Abstract
Cerebral sinus venous thrombosis (CSVT) is a rare phenomenon that can be seen with some frequency in young patients. CSVT is a multifactorial condition with gender-related specific causes, with a wide clinical presentation, the leading causes differ between developed and developing countries, converting CSVT in a condition characterized by a highly variable clinical spectra, difficult diagnosis, variable etiologies and prognosis that requires fine medical skills and a high suspicious index. Patients who presents with CSVT should underwent to CT-scan venography (CVT) and to the proper inquiry of the generating cause. This disease can affect the cerebral venous drainage and related anatomical structure. The symptoms may appear in relation to increased intracranial pressure imitating a pseudotumorcerebri. Prognosis depends on the early detection. Correcting the cause, generally the complications can be prevented. Mortality trends have diminished, and with the new technologies, surely it will continue. This work aims to review current knowledge about CSVT including its pathogenesis, etiology, clinical manifestations, diagnosis, and treatment.
Keywords: Brain, cerebral embolism and thrombosis, cerebral sinus venous thrombosis, cerebrovascular disease, neurosurgery, sinus thrombosis
Introduction
Cerebral sinus venous thrombosis (CSVT) is a rare form of venous thromboembolism (VTE).[1] CSVT represents almost 0.5%[2] -3%[2] of all the types of stroke, affecting predominantly younger people,[3] with an estimated incidence for adults of 3-4 per million, and for children 7 per million.[4]
In the pre-antibiotics era, the leading cause of CSVT were the septic processes, currently the aseptic form of CSVT is the most common cause.[5] An autopsy finding can represent 10% of death causes from cerebrovascular diseases.[6]
CSVT is characterized by a highly variable clinical spectra, difficult diagnosis, variable etiologies and prognosis. Of interest, in poor countries, there is an association with the puerperium, with no clear arguments, but probably related to factors such as inappropriate perinatal care, metabolic derangements, and infections associated to childbirth.[5] The peripartum-associated CSVT has been established to be of 11.6 cases per 100 000 deliveries.[7] There is sex predominance (hormonal?); 75% of all CSVT patients are women, with a 3:1 ratio compared to men.[8]
This work aims to review current knowledge about CSVT including its pathogenesis, etiology, clinical manifestations, diagnosis, and treatment.
Anatomy
Cerebral venous drainage is comprised of two systems, the superficial and the deep venous systems. The drained blood runs into the major dural sinuses: Superior sagittal sinus (SSS), inferior sagittal sinus (ISS), lateral sinus (LS), cavernous sinus and straight sinus, and then to the internal jugular vein (IJV). Due to its high proportion of anastomoses, the superficial venous system is difficult to diagnose in cases of occlusion, this system predominantly drain the SSS and the LS. Deep white matter and basal ganglia are drained by the deep venous system toward the vein of Galen. Many anastomoses can be found between these two cerebral venous systems.
Dural sinuses are divided classically into posterior superior (P-S) and anterior inferior groups (A-I). P-S comprises the SSS, ISS, LS, straight sinus, and occipital sinus. The A-I group comprises the superior and inferior petrosal sinuses and the cavernous sinus. Inside of dural sinuses are found the Pacchioni's or arachnoid granulations, which play an essential role in the cerebrospinal fluid (CSF) physiology. SSS is located anatomically in the attached margin of falxcerebri, and drains almost all cerebral cortex.
LS have two sections, the transverse and the sigmoid, which drain cerebellum, brain stem, and the posterior portions of brain hemispheres. In the skull base are located the cavernous sinuses, into these sinuses, run oculomotor, trochlear, the ophthalmic and maxillary branches of the trigeminal nerves, through the petrosal sinuses, the cavernous sinuses drain the blood to the IJV.
Pathogenesis
Clinical findings of CSVT can be product of two main mechanisms:
1. Occlusion in cerebral veins
This occlusion can cause outflow obstruction and venous congestion, increasing the capillary hydrostatic pressure[9] and then producing edema, but this not necessarily means focal venous infarctions.[10] Histological analysis reveals dilated and enlarged veins, edema and ischemic neuronal damage, petechial hemorrhages that can converge and transform into hematomas. Two types of edema can develop, cytotoxic and vasogenic edema, the magnetic resonance (MR) could differentiate the type of edema present during the CSVT event.[11,12]
2. Occlusion of venous sinuses
Derived from this phenomenon, the intracranial hypertension (ICH) is the predominant characteristic. Normally, the CSF drains into the SSS through the Pacchioni's or arachnoid granulations. When the thrombosis happens, the venous pressure raises due to delaying in the venous emptying, altering the CSF absorption, and thereby raising the intracranial pressure.[4,5]
The sufficiency of the collateral blood drainage will determine the symptoms. When collaterals are sufficient, symptoms are related to ICH; when they become insufficient, the venous congestion causes ischemia and infarctions.[5] In the case of younger patients, the CSVT spectrum can range from venous congestion detectable or not on neuroimaging, to the parenchymal cortical or subcortical ischemic injury,[9] lesser frequently is observed the CSVT-related sub-arachnoid and subdural hemorrhages.[9]
The International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) determined the frequency of the sites of SCVT as follows: Transverse sinus 86%, superior sagittal sinus 62%, straight sinus 18%, cortical veins 17%, jugular veins 12%, vein of Galen and internal brain veins 11%.[8]
Causes and Risk Factors
CSVT is a multifactorial condition with sex-related specific causes.[1] As in any thrombotic process, risk factors are associated with the classical Virchow triad of thrombogenesis: hypercoagulability, vessel wall damage and blood stasis.[13,14] It may be associated with inherited and acquired risks factors (see Table 1); however, this categorization is fairly artificial, because they have additive effects and CSVT is multifactorial.[15] By far, in developed countries, the most frequently associated factor is congenital thrombophilia.[2] Inherited prothrombotic risk factors include homocysteinemia, factor V Leiden homozygous mutation, G20210A prothrombin gene and Methylene- Tetra-Hydro-Folate-Reductase 677TT mutations, protein C and S and anti-thrombin III deficiency, and positive anti-cardiolipin or anti-phospholipid antibodies.[16,17]
Table 1.
Risk factors associated to CSVT
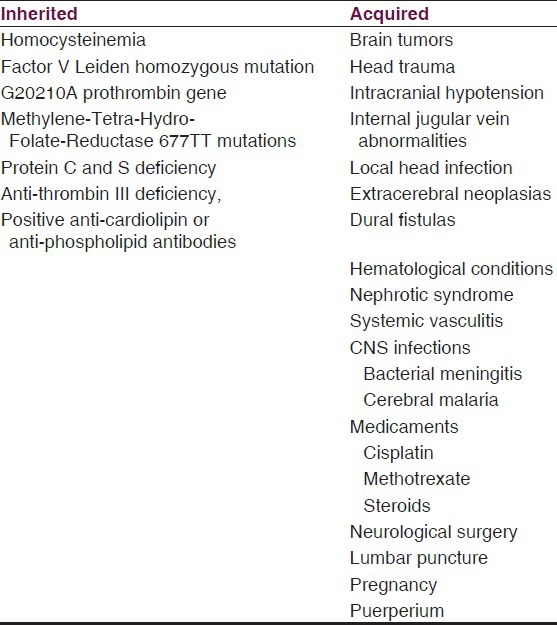
Acquired risks factors include all the usual causes of VTE and additionally causes such as brain tumors,[18] head trauma,[19] central nervous system infections (bacterial meningitis, cerebral malaria,[20,21,22,23] intracranial hypotension,[24,25,26,27,28,29] any local head infection,[2] extracerebral neoplasias,[30,31] dural fistulas,[32] hematological conditions,[33,34,35,36,37] nephrotic syndrome,[38,39,40,41] systemic vasculitis,[42,43,44] medicaments (cisplatin,[45] methotrexate,[46] steroids), neurological surgery, lumbar puncture,[47,48,49,50] pregnancy,[1,51,52,53,54] Puerperium.[3,55,56] The Table 1 summarizes the risk factors associated to CSVT.
