Abstract
The chemopreventive activity of green tea (GT) is limited by the low bioavailability and extensive methylation of GT polyphenols (GTPs) in vivo. We determined whether a methylation inhibitor quercetin (Q) will enhance the chemoprevention of prostate cancer in vivo. Androgen-sensitive LAPC-4 prostate cancer cells were injected subcutaneously into severe combined immunodeficiency (SCID) mice one week before the intervention. The concentration of GTPs in brewed tea administered as drinking water was 0.07% and Q was supplemented in diet at 0.2% or 0.4%. After 6-weeks of intervention tumor growth was inhibited by 3% (0.2% Q), 15% (0.4% Q), 21% (GT), 28% (GT+0.2% Q) and 45% (GT+0.4% Q) compared to control. The concentration of non-methylated GTPs was significantly increased in tumor tissue with GT+0.4% Q treatment compared to GT alone, and was associated with a decreased protein expression of catechol-O-methyltransferase and multidrug resistance-associated protein (MRP)-1. The combination treatment was also associated with a significant increase in the inhibition of proliferation, androgen receptor (AR) and phosphatidylinositol 3-kinases (PI3K)/Akt signaling, and stimulation of apoptosis. The combined effect of GT+0.4% Q on tumor inhibition was further confirmed in another experiment where the intervention started prior to tumor inoculation. These results provide a novel regimen by combining GT and Q to improve chemoprevention in a non-toxic manner and warrant future studies in humans.
Keywords: Green tea, quercetin, prostate cancer, catechol-O-methyltransferase, SCID mice
1. Introduction
Green tea (GT) is produced from the leaves of the plant Camellia sinensis. The major bioactive components of GT are GT polyphenols (GTPs) mainly including (-)-epigallocatechin (EGC), (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin (EC), and (-)-epicatechin-3-gallate (ECG), with EGCG as the major component [1]. The chemopreventive activity of GT has been well documented in cell culture studies in vitro and in animal models against several types of cancers including prostate cancer [2, 3]. However, evidence from human studies is inconsistent [2]. Several clinical trial studies have suggested a promising role of GT in the chemoprevention of prostate cancer. Bettuzzi et al. demonstrated a reduced incidence of prostate cancer in men with prostatic intraepithelial neoplasia after a one-year GT supplement intervention compared to a group of men receiving placebo [4]. Likewise, in a single-arm pre-prostatectomy trial of a GT supplement, McLarty et al. demonstrated a decrease in serum prostate-specific antigen (PSA) levels and decreased prostate tissue vascular endothelial growth factor and hepatocyte growth factor concentrations [5]. However, several other studies failed to find an association between GT consumption and risk of prostate cancer [2].
The anti-cancer potency of GT is limited by the low bioavailability of GTPs. The high doses of GTPs necessary in laboratory studies can hardly be achieved in humans by the consumption of GT alone. The absorption from the intestine and retention of GTPs in tissues is regulated by several transporters including the multidrug resistance-associated proteins (MRPs). Modulation of the MRP activity with combination treatments provides an opportunity to enhance the bioavailability of GTPs [6]. The anti-cancer potency of GT is also limited by the rapid biotransformation of GTPs in the body leading to enhanced excretion and reduced bioactivity [7, 8]. Upon uptake, the non-gallated GTPs such as EGC and EC undergo extensive glucuronidation and sulfation while the gallated GTPs EGCG and ECG are mainly present in the free form [9]. All GTPs are readily methylated by catechol-O-methyltransferase (COMT) leading to an increase in urine excretion [10]. COMT is a widely distributed intracellular enzyme [11]. Previously we found that approximately 50 percent of EGCG was present in methylated form (4″-O-methyl EGCG, 4″-MeEGCG) in human prostate tissues obtained at prostatectomy after consumption of 6 cups of GT daily for 3-5 weeks [12]. Methylation significantly decreased the anticarcinogenic activity of EGCG in cultured LNCaP prostate cancer cells and Jurkat cells [12, 13].
Quercetin (Q) is a flavonoid found in most edible vegetables and fruits particularly in onions, apples, and red wine. The inhibitory effect of Q on the activities of MRPs and COMT has been well documented [14-17]. Q itself has been shown to exhibit chemopreventive activities especially in prostate cancer [18]. We were able to demonstrate in vitro that the combined use of Q with GT significantly increased the cellular concentrations of non-methylated EGCG in prostate cancer LNCaP and PC-3 cells, leading to enhanced anti-proliferative effects [19]. The present study was designed to test the hypothesis that the combined effect of Q and GT in vivo leads to an increased anticarcinogenic effect in a xenograft prostate tumor mouse model using severe combined immunodecificency (SCID) mice and to elucidate the mechanisms of the increased anticarcinogenic effect of the combination treatment.
