Abstract
Background:
Corticosteroids (Cs) are used widely for their anti-inflammatory and immunosuppressive properties. They have the potential to cause dramatic improvement as well as produce equally dramatic adverse effects. The clinical misuse like over prescription of the drug should be avoided. Long-term administration may cause many adverse effects leading to impaired oral health. Oral health is usually not considered during management of patients on long-term corticosteroid therapy. The aim of this study was to assess the oral health status and radiological changes in the jaw bones of the patients under long-term corticosteroid therapy.
Materials and Methods:
Oral health of 100 patients under long-term corticosteroid therapy with a minimum of 3 months duration was compared with sex- and age-matched 100 healthy controls. The clinical examination included complete examination of the mouth and periodontal status. Radiographic evaluation of bone with the help of intra oral periapical radiograph and digital orthopantomograph and levels of serum calcium, alkaline phosphatase, and random blood sugar were assessed. ‘Chi-square test’, ‘Kolmogorov-Smirnov test’ and ‘Mann-Whitney U test’ were used for statistical analysis. P > 0.05 was considered significant.
Results:
Patients on steroids exhibited significantly higher levels of candidiasis and clinical attachment loss of the periodontal ligament, probing pocket depth. Bone density was significantly lower in the study group than that in the control group. Random blood glucose was significantly higher and significant lower levels of calcium were observed in patients on steroids.
Conclusion:
Long-term use of Cs may affect oral health adversely leading to candidiasis as well as impair bone metabolism leading to a considerable decrease in the mandibular bone mineral density.
Keywords: Bone mineral density, corticosteroids, oral manifestations, periodontal health
INTRODUCTION
Glucocorticoids were first introduced in the 1940s and have become a widely prescribed class of drugs[1] to treat a wide variety of medical disorders.[2] Corticosteroids (Cs) are used fundamentally as replacement therapy in patients with adrenal gland insufficiency, as immunesuppressive therapy, and as anti-inflammatory treatment.[3,4] In dentistry, they are used primarily to decrease post-operative edema, which may cause post-operative pain.[2,3,4,5,6] Also used to manage oral inflammatory diseases like oral lichen planus, pemphigus, pemphigoid, erythema multiforme, recurrent aphthous stomatitis, and allergic reactions.[2] Some studies have shown steroidal use in trismus,[4] mucocele, post-operative neuralgia, temporomandibular joint disorders, and Bells palsy. Steroids along with the broad spectrum antibiotics are used as a pulp capping agent due to its anti-inflammatory and anti-allergic property.[7]
The cortex of the adrenal gland produces both sex (androgens) and corticoid hormones,[1] the former is secreted in small quantity, with minimum importance in physiologic conditions,[8] the latter being divided into mineralocorticoid (aldosterone) and glucocorticoid (cortisol) steroids.[1,9] The adrenal gland normally produces about 24-30 mg of cortisol each day, but may produce up to 300 mg of cortisol during times of extreme stress. Cortisol secretion is regulated by circadian rhythm, a stress-related response and a negative feedback mechanism between the adrenals, pituitary and hypothalamus.[2] Glucocorticoids have a ‘permissive role’ affecting many physiological processes.[1]
Cs are chemically similar to endogenous cortisol.[10] When supraphysiologic doses of Cs (<30 mg cortisol equivalent) are administered for over 2 weeks, the hypothalamic-pituitary-adrenal (HPA) axis may become suppressed.[2,4] In patients with suppression of the HPA axis, extreme stress or sudden withdrawal of Cs may result in life threatening adrenal crisis due to the lack of endogenous cortisol required to maintain homeostasis.[2] By means of a dosage-time dependent effect, the use of Cs can provoke a secondary adrenal insufficiency or a series of adverse effects caused by the exaggeration of the physiological effects of these hormones.[8] Adverse effects being immune suppression, hypertension, increased fatty tissue (full moon facial appearance), cushing syndrome,[4] growth suppression increased intraocular pressure, cataracts,[2] hyperglycemia, osteoporosis, avascular osteonecrosis, myopathy (muscle weakness and wasting), peptic ulcers (decreases secretion of prostaglandin E2 PGE2 which protects the gastric mucosa), and water and electrolyte balance metabolic effects that result in edema.[2,4] Central nervous system CNS side effects include psychosis, euphoria, sleeplessness and restlessness.[2] Cs may also predispose to infections, acne, hirsutism,[2,4] weight gain, poor wound healing, thinning of the skin,[2] and amenorrhea.[4] Apart from the systemic manifestations, oral changes can also occur like candidiasis, periodontitis and may sometimes result in bony changes like changes in trabecular pattern in jaw bones.[9]
This study was intended to make an assessment of effects on oral health status and jaw bones if any in patients under long-term corticosteroid therapy. To create awareness and to educate these patients, for improving their oral health status.