The IJV represent the main outflow pathway for the cerebral venous system.[57] IJV abnormalities may change the hemodynamics of cerebral venous outflow, leading to insufficient venous drainage, and subsequently causing CSVT. In a study performed by Jia et al.,[14] found that among 51 consecutive CSVT cases with unknown etiologies, 61% of the cases (31/51) had an IJV abnormality; furthermore, almost all the CSVT occurred on the same side as the IJV lesions, which strongly suggests a close association between IJV abnormalities and CSVT. However, a predisposing factor may not be identified in up to 20–35% of cases.[17,58]
The marked sex preference is usually attributed to gender-specific risk factors, especially oral contraceptives use (OC) and to a lesser extent, pregnancy, puerperium, and the hormone replacement therapy. Conclusions have been obtained from researches in age groups that lack of these gender-specific risk factors (i.e., children and elderly patients), and have showed no gender predilection.[59,60] Furthermore, epidemiological studies performed before OC era do not show a difference in gender distribution.[60] Strikingly, evidence has shown that contraceptive products that delivers a lower systemic estrogen dose like NuvaRing® has at least as much prothrombotic potential as combined OC, doubting the theoretical benefits of non-oral route of steroid hormone delivery.[61] Case-control studies showed a significant association between use of OC and CSVT, which was confirmed in a meta-analysis (pooled OR, 5.59).[62]
In the ISCVT registry,[8] 44% of the patients had one risk factor, and congenital or genetic thrombophilia was present in 34.1% of the patients. Gender-specific risk factors were present in 65% of the women. Near to 85% of the patients with CSVT have prothrombotic risk factors or direct causes. Inherited or acquired thrombophilia and the OC use were the commonest risks factors.[8] In the group of acquired thrombophilia, the anti-phospholipid syndrome was the most frequent (5.9%), followed by hyperhomocystinemia.[8] OC use was in 54.3% of the patients;[8] thus, keeping relationship with the results of Bruijin et al,[63] 85% of women in their study used OC, from which 56% were third generation OC, containing gestodene or desogestrel.
The RENAMEVASC study (from a registry conducted between 2002 and 2004 in 25 Mexican hospitals) found that gender-specific risk factors explained the majority of cases, and as suboptimal the thrombophilia assessment and acute treatment. Those findings make a big difference regard registries conduced in developed countries, where is performed a standard protocol for thrombophilia and active seeking of oncology and hematology patients.[3]
The fluctuations of ICP during delivery and the hypercoagulability state of pregnancy due to enhanced platelet adhesion and to increased coagulation factors could be an explanation for the augmented risk of CSVT in the peripartum. In two clinical reports, one from the Hospital Militar Nueva Granada in Bogotá,[64] and another from the University Hospital of North Staffordshire,[65] both patients had persistent cephalea during more than two weeks after epidural anesthesia, with evidence of dural accident in one of the cases, unresponsive to blood patches, and with diagnosis of lately CSVT. The group of University Hospital of North Staffordshire proposes that obstetric units should have protocols for the management of post-puncture headache, which includes thromboprophylaxis for patients with low mobility and an earlier multidisciplinary approach with neurologists and radiologists when cephalea persists after dural blood patch.[65]
Not all CSVT involve venous infarction; SanzGallego et al.[10] studied the influence of risk factors in patients with CSVT regard the probability of developing venous infarction, they included 52 patients, and found that the most frequent risk factor in those who had venous infarction was thrombophilia (40.9%), whilst in those without venous infarction the use of OC predominated (26.7% of the total sample; 38% of females), with thrombophilic states only being detected in 16.5%. No cases of venous infarction were found in the group of patients with OC but without an associated thrombophilic state. They concluded that appears to be a different profile of risk factors in patients who develop venous infarction associated to CSVT, with thrombophilia prevailing. In conclusion, CSVT is a multi-factor consequence, and the identification of one of them should not prevent the intentional search for co-existing causes that may potentially increase the probability of recurrence.[3]
Clinical Manifestations
Clinical data of CSVT is extraordinarily variable, and such variability depends on different factors, such as the location and extension of the thrombosis, the extent of the venous occlusion, patients age, and the nature of the underlying disease or predisposing cause.[66] Although a few differences have been identified between men and women in the clinical presentation of CSVT, these differences are minor, and the diagnostic work up and treatment strategies are roughly similar in both groups.
In 30% of CSVT cases, it presents in an acute fashion and the symptoms appear in less than 48 h. In up to 50% of cases, it presents in a sub-acute fashion and the symptoms appear between 48 h and 30 days. The chronic form corresponds to 20% of cases, and the symptoms develop over a period greater than 30 days and up to 6 months,[5] see Table 2.
Table 2.
Presentation fashion of CSVT

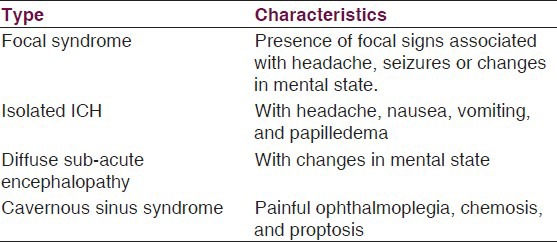
Clinical findings in CSVT usually are due to the impaired venous drainage, ICH, focal brain injury from venous ischemia/infarction or hemorrhage, or mix of them; however, it is not necessarily to be together at presentation.[13] Bousser et al.[2] described 4 clinical patterns for CSVT, see Table 3:
Table 3.
Clinical patterns of CSVT
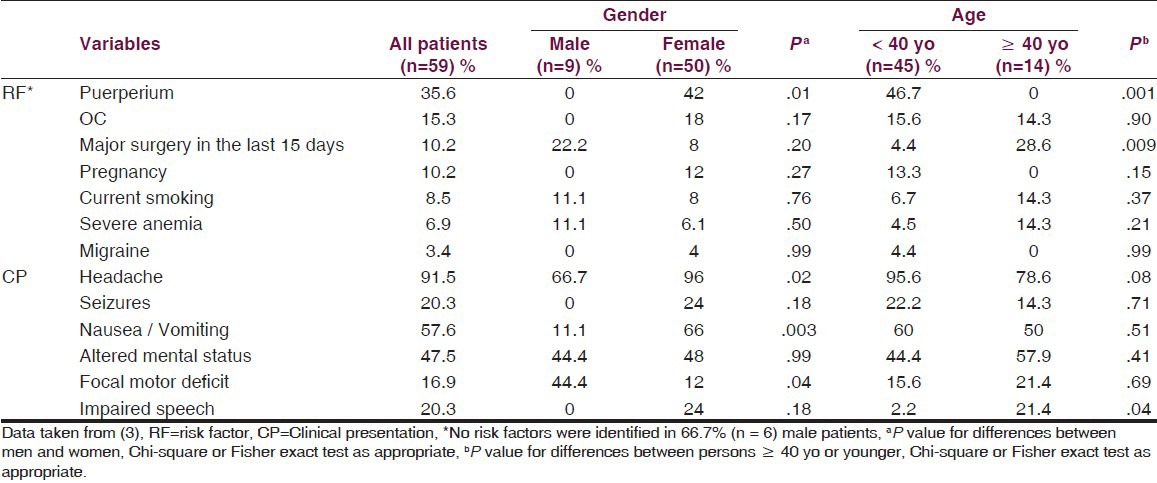
Headache is the most common symptom in CSVT. In the SCVT, it was present in almost 90% of patients; in fact, in CSVT, this symptom is more frequent than in cerebrovascular ischemic arterial disease. Its physiopathology lies in the ICH syndrome, in the venous walls distension or due to local inflammation or the blood leakage over the brain surface irritating dural sensitive fibers.[66] Similar headache frequency has been reported in other populations studied, see Table 4.[3,13]
Table 4.