2. Materials and Methods
2.1. Preparation of green tea and quercetin diet
GT was freshly prepared thrice a week on Monday, Wednesday and Friday by brewing one tea bag in 240 mL of boiling water (pH 3) for 5 minutes. Tea bags (authentic GT) were generously provided by Celestial Seasonings (Boulder, CO). The composition of GTPs in the brewed tea in mg/L was: EGC 204 ± 4, EGCG 388 ± 12, EC 44 ± 2, ECG 64 ± 7 and catechin 7 ± 1. GT was administered as drinking water ad libitum. Q (Sigma-Aldrich, St Louis, MO) was supplemented in AIN-93G diet at a concentration of 0.2% or 0.4% which was customized by Dyets Inc. (Bethlehem, PA).
2.2. Animal study
All procedures carried out in mice were approved by the UCLA Animal Research Committee. Male SCID mice (Charles River Laboratories) were bred in a pathogen-free colony. Mice were fed a sterilized AIN-93G diet (Dyets Inc.) and water for seven days. In the first study, mice were inoculated subcutaneously with 5×105 androgen-sensitive LAPC-4 prostate cancer cells. One week later when tumors reached a volume of around 10mm3 the intervention treatment started. Mice were randomly assigned to one of the six groups (n= 12 per group) including 1) control, receiving AIN-93G diet + water; 2) GT, receiving AIN-93G diet + brewed tea; 3) low dose (LD) Q, receiving 0.2% quercetin diet + water; 4) high dose (HD) Q, receiving 0.4% quercetin diet + water; 5) GT + LD Q, receiving 0.2% quercetin diet + brewed tea; and 6) GT + HD Q group, receiving 0.4% quercetin diet + brewed tea. Tumor size was measured twice a week with calipers. Tumor volume was calculated using the formula: length × width × height × 0.5236 [20]. Mouse body weight was measured once a week. After 6-weeks intervention all the mice were sacrificed and xenograft tumors were collected for biomarker analysis.
In a second study, the intervention treatment was started two weeks prior to the LAPC-4 cell inoculation. Mice were randomly assigned to four groups (n=10 per group) including the control, GT, 0.4% Q, and GT + 0.4% Q groups, receiving the same treatment as described in the first study, respectively. The treatment continued for 6 weeks after the tumor inoculation.
2.3. Analysis of tissue GTPs and Q
Five tumor samples were randomly selected from each group of the first intervention study. The method for the detection of tissue GTPs and Q was described previously [21]. Briefly, 150 mg of tumor tissues were homogenized and incubated with 1,000 units of β-glucuronidase (G7896, Sigma-Aldrich) and 40 units of sulfatase (S-9754, Sigma-Aldrich) buffered in 300 μL of 0.5 M phosphate buffer (pH 5.0) at 37°C for 45min to digest the conjugated forms into free forms. Then the homogenate was extracted by four times with 1mL of ethyl acetate and the pooled supernatant was dried in vacuum and reconstituted for high-performance liquid chromatography (HPLC) and CoulArray electrochemical detection (ESA, Chelmsford, MA).
2.4. Tissue microarray and immunohistochemical analysis of cell proliferation
The following mechanistic investigations focused on the combination treatment of 0.4% Q with GT using tumor tissues collected from the first intervention study, considering the stronger anti-tumor activity observed with this combination than 0.2% Q+GT treatment to provide a clearer picture of molecular changes. A section of each tumor was fixed in 10% phosphate buffered formalin and paraffin-embedded for tissue microarray and immunohistochemical determinations. Tissue microarray is a high-throughput technique where hundreds of tissue specimens (cores) are assembled in array fashion to allow multiplex histological analysis [22]. The tissue arrays were assembled by Dr. Jiaoti Huang's laboratory in the Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA. A semi-automated tissue arrayer (VTA-110CC, Veridiam, CA) was used to construct the tissue array blocks. A total of 6 cylindrical cores 1.0 mm in diameter were transferred from each donor block to two new paraffin (array) blocks. Four-micron sections were cut from each array for staining. The slides were prepared for immunohistochemistry by deparaffinizing and dehydrating using Hemo-De (Fisher Scientific, Pittsburgh, PA) and followed by an alcohol series and washing in PBS, pH 7.0. All samples were incubated in 3% H2O2 for 20 min at room temperature to eliminate endogenous peroxidase activity, and antigens was retrieved by boiling the slides in a microwave oven in 50 mM citrate buffer, pH 6.4 [23]. After blocking with goat serum the slides were incubated with monoclonal Ki67 antibody (DAKO North America Inc., Carpineteria, CA). The slides were counterstained with hematoxylin. Slides were digitized on a ScanScope AT (Aperio Technologies, Inc., Vista, CA) and morphimetric analysis performed with Definiens' Tissue Studio (Definiens Inc., Parsippany, NJ) to determine the percentage of Ki67 positive cells in a non-biased method. Briefly, using the pre-defined nuclear detection module and classification tool, positive and negative nuclei within each core were identified. Thresholds were set to classify hematoxylin stain for negative nuclei and DAB stain for positive nuclei. The data were exported to Excel for further statistical analysis. Scanning and analyses were performed through the Translational Pathology Core Laboratory, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA.