MATERIALS AND METHODS
Study group comprised of 100 patients under long-term systemically administered corticosteroid therapy with a minimum of 3 months duration who did not give any previous history of any oral diseases and did not have any previous history of immunodeficiency (acquired/congenital) were drawn from Department of Oral Medicine and Radiology, Narayana Dental College and Hospital, in-patients and out-patients from Departments of Pulmonology, Dermatology, Pediatrics, Nephrology and Neurology Narayana General and Super Specialty Hospital, Nellore. Control group comprised of 100 similar age and sex matched patients who were not suffering from any systemic diseases and not under any corticosteroid medication drawn from the outpatient department of Narayana Dental College and Hospital, Nellore.
In the 100 study group patients, 58 were males (mean age, 57.5 ± 8.21) and 42 were females (mean age, 54.21 ± 11.93) on steroids with mean dosage of 7.04 ± 3.56 mg, and mean duration of 10.58 ± 4.24 months. Control group consisted of sex- and age-matched 100 subjects including 53 males (mean age, 52.38 ± 10.55) and 47 females (mean age, 51.55 ± 12.60) who were not suffering from any systemic diseases and not under any corticosteroid medication.
For both the groups, the complete examination of the soft and hard tissues of the oral cavity was done. The number of remaining teeth except 3rd molars was recorded. Measurement of the periodontal status was done using the parameters like plaque index (PI), gingival index (GI) and clinical attachment loss (CAL) and probing pocket depth (PPD) was done to determine the level of the attachment and the depth of the gingival sulcus for all teeth present. Swab were collected from buccal mucosa and tongue by rolling the swab and transported into sterile screw-capped tube and cultured by standard protocol.
Radiographic evaluation was done using intra oral periapical radiograph (I.O.P.A.R.) (Kodak E-Speed Size 2 film) taken by bisecting angle technique in relation to 36,37,38 and 46,47,48 regions using intra oral X-ray machine (SATELEC X-MIND AC, Olgiate Olona [VA] Italy) and Orthopantomograph (OPG.) was taken using digital machine (Planmeca ProMax, Planmeca Oy, 00880 Helsinki, Finland).
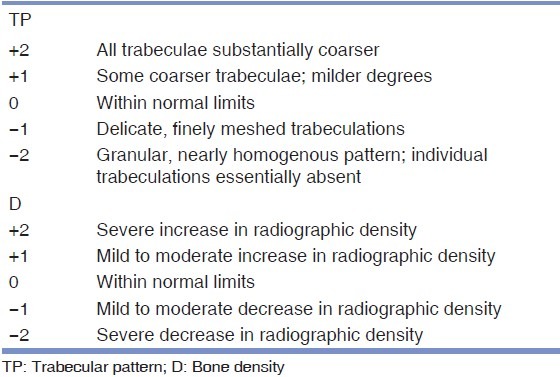
Intra oral periapical radiograph (I.O.P.A.R ) and digital Orthopantomographs (OPGs ) were evaluated by two experienced maxillofacial radiologists independently. I.O.P.A. radiographs were graded following the methodology adopted by Kelly et al.[11][Table 1] for the alterations in the trabecular pattern and overall density of the radiograph.
Table 1.
Criteria for intra oral periapical radiographic evaluation (adopted by Kelly[31])
5 ml of venous blood was drawn from each of both groups of patients. A portion of blood transferred into sodium fluoride coated tube for the estimation of random blood glucose by glucose oxidase method. The remaining blood was separated to obtain serum and stored at −20°C for analysis of alkaline phosphatase activity by rate mode (Kinetic Method) and calcium levels by end point photometry method by using automated chemistry analyzer (Human GMBH, Germany). The study protocol was approved by The Institutional Ethical Committee of Narayana Dental College, Nellore. Informed consent was taken from each patient.
The mean measures per patient were calculated and statistical analysis was done by using software SPSS, 11.5 version, P > 0.05 was considered significant.