Analysis of risk factors and clinical presentation of CSVT
Typically, the cephalea is described as diffuse and often progressive in severity over days to weeks,[13] but, in a minority of patients may present as a thunderclap headache, suggesting an hemorrhagic process (i.e., sub-arachnoid hemorrhage), oras migraine cephalea,[13] that difficult diagnosis, because can be confusing to distinguish due to its unilateral character, intermittent course and for the usual visual abnormalities.[66] Diffuse or anterior/posterior bilateral headache has been reported.[67]
Isolated headache without focal neurological findings or papilledema occurs in up to 25%-40% of patients with CSVT, confusing the diagnostic process.[13,37] Even in the absence of neurological focal signs suggestive of idiopathic ICH, in all patient with headache and papilledema or diplopia (caused by sixth nerve palsy), should be considered as differential diagnosis the CSVT.[13] Since ICH can be present in an isolated way, the idiopathic ICH should be considered among differential diagnosis; clinically, the main difference between ICH secondary to CSVT and idiopathic ICH is that the latter predominates in obese women.[5]
Near to 12-31.9% of patients have seizures as the presenting feature of CSVT, but 44.3% of patients may have seizures in the early stage of the disease.[68] Similar proportions have been found in other studies.[69,70] Focal sensory and motor deficits are very common and occasionally suggest the location site, especially when there is a paralysis of cranial nerves such as in IV paralysis.[5] Focal or generalized seizures are frequent, occurring in near 40% of patients.[13] In patients with seizures, Todd's paralysis can be presented in near 54% of cases.[69]
According to the SCVT, the course of clinical manifestations can be summarized as follows: intensive and progressive cephalea, the main symptom; seizures (39.3%); paresis (37.2%); papilledema (28.3%); altered mental state (22%), aphasia (19.1%), stupor or coma (13.9%), diplopia (13.5%), and visual deficits (13.2%).[8]
In cases of septic CSVT, the clinical pattern is characterized by cephalea, fever, ophtalmoplegia due to paralysis of oculomotor, abducens and trochlear nerves, all, making compatible with orbital cellulitis, which is the most common antecedent in these cases.
Diagnosis
This entity should be considered in all young women who have unusual headache that can be progressive in time, with focalized neurological deficits and without vascular risk factors. Because the variability in the clinical presentation, it should be excluded by using available neuroimaging studies when there is clinical suspicion. Due to the wide availability of magnetic resonance venography (MRV) and CT-scan venography (CTV), the invasive procedures (i.e., cerebral angiography and direct cerebral venography) are less commonly needed for making diagnosis. Generally, invasive modality of diagnosis is reserved when MRV or CTV results are inconclusive or if an endovascular procedure is being considered.[13]
CT-scan
Because of its wide use as the initial neuroimaging test in patients who present with new-onset neurological symptoms, the first study that should be carried out in the emergency department is a brain CT scan, with or without contrast. CT-scan will help us to discriminate many of the conditions that could mimic it.
In near to 40% of the cases is visualized on CT-scan generalized or localized hyperdensity areas, indicative of hemorrhagic infarctions, constituting the most common finding.[71] The hemorrhagic transformation of an infarcted area occasionally could generate intracranial hemorrhages, like sub-arachnoid ones.[5,72,73,74] The precise mechanism of development of SAH (sub-arachnoid hemorrhage) in patients with CSVT remains unknown; different pathophysiological explanations have been proposed:[72]
Cerebral venous thrombosis causes a local inflammatory response that increases the vascular permeability allowing for extravasation of blood into the sub-arachnoid space;
Venous parenchymal hemorrhagic infarct is a potential complication of CSVT and can rupture in certain cases into the sub-arachnoid space;
Extension of the dural sinus thrombosis into the superficial veins causing localized venous hypertension with dilatation of thin, fragile-walled cortical veins, which eventually rupture into the sub-arachnoid space.
Unenhanced CT will be normal in the majority of patients with a normal neurological examination, but is often abnormal in patients who do exhibit neurological signs.[75] The non-contrast-CT scan has low sensibility (25-56%), but the apparition of direct signs are highly specifics.[76,77,78] To diagnose CSVT, we can help of direct (visualization of thrombus in the affected vessel) and indirect (brain parenchyma damage from ischemia or vascular changes related to venous outflow disturbance) neuroradiological signs.[79]
Direct signs
There are three direct signs of CSVT in the CT-scan, the “string sign,, the “dense triangle sign,” and the “empty delta sign.”
The “string sign” is found in 25% of CSVT patients, it appears when there is cortical vein thrombosis in the non-contrast enhancing CT. It looks like elongated hyperdense image relating to the brain parenchyma. Slow flow can also produce it, thus it is a non-specific sign.
The “dense triangle sign” can be seen during the first 2 weeks in up to 60% of patients[5] and represents spontaneous SSS opacification from fresh coagulated blood.[80] Mimicking occurs in patients with increased hematocrit or dehydrated. Overall, this sign has been reported in only 2% of CSVT cases;[80] and the “empty delta (or empty triangle) sign,” seen after the contrast medium is administered, has been reported in between 10% and 35% of cases;[80] it is produced due to an intraluminal filling defect surrounded by contrast in the posterior portion of the SSS;[5] there is enhancement in the wall of this sinus that is outlining the hypodense clot within the lumen.[80] The enhancement is occurring in collateral veins in the sinus wall surrounding the clot;[78,81,82] usually is associated to poor prognosis. This sign can be mimicked by many conditions like high splitting of the superior sagittal sinus, subdural hematoma, sub-arachnoid hemorrhage, epidural abscesses, and by the presence of fenestrations and septae within the sinus.
Indirect signs
Indirect signs of CSVT are much more commonly visualized on CT-scan than direct signs;[80] they are not specific, but they should draw attention and prompt a search for direct visualization of thrombi, whether in sinus or in cortical areas.[79] In patients with septic, lateral sinus thrombosis can be found erosion in the middle ear structures and changes in the mastoid region.[83] Early changes are often subtle, with brain edema and swelling of the gyri.[79] Bilateral parasagittal hemispheric lesions are suggestive of SSS thrombosis;[79] when the transverse sinus is compromised, ipsilateral temporo-occipital and cerebellar lobe lesions, hydrocephalus and compression of the fourth ventricle can be present.[5,79]
White matter hypointensity without contrast enhancement suggestive of cerebral edema, ventricles with reduced size secondary to cerebral edema are also indirect signs of CSVT.[5,79] In close than 40% of CSVT patients can be present secondary strokes associated to focal or diffuse edema, brain furrow effacement, and strengthening the falx or tentorium. Venous infarction manifests as a low-attenuation lesion with or without sub-cortical hemorrhage.[79] Strokes secondary to CSVT can be bleeding or non-bleeding and usually affect structures near the damaged site.[5]
Magnetic resonance (MRI)
Imaging of the cerebral venous system by direct visualization of a thrombus within the vessel,[84] constitutes the primary findings on MRI, can be seen like an absence of a flow void and the presence of altered signal intensity in the sinus.[75] The MRI will vary depending on the age of the thrombus. In the acute and sub-acute stages, the signal characteristic alters according to the presence of blood breakdown products.
T2 imaging sequences may be an important diagnostic aid in acute-stage thrombosis, when the signal intensities on T1- and T2-weighted images may be more subtle.
The presence of paramagnetic blood breakdown products (i.e., deoxyhemoglobin and methemoglobin) produces blooming artifacts in the thrombosed segments.
An MRI can even be normal in up to 30% of patients.[84] The presence of thalamic edema is highly suggestive of deep venous occlusion; this is an alarming finding, because the patient may deteriorate quickly to coma. Parenchymal hemorrhage can be seen in up to 30% of the CSVT cases. In patients with SSS thrombosis, typically is the finding of flame-shaped, irregular zones of lobar hemorrhage in the parasagittal frontal and parietal lobes. This should prompt additional imaging evaluation with MRV or CTV. The transverse sinus thrombosis can be seen as hemorrhage lesions in temporal or occipital lobes. MRI with T2 sequences is sensitive in the depiction of zones of parenchymal hemorrhage.[85] MRV and CTV have equivalent sensitivity and specificity for demonstration of the thrombosed segment.[85]
CT and MR venography
Due to its vascular detail and ease interpretation, CTV can provide a rapid and reliable diagnosis of CSVT.[79] CTV has proved to be a reliable method to investigate the structure of the cerebral veins, with a reported sensitivity of 95% with multiplanar reformatted (MPR) images when compared with digital subtraction angiography (DSA) as the gold standard.
