Abstract
Oral submucous fibrosis (OSMF) is a potentially malignant oral condition effectively linked to the causative habit of chewing areca nut. Since its first description in the 1950s, numerous epidemiological, biochemical, histological, and genetic studies have been reported. While most studies point out to the cause and effect of areca nut, co-additive factors are also implicated in the progression and malignant transformation of this condition. Biochemical investigations have concentrated on outlining such changes in the blood, serum or tissues of these patients and have given insights on the possible pathogenesis of OSMF. This article attempts to compile details of biochemical investigations in OSMF and summarize and infer on the findings.
Keywords: Biochemical parameters, immunology, malignant transformation, oral submucous fibrosis, trace elements
INTRODUCTION
Oral submucous fibrosis (OSMF) has been described as “an insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although, occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic change of the lamina propria, with epithelial atrophy leading to stiffness of the oral mucosa and causing trismus and inability to eat.”[1]
The condition has been aptly described as a “potentially malignant disorder”[2] in view of the high rate of malignant transformation. In addition, it shares a unique predisposition of occurrence in the Indian subcontinent, parts of Asia and among individuals of Indian origin abroad. The association of the habit of consuming betel nut (areca catechu) has now been widely recognized as causative. Reports of OSMF occurring in Asians predisposed to the habit have supported this contention.[3]
As is the norm with other disease afflictions, wide ranges of investigations have been carried out in the condition to identify the causation and pathogenesis. Biochemical investigations of blood, serum, and tissues have been the earliest form of interventions. Such investigations have largely helped to localize parameters that predispose to the development of the condition, modify its behavior and prognosticate on its malignant transformation potential.
The present review attempts to collate the biochemical investigation carried out in OSMF and relates the importance of the parameters evaluated.
METHODOLOGY
Data were gleaned from a literature search of available medical and dental databases including PubGet, Research gate, Ovid, PubMed, Medline, Cochrane and non-medical search engines such as Wikipedia and Google. The search phrases included the main set “OSMF” with defined subsets such as “biochemical parameters of,” malignant transformation of,” “trace elements in,” blood, and serum investigations in” etc.
RESULTS
The retrieved data were grouped into (1) serum iron and related compounds, (2) trace elements, (3) antioxidants, (4) immunological investigations, (5) genetic parameters, and (6) others including lipids, glycoconjugates, and enzymes.
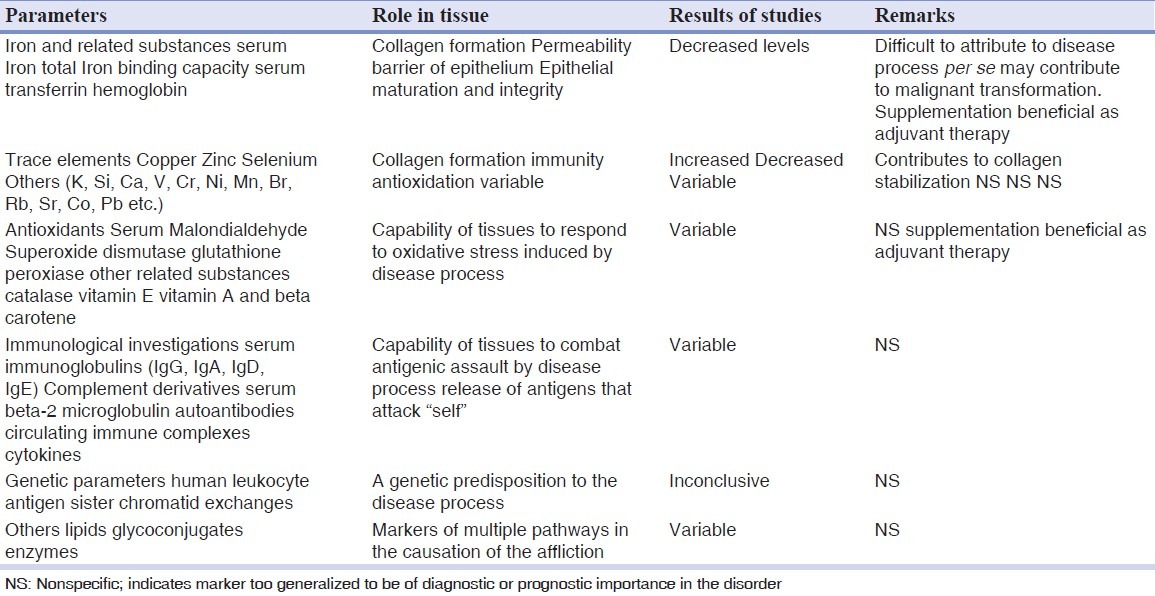
Table 1 lists the comprehensive collection of the broad parameters, individual components, and observations from the respective studies of the biochemical parameters assayed in OSMF.
Table 1.