RESULTS
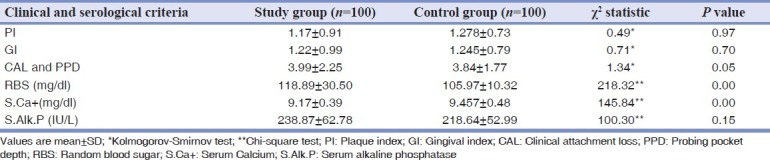
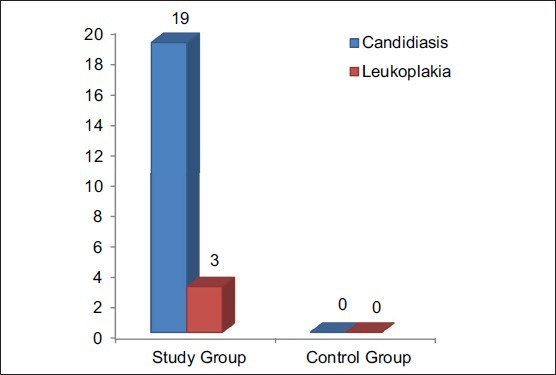
Between study and control groups, Chi-square test (χ2 ) was administered for the prevalence of candidiasis; the value obtained was 53.83, which was found to be highly significant [Figure 1]. Using Kolmogorov-Smirnov test for prevalence of periodontal health status, the values obtained were 0.49 for PI, and 0.71 for GI, which were found to be not significant and 1.34 for CAL and PPD, which was found to be slightly significant [Table 2].
Figure 1.
Comparison of oral mucosal lesions between study and control groups
Table 2.
Comparison of periodontal health status, random blood sugar, serum calcium and alkaline phosphatase levels in the study and control groups
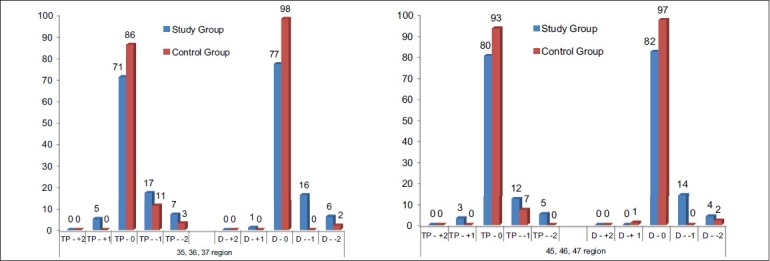
Using Mann-Whitney U test between study group and control groups for trabecular pattern and overall density of the bone in relation to 35,,36,37 and 45,46,47 region, the values obtained were 4702.0 for trabecular pattern in relation to 35,36,37 region, 4622.0 for trabecular pattern in relation to 45,46,47 region, which were found to be not significant and 4065.0 for overall density of the bone in relation to 35,36,37 region and 4173.0 for overall density of the bone in relation to 45,46,47 region [Figure 2] which were found to be significant.
Figure 2.
Comparison of trabecular pattern and bone density changes in intra oral periapical radiograph in relation to 35,36,37 and 45,46,47 regions between patient and control groups
Using Chi-square test for the significance of random blood sugar (RBS) levels, serum calcium (S.Ca+) levels, serum alkaline phosphatase (S.ALP) levels between two groups were obtained. RBS level in the study group was significantly higher than the control group (P = 0.00). S.Ca+ level in the study group was significantly lower compared with those in the control group (P = 0.00). The S.ALP level in the study group was not statistically significant (P = 0.15) [Table 2].
DISCUSSION
Cs can be employed locally or systemically. Topical application is the choice in cases of orally localized lesions.[8,12] In these types of lesions, it is the local action whereas the systemic effect is avoided to its maximum. Absorption occurs at a lesser amount than in other routes. Systemic route of administration is the choice in extensive lesions with aggressive symptomatology, with extensive affectation in other areas of the organism, in those cases wherein a satisfactory result is not achieved by using a local treatment[8] and to treat a wide variety of medical disorders.[2] It has been shown that systemic administration of long-term use of Cs may lead to adrenal suppression,[13] immunosuppression, central obesity, hyperglycaemia, increased susceptibility to infection, reduction of bone mineral density (BMD) and increased risk of osteoporosis.[2,9] Inhaled Cs have a considerably better safety profile than oral steroids.[14]
Apart from the systemic manifestations, oral changes can also occur like oropharyngeal candidiasis,[9,13] periodontitis and bony changes in trabecular pattern of jaw bones.[9] Patients on Cs should be monitored for yeast superadded infection.[15] In our study, the clinical examination of the patient of the control group were free of any oral mucosal lesions and among the study group, 78% were free of oral mucosal lesions, candidiasis [Figures 3 and 4] was observed in 19%, and leukoplakia in 3%. One of the etiological factors for leukoplakia was tobacco, and in our study, leukoplakia was only seen in patients with chronic obstructive pulmonary disease, so it has excluded. The prevalence of candidiasis was found to be highly significant (P = 0.00) [Figure 1]. Digital OPG showed slight significance in periodontitis between the two groups [Figure 5].