Some disadvantages of conventional CTV are the time-consuming and operator-dependent editing needed to remove over projecting bone for the angiographic display of intracranial vessels,[79] radiation exposure, and problems related to use of contrast in the setting of poor renal function, or in patients with contrast material allergy, because of these concerns, MRV have being preferred to CTV. CTV is much more useful in sub-acute or chronic situations because of the varied density in thrombosed sinus. As previously mentioned, the dense cortical bone adjacent to dural sinus made prone bone artifact interfering with the visualization of enhanced dural sinus.
The combination of abnormal sinus signal and the corresponding absence of venous flow in the MRV confirm the diagnosis, but in front of a chronic CSVT with unclear MRI, the angiography could be the first diagnostic tool.[86,87] Besides of allow the CSVT diagnosis, the MRI and the MRI angiography obviate the use of invasive angiography, and furthermore, they are useful for the follow up of CSVT patients.[88]
CT-contrasted angiography and the Gadolinium-enhanced MRI or the MRI angiography has 100% sensitivity and specificity for identification of CSVT;[88,89] thus, CTV is at least equivalent to MRV in the diagnosis of CSVT.
MRV features include non-visualization of the vessel (i.e. no flow), flow defect, and presence of collaterals at the site of occlusion,[84] see Figure 1. In the first 5 days, the thrombus is highly hypointense in T2- weighted MRI and isointense in T1- weighted MRI. Until the 15th day, the hyperintense thrombus signal in T1- and a T2- weighted image persists. This could be the most important period of time because is when most diagnosis are made. The thrombus signal diminishes at the third week, and the imagenological evolution is toward the re-establishment of the blood flow or to a residual blood clot. Venous infarction on MRI is a significant predictor of clinical deterioration in patients with CSVT (P = 0.001).[90] Diffusion-weighted image (DWI) hyperintensity indicating diffusion restriction is also predictive of clinical deterioration in patients with CSVT.[90]
Figure 1.
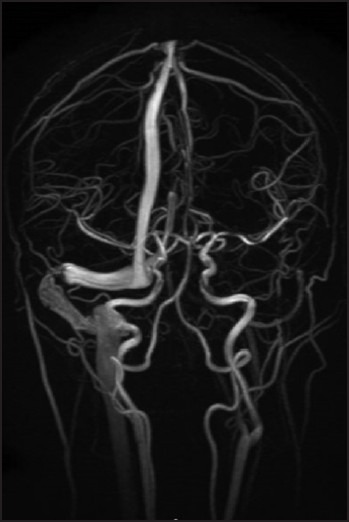
3D-MIP (reformatted by 2D-TOF) sequence of a 40-year-old female patient with CSVT. Sequence obtained after 2 months does not show changes at the level of straight sinus; the transverse sinus is still absent of flow near the confluence of the sinuses.
Ultrasound
Intravascular ultrasound (IVUS) is an important diagnostic tool in many interventions; its application in the management of diseases of the cerebral venous system remains an unexplored territory. In a recent case presentation, Mokim et al.[91] used IVUS in 3 patients, in whom digital subtraction angiography failed to discriminate thrombosis from structural parietal stenosis. It has been reported that MRV has clear limitations; distinction between thrombosis and simple flow gaps using MRV may, therefore, be difficult. The main limitation of that report is clearly the small number of cases. However, that experience, in addition to others in literature, suggests that IVUS could have an expanded use in the future, participating in better diagnosis of cerebral intravascular pathologies and helping in the monitoring of intravascular treatments using tissue plasminogen activator (tPA), angioplasty, or stent application.[92] Main problems that must to solve ultrasound technology for being useful in CSVT is to establish hemodinamically relevant alterations, that are highly mutable due to the variability of cerebral venous system anatomy, furthermore normal venous blood flow velocities often do not necessarily exclude the diagnosis of CSVT. Transfontanellar ultrasound may be used to evaluate pediatric patients with open anterior or posterior fontanels.[13]
Treatment
Treatment strategies are aimed to control or resolve the underlying pathology, controlling ICH and treatment of seizures or focal deficits caused by cerebral edema or infarction.[93] Anti-coagulation is used almost universally and in selected cases, endovascular and surgical techniques have been employed to remove the clot. Surgical techniques are further used to treat the sequelae of CSVT such as hydrocephalus, ICH, hemorrhagic stroke, among others.
Medical Management
General measures
General measures like proper headboard inclination, adequate oxygenation, and protection of airway due to risk of bronchoaspiration are recommended. Seizures can be present in more than 30% of CSVT patients.[94] Current CSVT guidelines state that because seizures increase the risk of anoxic damage, anti-convulsant treatment after even a single seizure is reasonable.[13] Patients who initially present seizures, hemorrhage, targeting data or thrombosis in the cortical veins are candidates for anti-convulsive drugs.[5] Ferro et al.[95] found that CSVT patients with supratentorial lesions had a higher risk for both presenting and early seizures, whereas patients with presenting seizures had a higher risk of recurrent seizures within 2 weeks, supporting the prescription of anti-epileptic drugs in acute CSVT patients with supratentorial lesions who present with seizures.
ICH is a complication that can be resolved or treated early attacking the thrombotic event (anti-coagulation or thrombolysis), and with alter invasive procedures such as removal of CSF with lumbar puncture, until achieve a normal closing pressure. Unfortunately, as happens with seizures prophylaxis, no randomized trials are available to clarify the optimal treatment. In the case of septic causal factors, the use of appropriate antibiotics and the drainage of the infectious focus are recommended.[13] The use of steroids are not recommended, because they can generate further hypercoagulability and are associated to poor prognosis.[96]
Anticoagulation
Heparin has been used to treat CSVT since 1941.[93] The use of heparin and oral anticoagulants (OA) is based basically on the rationale of reversing the causal thrombotic process and on preventing complications. Due to the presence of a hemorrhagic element in 40% of CSVT, the administration of anticoagulant treatment stills controversial.[5]
In the metanalysis performed by Coutinho et al.,[97] where only were included the only two random studies with the minimum methodological standards, heparin was associated with an absolute reduction in mortality of 13% (CI 95%: 27-1%; P = .08) and a reduction in absolute risk of death or dependence of 15%, without causing an increase in new hemorrhagic lesions, also was observed a greater frequency of pulmonary embolism in those patients who did not receive anticoagulation.
Further evidence for support the use of heparin derive from the finding that 39% of cases in the SCVT cohort had intracerebral hemorrhage before treatment, no prognosis worsening was observed in the 83% of all patients treated with heparin.[8] Other concern related to heparin is that currently there are no precise instructions on which type of heparin to use [Unfractioned heparin (UFH) vs. Low Molecular Weigh Heparin (LMWH)]. The “European Federation of Neurological Societies” (EFNS) guideline recommends LMWH because of practical advantages. The main advantage of this heparin use is that it is easy to antagonize in situations such as the need for surgical intervention[5,98] and based upon data from randomized trials in leg-vein thrombosis,[99] but in the ISCVT, the UFH was used in almost the majority of patients.[8] An international survey performed with the aim to determine how physicians worldwide treat CSVT showed that physicians worldwide consider heparin the primary therapy for CVT patients. However, while evidence-based guidelines advocate the use of LMWH, the majority uses UFH.[100] Other advantages of LMWH in therapeutic doses are that it provides more steady anticoagulation and does not require dosage adjustment based on coagulation times.[5] UFH is administered intravenously at an initial dose of 5000 UI, and then the infusion is maintained at 1000 UI/h or a response dose to achieve an activated partial thromboplastin time of 60-80s.[101]
Guenther et al.[5] recommends that after the acute phase of CSVT, to use OA unless there is a clear contraindication and in cases of CSVT associated with a transient risk factor such as infection, trauma or pregnancy, a treatment period of 3 months is enough, but in the case of being present a greater risk of recurrence, such as pro-thrombotic states, the duration of anticoagulation should be longer (6-12 months). Also recommend a suggest maintaining anticoagulation with an international normalized ratio (INR) of between 2.0 and 3.0.