2.5. Western blot analysis of protein expression
Total protein was extracted from tumor tissues using RIPA lysis buffer (Santa Cruz, CA) according to manufacturer's instruction. The Western blot procedure was described previously [21]. Briefly, 50 μg of protein was separated on a 4-12% Bis-Tris gel (Invitrogen, Carlsbad, CA), electrotransferred to nitrocellulose membranes and blocked in Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk for 1 hour at room temperature. Membranes were incubated with primary antibodies for the detection of COMT (sc-25844), MRP1 (sc-7773-R), Bax (sc-493), Bcl-2 (sc-509), AR (sc-815), PSA (sc-7638, Santa Cruz, CA), Akt (#4685) and p-Akt (#4058, Cell signaling, Danvers, MA), respectively. GAPDH protein served as loading control. Images were visualized and quantified using a ChemiDoc XRS chemiluminescence detection system (Bio-Rad Laboratories, Hercules, CA).
2.6. Quantitative real-time PCR analysis of COMT and MRP1 gene expression
The primers and fluorogenic probes for the real-time polymerase chain reaction (PCR) analysis of COMT and MRP1 mRNA expression were provided by TaqMan Gene Expression Assay kit (ID: Hs02511558_s1 for COMT and Hs02514110_s1 for MRP1) (Applied Biosystems, Foster City, CA). The PCR procedure was described previously [24]. Briefly, mRNA was transcribed to cDNA by SuperScript III Reverse Transcriptase (Invitrogen). The final volume of the 20 μL real-time PCR mixture contained 2 μL of cDNA template, 1 μL of 20× primer and probe mixture, 10 μL of 2× TaqMan Universal PCR MasterMix (Applied Biosystems), and 7 μL of nuclease-free water. PCR amplification was performed on a 7900HT Fast Real-Time System (Applied Biosystems). Each sample was in triplicate, and non-template negative controls were included in each run. The 2−(ΔΔCt) method was used to normalize the expression of COMT in each sample to GAPDH expression and to compare to the average ΔCt value.
2.7. Statistical analysis
SPSS (Version 20, Chicago, IL) was used for statistical analyses. Data were expressed as mean ± standard deviation (SD). Comparison of means was performed by two independent samples t-test, or one-way analysis of variance with Tukey's posttest. Differences were considered significant if P<0.05.
3. Results
3.1. GT and Q in combination enhanced the inhibition of tumor growth
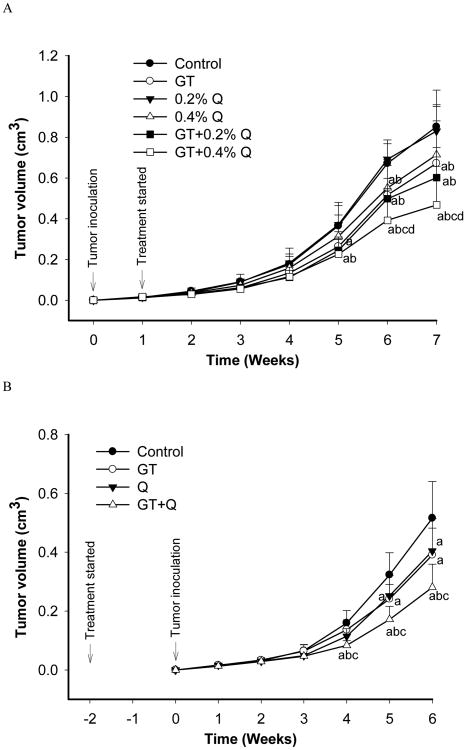
In the first experiment the intervention treatment started one week post tumor inoculation when the tumors reached a size of 10mm3, and lasted for six weeks. A significant inhibition of tumor growth by 38% was observed 5 weeks after the tumor inoculation (4 weeks post intervention) by the co-treatment of GT and 0.4% Q as compared to control (Figure 1A). During the next two weeks GT+0.4% Q treatment demonstrated a significantly stronger inhibition of tumor growth than GT or Q alone. The tumor growth was inhibited by 3% (0.2% Q), 15% (0.4% Q), 21% (GT), 28% (GT+0.2% Q) and 45% (GT+0.4% Q) 7 weeks post tumor inoculation (6 weeks post intervention) compared to control (Figure 1A). In the second study the pre-treatment started two weeks earlier than tumor inoculation. The combination treatment of GT and 0.4% Q demonstrated a stronger inhibition of tumor growth than GT or Q alone after 4-weeks of tumor inoculation, and inhibited the tumor growth by 47% compared to control (Figure 1B). At the end of the intervention the tumor growth was inhibited by 24% (0.4% Q), 21% (GT), and 46% (GT+0.4% Q) compared to control (Figure 1B). There was no difference in the amount of daily consumption of food or water among groups during the interventions. There was no intervention-related weight loss in the treatment groups as compared to control. Mice were physically active with smooth fur during the interventions.
Fig. 1.
GT and Q in combination enhanced the inhibition of xenograft tumor growth in SCID mice. SCID mice were inoculated subcutaneously with 5×105 androgen-sensitive LAPC-4 prostate tumor cells. The intervention treatments started either one week after the inoculation when tumors were established (A, n=12 per group) or two weeks before the inoculation (B, n=10 per group). Tumor size was measured using caliper and volume calculated using the formula: length × width × height × 0.5236. Data are presented as mean ± SD. Letters represent significant difference between groups (P<0.05) when compared to a- control group; b- 0.2% Q group; c- 0.4% Q group; and d- GT group.