Figure 3.
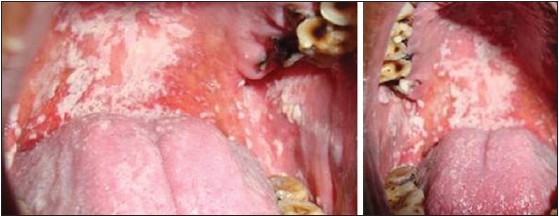
Patient under long-term corticosteroid therapy showing oral candidiasis on the palate and buccal mucosa
Figure 4.
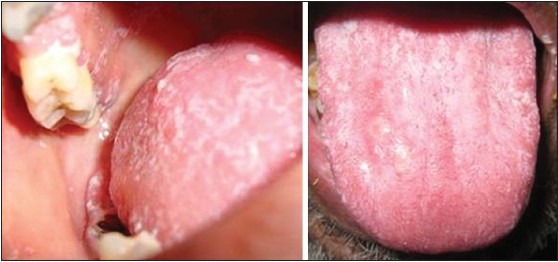
Patient under long-term corticosteroid therapy showing oral candidiasis on the tongue
Figure 5.
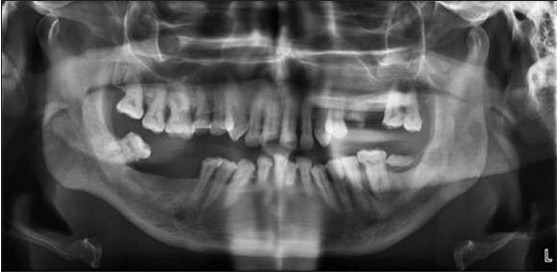
Orthopantomograph showing the periodontal condition of the patient under long-term corticosteroid therapy
Significantly greater loss of attachment with no significant differences in PI and gingival bleeding was reported in osteoporotic women.[16] Ronderos et al. has stated that the clinical attachment level was associated with lowered femoral BMD.[17] In our study, the mean ± SD of PI, GI and CAL and PPD for study group were 1.17 ± 0.91, 1.22 ± 0.99 and 3.99 ± 2.25 respectively and for the control group 1.278 ± 0.73, 1.245 ± 0.79 and 3.84 ± 1.77 respectively. No significant differences in PI, GI were found between the study and healthy control subjects. CAL and PPD, which was found to be slightly significant (P = 0.05) [Table 2].
Cs has effect on electrolyte and water balance. Cs stimulates reabsorption of sodium and excretion of potassium, calcium, and hydrogen ions. Thus, prolonged corticosteroid use may lead to hypernatremia, hypokalemia, hypocalcemia and alkalosis.[2] Long-term use of Cs significantly decreases calcium absorption and increases bone loss and fracture rates.[18] Corticosteroid-induced bone loss is biphasic with a rapid initial phase of approximately 10-15% during the first few months and a slower phase of approximately 2-5% annually.[19,20,21,22] This bone loss, which can be substantial and rapid, is related to both dose and duration of corticosteroid therapy and occurs more rapidly in trabecular bone than in cortical bone.[21,22,23,24] Daily prednisone doses of ≥7.5 mg cause significant bone loss and a doubling in the risk of fracture; if continued for ≥6 months.[20,21,22] Our patients were under prednisolone (n = 70), methyl prednisolone (n = 8), dexamethasone (n = 10), betamethasone (n = 9) and triamcinolone acetonide (n = 3) with the average dose of 7.04 (±3.56) mg and average duration of 10.58 (±4.24) months. In our study, there were no corticosteroid-induced fractures, though there was more number of patients under long-term prednisolone. However, even lower doses (e.g., 6.3 mg/day) of inhaled steroids may also induce bone loss.[20,21,25] Deepak et al. in their case report stated that there was nasal septal perforation in a patient with allergic bronchopulmonary aspergillosis and rhinitis due to prolonged use of inhaled corticosteroid spray.[26]
Tooth loss is influenced by many factors; however, mineral density of the mandible may also play a role in the stability of teeth in the alveolar socket. Hence, a decrease in the BMD may contribute to the resorption of tooth-supporting alveolar bone.[27] The decreased BMD of the jaws may be particularly important as they are exposed to constant masticatory forces. Impaired bone density of the jaws may be a risk factor for tooth loss. Klemetti et al. has stated that individuals with high skeletal BMD appear to retain their teeth with deep periodontal pockets, as opposed to those with low BMD.[28] Komerik et al. has stated that patients with low BMD in various sites of the body skeleton had less teeth compared with the normal population.[27] In our study, though the bone density was low, prevalence of missing teeth among study and control groups was found to be not significant. Olgaard et al. has stated that although significant reductions of the bone mineral content were observed in corticosteroid treated-nephrotic patients, the bone decay rates were significantly different at the mandible, forearm and lumbar spine.[29]