Endovascular and Surgical Management
Thrombolysis
Thrombolytic agents, applied locally with endovascular jugular or femoral access, have been used since 1971. Endovascular thrombolysis is generally reserved for severe cases, but no randomized trials have been performed.[100] It seems that both endovascular thrombolysis and decompressive craniectomy are increasingly used. In the ISCVT study, 2% of patients received thrombolysis and 1% of patients received decompressive craniectomy;[8] in the international survey mentioned above, 43% of physicians had used either therapy during the last 5 years.[8,100] In the two largest series where fibrinolytic agents were used, blood flow was restored in the majority of cases (71.4%).[102,103] Apparently local fibrinolytic treatment restores blood flow more quickly and efficiently than heparin, but carries the risk of hemorrhage. Currently, there have been no clear indications for the use of local or systemic thrombolytic agents due to the lack of conclusive studies supporting it.[5] Mechanical techniques (i.e., extracting the clot with waves) reduce the required thrombolytic dosage and, therefore, decrease the risk of intracranial hemorrhage. Thrombolysis is an option for providing rapid recanalization. Local thrombolysis involves removal of the thrombus and can restore the patency of the involved sinuses. Local intra-sinus thrombolysis can be an effective and relatively safe treatment for acutely deteriorating patients who have not responded to conventional anticoagulant therapy.[104] Local thrombolysis is a safe and effective treatment modality for patients suffering from progressive CSVT.[104] In general, thrombolytic therapy is used if clinical deterioration continues despite anticoagulation or if a patient has ICH that evolves despite other management approaches.[13]
Decompressive craniectomy (DC)
In patients with ICH who have minimal or none response to the initial treatment, should be considered DC. The rationale is to provide a new space to the brain to diminish the pressure. Coutinho et al.[105] and Théaudin et al.,[106] in cases with CSVT and unfavorable evolution, showed that DC saved patients life, and also improved functional prognosis even in patients with bilateral pupil dilation. In a recent retrospective study, among 34 patients (the largest series currently) who underwent decompressive craniectomy, 26 (76.4%) achieved a favorable outcome (GOS ≥ 4).[107] In the ISCVT cohort, DC was used in only 9 patients (1.4% of cases), and in the RENEMEVASC cohort, DC was performed in 2 (3%) patients. These reflect how little this measure is used in daily practice. Surgical thrombectomy is needed uncommonly, but may be considered if severe neurological or visual deterioration occurs despite maximal medical therapy.[13]
Outcome and Prognosis
Fifty years ago, CSVT was a mortal condition, but with the introduction of neuroimaging, the mortality rates have become minimal. A case fatality rate of 3% in the RENEMEVASC registry[3] is considerably lower than other studies that report range between 6% to 27% in elderly patients.[8,62,108,109,110,111] Although CSVT may have a lower case fatality compared to arterial strokes, is important to predict the subgroup of patients with CSVT who are at risk of clinical deterioration. Yii et al.,[90] in a retrospective analysis of 106 CSVT patients with the aim to investigate the imaging predictors of clinical deterioration, founded that patients with venous infarcts and hyperintensity on DWI MRI were at increased risk of clinical deterioration, suggesting an essential need for close monitoring of these patients. In the RENEMEVASC registry, 37 (63%) patients attained functional independence (mRS, 0-2) at hospital discharge, 20 (34%) were dependent. At 30-day follow up, 43 (72.9%) achieve functional independence, and 14 (23.7%) persist dependent. In the ISCVT registry, only 5.1% of patients presented serious residual disability, while 70-85% of patients presented a complete recovery 2 months after follow-up. Recurrence of thrombosis is specially high for patients with pro-thrombotic risks factors or for those who have deep venous thrombosis in lower limbs, the range can go from 0% in the first year, up to 12% at 6.5 years.[5,112,113]
Conclusion
CSVT is a multifactorial condition with gender-related specific causes, with a wide clinical presentation, furthermore, the leading causes differ between developed and developing countries, i.e., congenital thrombophilia vspuerperium or OC use respectively.
CSVT is a condition characterized by a highly variable clinical spectra, difficult diagnosis, variable etiologies and prognosis that requires fine medical skills and a high suspicious index. Patients who present with CSVT should underwent to CVT and to the proper inquiry of the generating cause. It is imperative an intentional search for co-existing causes, as well as the management of post-puncture headache.
Correcting the cause, generally the complications can be prevented. Mortality trends have diminished, and with the new technologies, surely it will continue. Treatment strategies are aimed at treating the underlying pathology, controlling ICH, and management of seizures or focal deficits caused by cerebral edema or infarction.
Fifty years ago, CSVT was a mortal condition, but with the introduction of neuroimaging, the mortality rates have become minimal; however, it is needed further impulse because still persist great blanks, especially regard to clarification of its management.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bousser MG, Crassard I. Cerebral venous thrombosis, pregnancy and oral contraceptives. Thromb Res. 2012;130(Suppl):S19–22. doi: 10.1016/j.thromres.2012.08.264. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG, Ferro JM. Cerebral venous thrombosis: An update. Lancet Neurol. 2007;6:162–70. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Sandoval JL, Chiquete E, Bañuelos-Becerra LJ, Torres-Anguiano C, González-Padilla C, Arauz A, et al. Cerebral venous thrombosis in a Mexican multicenter registry of acute cerebrovascular disease: The RENAMEVASC study. J Stroke Cerebrovasc Dis. 2012;21:395–400. doi: 10.1016/j.jstrokecerebrovasdis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–8. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 5.Guenther G, Arauz A. Cerebral venous thrombosis: A diagnostic and treatment update. Neurologia. 2011;26:488–98. doi: 10.1016/j.nrl.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee AK, Varma M, Vasista RK, Chopra JS. Cerebrovascular disease in north-west India: A study of necropsy material. J Neurol Neurosurg Psychiatry. 1989;52:512–5. doi: 10.1136/jnnp.52.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–82. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 8.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–70. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 9.Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010;21:511–27. doi: 10.1016/j.nec.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanz Gallego I, Fuentes B, Martínez-Sánchez P, Díez Tejedor E. Do cerebral venous thrombosis risk factors influence the development of an associated venous infarction? Neurologia. 2011;26:13–9. doi: 10.1016/j.nrl.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Corvol JC, Oppenheim C, Manaï R, Logak M, Dormont D, Samson Y, et al. Diffusion-weighted magnetic resonance imaging in a case of cerebral venous thrombosis. Stroke. 1998;29:2649–52. doi: 10.1161/01.str.29.12.2649. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa T, Abe O, Tsuchiya K, Okubo T, Tobe K, Masumoto T, et al. Diffusion-weighted magnetic resonance imaging of dural sinus thrombosis. Neuroradiology. 2002;44:481–8. doi: 10.1007/s00234-002-0772-4. [DOI] [PubMed] [Google Scholar]