3.2. Increased bioavailability and decreased methylation of GTPs
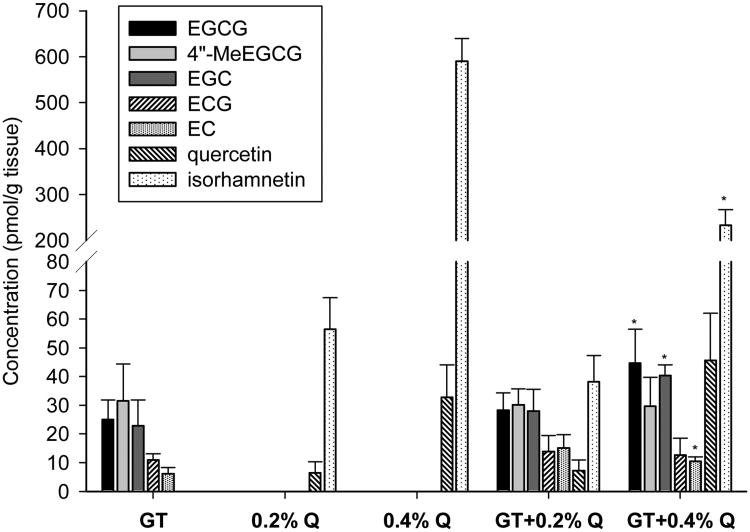
The concentrations of GTPs, Q and their metabolites in the xenograft tumor tissues were detected using HPLC and electrochemical detection. There were no GTPs, Q or their metabolites detectable in the control group. All the major GTP components including EGCG, its methyl metabolite 4″-MeEGCG, EGC, ECG and EC existed in the tumor tissues treated with GT or GT + Q (Figure 2). The co-treatment of 0.4% Q with GT significantly increased the tissue concentrations of total GTPs by 1.5 fold compared to GT alone. The concentration of non-methylated EGCG was increased by 1.8 fold, while the percentage of 4″-MeEGCG in total EGCG was decreased from 56% to 40%. In addition, the methylation of Q was decreased from 95% to 84% as represented by the percentage of its major methyl metabolite isorhamnetin by the combination of GT and 0.4% Q compared to Q alone (Figure 2). However, the co-treatment of 0.2% Q with GT did not change the tissue concentrations of GTPs compared to GT alone (Figure 2).
Fig. 2.
GTPs, Q and their metabolites in tumor tissues. Five tumor samples were randomly selected from each groups of the first intervention study. Tumor tissues were homogenized, enzyme treated, and GTPs, quercetin and their metabolites detected by HPLC and Coularray electrochemical detection. Data are presented as mean ± SD. *P<0.05 compared to individual treatment of GT or Q.
3.3. Increased inhibition of COMT protein and mRNA expression and MRP1 protein expression
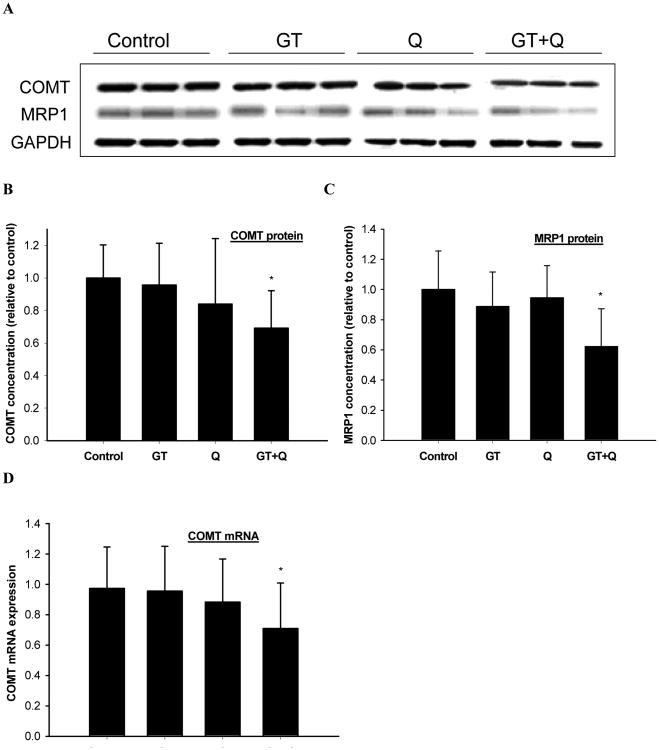
The individual treatment of GT or 0.4% Q did not significantly change the protein expression of COMT or MRP1 in the tumor tissues (Figure 3A, B, C). Whereas a significant decrease in both COMT and MRP1 protein expression was observed with the combination treatment of GT and Q compared to control (Figure 3A, B, C). Real-time qPCR analysis showed that the mRNA level of COMT in the tumor tissues was also significantly decreased by GT plus Q treatment while there was no effect with the individual treatments compared to control (Figure 3D). However the MRP1 mRNA expression was not changed by GT, Q or their combination (data not shown).