Bone mineral densityBMD, g/cm2 is a strong determinant of bone strength, but there is an overlap between BMD values of fractured and non-fractured populations. Nearly half of the fractures occur in patients with BMD which does not reach the osteoporotic threshold. The force in a fall is a strong risk factor that is not captured by BMD. In order to assess properly fracture risk, other factors are important to be taken into account such as clinical risk factors as well as macro- and micro-architecture of bone.[30] The magnitude of the risk of corticosteroid-induced fractures was related to daily corticosteroid dose.[21,23,31] Women using oral Cs had significantly lower bone mineral densities at the midshaft of radius, hip, and spine compared with women who had never used Cs.[18]
Osteoporosis is defined by the World Health Organization as a disease characterized by reduced bone mass and micro-architectural deterioration of bone tissue, with consequent bone fragility and susceptibility to fractures. Such criteria have not been defined for men, who have larger bones with thicker cortices, although their density and trabecular architecture is similar to that of women.[32] The influence of Cs therapy on the trabecular bone is considered predominant versus cortical bone. Fractures are especially more frequent at trabecular site such as the spine. The BMD decrease during Cs therapy is found both at trabecular and cortical sites.[33] Osteoporosis is one of the serious adverse effects of long-term Cs. Disturbed bone metabolism in osteoporosis leads to low BMD.[27] Trabecular thickness, trabecular separation, star volume, and bone volume/trabecular volume are now assessable by different techniques. Bone microarchitecture can be measured in three dimensions using high-resolution quantitative computed tomography (HR-QCT).[30] BMD was measured by dual-energy X-ray absorptiometry (DEXA).[33,34] Cancellous bone microarchitecture is a key determinant of bone strength but cannot be measured using DEXA. To meet the need for a clinical tool capable of assessing bone microarchitecture, the trabecular bone score (TBS) was developed. It is a noninvasive method for assessing bone microarchitecture. The TBS is a texture parameter that evaluates pixel gray-level variations in DEXA images. The TBS variations may reflect bone microarchitecture. In a retrospective study, among 140 women aged 55.9 ± 14 years with rheumatoid arthritis (mean duration, 15.2 ± 2 years), the mean TBS was significantly lower in the 94 patients taking glucocorticoids (1.19 ± 0.11) than in the 46 other patients (1.23 ± 0.09) (P = 0.03). In this population, no significant differences were found for BMD values or vertebral fracture prevalence.[35] A reduction in bone mass may be suspected from plain radiographs.[22] Digital radiographs can be used to provide bone texture parameters.[30] In our study, I.O.P.A. radiographs and digital OPG were taken and showed no significant difference in the trabecular pattern of the mandibular bone between patients on long-term corticosteroid therapy and healthy control subjects. The density of the mandibular bone in relation to 35,36,37 and 45,46,47 regions in patients on long-term corticosteroid treatment was significant especially at the score of −1 i.e., mild to moderate decrease in radiographic density [Figures 2 and 6] than that in the control subjects (P = 0.00) which implies that the long-term use of Cs may lead to a marked reduction in the mineral density of the mandibular bone.
Figure 6.
