. NO mediates estrogen-induced vasodilation (36), prevents acute mechanical or electrical damage to the endothelium (37), and defends against age-related hypertension (the latter mediated through ERβ) (38). NO is produced from the rapid activation of the small G protein subunit, Gαi, by ER (39) and resulting stimulation of Erk and phosphoinositide 3-kinase (PI3K) (40). This signaling enhances the activity of the NO synthase enzyme, generating NO that acts both at the adjacent vascular smooth muscle layer and the endothelial lining of arteries.
As an additional function, ERβ has been implicated to protect female hearts from acute ischemia/reperfusion injury through PI3K/AKT signaling (41). Cardiomyocyte survival and function may be maintained from the resulting decreased caspases 3 and 8 levels and to increased Bcl-2 protein in the heart. Recent work from Murphy and co-workers (42) showed sex differences in the responses of rodent female vs. male cardiomyocytes and hearts to ischemia/reperfusion injury. The authors demonstrated decreased reactive oxygen species (ROS) generation only in the female cells/hearts due in part to PI3K-induced phosphorylation and decreased DNA damage from the activation of aldehyde dehydrogenase, an enzyme that detoxifies ROS-generated adducts (42). Furthermore, only the female cardiomyocyte mitochondria showed a protein kinase C-dependent phosphorylation and inhibition of α-ketoglutarate dehydrogenase. This enzyme produces ROS in response to ischemia/reperfusion injury in the heart. Whether the ER mediates these changes in female heart is not clear.
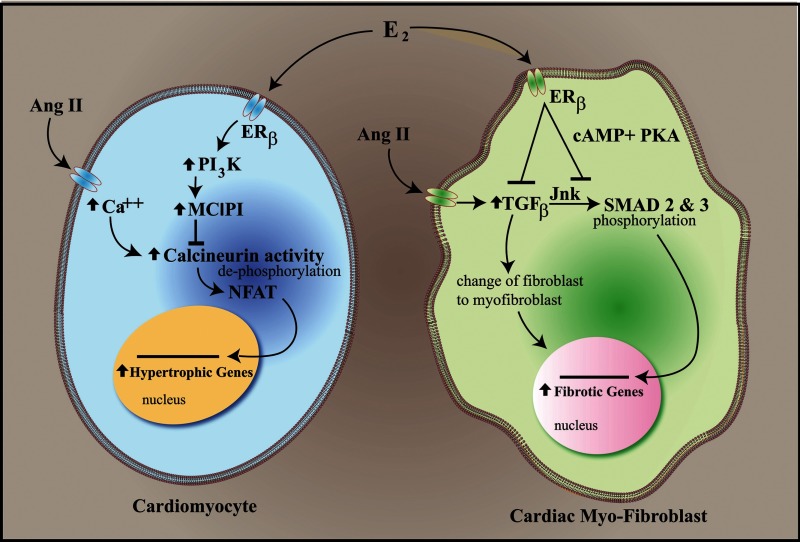
Estrogen protection from angiotensin II induced and other models of cardiac hypertrophy and fibrosis has been convincingly shown by several groups (43–45). Protection results from membrane ERβ stimulating a PI3K-dependent activation of the modulatory calcineuron interacting protein 1 gene, resulting in modulatory calcineuron interacting protein 1 protein production that blocks angiotensin activation of the calcineurin phosphatase (46). As a result, dephosphorylation of the important transcription factors, nuclear factor of activated T cells (NFAT)c2 and NFATc3, does not occur, and these proteins are sequestered in the cytoplasm. NFAT sequestration results in the prevention of cardiac gene trans-activation that produces hypertrophy (Fig. 2). Regarding cardiac fibrosis, membrane ERβ signaling in fibroblasts through cAMP and protein kinase A blocks the up-regulation of TGF-β production and subsequent c-Jun N-terminal kinase signaling. As a result, the key TGF-β-responsive transcription factors, SMAD2 and SMAD3, are not phosphorylated and do not traffic to the nucleus. Thus, key genes, such as collagens I and III, are not activated, preventing the in vivo fibrotic phenotype (Fig. 2) (47).
Fig. 2.