Fig. 3.
Enhanced inhibition of COMT protein and mRNA expression and MRP1 protein expression by GT and Q in combination. The protein expression of COMT (A, B) and MRP1 (A, C) in tumor tissues (n=10 per group) was detected by Western blot and COMT mRNA expression (D) by quantitative real-time PCR. Data are presented as mean ± SD. *P<0.05 compared to control.
3.4. Increased inhibition of proliferation and stimulation of apoptosis
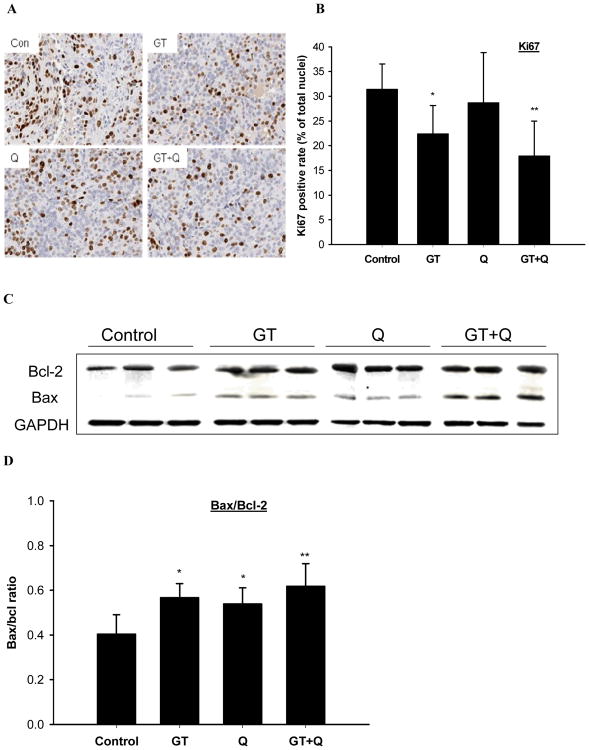
Tissue microarray analysis demonstrated that the individual treatment with GT significantly inhibited Ki67 protein expression by 29% compared to control, while Q alone did not show a significant effect (Figure 4A, B). The combination treatment of GT and Q inhibited Ki67 expression by 43% compared to control (Figure 4A, B). The apoptosis stimulatory effect was assessed based on the ratio of Bax to Bcl-2 protein expression in the tumor tissues. There was no significant difference in Bcl-2 protein expression among groups, whereas the ratio of Bax/Bcl-2 was significantly increased by 1.4-fold and 1.3-fold with the individual treatment of GT and Q, respectively (Figure 4C, D). The combination treatment of GT and Q demonstrated a trend to further increase the ratio of Bax/Bcl-2 compared to individual treatments (Figure 4C, D).
Fig. 4.
Increased inhibition of proliferation and induction of apoptosis by combing GT and Q. A section of tumor tissue was formalin-fixed and paraffin-embedded for tissue microarray and immunohistochemical detections. Slides were cut from the arrays and incubated with primary Ki67 antibodies for Ki67 (A, B). The slides were counterstained with hematoxylin. Nuclei were stained in blue and Ki67 in brown color. The positive rates of Ki67 nuclear staining are presented as mean ± SD. The protein expression of Bax and Bcl-2 in tumor tissues was detected by Western blot (C). The ratios of Bax to Bcl-2 are presented as mean ± SD (D). n=10 per group. *P<0.05, **P<0.01 compared to control.
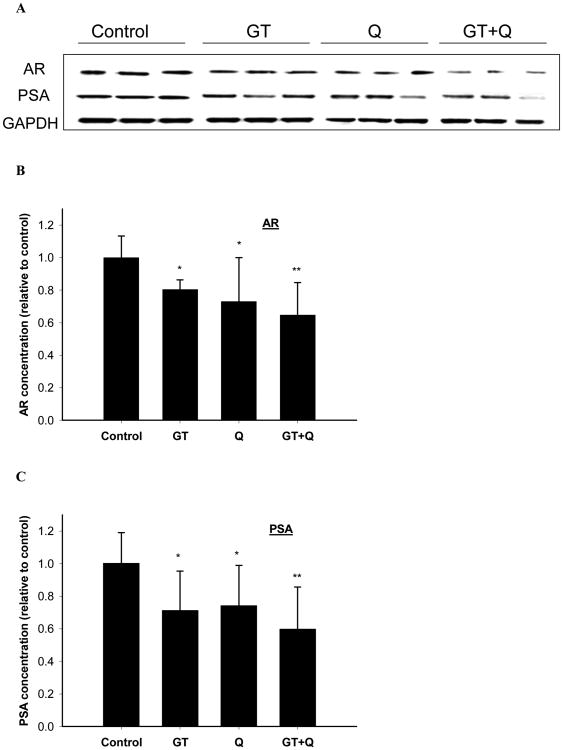
3.5. Inhibition of AR signaling
Both GT and 0.4% Q treatments significantly inhibited the protein expression of AR in the tumor tissues (Figure 5A, B). The co-treatment of GT and Q demonstrated a trend to increase the inhibition of AR expression compared to the individual treatments (Figure 5A, B). Consistent with the AR changes the PSA protein expression in the tumor tissues was also significantly decreased by GT and Q alone, and further inhibited by the combination of GT and Q (Figure 5A, C).