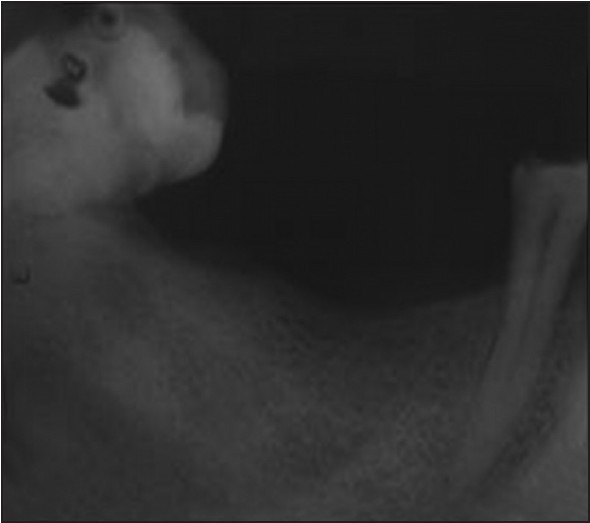
Intra oral periapical radiograph in relation to 45,46,47 region of a patient under long-term corticosteroid therapy showing mild to moderate decrease in the radiographic density
Glucocorticoids play a critical role in the body's response to stress. Stress results in the release of cytokines, and in particular the cytokine interleukin-1, which causes cortisol levels to rise thereby mobilizing the body's glycogen and fat stores.[1] Cs have effect on carbohydrate and protein metabolism. Cs decreases the peripheral utilization of glucose, stimulates gluconeogenesis in the liver from amino acids and inhibits protein synthesis in the muscle. This elevates blood glucose and liver glycogen.[2] Higher doses from the oral versus IV route of administration of Cs have been shown to increase the risk of hyperglycemia.[36] In our study, 83 patients were on oral route of administration. RBS level in the study group was 118.89 ± 30.50 mg/dl as opposed to 105.97 ± 10.32 mg/dl in the control group, and the difference was statistically significant (P = 0.00) [Table 2].
Glucocorticoid treatment, which suppresses disease activity, is also known to affect calcium and bone metabolism.[37] Demineralization of the bone by long-term corticosteroid treatment was stated to be mainly due to the reduced intestinal absorption of calcium.[38] Bone metabolism includes serum carboxypropeptide of type I procollagen, S.ALP and urinary hydroxyproline reported to be abnormal.[39] A slight but significant negative correlation indicating an increasing alkaline phosphatase activity with decreasing bone mass.[40] Studies of biochemical markers of bone metabolism have shown less consistent results in S.ALP.[14] In our study, S.Ca+ level in the study group was significantly lower compared with those in the control group (P = 0.00) [Table 2]. Significantly low levels of calcium found in this study may also reflect the disturbance of calcium absorption. Therefore, a diet rich in calcium and vitamin D can be helpful to maintain adequate bone density in this group of patients. The S.ALP level in the study group was 238.87 ± 62.78 IU/L and in the control group 218.64 ± 52.99 IU/L, the difference were not statistically significant (P = 0.15) [Table 2].
In conclusion, the present study provides evidence suggesting that long-term corticosteroid treatment may affect oral health adversely leading to candidiasis as well as impair bone metabolism leading to a considerable decrease in the mandibular BMD. Regular dental check-up and measurement of BMD of the mandible may be advised for patients receiving long-term corticosteroid treatment to see any changes in the oral health status and to estimate the risk factors for osteoporosis. However, it is difficult to draw a conclusion on the association of oral Cs with tooth loss and periodontal status. Further studies on the prevalence of radiographic changes between plain radiographs and DEXA, HR-QCT, TBS are required to draw definitive conclusions on the relationship between health of dentition and the mineral status of the jaws in patients under long-term corticosteroid treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gibson N, Ferguson JW. Steroid cover for dental patients on long-term steroid medication: Proposed clinical guidelines based upon a critical review of the literature. Br Dent J. 2004;197:681–5. doi: 10.1038/sj.bdj.4811857. [DOI] [PubMed] [Google Scholar]
- 2.Hargitai LI, Sherman CR. Corticosteroids in dentistry. Clin update. 2001;23:11–2. [Google Scholar]
- 3.Bennett PN, Brown MJ. Clinical Pharmacology. 9th ed. New Delhi: Reed Elsevier; 2003. p. 664. [Google Scholar]
- 4.Ata-Ali J, Ata-Ali F, Peñarrocha-Oltra D, Peñarrocha M. Corticosteroids use in controlling pain, swelling and trismus after lower third molar surgery. J Clin Exp Dent. 2011;3:e469–75. [Google Scholar]