Estrogen repression of pathological gene transcription in the heart. E2 signals from the membrane ERβ to the alteration of transcription factor dephosphorylation (cardiac myocytes) or phosphorylation (cardiac fibroblasts) in cytoplasm. This prevents the transcription factors (NFAT and SMAD) from trafficking to the nucleus, thus preventing the induction of hypertrophic and fibrosis-inducing gene transcription in cardiomyocytes and cardiac myo-fibroblasts, respectively. Ang, Angiotensin; MCIP, modulator of calcium-induced phosphatase; PKA, protein kinase A; Jnk, c-Jun N-terminal kinase.
Estrogen inhibition of the genes that produce cardiac hypertrophy and fibrosis identifies a novel mechanism by which rapid signaling from the membrane prevents pathobiology. Membrane ER signaling to the posttranslational alteration of transcription factor phosphorylation dictates whether the proteins trans-locate to the nucleus and trans-activate the key target genes. Interestingly, both dephosphorylation and phosphorylation are regulated by membrane ER signaling, widening the many potential applications for this novel mechanism of gene repression (Fig. 2). It is also likely that in target organs such as brain and bone, kinase activation by membrane steroid receptors phosphorylates and promotes transcription factor import into the nucleus, thereby stimulating gene expression that is important to normal function and development.
Extranuclear Estrogen Effects in Breast Cancer
Similar to progesterone in breast cancer and androgens in prostate cancer, extranuclear ER signaling modulates intranuclear ER signaling to control proliferation, migration, and invasion. One important regulator of both extranuclear and intranuclear estrogen signaling in breast cancer cells is proline-, glutamic acid-, and leucine-rich protein-1 (PELP-1). PELP-1 has multiple functions as a scaffold for membrane-localized ER and AR interactions with signaling molecules such as Src and possibly even G proteins (48–50). Further, PELP-1 acts as a coactivator for nuclear sex steroid receptors (51). Recent studies have indicated that extranuclear ER rapid signaling in breast cancer cells promotes cytoskeletal reorganization and cell migration (52). This occurs when ER activates Src kinase to enlist a PELP-1/integrin-linked kinase signaling module involving PI3K. These components were required for the cell biological effects from 17-β-estradiol (E2)/membrane ER signaling. In the nucleus, PELP-1 is phosphorylated by cyclin-dependent kinases at serine 991, resulting in the up-regulation of E2F1 trans-activated genes. In this way, PELP-1 contributes to cell cycle progression in breast cancer (53). Interestingly, PELP-1 also collaborates with the histone lysine demethylase, KDM1, altering the histone substrate preference for KDM1 to histone 3, lysine 9, thereby altering the histone code at ERα target genes (54).
Estrogen Signaling Through Erk Impacts Nuclear ER Transcription
Madak-Erdogan et al. (55) have further refined the understanding as to how Erk participates in gene regulation induced by estrogen. These investigators describe the ability of nuclear ERα to recruit predominantly Erk2 to ER-binding sites throughout the genome of MCF-7 cells. This recruitment required Erk2 activation by E2, presumably from membrane ERα binding, establishing a collaborative basis between the receptor pools for gene regulation. The findings extend the initial description by Beato and co-workers (28) that membrane PR signaling through Erk is responsible for the vast majority of genes that were induced by nuclear PR in breast cancer cells, mediated in part by nuclear Erk/nuclear PR recruiting additional kinases that removed repressive protein complexes from histones at activated genes (56).
In addition, Madak-Erdogan et al. (55) found that the cAMP-response element-binding protein and transcription factor was also recruited to overlapping sites of ER and Erk2 binding to chromatin, and all three were needed to induce cell cycle/proliferation genes. How Erk precisely contributes to the regulation of the target genes was not determined. Further, whether Erk is recruited to the promoters, enhancer elements, or introns of repressed genes is currently unknown.
Estrogen Regulation of Insulin Secretion and Action
The ERα knockout (KO) mouse has a phenotype of insulin resistance and glucose intolerance but not fully developed diabetes mellitus (57). Recent studies have implicated E2 and ERα as modulating the long-term ability of glucose to stimulate insulin production and release from pancreatic β-cells (58). This may arise in part from ER-induced preservation of islet viability during various stresses that result in significant apoptosis of β-cells (59). Furthermore, a pancreas-specific ERα KO mouse showed loss of glucose-induced insulin gene up-regulation (60). Preservation of insulin gene transcription by E2 in wild-type mice required extranuclear ER signaling to Src and Erk kinases, increasing the binding of the NeuroD1 transcription factor to the insulin promoter.