Fig. 5.
Enhanced inhibition of AR and PSA protein expression by GT plus Q treatment. The protein expression of AR (A, B) and PSA (A, C) in tumor tissues (n=10 per group) was detected by Western blot. Data are presented as mean ± SD. *P<0.05, **P<0.01 compared to control.
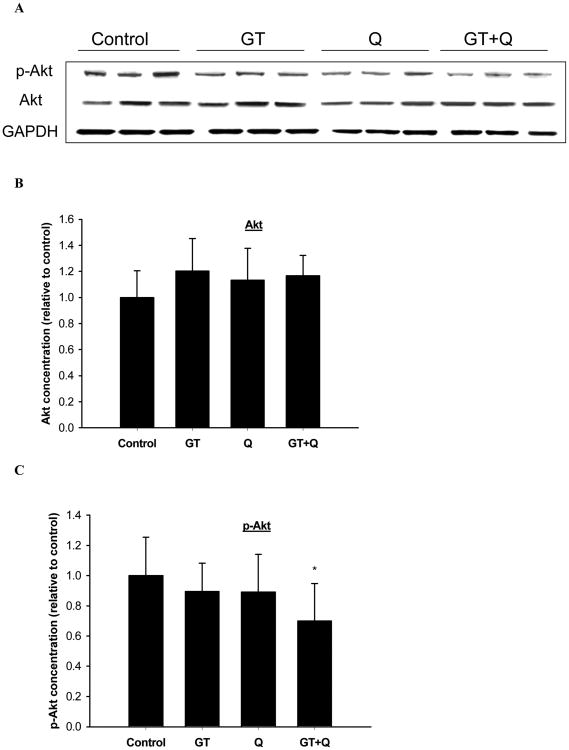
3.6. Inhibition of PI3K/Akt pathway
GT and Q alone or in combination did not change the protein expression of Akt significantly (Figure 6A, B). The phosphorylation of Akt was slightly but not significantly inhibited by the individual treatment of GT or Q. However, GT and Q in combination exhibited a significant inhibition of Akt phosphorylation compared to control (Figure 6A, C).
Fig. 6.
Decreased phosphorylation of Akt by GT plus Q treatment. The protein expression of Akt (A, B) and phosphor-Akt (A, C) in tumor tissues (n=10 per group) was detected by Western blot. Data are presented as mean ± SD. *P<0.05 compared to control.
4. Discussion
Our results presented here demonstrated that the combined treatment with GT and Q enhanced their chemopreventive activities in vivo. Natural compounds are promising sources for the development of non-toxic antitumor agents. However, due to low bioavailability, we may not be able to translate the in vitro findings to clinical application [25]. As demonstrated in our recently completed pre-prostatectomy GT trial, only a small amount of EGCG was detectable in prostate tissue after daily consumption of 6 cups of GT for 3-5 weeks [12]. A promising strategy to enhance the efficacy of these compounds is to administer a combination of natural compounds [19, 21]. In previous cell culture experiments, we were able to demonstrate that the combined use of Q with EGCG dramatically increased the cellular absorption and decreased the methylation of EGCG in prostate cancer LNCaP and PC-3 cells leading to enhanced antiproliferative effect [19]. The present study confirmed that the combination of GT and Q in vivo also increased the anticarcinogenic effect in a dose-dependent manner. In addition, our results indicate that the administration of the combination treatment prior to injecting the tumor cells may provide an earlier and enhanced effect on tumor inhibition.
The effect of the combination treatment was related to the concentration of GTPs in tumor tissue, which in turn was dependent on the Q dose. The dose of GT used in this study is equivalent to the consumption of 5-6 cups of green tea per day for an adult human. This estimate is based on the observation that the consumption of 5-6 cups of tea daily achieved similar tissue concentrations in human prostate compared to tissue in mice consuming the same brewed GT [12, 24]. Q dose would be equivalent to 1.0g (low dose) and 2.0g (high dose) per day for an adult based on blood concentrations of Q and its metabolites as observed in the present study (data not shown) relative to that from a human study [26]. The consumption of 1000 mg of Q per day was not associated with any adverse effects in humans [26, 27]. A pilot clinical trial is ongoing to determine the Q concentration necessary in humans to increase the bioavailability of EGCG. Our results showed that the combination treatment decreased the protein expression of MRP1 in tumor tissues. However, no changes of mRNA expression of MRP1 were observed, indicating that post-transcriptional regulation such as microRNA (miRNA) may be responsible. Many polyphenols including GT and Q have been shown to modulate the expression of miRNA, a class of small non-coding RNAs that interact with mRNA to regulate the gene expression post-transcriptionally [28-30]. Several other investigators demonstrated the inhibitory effects of Q on the activities of transport-regulating proteins such as p-glycoprotein and MRPs [14, 17], leading to an increased absorption of GTPs from the intestinal tract and retention in the tissues. Although Q is extensively methylated, sulfated, or glucuronidated upon uptake [31] it has been demonstrated that these Q metabolites, such as isorhamnetin and 7-O-glucuronosyl quercetin exhibited equal or stronger inhibition on the activities of MRPs compared to Q [32]. Considering the importance of MRPs in the development of chemoresistance during chemotherapy, GT and Q may also be good candidates to be combined with chemotherapy drugs to reduce drug resistance and enhance therapeutic efficacy.