Biochemical parameters investigated in oral submucous fibrosis at a glance
Serum iron and related compounds
The role of iron in the development, maintenance, and defense abilities of the oral mucosa has been well-documented. Iron is a necessary trace element found in nearly all living organisms. Iron-containing enzymes and proteins, often containing heme prosthetic groups, participate in many biological oxidations and in transport. Examples of proteins found in higher organisms include hemoglobin, cytochrome, and catalase.[4]
In the oral mucosa, iron plays a part in the maturation of epithelium via the action of cytochrome oxidase and is also thought to have a role in the maintenance of the permeability barrier.[5]
The role of iron in collagen synthesis is well-established. Iron is required for collagen synthesis by enzymes proline hydroxylase and peptidyl lysine hydroxylase in hydroxylation of proline and lysine respectively. Iron is a co-factor along with molecular oxygen alpha-Ketoglutarate and ascorbic acid that is used by peptidyl proline hydroxylase in this process.
Iron has been evaluated in various forms in OSMF. Hemoglobin levels, serum iron, total iron binding capacity, and serum transferrin has been assessed. In addition, iron has also been estimated in the tissue samples of patients with OSMF.
The occurrence of anemia in the Indian subcontinent is endemic and is linked to dietary factors and physiological situations. In addition, the decreased intake of food resultant from OSMF symptoms adds to the problem. Thus, iron deficiency in OSMF patients is a vicious cycle that potentiates the condition and predisposes to malignant transformation.
OSMF had been earlier described as “sideropenic dysphagia.” The author attributed the generalized occurrence of anemia in patients with OSMF to predisposition of the condition in patients consuming areca nuts. His “seed and soil theory” was one of the earlier attempts to implicate iron deficiency in the etiopathogenesis of the condition.[6]
Varying degrees of iron deficiency in almost all forms of iron presentation in the human body have been reported.[7,8,9,10,11,12,13] The role of iron has been commented on two aspects of the disease process. Deficiency of iron has been postulated to predispose the individual consuming areca nuts to the development of OSMF. Changes in epithelial maturation and integrity due to iron deficiency have been implicated in the malignant transformation of the condition.[6,8]
Iron supplementations have formed the mainstay of treatment modalities of OSMF. Prospective studies of the role of iron supplementation in the diets of patients with OSMF conducted by Maher et al.[14] in Pakistan have conclusively proven its efficacy.
Trace elements
Copper
Copper has diverse roles in biological electron transport and oxygen transportation. Copper is a component of other proteins associated with the processing of oxygen. In cytochrome c oxidase, which is required for aerobic respiration, copper, and iron cooperate in the reduction of oxygen. Copper is also found in many superoxide dismutases (SOD), proteins that detoxify superoxides by converting it to oxygen and hydrogen peroxide. Copper is a component of the areca nut in all forms, which is implicated in the etiology of OSMF.
Of late, a renewed interest in the role of copper as a mediator of fibrosis in OSMF has been postulated. Trivedy et al.[15,16,17] have carried out a series of studies on the estimation of copper in areca nut, in sera and tissue of OSMF patients. Copper content of areca nut was found to be more than the content in nuts in snacks consumed by the population. When whole saliva from volunteers chewing pan parag (a proprietary form of areca with additives) was analyzed copper concentrations were found to be high, indicating release, and absorption of copper. When comparing tissue and serum copper levels in patients chewing areca nut preparations, they found a consistently higher level of copper in the tissue while copper concentrations, ceruloplasmin, and urinary copper were within standard reference range. The authors postulate a strong role for this element in the etiology of OSMF. It has been suggested that copper regulates the production of fibrosis via the enzyme lysyl oxidase, which results in crosslinking of collagen and subsequent resistance to degradation, by enzymes. In a study, estimation of copper and zinc in sera of OSMF was carried out. The authors found decreased levels of copper and zinc in OSMF cases with an increase in copper/zinc ratio. This suggests depletion of zinc at a faster rate than copper in OSMF. Most other studies from all around the world share similar results.[18,19,20,21,22,23,24]
Selenium
Selenium is an antioxidative nutrient that forms an integral part of enzymes glutathione peroxidase, type I iodothyronine deiodinase, metalloprotein, fatty acid binding protein and selenoprotein P. The role of this trace element has been studied extensively in oral potentially malignant conditions and oral cancer.
Rajendran et al.[25] estimated the levels of cadmium, selenium, chromium, magnesium, and calcium in the sera of patients with oral leukoplakia, OSMF, and squamous cell carcinoma. No appreciable changes were noted in OSMF, but oral leukoplakias and oral cancers showed a decrease. Other studies, where estimations of multiple trace elements were carried out have not revealed any significant alterations of this trace element in OSMF.[7]
While, the role of oxidative stress is well-established in the pathogenesis of OSMF, estimations of individual entities that modulate the process may not be reflective of their proposed role. This may be due to the fact that the process of oxidative stress has many pathways and the detection or expression of any one element or enzyme may not necessarily be involved.