- 13.Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–92. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 14.Jia LY, Hua Y, Ji XM, Liu JT. Correlation analysis of internal jugular vein abnormalities and cerebral venous sinus thrombosis. Chin Med J (Engl) 2012;125:3671–4. [PubMed] [Google Scholar]
- 15.De Freitas GR, Bogousslavsky J. Risk factors of cerebral vein and sinus thrombosis. Front Neurol Neurosci. 2008;23:23–54. doi: 10.1159/000111259. [DOI] [PubMed] [Google Scholar]
- 16.Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, et al. Management of stroke in infants and children: A scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–91. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 17.Wysokinska EM, Wysokinski WE, Brown RD, Karnicki K, Gosk-Beirska I, Grill D, et al. Thrombophilia differences in cerebral venous sinus and lower extremity deep venous thrombosis. Neurology. 2008;70:627–33. doi: 10.1212/01.wnl.0000297195.97325.a8. [DOI] [PubMed] [Google Scholar]
- 18.Brown A. Preventing venous thromboembolism in hospitalized patients with cancer: Improving compliance with clinical practice guidelines. Am J Health Syst Pharm. 2012;69:469–81. doi: 10.2146/ajhp110187. [DOI] [PubMed] [Google Scholar]
- 19.Dobbs TD, Barber ZE, Squier WL, Green AL. Cerebral venous sinus thrombosis complicating traumatic head injury. J Clin Neurosci. 2012;19:1058–9. doi: 10.1016/j.jocn.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan A, Karnad DR, Limaye U, Siddharth W. Cerebral venous and dural sinus thrombosis in severe falciparum malaria. J Infect. 2004;48:86–90. doi: 10.1016/s0163-4453(03)00130-0. [DOI] [PubMed] [Google Scholar]
- 21.Jia M, Xiong N, Huang J, Wang Y, Zhang X, Zhang Z, et al. Japanese encephalitis accompanied by cerebral venous sinus thrombosis: A case report. BMC Neurol. 2012;12:43. doi: 10.1186/1471-2377-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzoleni R, Piette T, Lucas C, Seeldrayers P. Cerebral venous thrombosis during acute herpes simplex menigoradicultis. What is the pathophysiological mechanism? Rev Neurol (Paris) 2012;168:379–80. doi: 10.1016/j.neurol.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Price K, Wilson L, Tsegaye M. A case of craniocervical abscess with sinus thrombosis in Lemierre's syndrome. Br J Neurosurg. 2012;26:426–8. doi: 10.3109/02688697.2011.625062. [DOI] [PubMed] [Google Scholar]
- 24.Costa P, Del Zotto E, Giossi A, Volonghi I, Poli L, Frigerio M, et al. Headache due to spontaneous intracranial hypotension and subsequent cerebral vein thrombosis. Headache. 2012;52:1592–6. doi: 10.1111/j.1526-4610.2012.02261.x. [DOI] [PubMed] [Google Scholar]
- 25.Fabricius J, Klotz JM, Hofmann E, Behr R, Neumann-Haefelin T. Cerebral venous thrombosis and subdural haematoma: Complications of spontaneous intracranial hypotension. Fortschr Neurol Psychiatr. 2012;80:599–601. doi: 10.1055/s-0032-1312977. [DOI] [PubMed] [Google Scholar]
- 26.Mao YT, Dong Q, Fu JH. Delayed subdural hematoma and cerebral venous thrombosis in a patient with spontaneous intracranial hypotension. Neurol Sci. 2011;32:981–3. doi: 10.1007/s10072-011-0715-0. [DOI] [PubMed] [Google Scholar]
- 27.Seiler R, Hamann GF. Sinus venous thrombosis as complication of a spontaneous intracranial hypotension. Nervenarz. 2009;80:963–6. doi: 10.1007/s00115-009-2691-7. [DOI] [PubMed] [Google Scholar]
- 28.Tian C, Pu C. Dural enhancement detected by magnetic resonance imaging reflecting the underlying causes of cerebral venous sinus thrombosis. Chin Med J (Engl) 2012;125:1513–6. [PubMed] [Google Scholar]
- 29.Yoon KW, Cho MK, Kim YJ, Lee SK. Sinus thrombosis in a patient with intracranial hypotension: A suggested hypothesis of venous stasis. a case report. Interv Neuroradiol. 2011;17:248–51. doi: 10.1177/159101991101700218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eudo C, Petit A, Mondon K, Rippault H, Dardaine V, Constans T, et al. Cerebral venous thrombosis in an individual with multiple myeloma treated with lenalidomide. J Am Geriatr Soc. 2011;59:2371–2. doi: 10.1111/j.1532-5415.2011.03688.x. [DOI] [PubMed] [Google Scholar]
- 31.May T, Rabinowe SN, Berkowitz RS, Goldstein DP. Cerebral venous sinus thrombosis presenting as cerebral metastasis in a patient with choriocarcinoma following a non-molar gestation. Gynecol Oncol. 2011;122:199–200. doi: 10.1016/j.ygyno.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Miki Y, Tomiyama M, Arai A, Kimura T, Suzuki C, Nunomura J, et al. Cerebral venous thrombosis with dural arteriovenous fistulas and antiphospholipid syndrome: A case report. Neurol Sci. 2010;31:237–8. doi: 10.1007/s10072-009-0166-z. [DOI] [PubMed] [Google Scholar]
- 33.Beslow LA, Abend NS, Smith SE. Cerebral sinus venous thrombosis complicated by cerebellar hemorrhage in a child with acute promyelocytic leukemia. J Child Neurol. 2009;24:110–4. doi: 10.1177/0883073808321057. [DOI] [PubMed] [Google Scholar]
- 34.Giordano P, Cecinati V, Grassi M, Del Vecchio GC, Dicuonzo F, Palma M, et al. Magnetic resonance imaging screening of cerebral thromboembolic events in children with acute lymphoblastic leukemia: A pilot study. Neuropediatrics. 2011;42:55–9. doi: 10.1055/s-0031-1279726. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey AL, Higgins JN, Beer PA, Craig JIO, Vassiliou GS. In situ thrombolysis for cerebral venous thrombosis complicating anti-leukemic therapy. Leuk Res. 2011;35:1127–9. doi: 10.1016/j.leukres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 36.González García H, Sacoto Erazo G, Moreno Gómez E, Blanco Quirós A, Fernández Abril MC, Alvarez Guisasola FJ. Cerebral sinovenous thrombosis in a girl with acute lymphoblastic leukaemia carrying the prothrombin G20210A variant. An Pediatr (Barc) 2013;78:263–7. doi: 10.1016/j.anpedi.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Wang TY, Yen HJ, Hung GY, Hsieh MY, Tang RB. A rare complication in a child undergoing chemotherapy for acute lymphoblastic leukemia: Superior sagittal sinus thrombosis. J Chin Med Assoc. 2011;74:183–7. doi: 10.1016/j.jcma.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Friemel SP, Mackey DW, Fenves AZ, Hise JH, Cheung EH, Stone MJ. Nephrotic syndrome presenting as dural sinus thrombosis. Am J Med. 2002;113:258–60. doi: 10.1016/s0002-9343(02)01167-1. [DOI] [PubMed] [Google Scholar]
- 39.Al Fakeeh KN, Al Rasheed SA. Cerebral venous thrombosis in the nephrotic syndrome. Saudi J Kidney Dis Transpl. 2000;11:59–63. [PubMed] [Google Scholar]
- 40.Costa P, Biscoito L, Vieira M, Marçal M, Camilo C, Neto L, et al. Endovascular thrombolysis for massive cerebral venous thrombosis in a teenager with nephrotic syndrome. Acta Med Port. 2010;23:1141–6. [PubMed] [Google Scholar]
- 41.Xu H, Chen K, Lin D, Dai L, Chen H, Xu Z. Cerebral venous sinus thrombosis in adult nephrotic syndrome. Clin Nephrol. 2010;74:144–9. doi: 10.5414/cnp74144. [DOI] [PubMed] [Google Scholar]
- 42.Lizarazo-Barrera JC, Jacobelli S, Mellado P, González S, Massardo L. Extensive cerebral vein thrombosis as first manifestation of Behçet's disease. Report of one case. Rev Med Chil. 2010;138:746–51. doi: 10.4067/s0034-98872010000600013. [DOI] [PubMed] [Google Scholar]