Interestingly, ERβ also may modulate insulin secretion, perhaps in the acute situation. E2 binding to ERβ causes increased calcium flux that inhibits the ATP-regulated potassium channel function in pancreatic β-cells, leading to enhanced insulin secretion (61). This may occur indirectly, because ERβ appears to activate the natriuretic peptide GC-A receptor in some fashion, required for the stimulation of insulin secretion from the islet.
It is currently unclear, however, as to when the modulatory effects of estrogen are most important. The pancreatic-specific ERα KO mouse (60) was normal with regard to glucose homeostasis but was not evaluated in the setting of high-fat or glucose diets or in response to obesity or during pregnancy. Similarly, it is not clear under what circumstances ERβ function in islets may be important, but perhaps there is resistance to sex steroid function contributing to the glucose intolerance of late term pregnancy.
Peroxisome Proliferator-Activated Receptor γ (PPARγ) Effects in the Kidney
PPARγ is an orphan nuclear receptor that plays an important role in adipose, vascular, and immune cell development and function. Unlike steroid receptors, the physiologic ligands for PPARγ have not been well characterized but may be some form of fatty acid, phospholipid, or prostaglandin that binds with low affinity. However, members of the thiazolidinedione (TZD) family of pharmacological agents, such as rosiglitazone and pioglitazone, bind with high affinity to PPARγ and are used as insulin sensitizers in type 2 diabetes mellitus. Notably, recent work suggests that, as with classical steroid receptors, TZD signal through membrane-localized PPARγ in the renal proximal tubule to trans-activate the EGFR, stimulate Src and Erk signaling, and ultimately promote the rapid absorption of bicarbonate (62). Interestingly, this TZD effect is seen in rat, rabbit, and human proximal tubules but not in mice, because EGFR-Src signaling is constitutively active in murine kidneys. Perhaps these rapid extranuclear PPARγ signaling events, in collaboration with PPARγ-mediated transcriptional up-regulation of sodium transporters, play an important role in promoting the edema often associated with TZD use.
Of note, the retinoic acid receptor mediates activation of PI3K and subsequent induction of the sodium/iodide symporter in MCF-7 breast cancer cells, suggesting that additional orphan nuclear receptors may exist in extranuclear pools (63).
Summary
Recent work emphasizes the critical cross talk that occurs between extranuclear and intranuclear steroid signaling to regulate important physiologic processes. Given this tight relationship, dissecting the relative contribution of these compartmentalized signals for each process will continue to be difficult. However, new reagents, such as the estrogen dendrimeric compound (37) that binds only to the membrane pool of ER, and novel in vivo models, such as the membrane-only ERα mouse (64), offer exciting strategies that will help separate extranuclear from intranuclear signaling. These tools will justify targeting of specific receptor pools to produce favorable biology but avoid unwanted pathological effects of steroids, such as promoting hormone-responsive cancers. Notably, comparable models and reagents for other sex steroid receptors are also being developed. Furthermore, extranuclear signaling by glucocorticoid and mineralocorticoid receptors has been shown to impact various in vivo models, justifying unique reagent development for these areas. Finally, the presence of rapid signaling receptors for PPARγ and perhaps retinoic acid suggests that defining extranuclear pools of “orphan” receptors could lead to identifying endogenous ligands that have remained elusive.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- E2
- 17-β-estradiol
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- KO
- knockout
- mPR
- membrane progesterone receptor
- NFAT
- nuclear factor of activated T cells
- NO
- nitric oxide
- PELP-1
- proline-, glutamic acid-, and leucine-rich protein-1
- PI3K
- phosphoinositide 3-kinase
- PPARγ
- peroxisome proliferator-activated receptor γ
- PR
- progesterone receptor
- ROS
- reactive oxygen species
- TZD
- thiazolidinedione.