Zinc
Zinc is an essential trace element, necessary for plants, animals, and microorganisms. Zinc is found in nearly 100 specific enzymes, serves as structural ions in transcription factors. It is “typically the second most abundant transition metal in organisms” after iron and it is the only metal, which appears in all enzyme classes.[26]
In humans, zinc plays “ubiquitous biological roles.” It interacts with “a wide range of organic ligands” and has roles in the metabolism of RNA and DNA, signal transduction, and gene expression. It also regulates apoptosis. About 10% of human proteins are bound to zinc.[27]
In blood plasma, zinc is bound to and transported by albumin and transferrin. Since transferrin also transports iron, excessive iron reduces zinc absorption, and vice-versa. A similar reaction occurs with copper. The concentration of zinc in blood plasma stays relatively constant regardless of zinc intake. Cells in the salivary gland, prostate, immune system, and intestine use zinc signaling as one way to communicate with other cells.[28]
The role of zinc as a trace element in OSMF has been evaluated in many studies. Reduced levels of zinc were found in most studies and were attributed to the decreased immunity status of individuals.[9,29,30] Interestingly, copper/zinc ratios have been consistently used as indicators of immune supplementation in potentially malignant oral disorders and oral malignancies. An elevation of the ratio has been consistently reported.[9,18]
Other trace elements
Various other trace elements have been evaluated in OSMF, including K, Si, Ca, V, Cr, Ni, Mn, Br, Rb, Sr, Co, and Pb. In an analysis on 16 trace elements in OSMF, Ray et al.[30] found gross depletion of Zn, Br, and Fe while Mn and Co showed an increase in blood concentrations. Paul et al.[9] evaluated 68 bioinorganic elements in their study on OSMF. The authors based their study on the premise that in the OSMF, in addition to deleterious oral habits, malnutrition, and possible genetic predisposition, altered bioelemental status is also likely to play an important role in the pathogenesis. They reported significant alterations in the bioelemental profiles indicating a homeostatic imbalance. It was suggested that these bioelemental alterations leading to homeostatic imbalance might be considered as an important biological event in the pathogenesis of OSF.
The role of trace elements in OSMF is largely related to the malnutrition status of individuals. In a country like India, where malnutrition is an established entity, the specificity of the results of such studies gets diluted. Nevertheless, institution of nutritional supplements, especially of the trace elements in diets of individuals afflicted with the condition may be beneficial. Positive reports emerge from literature in such interventions. In studies conducted in Pakistan, Maher et al.[14] introduced dietary supplements of essential and trace elements in a cohort group of individuals suffering from OSMF. The authors observed substantial improvement in the condition of the patients, especially in symptom amelioration and increase in the interincisal distance; though the resolution of the disease affliction did not occur.
In an interesting study, Xie et al.[31] evaluated the concentrations of trace elements in the “intranuclei and extranuclei (cytoplasm)” of epithelial cells in OSMF using electron probe micro-analysis. The elements assayed included Cu, Zn, S, As, Se, and Mo. The concentrations of Cu, Zn, S, and As were significantly lower while those of Se and Mo were found to be higher than in normal buccal mucosa. The authors state that the high levels of Se and low levels of Cu and Zn severely damage the epithelial cells predisposing them to cancer development.
Antioxidants
Studies have shown that the process of carcinogenesis occurs by generation of reactive oxygen species (ROS),[32] which act by initiating lipid peroxidation (LPO).[33] Prevention against LPO mediated damage is carried out by non-enzymatic antioxidants, especially beta-carotene and Vitamin E and enzymatic antioxidant like SOD.[34]
Antioxidants and LPO by products that have been evaluated in OSMF include serum malondialdehyde (MDA), enzymes SOD, glutathione peroxidase and related derivatives, catalase, beta-carotene, and Vitamins A and E.
Serum MDA
ROS degrade polyunsaturated lipids, forming MDA. This compound is a reactive aldehyde and is one of the many reactive electrophile species that cause toxic stress in cells and form covalent protein adducts referred to as advanced lipoxidation end-products. The production of this aldehyde is used as a biomarker to measure the level of oxidative stress in an organism.[35]
SOD
SODs are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen.
Glutathione peroxidase
Glutathione peroxidase is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
Betacarotene and vitamin A
β-Carotene is a strongly colored red-orange pigment abundant in plants and fruits. In nature, β-carotene is a precursor (inactive form) to vitamin A via the action of beta-carotene 15,15’-monooxygenase. Vitamin A functions in a very different role as an irreversibly oxidized form of retinol known as retinoic acid, which is an important hormone-like growth factor for epithelial and other cells. Vitamin A, and more specifically, retinoic acid, appears to maintain normal skin and oral mucosal health by switching on genes and differentiating keratinocytes into mature epithelial cells.