- 5.Sortino F, Cicciù M. Strategies used to inhibit postoperative swelling following removal of impacted lower third molar. Dent Res J (Isfahan) 2011;8:162–71. doi: 10.4103/1735-3327.86031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander RE, Throndson RR. A review of perioperative corticosteroid use in dentoalveolar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:406–15. doi: 10.1067/moe.2000.109778. [DOI] [PubMed] [Google Scholar]
- 7.Kallali B, Singh K, Thaker V. Corticosteroids in dentistry. J Indian Acad Oral Med Radiol. 2011;23:128–31. [Google Scholar]
- 8.Llamas-Martínez S, Esparza-Gómez GC, Moreno-López LA, Cerero-Lapiedra R. Corticoids: Their use in the pathology of the oral mucosa. Med Oral. 2003;8:248–59. [PubMed] [Google Scholar]
- 9.Tripathi KD. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers, Medical publishers (P) Ltd; 2008. [Google Scholar]
- 10.Clarence L Trummel. Pharmacology and Therapeutics for Dentistry of Special Drug Groups. 5th ed. St Louis: Elsevier science, Health science division; 1997. [Google Scholar]
- 11.Kelly WH, Mirahmadi MK, Simon JH, Gorman JT. Radiographic changes of the jawbones in end stage renal disease. Oral Surg Oral Med Oral Pathol. 1980;50:372–81. doi: 10.1016/0030-4220(80)90423-5. [DOI] [PubMed] [Google Scholar]
- 12.González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Patol Oral Cir Bucal. 2010;15:e827–31. [PubMed] [Google Scholar]
- 13.Reddi SP, Shafer AT. Oral premalignant lesions: Management considerations. Oral Maxillofac Surg Clin North Am. 2006;18:425–33. doi: 10.1016/j.coms.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewski AF, Lewis SA, Green DJ, Maslanka W, Burrell H, Tattersfield AE. Cross sectional investigation of the effects of inhaled corticosteroids on bone density and bone metabolism in patients with asthma. Thorax. 1997;52:853–60. doi: 10.1136/thx.52.10.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: A consensus approach. J Am Dent Assoc. 2003;134:200–7. doi: 10.14219/jada.archive.2003.0134. [DOI] [PubMed] [Google Scholar]
- 16.von Wowern N, Klausen B, Kollerup G. Osteoporosis: A risk factor in periodontal disease. J Periodontol. 1994;65:1134–8. doi: 10.1902/jop.1994.65.12.1134. [DOI] [PubMed] [Google Scholar]
- 17.Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: Cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol. 2000;27:778–86. doi: 10.1034/j.1600-051x.2000.027010778.x. [DOI] [PubMed] [Google Scholar]
- 18.Marystone JF, Barrett-Connor EL, Morton DJ. Inhaled and oral corticosteroids: Their effects on bone mineral density in older adults. Am J Public Health. 1995;85:1693–5. doi: 10.2105/ajph.85.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEvoy CE, Ensrud KE, Bender E, Genant HK, Yu W, Griffith JM, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704–9. doi: 10.1164/ajrccm.157.3.9703080. [DOI] [PubMed] [Google Scholar]
- 20.Jehle PM, Jehle DR. Use of corticosteroids in nephrology-risk and prevention of osteoporosis induction. Nephrol Dial Transplant. 2000;15:565–8. doi: 10.1093/ndt/15.5.565. [DOI] [PubMed] [Google Scholar]
- 21.Dubois EF, Röder E, Dekhuijzen PN, Zwinderman AE, Schweitzer DH. Dual energy X-ray absorptiometry outcomes in male COPD patients after treatment with different glucocorticoid regimens. Chest. 2002;121:1456–63. doi: 10.1378/chest.121.5.1456. [DOI] [PubMed] [Google Scholar]
- 22.Roy D, O’Neill T. Corticosteroid-induced osteoporosis: Prevention and treatment. Arthritis Res Campaign (arc) 2005:95–8. [Google Scholar]
- 23.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 24.LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:39–51. doi: 10.1016/0169-6009(91)90139-q. [DOI] [PubMed] [Google Scholar]
- 25.Ishizuka T, Yoshii A, Hisada T, Tsukagoshi H, Okayama Y, Iizuka K, et al. Effects of fluticasone propionate on bone mineral density in patients with persistent bronchial asthma. Intern Med. 2002;41:798–804. doi: 10.2169/internalmedicine.41.798. [DOI] [PubMed] [Google Scholar]