References
- 1. Hammes SR, Levin ER. 2007. Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 2. Smith LD, Ecker RE. 1969. Role of the oocyte nucleus in physiological maturation in Rana pipiens. Dev Biol 19:281–309 [DOI] [PubMed] [Google Scholar]
- 3. Maller JL, Krebs EG. 1980. Regulation of oocyte maturation. Curr Top Cell Regul 16:271–311 [DOI] [PubMed] [Google Scholar]
- 4. Hammes SR. 2004. Steroids and oocyte maturation–a new look at an old story. Mol Endocrinol 18:769–775 [DOI] [PubMed] [Google Scholar]
- 5. Lutz LB, Kim B, Jahani D, Hammes SR. 2000. G protein βγ subunits inhibit nongenomic progesterone-induced signaling and maturation in Xenopus laevis oocytes. Evidence for a release of inhibition mechanism for cell cycle progression. J Biol Chem 275:41512–41520 [DOI] [PubMed] [Google Scholar]
- 6. Tian J, Kim S, Heilig E, Ruderman JV. 2000. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci USA 97:14358–14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bayaa M, Booth RA, Sheng Y, Liu XJ. 2000. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc Natl Acad Sci USA 97:12607–12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. 2004. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod 71:366–373 [DOI] [PubMed] [Google Scholar]
- 9. Gill A, Jamnongjit M, Hammes SR. 2004. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol 18:97–104 [DOI] [PubMed] [Google Scholar]
- 10. Gill A, Hammes SR. 2007. Gβγ signaling reduces intracellular cAMP to promote meiotic progression in mouse oocytes. Steroids 72:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li M, Ai JS, Xu BZ, Xiong B, Yin S, Lin SL, Hou Y, Chen DY, Schatten H, Sun QY. 2008. Testosterone potentially triggers meiotic resumption by activation of intra-oocyte SRC and MAPK in porcine oocytes. Biol Reprod 79:897–905 [DOI] [PubMed] [Google Scholar]
- 12. Ning G, Ouyang H, Wang S, Chen X, Xu B, Yang J, Zhang H, Zhang M, Xia G. 2008. 3′,5′-cyclic adenosine monophosphate response element binding protein up-regulated cytochrome P450 lanosterol 14alpha-demethylase expression involved in follicle-stimulating hormone-induced mouse oocyte maturation. Mol Endocrinol 22:1682–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sen A, Hammes SR. 2010. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 24:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu Y, Bond J, Thomas P. 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasar M, DeFranco DB, Hammes SR. 2006. Paxillin regulates steroid-triggered meiotic resumption in oocytes by enhancing an all-or-none positive feedback kinase loop. J Biol Chem 281:39455–39464 [DOI] [PubMed] [Google Scholar]
- 17. Meizel S, Turner KO. 1991. Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol 77:R1–R5 [DOI] [PubMed] [Google Scholar]
- 18. Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. 1991. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl 12:323–330 [PubMed] [Google Scholar]
- 19. Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. 1999. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology 140:5999–6002 [DOI] [PubMed] [Google Scholar]
- 20. Lösel R, Breiter S, Seyfert M, Wehling M, Falkenstein E. 2005. Classic and non-classic progesterone receptors are both expressed in human spermatozoa. Horm Metab Res 37:10–14 [DOI] [PubMed] [Google Scholar]
- 21. Tubbs C, Thomas P. 2009. Progestin signaling through an olfactory G protein and membrane progestin receptor-alpha in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology 150:473–484 [DOI] [PubMed] [Google Scholar]
- 22. Lishko PV, Botchkina IL, Kirichok Y. 2011. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471:387–391 [DOI] [PubMed] [Google Scholar]
- 23. Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. 2011. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471:382–386 [DOI] [PubMed] [Google Scholar]
- 24. Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. 2007. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol 21:359–375 [DOI] [PubMed] [Google Scholar]
- 25. Faivre EJ, Lange CA. 2007. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol 27:466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skildum A, Faivre E, Lange CA. 2005. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol 19:327–339 [DOI] [PubMed] [Google Scholar]
- 27. Daniel AR, Lange CA. 2009. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci USA 106:14287–14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vicent GP, Nacht AS, Zaurín R, Ballaré C, Clausell J, Beato M. 2010. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol 24:2088–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hagan CR, Regan TM, Dressing GE, Lange CA. 2011. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol Cell Biol 31:2439–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu XD, Goglia L, Sanchez AM, Flamini M, Giretti MS, Tosi V, Genazzani AR, Simoncini T. 2010. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Cancer 17:431–443 [DOI] [PubMed] [Google Scholar]
- 31. Sen A, O'Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. 2010. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem 285:28787–28795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Wang JP, Santen RJ, Kim TH, Park H, Fan P, Yue W. 2010. Estrogen stimulation of cell migration involves multiple signaling pathway interactions. Endocrinology 151:5146–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auricchio F, Migliaccio A, Castoria G. 2008. Sex-steroid hormones and EGF signalling in breast and prostate cancer cells: targeting the association of Src with steroid receptors. Steroids 73:880–884 [DOI] [PubMed] [Google Scholar]