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. It is a fat-soluble antioxidant that stops the production of ROS formed when fat undergoes oxidation.
In a study, Gupta et al.[36] evaluated antioxidant parameters that included serum malonaldehyde (MDA), SOD, beta-carotene and Vitamin E in patients with OSMF. Plasma MDA level was found to be increased in all grades of OSMF cases, beta carotene level was found to be decreased in all grades of OSMF cases. Plasma Vitamin E level was found to be decreased in grade II and III OSMF cases, but not in grade I cases. Enzymatic antioxidant defense as assessed by SOD activity did not show any significant change in any stage of the disease.
The findings were largely concurrent in another study.[37] Increase in MDA levels proportional to grades of OSMF were noted. In contrast, levels of SOD and vitamin A were found to be decreased in progressive grades of OSMF.
In addition to SOD, the enzyme glutathione peroxidase, also a marker of oxidative stress was assessed in OSMF. There was an increase in levels of both the enzymes indicating tissue oxidative stress due to the disease.[38]
Assessing a host of oxidative stress markers Subapriya et al.[39] found reduced levels of antioxidants due to enhanced LPO during the disease progress. They examined the blood levels of lipid peroxides and the antioxidants SOD, catalase, reduced glutathione, glutathione peroxidase, and glutathione-S-transferase in oral pre-cancer, pre-operative, post-operative, and recurrent oral cancer.
These studies indicate that OSMF or the products associated in its etiology (areca nut and additives) induce oxidative stress on the tissues. Thus, supplemental therapy with antioxidants would be beneficial in the therapy of the condition.
Immunological mediation
Immunological mediation is a well-established factor in the pathogenesis of human disorders. Predictably, assessment of immunological parameters has been one of the earliest investigations in OSMF. Evaluation of immunoglobulins (Ig), autoantibodies, cytokines, complement derivatives, and circulating immune complexes has been carried out in OSMF.
Igs
Elevated levels of major Igs were noted in patients with OSMF by Gupta et al., this being one of the earliest recorded studies on the subject in India.[40] A similar increase in salivary and serum IgG and IgA has been reported in another study.[41] Correlating the Ig levels to total serum protein, the authors found a significant drop in the latter in all patients with OSMF. Based on the findings a nutritional basis for the disease was suggested. Concurrent studies report similar increases in IgG, IgA, and IgM.[42] Anil et al.[43] evaluated the tumor marker serum beta-2 microglobulin in oral cancer, oral lichen planus and OSMF patients matched with controls. A significant increase in the marker in OSMF and oral cancer patients was noted with little or no increase in the oral lichen planus group. The authors postulated this finding with the theory of increased production or impaired excretion of the protein. The correlation of the levels with oral cancer pointed to the high malignant potential of OSMF.
Cytokines
Cytokines are byproducts of cellular reactions involved in defense. They are the mediators through, which tissue reactions occur and symptoms are reflected.
Haque et al.,[44] evaluated levels of several cytokines in OSMF patients. The cytokines studied included: Interleukin-1beta, interleukin-6, interleukin-8, tumor necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma). Controls included genetic relatives and a matched population of Indians and Caucasians. A significant increase of all cytokines with a decrease of IFN-gamma was noted in patients. While, no differences in levels were seen in genetic relatives, they were more than the matched population. Mediation by pro-inflammatory cytokines and modulation by IFN-gamma were postulated as possible pathways for the disease.
In another study, Hsu et al.[45] evaluated the effects of arecoline on the levels of IL-2, TGF-beta, TNF-alpha and IFN-gamma in patients with OSMF, mucosal disorders, and oral cancers. They found decreased levels of all the cytokines in OSMF patients as compared with subjects that indulged in the habit of betel nut consumption, but without oral lesions. The decrease in IFN-gamma is consistent with the above study.
Cytokines have also been evaluated as indicators to therapy in various potentially malignant disorders. In a study on Chinese patients, Sun et al.[46] used interleukin-6 levels as indicators of therapy with levamisole and traditional Chinese herbs. Interleukin-6 levels were higher in OSMF as compared with controls, but much lower than other mucosal disorders.
Mediation by cytokines as a possible pathway in OSMF seems to be the impression from the above studies. Yet, cytokine production and its effects on the oral tissues is a normal phenomenon in all reactions ranging from inflammations to oral cancer. The specificity of this tissue reaction to OSMF is not elaborated and forthcoming.
Autoantibodies
A high incidence of autoantibodies including, antinuclear, anti-smooth muscle, anti-gastric parietal cell, anti-thyroid microsomal has been demonstrated in a Taiwanese study.[47] The authors opine that altered auto-antigens released from arecoline ingredients-damaged cells may induce autoantibody production. The trauma caused to the oral and gastric mucosa from ingestion of the betel nut may increase absorption and help in the process. The authors further stressed the role of human leukocyte antigen (HLA) predisposition may speed the process.