The important role of catechol O-methylation of GTPs in cancer prevention has been demonstrated in several studies. Due to a common polymorphism of COMT its activity can vary by 3 to 4-fold [33]. A case control study in Asian-American women provided evidence that the risk of breast cancer was significantly reduced only among tea drinkers possessing at least one low-activity COMT allele [34]. We found earlier that EGCG was extensively methylated in human prostate tissues obtained from prostatectomy and in mouse tissues after GT consumption [12, 24]. In cell culture experiments methylation significantly decreased the anticancer activities of EGCG as shown by our laboratory and other investigators [12, 13]. Previously we demonstrated in vitro that the combination of GT and Q significantly decreased the activity and protein expression of COMT in various cancer cell lines [19, 21]. This inhibition of COMT was associated with a decrease in EGCG methylation and increase in the antiproliferative activity. Similarly, Landis-Piwowar et al. demonstrated that EGCG treatment in breast cancer cells of lower COMT activity led to stronger proteasome inhibition and apoptosis induction [35]. The present study confirmed the inhibition of COMT in vivo both in mRNA and protein expression by the combination treatment of GT and Q, which may contribute to the increased concentrations of non-methylated EGCG in tumor tissues and supports the important role of COMT in GT chemoprevention.
Cancer results from a multistage process with distinct molecular and cellular alterations. Therefore, treatments targeting many concerted processes may be advantageous in cancer prevention, therapy and reducing resistance to the treatment [36]. Natural compounds such as GT and Q target multiple events and signaling pathways throughout the stages of carcinogenesis [3, 37, 38]. In combination these compounds may increase the anticarcinogenic activity by expanding the coverage of molecular targets. The androgen receptor (AR) signaling pathway plays a critical role in prostate tumor growth and progression, thus it is an important target in prostate cancer prevention and treatment [39]. Nevertheless, there are other signaling pathways particularly the phosphatidylinositol 3-kinases/Akt/ mammalian target of rapamycin (PI3K/Akt/mTOR) pathway that crosstalk with AR signaling and may directly regulate the expression and activation of AR [40]. The upregulation and activation of PI3K/Akt/mTOR pathway is thought to play an important role in prostate cancer due to the decreased expression or loss of the negative regulator, tumor suppressor phosphatase and tensin homolog (PTEN) [40]. Akt is activated after phosphorylation by phosphorylated PI3K and in turn activates its substrates, one being mTOR, which leads to increased cell proliferation and survival [40]. Therefore, an effective intervention strategy in prostate cancer may need to target both AR and PI3K/Akt/mTOR signaling pathways. Both GT and Q inhibit AR signaling through multiple mechanisms including the decrease of AR expression and its nuclear translocation [41-43]. The combined use of GT and Q in the present study demonstrated an increasing ability to inhibit AR expression compared to individual treatments. In addition, the phosphorylation of Akt was significantly inhibited by the combination treatment while only a slight but non-significant decrease by the individual treatments. Further evidence of a stronger inhibition of AR and PI3K/Akt signaling was also provided through increased inhibition of AR-mediated PSA expression in tumor tissues from mice treated with GT+Q. Similar effects were demonstrated by a recent study that combined mTOR inhibition (everolimus) with an anti-androgen (bicalutamide) to block both pathways, resulting in tumor growth was statistically significantly reduced [44]. In addition to their applications in cancer prevention, GT and Q may be ideal candidates to be combined with anti-androgens to enhance the therapeutic efficacy in a less-toxic manner in the treatment of advanced prostate cancer.
In summary, we have demonstrated that a novel regimen of combining GT and Q significantly enhanced the tumor inhibitory effect in vivo. This was associated with an increased bioavailability of non-methylated GTPs and enhanced antiproliferative and proapoptotic effect. These results warrant future human intervention studies to confirm the combined effect of GT and Q in prostate cancer prevention and treatment.