- 34. Migliaccio A, Castoria G, Di Domenico M, Ciociola A, Lombardi M, De Falco A, Nanayakkara M, Bottero D, De Stasio R, Varricchio L, Auricchio F. 2006. Crosstalk between EGFR and extranuclear steroid receptors. Ann NY Acad Sci 1089:194–200 [DOI] [PubMed] [Google Scholar]
- 35. Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, Baldi A, Barone MV, Appella E, Auricchio F. 2007. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene 26:6619–6629 [DOI] [PubMed] [Google Scholar]
- 36. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. 2005. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and β. J Biol Chem 280:19704–19710 [DOI] [PubMed] [Google Scholar]
- 37. Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. 2010. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120:2319–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. 2002. Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295:505–508 [DOI] [PubMed] [Google Scholar]
- 39. Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. 2007. Direct interactions with G alpha i and Gβγ mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol 21:1370–1380 [DOI] [PubMed] [Google Scholar]
- 40. Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. 1999. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang T, Tang W, Sun S, Ristagno G, Xu T, Weil MH. 2009. Improved outcomes of cardiopulmonary resuscitation in rats with myocardial infarction treated with allogenic bone marrow mesenchymal stem cells. Crit Care Med 37:833–839 [DOI] [PubMed] [Google Scholar]
- 42. Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. 2010. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106:1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Eickels M, Grohé C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 2001. 17β-Estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104:1419–1423 [DOI] [PubMed] [Google Scholar]
- 44. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. 2008. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology 149:3361–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. 2008. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res 77:774–781 [DOI] [PubMed] [Google Scholar]
- 46. Pedram A, Razandi M, Aitkenhead M, Levin ER. 2005. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. 2010. Estrogen receptor-β prevents cardiac fibrosis. Mol Endocrinol 24:2152–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. 2004. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol 18:1096–1108 [DOI] [PubMed] [Google Scholar]
- 49. Haas D, White SN, Lutz LB, Rasar M, Hammes SR. 2005. The modulator of nongenomic actions of the estrogen receptor (MNAR) regulates transcription-independent androgen receptor-mediated signaling: evidence that MNAR participates in G protein-regulated meiosis in Xenopus laevis oocytes. Mol Endocrinol 19:2035–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. 2004. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res 64:7156–7168 [DOI] [PubMed] [Google Scholar]
- 51. Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. 2004. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res 64:6416–6423 [DOI] [PubMed] [Google Scholar]
- 52. Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK. 2010. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res 70:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nair BC, Nair SS, Chakravarty D, Challa R, Manavathi B, Yew PR, Kumar R, Tekmal RR, Vadlamudi RK. 2010. Cyclin-dependent kinase-mediated phosphorylation plays a critical role in the oncogenic functions of PELP1. Cancer Res 70:7166–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nair SS, Nair BC, Cortez V, Chakravarty D, Metzger E, Schüle R, Brann DW, Tekmal RR, Vadlamudi RK. 2010. PELP1 is a reader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep 11:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M. 2006. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell 24:367–381 [DOI] [PubMed] [Google Scholar]
- 57. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mauvais-Jarvis F. 2011. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. 2006. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. 2010. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA 107:13057–13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soriano S, Ripoll C, Fuentes E, Gonzalez A, Alonso-Magdalena P, Ropero AB, Quesada I, Nadal A. 2011. Regulation of K(ATP) channel by 17β-estradiol in pancreatic β-cells. Steroids 76:856–860 [DOI] [PubMed] [Google Scholar]
- 62. Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, Shirai A, Nakamura M, Iso-O N, Li Y, Hara M, Tsukamoto K, Moriyama N, Kudo A, Kawakami H, Yamauchi T, Kubota N, Kadowaki T, Kume H, Enomoto Y, Homma Y, Seki G, Fujita T. 2011. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARγ-dependent nongenomic signaling. Cell Metab 13:550–561 [DOI] [PubMed] [Google Scholar]
- 63. Ohashi E, Kogai T, Kagechika H, Brent GA. 2009. Activation of the PI3 kinase pathway by retinoic acid mediates sodium/iodide symporter induction and iodide transport in MCF-7 breast cancer cells. Cancer Res 69:3443–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. 2009. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 284:3488–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]