Genetic parameters in OSMF
The deposition of collagen fibers has long been related to genetic mechanisms. Initial investigations centered around genetic parameters evaluated from blood and serum of patients with OSMF. These include sister chromatid exchanges (SCEs) and HLA genotypes.
SCEs
SCE is the exchange of genetic material between two identical sister chromatids.
During the S-phase of the cell cycle, DNA is replicated and each chromosome is present in a duplicated state with the two genetically identical chromatids joined together at the centromere. These two sister chromatids are readily apparent in late prophase or early metaphase of mitosis.
SCE is the process wherein the two sister chromatids break and rejoin with one another, physically switching positions on the chromosome. Because the exchanges occur with tremendous precision with respect to the DNA sequence, and the sister chromatids are genetically identical, no information is altered during the exchange. Such exchanges are natural events during cell replication with each cell typically undergoing three to four SCEs during each replication cycle.
The reason for the SCE is not known, but it is required and used as a mutagenic testing of many products. Four to five SCE per chromosome pair, per mitosis is in the normal distribution, 14-100 exchanges is not normal and presents a danger to the organism.
Studies on SCEs in OSMF indicate increased frequency expression in the condition along with oral cancer as matched with controls. Ghosh et al.[48] evaluated SCEs in patients with OSMF and those consuming areca nut products and tobacco and found a significantly higher expression than controls. Interestingly levels in patients with combined habits of smoking and chewing were higher than in the other groups. It is difficult to assess whether the mutagenic indicator expression was due to the effects of the habits or the disease process. In another study,[49] patients with OSMF and those chewing betel nuts alone were assessed for SCEs. Expression was higher in both the groups as compared with controls. The authors rightly opine that the habit of chewing betel nut is the primary mutagenic factor and the tissue change of OSMF probably follows. The malignant transformation of the condition is also probably a result of this mutagenic effect.
HLA
The HLA system is the name of the major histocompatibility complex in humans. The super locus contains a large number of genes related to the immune system function in humans. This group of genes resides on chromosome 6 and encodes cell-surface antigen-presenting proteins and many other genes. The major HLA antigens are essential elements for immune function. They are important in disease defense and organ transplant rejections. They may protect against or fail to protect (if down regulated by an infection) against cancers and may mediate autoimmune disease (e.g., Type I diabetes, coeliac disease).
Impairment of the immune system has long been propagated as a mechanism in the pathogenesis of OSMF. The geographical location and the almost definite association with habit of chewing betel nut have led to suggestions of an autoimmune basis for the condition. Assessment of HLA antibodies in patients with OSMF lends some credence to this suggestion. In one of the earliest studies on HLA prototypes, Canniff et al.[50] evaluated HLA type A10 and DR3 in OSMF patients. The authors found conclusive evidence of raised expression of these prototypes in OSMF patients. They opine “the results support the concept that OSF is a chronic autoimmune disease, initiated by constituents of betel nut, and occurring in genetically susceptible individuals.” It was also suggested by them that genes situated in the HLA region are important determinants of genetic susceptibility in OSF. In contrast, in studies on larger samples of betel nut chewers in South African subjects of Indian origin, van Wyk et al.[51] could not find any significant association of HLA antigens and have discarded the hypothesis of autoimmunity for this disorder.
While, it may be plausible that consumption of betel nut may predispose to condition, the basis for autoimmunity seems not to have much laboratory evidence. At best, OSMF is a cause and effect disorder related to the habit, rather than a disease of genetic susceptibility.
Other biochemical parameters
Lipid profile
Changes in lipid profile have long been associated with malignancies as lipids play a key role in maintenance of cell integrity. Studies on lipid profiles of OSMF patients have consistently revealed lower levels compared to controls. Two studies.[52,53] were available in the literature on the role of lipids in OSMF. These studies analyzed a range of lipids including total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, very low density lipoprotein cholesterol, triglycerides, Apo-A1, Apo-B and LPa in the sera of patients with OSMF. A consistent observation of lower lipid levels in OSMF as compared to controls was found in both studies. The inference was unanimous that lower levels of plasma cholesterol and other lipid constituents in patients might be due to their increased utilization by neoplastic cells for new membrane biogenesis.
Lactate dehydrogenase
Tissue breakdown releases Lactate dehydrogenase (LDH); and therefore, LDH can be measured as a surrogate for tissue breakdown, e.g., hemolysis. Other disorders indicated by elevated LDH include cancer, meningitis, encephalitis, acute pancreatitis, and human immunodeficiency virus. In the two studies.[54,55] found in the literature relating to this enzyme, elevated levels were seen in OSMF patients indicating evidence of tissue breakdown.