- 43.Teresa Sartori M, Briani C, Munari M, Amistà P, Pagnan A, Zampieri P. Cerebral venous thrombosis as a rare onset of Churg-Strauss syndrome. Thromb Haemost. 2006;96:90–2. doi: 10.1160/TH06-02-0084. [DOI] [PubMed] [Google Scholar]
- 44.Ahbeddou N, Benomar A, Rasmouni K, Quessar A, Ouhabi H, Ait Ben Haddou E, et al. Cerebral venous thrombosis and acute polyradiculoneuritis revealing systemic lupus erythematosus. Rev Neurol (Paris) 2010;166:458–63. doi: 10.1016/j.neurol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Karam C, Koussa S. Cerebral dural sinus thrombosis following cisplatin chemotherapy. J Clin Neurosci. 2008;15:1274–5. doi: 10.1016/j.jocn.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Bienfait HP, Gijtenbeek JM, Van den Bent MJ, De Bruin HG, Voogt PJ, Pillay M. Cerebral venous and sinus thrombosis with cerebrospinal fluid circulation block after the first methotrexate administration by lumbar puncture. Neuroradiology. 2002;44:929–32. doi: 10.1007/s00234-002-0854-3. [DOI] [PubMed] [Google Scholar]
- 47.Casado-Menéndez I, Uría DF, Jiménez L. Cerebral venous thrombosis as a complication following a diagnostic lumbar puncture. Rev Neurol. 2011;52:252–3. [PubMed] [Google Scholar]
- 48.Ferrante E, Spreafico C, Regna-Gladin C, Protti A. Images from Headache. Cerebral venous thrombosis complicating lumbar puncture. Headache. 2009;49:276–7. doi: 10.1111/j.1526-4610.2008.001327.x. [DOI] [PubMed] [Google Scholar]
- 49.Pfeilschifter W, Neumann-Haefelin T, Hattingen E, Singer OC. Cortical venous thrombosis after a diagnostic lumbar puncture. Nervenarzt. 2009;80:1219–21. doi: 10.1007/s00115-009-2834-x. [DOI] [PubMed] [Google Scholar]
- 50.Presicci A, Garofoli V, Simone M, Campa MG, Lamanna AL, Margari L. Cerebral venous thrombosis after lumbar puncture and intravenous high dose corticosteroids: A case report of a childhood multiple sclerosis. Brain Dev. 2013;35:602–5. doi: 10.1016/j.braindev.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Skeik N, Stark MM, Tubman DE. Complicated cerebral venous sinus thrombosis with intracranial hemorrhage and mastoiditis. Vasc Endovascular Surg. 2012;46:585–90. doi: 10.1177/1538574412457473. [DOI] [PubMed] [Google Scholar]
- 52.Alvis JS, Hicks RJ. Pregnancy-induced acute neurologic emergencies and neurologic conditions encountered in pregnancy. Semin Ultrasound CT MR. 2012;33:46–54. doi: 10.1053/j.sult.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Gao H, Yang BJ, Jin LP, Jia XF. Predisposing factors, diagnosis, treatment and prognosis of cerebral venous thrombosis during pregnancy and postpartum: A case-control study. Chin Med J (Engl) 2011;124:4198–204. [PubMed] [Google Scholar]
- 54.Munira Y, Sakinah Z, Zunaina E. Cerebral venous sinus thrombosis presenting with diplopia in pregnancy: A case report. J Med Case Rep. 2012;6:336. doi: 10.1186/1752-1947-6-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo XB, Fu Z, Song LJ, Guan S. Local thrombolysis for patients of severe cerebral venous sinus thrombosis during puerperium. Eur J Radiol. 2013;82:165–8. doi: 10.1016/j.ejrad.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 56.McCaulley JA, Pates JA. Postpartum cerebral venous thrombosis. Obstet Gynecol. 2011;118:423–5. doi: 10.1097/AOG.0b013e318212fca2. [DOI] [PubMed] [Google Scholar]
- 57.Menegatti E, Zamboni P. Doppler haemodynamics of cerebral venous return. Curr Neurovasc Res. 2008;5:260–5. doi: 10.2174/156720208786413442. [DOI] [PubMed] [Google Scholar]
- 58.Diakou M, Kostadima V, Giannopoulos S, Zikou AK, Argyropoulou MI, Kyritsis AP. Cerebral venous thrombosis in an adolescent with ulcerative colitis. Brain Dev. 2011;33:49–51. doi: 10.1016/j.braindev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 59.deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 60.Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Cantú C, Bousser MG, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40:2356–61. doi: 10.1161/STROKEAHA.108.543884. [DOI] [PubMed] [Google Scholar]
- 61.Kolacki C, Rocco V. The combined vaginal contraceptive ring, nuvaring, and cerebral venous sinus thrombosis: A case report and review of the literature. J Emerg Med. 2012;42:413–6. doi: 10.1016/j.jemermed.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Dentali F, Gianni M, Crowther MA, Ageno W. Natural history of cerebral vein thrombosis: A systematic review. Blood. 2006;108:1129–34. doi: 10.1182/blood-2005-12-4795. [DOI] [PubMed] [Google Scholar]
- 63.De Bruijn SF, Stam J, Koopman MM, Vandenbroucke JP. Case-control study of risk of cerebral sinus thrombosis in oral contraceptive users and in [correction of who are] carriers of hereditary prothrombotic conditions. The Cerebral Venous Sinus Thrombosis Study Group. BMJ. 1998;316:589–92. doi: 10.1136/bmj.316.7131.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia AH, De La Barrera Padilla H, Vega Torres G. Trombosis de senos venosos posterior a analgesia peridural para trabajo de parto. Rev Colomb Anestesiol. 2005;33:285–8. [Google Scholar]
- 65.Ghatge S, Uppugonduri S, Kamarzaman Z. Cerebral venous sinus thrombosis following accidental dural puncture and epidural blood patch. Int J Obstet Anesth. 2008;17:267–70. doi: 10.1016/j.ijoa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Rangel Guerra R. Avances recientes en el diagnóstico y tratamiento de la trombosis venosa cerebral. Medicina Universitaria. 2002;4:15–27. [Google Scholar]
- 67.Timóteo Â, Inácio N, Machado S, Pinto AA, Parreira E. Headache as the sole presentation of cerebral venous thrombosis: A prospective study. J Headache Pain. 2012;13:487–90. doi: 10.1007/s10194-012-0456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalita J, Chandra S, Misra UK. Significance of seizure in cerebral venous sinus thrombosis. Seizure. 2012;21:639–42. doi: 10.1016/j.seizure.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Masuhr F, Busch M, Amberger N, Ortwein H, Weih M, Neumann K, et al. Risk and predictors of early epileptic seizures in acute cerebral venous and sinus thrombosis. Eur J Neurol. 2006;13:852–6. doi: 10.1111/j.1468-1331.2006.01371.x. [DOI] [PubMed] [Google Scholar]
- 70.Gosk-Bierska I, Wysokinski W, Brown RD, Karnicki K, Grill D, Wiste H, et al. Cerebral venous sinus thrombosis: Incidence of venous thrombosis recurrence and survival. Neurology. 2006;67:814–9. doi: 10.1212/01.wnl.0000233887.17638.d0. [DOI] [PubMed] [Google Scholar]
- 71.Fischer C, Goldstein J, Edlow J. Cerebral venous sinus thrombosis in the emergency department: retrospective analysis of 17 cases and review of the literature. J Emerg Med. 2010;38:140–7. doi: 10.1016/j.jemermed.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 72.Kato Y, Takeda H, Furuya D, Nagoya H, Deguchi I, Fukuoka T, et al. Subarachnoid hemorrhage as the initial presentation of cerebral venous thrombosis. Intern Med. 2010;49:467–70. doi: 10.2169/internalmedicine.49.2789. [DOI] [PubMed] [Google Scholar]
- 73.Oppenheim C, Domigo V, Gauvrit JY, Lamy C, Mackowiak-Cordoliani MA, Pruvo JP, et al. Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol. 2005;26:614–7. [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma S, Sharma N, Yeolekar ME. Acute subarachnoid hemorrhage as initial presentation of dural sinus thrombosis. J Neurosci Rural Pract. 2010;1:23–5. doi: 10.4103/0976-3147.63097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mortimer AM, Bradley MD, Stoodley NG, Renowden SA. Thunderclap headache: Diagnostic considerations and neuroimaging features. Clin Radiol. 2013;68:e101–13. doi: 10.1016/j.crad.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Teasdale E. Cerebral venous thrombosis: Making the most of imaging. J R Soc Med. 2000;93:234–7. doi: 10.1177/014107680009300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vijay RK. The cord sign. Radiology. 2006;240:299–300. doi: 10.1148/radiol.2401031739. [DOI] [PubMed] [Google Scholar]