Acknowledgments
This work was supported by the Department of Defense [W81XWH-10-1-0298 to P.W. ]; and the National Institutes of Health [RO3 CA150047-01 and RO1 CA116242 to S.H., U54 CA143931-01 to J.V., and P50CA092131 to J.S. ]
Abbreviations
- AR
androgen receptor
- COMT
catechol-O-methyltransferase
- GT
green tea
- GTPs
green tea polyphenols
- EC
(-)-epicatechin, ECG, (-)-epicatechin-3-gallate
- EGC
epigallocatechin
- EGCG
(-)-epigallocatechin-3-gallate
- HD
high dose
- HPLC
high-performance liquid chromatography
- LD
low dose
- 4″-MeEGCG
4″-O-methyl EGCG
- MRP
multidrug resistance-associated protein
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinases
- PSA
prostate-specific antigen
- Q
quercetin
- SCID
severe combined immunodeficiency
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003:523–524. 201–8. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 2.Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: green tea versus black tea. Molecular nutrition & food research. 2011;55:905–20. doi: 10.1002/mnfr.201000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CS, Wang X. Green tea and cancer prevention. Nutrition and cancer. 2010;62:931–7. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 4.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 5.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 6.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Molecular pharmaceutics. 2007;4:819–25. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho M, Jerónimo C, Valentão P, Andrade PB, Silva BM. Green tea: A promising anticancer agent for renal cell carcinoma. Food Chemistry. 2010;122:49–54. [Google Scholar]
- 9.Henning SM, Choo JJ, Heber D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J Nutr. 2008;138:1529S–34S. doi: 10.1093/jn/138.8.1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, et al. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int J Mol Epidemiol Genet. 2010;1:114–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer research. 1998;58:2269–77. [PubMed] [Google Scholar]
- 12.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3:985–93. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 14.van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, Bladeren PJ, et al. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69:699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab Dispos. 2004;32:497–504. doi: 10.1124/dmd.32.5.497. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Naidu PS, Kulkarni SK. Quercetin potentiates L-Dopa reversal of drug-induced catalepsy in rats: possible COMT/MAO inhibition. Pharmacology. 2003;68:81–8. doi: 10.1159/000069533. [DOI] [PubMed] [Google Scholar]
- 17.Kim KA, Park PW, Park JY. Short-term effect of quercetin on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein, in healthy volunteers. Eur J Clin Pharmacol. 2009;65:609–14. doi: 10.1007/s00228-009-0627-6. [DOI] [PubMed] [Google Scholar]
- 18.Ma ZS, Huynh TH, Ng CP, Do PT, Nguyen TH, Huynh H. Reduction of CWR22 prostate tumor xenograft growth by combined tamoxifen-quercetin treatment is associated with inhibition of angiogenesis and cellular proliferation. Int J Oncol. 2004;24:1297–304. [PubMed] [Google Scholar]
- 19.Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer. 2012;64:580–7. doi: 10.1080/01635581.2012.661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Heber D, Henning SM. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food & function. 2012;3:635–42. doi: 10.1039/c2fo10254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature medicine. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 23.Blancato J, Singh B, Liu A, Liao DJ, Dickson RB. Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer. 2004;90:1612–9. doi: 10.1038/sj.bjc.6601703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henning SM, Wang P, Said J, Magyar C, Castor B, Doan N, et al. Polyphenols in brewed green tea inhibit prostate tumor xenograft growth by localizing to the tumor and decreasing oxidative stress and angiogenesis. The Journal of nutritional biochemistry. 2012;23:1537–42. doi: 10.1016/j.jnutbio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(1):S139–51. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 26.Cialdella-Kam L, Nieman DC, Sha W, Meaney MP, Knab AM, Shanely RA. Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. The British journal of nutrition. 2013;109:1923–33. doi: 10.1017/S0007114512003972. [DOI] [PubMed] [Google Scholar]
- 27.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54:960–3. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 28.Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free radical biology & medicine. 2013 doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Boesch-Saadatmandi C, Wagner AE, Wolffram S, Rimbach G. Effect of quercetin on inflammatory gene expression in mice liver in vivo - role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;65:523–30. doi: 10.1016/j.phrs.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Milenkovic D, Deval C, Gouranton E, Landrier JF, Scalbert A, Morand C, et al. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: a new mechanism of the action of polyphenols. PloS one. 2012;7:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–52. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 32.van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, Cnubben NH, et al. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem Pharmacol. 2007;74:345–51. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–22. [PubMed] [Google Scholar]
- 34.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]
- 35.Landis-Piwowar K, Chen D, Chan TH, Dou QP. Inhibition of catechol-Omicron-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (-)-EGCG. Oncol Rep. 2010;24:563–9. doi: 10.3892/or_00000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharmaceutical research. 2010;27:950–61. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 37.Dou QP. Molecular mechanisms of green tea polyphenols. Nutrition and cancer. 2009;61:827–35. doi: 10.1080/01635580903285049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evidence-based complementary and alternative medicine : eCAM. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittmaster RS. Chemoprevention of prostate cancer. Acta Oncol. 2011;50(1):127–36. doi: 10.3109/0284186X.2010.527367. [DOI] [PubMed] [Google Scholar]
- 40.Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–49. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren F, Zhang S, Mitchell SH, Butler R, Young CY. Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene. 2000;19:1924–32. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 42.Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schayowitz A, Sabnis G, Goloubeva O, Njar VC, Brodie AM. Prolonging hormone sensitivity in prostate cancer xenografts through dual inhibition of AR and mTOR. Br J Cancer. 2010;103:1001–7. doi: 10.1038/sj.bjc.6605882. [DOI] [PMC free article] [PubMed] [Google Scholar]