Serum glycoconjugates
The changes in lipid profile have long been associated with cancer because lipids play a key role in maintenance of cell integrity. A glycoconjugate is a molecule in which one or more glycan units are covalently linked to a non-carbohydrate entity. Glycoconjugates are very important compounds in biology and consist of many different categories such as glycoproteins, glycopeptides, peptidoglycans, glycolipids, and lipopolysaccharides. They are involved in cell-cell interactions, including cell-cell recognition, and cell-matrix interactions.
Evaluation of serum glycoconjugates is frequently carried out in tumors and is an indicator of the metastatic potential of the tumor. Serum glycoconjugates have been evaluated in OSMF and other oral potentially malignant conditions including oral cancer. Baxi et al.[56] evaluated serum glyconjugates (serum sialic acid, lipid bound sialic acid, mucoid proteins and hexoses) in oral precancerous conditions including OSMF and found elevated levels of the entities when compared to controls and chewers. There was a progressive increase of the markers with increasing grade of malignancy. Though no specific inferences were drawn relating to the elevated levels in OSMF, the authors have postulated a role for these biochemical investigations in monitoring of the lesions. Other studies showed related findings of increased levels in OSMF without alluding to the probable role of the marker in the pathogenesis of the disease.[52,57,58]
It is feasible that increased expression of serum glyconjugates may indicate a tendency toward malignant transformation in OSMF, especially in view of the potential of the markers in cell-to cell interactions.
CONCLUSION
Biochemical investigations in OSMF have thus yielded confusing results. At best they are indicators of disease progress or intermediary pathways in the pathogenesis. They are parts of the puzzle that the disorder reflects and their analysis may serve as adjuncts in defining the broad spectrum of the causation of this potentially malignant condition.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 3.Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. Mill Valley: University Science Books; 1994. [Google Scholar]
- 5.Rennie JS, MacDonald DG, Dagg JH. Iron and the oral epithelium: A review. J R Soc Med. 1984;77:602–7. doi: 10.1177/014107688407700714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramanathan K. Oral submucous fibrosis - An alternative hypothesis as to its causes. Med J Malaysia. 1981;36:243–5. [PubMed] [Google Scholar]
- 7.Khanna SS, Karjodkar FR. Circulating immune complexes and trace elements (Copper, Iron and Selenium) as markers in oral precancer and cancer: A randomised, controlled clinical trial. Head Face Med. 2006;2:33. doi: 10.1186/1746-160X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendran R, Vasudevan DM, Vijaykumar T. Serum iron and proteins in oral submucous fibrosis (OSMF) Annals Dent. 1990;49:23–5. [PubMed] [Google Scholar]
- 9.Paul RR, Chatterjee J, Das AK, Dutta SK, Roy D. Zinc and iron as bioindicators of precancerous nature of oral submucous fibrosis. Biol Trace Elem Res. 1996;54:213–30. doi: 10.1007/BF02784433. [DOI] [PubMed] [Google Scholar]
- 10.Tadakamadla J, Kumar S, GP M. Evaluation of serum copper and iron levels among oral submucous fibrosis patients. Med Oral Patol Oral Cir Bucal. 2011;16:e870–3. doi: 10.4317/medoral.17083. [DOI] [PubMed] [Google Scholar]
- 11.Anuradha CD, Devi CS. Serum protein, ascorbic acid & iron & tissue collagen in oral submucous fibrosis - A preliminary study. Indian J Med Res. 1993;98:147–51. [PubMed] [Google Scholar]
- 12.Gupta PC, Hebert JR, Bhonsle RB, Sinor PN, Mehta H, Mehta FS. Dietary factors in oral leukoplakia and submucous fibrosis in a population-based case control study in Gujarat, India. Oral Dis. 1998;4:200–6. doi: 10.1111/j.1601-0825.1998.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 13.Hebert JR, Gupta PC, Bhonsle RB, Mehta H, Zheng W, Sanderson M, et al. Dietary exposures and oral precancerous lesions in Srikakulam District, Andhra Pradesh, India. Public Health Nutr. 2002;5:303–12. doi: 10.1079/PHN2002249. [DOI] [PubMed] [Google Scholar]
- 14.Maher R, Aga P, Johnson NW, Sankaranarayanan R, Warnakulasuriya S. Evaluation of multiple micronutrient supplementation in the management of oral submucous fibrosis in Karachi, Pakistan. Nutr Cancer. 1997;27:41–7. doi: 10.1080/01635589709514499. [DOI] [PubMed] [Google Scholar]