- 78.Virapongse C, Cazenave C, Quisling R, Sarwar M, Hunter S. The empty delta sign: Frequency and significance in 76 cases of dural sinus thrombosis. Radiology. 1987;162:779–85. doi: 10.1148/radiology.162.3.3809494. [DOI] [PubMed] [Google Scholar]
- 79.Mahmoud M, Elbeblawy M. The role of multidetector CT venography in diagnosis of cerebral venous sinus thrombosis. Res J Med Med Sci. 2009;4:284–9. [Google Scholar]
- 80.Kim BS, Do HM, Marks MP. Diagnosis and Management of Cerebral Venous and Sinus Thrombosis. Semin Cerebrovasc Dis Stroke. 2004;4:205–16. [Google Scholar]
- 81.Chen HM, Chen CC, Tsai FY, Shy CG, Wu CH, Chen WS, et al. Cerebral sinovenous thrombosis. Neuroimaging diagnosis and clinical management. Interv Neuroradiol. 2008;14(Suppl 2):35–40. doi: 10.1177/15910199080140S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee EJ. The empty delta sign. Radiology. 2002;224:788–9. doi: 10.1148/radiol.2243990978. [DOI] [PubMed] [Google Scholar]
- 83.Levine HR, Ha KY, O’Rourke B, Owens FD, Doughty KE, Opatowsky MJ. A pictorial review of complications of acute coalescent mastoiditis. Proc (Bayl Univ Med Cent) 2012;25:372–3. doi: 10.1080/08998280.2012.11928882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Damarawy EA, El-Nekiedy AE, Fathi AM, Eissa AE, Darweesh RM. Role of magnetic resonance venography in evaluation of cerebral veins and sinuses occlusion. Alex J Med. 2012;48:29–34. [Google Scholar]
- 85.Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: Current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. 2006;26(Suppl 1):S19–41. doi: 10.1148/rg.26si055174. [DOI] [PubMed] [Google Scholar]
- 86.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: Normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21:74–8. [PMC free article] [PubMed] [Google Scholar]
- 87.Isensee C, Reul J, Thron A. Magnetic resonance imaging of thrombosed dural sinuses. Stroke. 1994;25:29–34. doi: 10.1161/01.str.25.1.29. [DOI] [PubMed] [Google Scholar]
- 88.Lafitte F, Boukobza M, Guichard JP, Hoeffel C, Reizine D, Ille O, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT) Clin Radiol. 1997;52:672–9. doi: 10.1016/s0009-9260(97)80030-x. [DOI] [PubMed] [Google Scholar]
- 89.Linn J, Ertl-Wagner B, Seelos KC, Strupp M, Reiser M, Brückmann H, et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol. 2007;28:946–52. [PMC free article] [PubMed] [Google Scholar]
- 90.Yii IY, Mitchell PJ, Dowling RJ, Yan B. Imaging predictors of clinical deterioration in cerebral venous thrombosis. J Clin Neurosci. 2012;19:1525–9. doi: 10.1016/j.jocn.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Mokin M, Kan P, Abla AA, Kass-Hout T, Snyder KV, Levy EI, et al. Intravascular ultrasound in the evaluation and management of cerebral venous disease. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 92.Raftopoulos C. Intravascular ultrasound: A plus in the management of cerebral venous intravascular problems? World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.06.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 93.Etages A, Chan H. Current intervention strategies for Cerebral Venous Sinus Thrombosis. Int J Neurosurg. 2007;4 ??? [Google Scholar]
- 94.Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992;10:87–111. [PubMed] [Google Scholar]
- 95.Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F. Early seizures in cerebral vein and dural sinus thrombosis: Risk factors and role of antiepileptics. Stroke. 2008;39:1152–8. doi: 10.1161/STROKEAHA.107.487363. [DOI] [PubMed] [Google Scholar]
- 96.Canhão P, Cortesão A, Cabral M, Ferro JM, Stam J, Bousser MG, et al. Are steroids useful to treat cerebral venous thrombosis? Stroke. 2008;39:105–10. doi: 10.1161/STROKEAHA.107.484089. [DOI] [PubMed] [Google Scholar]
- 97.Coutinho J, De Bruijn SF, Deveber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011:CD002005. doi: 10.1002/14651858.CD002005.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41:2575–80. doi: 10.1161/STROKEAHA.110.588822. [DOI] [PubMed] [Google Scholar]
- 99.Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–35. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 100.Coutinho JM, Seelig R, Bousser MG, Canhão P, Ferro JM, Stam J. Treatment variations in cerebral venous thrombosis: An international survey. Cerebrovasc Dis. 2011;32:298–300. doi: 10.1159/000330646. [DOI] [PubMed] [Google Scholar]
- 101.Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 102.Kim SY, Suh JH. Direct endovascular thrombolytic therapy for dural sinus thrombosis: Infusion of alteplase. AJNR Am J Neuroradiol. 1997;18:639–45. [PMC free article] [PubMed] [Google Scholar]
- 103.Horowitz M, Purdy P, Unwin H, Carstens G, Greenlee R, Hise J, et al. Treatment of dural sinus thrombosis using selective catheterization and urokinase. Ann Neurol. 1995;38:58–67. doi: 10.1002/ana.410380112. [DOI] [PubMed] [Google Scholar]
- 104.Mohammadian R, Sohrabi B, Mansourizadeh R, Mohammadian F, Nazempour A, Farhoudi M, et al. Treatment of progressive cerebral sinuses thrombosis with local thrombolysis. Interv Neuroradiol. 2012;18:89–96. doi: 10.1177/159101991201800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: Consecutive case series and review of the literature. Stroke. 2009;40:2233–5. doi: 10.1161/STROKEAHA.108.543421. [DOI] [PubMed] [Google Scholar]
- 106.Théaudin M, Crassard I, Bresson D, Saliou G, Favrole P, Vahedi K, et al. Should decompressive surgery be performed in malignant cerebral venous thrombosis?: A series of 12 patients. Stroke. 2010;41:727–31. doi: 10.1161/STROKEAHA.109.572909. [DOI] [PubMed] [Google Scholar]
- 107.Rajan Vivakaran TT, Srinivas D, Kulkarni GB, Somanna S. The role of decompressive craniectomy in cerebral venous sinus thrombosis. J Neurosurg. 2012;117:738–44. doi: 10.3171/2012.6.JNS11102. [DOI] [PubMed] [Google Scholar]
- 108.Daif A, Awada A, Al-Rajeh S, Abduljabbar M, Al Tahan AR, Obeid T, et al. Cerebral venous thrombosis in adults. A study of 40 cases from Saudi Arabia. Stroke. 1995;26:1193–5. doi: 10.1161/01.str.26.7.1193. [DOI] [PubMed] [Google Scholar]
- 109.Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke. 2005;36:1927–32. doi: 10.1161/01.STR.0000177894.05495.54. [DOI] [PubMed] [Google Scholar]
- 110.Khealani BA, Wasay M, Saadah M, Sultana E, Mustafa S, Khan FS, et al. Cerebral venous thrombosis: A descriptive multicenter study of patients in Pakistan and Middle East. Stroke. 2008;39:2707–11. doi: 10.1161/STROKEAHA.107.512814. [DOI] [PubMed] [Google Scholar]
- 111.Koopman K, Uyttenboogaart M, Vroomen PC, Van der Meer J, De Keyser J, Luijckx GJ. Long-term sequelae after cerebral venous thrombosis in functionally independent patients. J Stroke Cerebrovasc Dis. 2009;18:198–202. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 112.De Bruijn SF, De Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70:105–8. doi: 10.1136/jnnp.70.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenet G, Kirkham F, Niederstadt T, Heinecke A, Saunders D, Stoll M, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: A multicentre cohort study. Lancet Neurol. 2007;6:595–603. doi: 10.1016/S1474-4422(07)70131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