- 26.Deepak D, Panjabi C, Gudwani S, Chaudhary N, Shah A. Nasal septal perforation in a patient with allergic bronchopulmonary aspergillosis and rhinitis on long term corticosteroids. Asian Pac J Allergy Immunol. 2001;19:287–90. [PubMed] [Google Scholar]
- 27.Komerik N, Akkaya A, Yildiz M, Buyukkaplan US, Kuru L. Oral health in patients on inhaled corticosteroid treatment. Oral Dis. 2005;11:303–8. doi: 10.1111/j.1601-0825.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 28.Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeleton and advanced periodontal disease. J Clin Periodontol. 1994;21:184–8. doi: 10.1111/j.1600-051x.1994.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 29.Olgaard K, Storm T, van Wowern N, Daugaard H, Egfjord M, Lewin E, et al. Glucocorticoid-induced osteoporosis in the lumbar spine, forearm, and mandible of nephrotic patients: A double-blind study on the high-dose, long-term effects of prednisone versus deflazacort. Calcif Tissue Int. 1992;50:490–7. doi: 10.1007/BF00582160. [DOI] [PubMed] [Google Scholar]
- 30.Kolta S, Paratte S, Amphoux T, Persohn S, Campana S, Skalli W, et al. Bone texture analysis of human femurs using a new device (BMA™) improves failure load prediction. Osteoporos Int. 2012;23:1311–6. doi: 10.1007/s00198-011-1674-2. [DOI] [PubMed] [Google Scholar]
- 31.Koch CA, Layton KF, Kallmes DF. Outcomes of patients receiving long-term corticosteroid therapy who undergo percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2007;28:563–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Flöter M, Bittar CK, Zabeu JL, Carneiro AC. Review of comparative studies between bone densitometry and quantitative ultrasound of the calcaneus in osteoporosis. Acta Reumatol Port. 2011;36:327–35. [PubMed] [Google Scholar]
- 33.Lespessailles E, Siroux V, Poupon S, Andriambelosoa N, Pothuaud L, Harba R, et al. Long-term corticosteroid therapy induces mild changes in trabecular bone texture. J Bone Miner Res. 2000;15:747–53. doi: 10.1359/jbmr.2000.15.4.747. [DOI] [PubMed] [Google Scholar]
- 34.Le Corroller T, Halgrin J, Pithioux M, Guenoun D, Chabrand P, Champsaur P. Combination of texture analysis and bone mineral density improves the prediction of fracture load in human femurs. Osteoporos Int. 2012;23:163–9. doi: 10.1007/s00198-011-1703-1. [DOI] [PubMed] [Google Scholar]
- 35.Bousson V, Bergot C, Sutter B, Levitz P, Cortet B. Scientific Committee of the Groupe de Recherche et d’Information sur les Ostéoporoses. Trabecular bone score (TBS): Available knowledge, clinical relevance, and future prospects. Osteoporos Int. 2012;23:1489–501. doi: 10.1007/s00198-011-1824-6. [DOI] [PubMed] [Google Scholar]
- 36.Tashkin DP. Oral vs IV corticosteroids for in-hospital treatment of COPD exacerbations. Chest. 2007;132:1728–9. doi: 10.1378/chest.07-1622. [DOI] [PubMed] [Google Scholar]
- 37.Lange U, Teichmann J, Müller-Ladner U, Strunk J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: A prospective open-label pilot study. Rheumatology (Oxford) 2005;44:1546–8. doi: 10.1093/rheumatology/kei082. [DOI] [PubMed] [Google Scholar]
- 38.Gennari C. Differential effect of glucocorticoids on calcium absorption and bone mass. Br J Rheumatol. 1993;32(Suppl 2):11–4. doi: 10.1093/rheumatology/32.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- 39.Luengo M, del Río L, Pons F, Picado C. Bone mineral density in asthmatic patients treated with inhaled corticosteroids: A case-control study. Eur Respir J. 1997;10:2110–3. doi: 10.1183/09031936.97.10092110. [DOI] [PubMed] [Google Scholar]
- 40.Hulth AG, Nilsson BE, Westlin NE, Wiklund PE. Alkaline phosphatase in women with osteoporosis. Acta Med Scand. 1979;206:201–3. doi: 10.1111/j.0954-6820.1979.tb13494.x. [DOI] [PubMed] [Google Scholar]