- 15.Trivedy C, Baldwin D, Warnakulasuriya S, Johnson N, Peters T. Copper content in Areca catechu (betel nut) products and oral submucous fibrosis. Lancet. 1997;349:1447. doi: 10.1016/S0140-6736(97)24020-1. [DOI] [PubMed] [Google Scholar]
- 16.Trivedy C, Meghji S, Warnakulasuriya KA, Johnson NW, Harris M. Copper stimulates human oral fibroblasts in vitro: A role in the pathogenesis of oral submucous fibrosis. J Oral Pathol Med. 2001;30:465–70. doi: 10.1034/j.1600-0714.2001.030008465.x. [DOI] [PubMed] [Google Scholar]
- 17.Trivedy CR, Warnakulasuriya KA, Peters TJ, Senkus R, Hazarey VK, Johnson NW. Raised tissue copper levels in oral submucous fibrosis. J Oral Pathol Med. 2000;29:241–8. doi: 10.1034/j.1600-0714.2000.290601.x. [DOI] [PubMed] [Google Scholar]
- 18.Varghese I, Sugathan CK, Balasubramoniyan G, Vijayakumar T. Serum copper and zinc levels in premalignant and malignant lesions of the oral cavity. Oncology. 1987;44:224–7. doi: 10.1159/000226482. [DOI] [PubMed] [Google Scholar]
- 19.Pillai KG, Burde KN. Increased copper level in oral mucosal tissue of patients with submucous fibrosis and who chew areca nut products. West Indian Med J. 2005;54:270–1. doi: 10.1590/s0043-31442005000400014. [DOI] [PubMed] [Google Scholar]
- 20.Rooban T, Saraswathi TR, George A, Joshua E, Ranganathan K. Cytological study of copper in oral submucous fibrosis. Indian J Dent Res. 2004;15:129–32. [PubMed] [Google Scholar]
- 21.Meghji S, Haque MF, Harris M. Oral submucous fibrosis and copper. Lancet. 1997;350:220. doi: 10.1016/s0140-6736(05)62388-4. [DOI] [PubMed] [Google Scholar]
- 22.Raja KB, Hazarey VK, Peters TJ, Warnakulasuriya S. Effect of areca nut on salivary copper concentration in chronic chewers. Biometals. 2007;20:43–7. doi: 10.1007/s10534-006-9013-3. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran R, Kumari KR, Kumar AS. Liver ultrasound and faecal copper estimation in oral submucous fibrosis. Indian J Dent Res. 2003;14:13–21. [PubMed] [Google Scholar]
- 24.Shakya S, Ongole R, Sumanth KN. Copper content of various constituents of betel quid. Indian J Dent Res. 2009;20:516–7. doi: 10.4103/0970-9290.59432. [DOI] [PubMed] [Google Scholar]
- 25.Rajendran R. Serum levels of some trace and bulk element in OSMF. J Indian Dent Assoc. 1992;631:251–4. [Google Scholar]
- 26.Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 27.Hambidge KM, Krebs NF. Zinc deficiency: A special challenge. J Nutr. 2007;137:1101–5. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 28.Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59:541–52. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 29.Khademi H, Shaikhiany J. Comparison of serum zinc levels in recurrent apthous stomatitis patients and normal individuals. Dent Res J. 2006;2:1–5. [Google Scholar]
- 30.Ray JG, Ghosh R, Mallick D, Swain N, Gandhi P, Ram SS, et al. Correlation of trace elemental profiles in blood samples of Indian patients with leukoplakia and oral submucous fibrosis. Biol Trace Elem Res. 2011;144:295–30. doi: 10.1007/s12011-011-9091-0. [DOI] [PubMed] [Google Scholar]
- 31.Xie X, Tang Z, Liu S. Changes in trace elements in epithelial cells of oral submucous fibrosis. Hunan Yi Ke Da Xue Xue Bao. 1999;24:504–6. [PubMed] [Google Scholar]
- 32.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 33.Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 34.Sen CK. Oxygen toxicity and antioxidants: State of the art. Indian J Physiol Pharmacol. 1995;39:177–96. [PubMed] [Google Scholar]
- 35.Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–7. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Reddy MV, Harinath BC. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J Clin Biochem. 2004;19:138–41. doi: 10.1007/BF02872409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metkari SB, Tupkari JV, Barpande SR. An estimation of serum malondialdehyde, superoxide dismutase and vitamin a in oral submucous fibrosis and its clinicopathologic correlation. J Oral Maxillofac Pathol. 2007;11:23–7. [Google Scholar]
- 38.Uikey AK, Hazarey VK, Vaidhya SM. Estimation of serum antioxidant enzymes superoxide dismutase and glutathione peroxidase in oral submucous fibrosis: A biochemical study. J Oral Maxillofac Pathol. 2003;7:44–5. [Google Scholar]
- 39.Subapriya R, Kumaraguruparan R, Nagini S, Thangavelu A. Oxidant-antioxidant status in oral precancer and oral cancer patients. Toxicol Mech Methods. 2003;13:77–81. doi: 10.1080/15376510309825. [DOI] [PubMed] [Google Scholar]
- 40.Gupta DS, Gupta M, Oswal RH. Estimation of major immunoglobulin profile in oral submucous fibrosis by radial immunodiffusion. Int J Oral Surg. 1985;14:533–7. doi: 10.1016/s0300-9785(85)80060-0. [DOI] [PubMed] [Google Scholar]
- 41.Patidar KA, Parwani RN, Wanjari SP. Correlation of salivary and serum IgG, IgA levels with total protein in oral submucous fibrosis. J Oral Sci. 2011;53:97–102. doi: 10.2334/josnusd.53.97. [DOI] [PubMed] [Google Scholar]
- 42.Shah N, Kumar R, Shah MK. Immunological studies in oral submucous fibrosis. Indian J Dent Res. 1994;5:81–7. [PubMed] [Google Scholar]
- 43.Anil S, Beena VT, Nair RG, Vijayakumar T. Evaluation of serum beta 2-microglobulin in premalignant and malignant lesions of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:750–2. doi: 10.1016/s1079-2104(05)80311-7. [DOI] [PubMed] [Google Scholar]
- 44.Haque MF, Meghji S, Khitab U, Harris M. Oral submucous fibrosis patients have altered levels of cytokine production. J Oral Pathol Med. 2000;29:123–8. doi: 10.1034/j.1600-0714.2000.290304.x. [DOI] [PubMed] [Google Scholar]
- 45.Hsu HJ, Chang KL, Yang YH, Shieh TY. The effects of arecoline on the release of cytokines using cultured peripheral blood mononuclear cells from patients with oral mucous diseases. Kaohsiung J Med Sci. 2001;17:175–82. [PubMed] [Google Scholar]
- 46.Sun A, Chia JS, Chang YF, Chiang CP. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196–203. doi: 10.1034/j.1600-0714.2002.310402.x. [DOI] [PubMed] [Google Scholar]
- 47.Chiang CP, Hsieh RP, Chen TH, Chang YF, Liu BY, Wang JT, et al. High incidence of autoantibodies in Taiwanese patients with oral submucous fibrosis. J Oral Pathol Med. 2002;31:402–9. doi: 10.1034/j.1600-0714.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh PK, Madhavi R, Guntur M, Ghosh R. Sister chromatid exchanges in patients with oral submucous fibrosis. Cancer Genet Cytogenet. 1990;44:197–201. doi: 10.1016/0165-4608(90)90047-e. [DOI] [PubMed] [Google Scholar]
- 49.Lin T, Huang S, Tang J. A study on the frequency of sister chromatid exchanges in patients with oral submucous fibrosis in peripheral blood lymphocytes. Hua Xi Kou Qiang Yi Xue Za Zhi. 1997;15:16–7. [PubMed] [Google Scholar]
- 50.Canniff JP, Batchelor JR, Dodi IA, Harvey W. HLA-typing in oral submucous fibrosis. Tissue Antigens. 1985;26:138–42. doi: 10.1111/j.1399-0039.1985.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 51.van Wyk CW, Grobler-Rabie AF, Martell RW, Hammond MG. HLA-antigens in oral submucous fibrosis. J Oral Pathol Med. 1994;23:23–7. doi: 10.1111/j.1600-0714.1994.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 52.Patel PS, Shah MH, Jha FP, Raval GN, Rawal RM, Patel MM, et al. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J Cancer. 2004;41:25–31. [PubMed] [Google Scholar]
- 53.Mehrotra R, Pandya S, Chaudhary AK, Singh HP, Jaiswal RK, Singh M, et al. Lipid profile in oral submucous fibrosis. Lipids Health Dis. 2009;8:29. doi: 10.1186/1476-511X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muralidhar M, Raghavan MR, Bailoor DN, Kamath VV. Evaluation of serum lactate dehydrogenase (LDH) in oral premalignant and malignant lesions. Ann Dent. 1988;47:11–5. [PubMed] [Google Scholar]
- 55.Langvad E, Zachariah J, Pindborg JJ. Lactate dehydrogenase isoenzyme patterns in leukoplakia, submucous fibrosis and carcinoma of the oral mucosa in south Indians. Acta Pathol Microbiol Scand A. 1970;78:509–15. doi: 10.1111/j.1699-0463.1970.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 56.Baxi BR, Patel PS, Adhvaryu SG. A report on clinical importance of serum glycoconjugates in oral cancer. Ind J Clin Biochem. 1990;5:139–44. [Google Scholar]
- 57.Rajpura KB, Patel PS, Chawda JG, Shah RM. Clinical significance of total and lipid bound sialic acid levels in oral pre-cancerous conditions and oral cancer. J Oral Pathol Med. 2005;34:263–7. doi: 10.1111/j.1600-0714.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 58.Raval GN, Patel DD, Parekh LJ, Patel JB, Shah MH, Patel PS. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral Dis. 2003;9:119–28. doi: 10.1034/j.1601-0825.2003.01795.x. [DOI] [PubMed] [Google Scholar]


