Abstract
A total of 87 patients were enrolled and bronchoalveolar lavage fluid (BALF) samples were obtained from all subjects. A significant difference was found in BALF VEGF-C level between patients with squamous cell carcinoma and benign diseases (P = 0.043). In addition, the concentration of NSE in BALF form the malignant group was significantly higher compared with that of the benign groups (P = 0.018). However, no statistical difference was observed in BALF CEA (P = 0.375) or CYFRA21-1 (P = 0.838) between lung cancer patients and nonmalignant controls. With a cut-off value of 2.06 ng/ml, NSE had a sensitivity of 72.9%, a specificity of 69.2%, respectively, in predicting the malignant nature of pulmonary mass. Our study observed that the level of VEGF-C was increased in BALF of patients with squamous cell carcinoma. Moreover, we found that NSE was significantly higher in BALF of lung cancer patients than in benign diseases.
Fiberoptic bronchoscopy is currently the most commonly used methods to distinguish lung cancer from benign diseases1,2. Patients suspected of malignancy often undergo bronchoscope examination and bronchial washing are traditionally used3. Changes of cytokines in bronchoalveolar lavage fluid (BALF) reflect immunologic reactions of the lung in pulmonary malignancies4. Thus, detection of biomarkers in BALF might serve as an important adjunct to bronchoscopy for differential diagnosis of lung cancer5,6,7,8,9,10,11. In our previous study, we found that the level of vascular endothelial growth factor (VEGF) was significantly increased in both serum and BALF among patients with lung cancer12. Moreover, measurement of VEGF concentration in BALF might be helpful for lung cancer diagnosis12. VEGF-C, a member of the VEGF family, specifically modulates many endothelial cell functions, especially involving angiogenesis and lymphangiogenesis of different human cancers13,14,15. There is evidence that the high level of VEGF-C in peripheral blood indicated a high probability of lung cancer14,15. However, to the best of our knowledge, the expression of VEGF-C in BALF of lung cancer patients has not yet been examined. On the other hand, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin 19 fragment (CYFRA21-1) were suggested in published studies as possible tumor markers for pulmonary malignancy due to their higher sensitivity in different pathological type lung cancer16,17,18. Nevertheless, only a few studies with limited samples have investigated these tumor markers in BALF of lung cancer. Based on the above evidence, the present study was designed to assess the usefulness of VEGF-C, CEA, NSE, and CYFRA21-1 in BALF for differential diagnosis of lung cancer.
Results
VEGF-C level in BALF
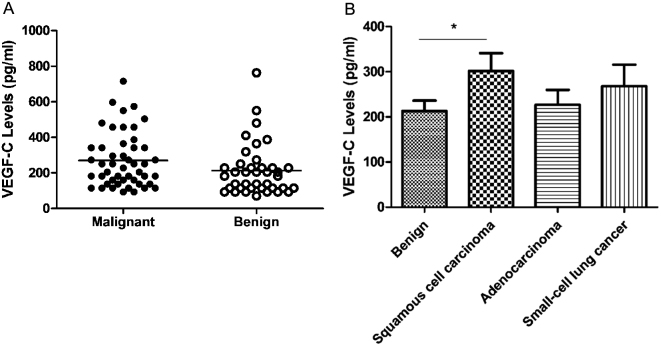
The level of BALF VEGF-C was higher among patients with lung cancer than patients with benign diseases, but the difference did not reach statistical significance (269.9 ± 22.0 pg/ml versus 212.9 ± 23.2 pg/ml, P = 0.08; Fig. 1A). When the lung cancer cases were categorized by tumor histology, however, a statistically significant difference was found between patients with squamous cell carcinoma and patients with benign diseases (302.0 ± 39.1 pg/ml versus 212.9 ± 23.2 pg/ml, P = 0.043; Fig. 1B), while no significant difference was found between malignant and benign groups with respect to adenocarcinoma or small cell carcinoma (data not shown).
Figure 1. Comparison of VEGF-C level in bronchoalveolar lavage fluid from benign and malignant groups.
No significant difference of VEGF-C level was found between malignant and nonmalignant groups (P = 0.08) (A). The level of VEGF-C was significantly higher in patients with squamous cell carcinoma than in those with benign diseases (P = 0.043) (B); Horizontal lines represent the mean values.
CEA, NSE, and CYFRA21-1 levels in BALF
As shown in Figure 2A, the concentration of NSE in malignant group was significantly higher compared with benign groups (6.1 ± 0.8 ng/ml versus 3.3 ± 0.8 ng/ml, P = 0.018). However, no significant difference was observed in BALF CEA (32.6 ± 6.9 ng/ml versus 24.8 ± 4.8 ng/ml, P = 0.375; Fig. 2B) or CYFRA21-1 (52.5 ± 8.0 ng/ml versus 55.4 ± 12.3 ng/ml, P = 0.838; Fig. 2C) between lung cancer patients and non-cancer controls.
Figure 2. Comparison of CEA, NSE, and CYFRA21-1 levels in bronchoalveolar lavage fluid between benign and malignant groups.
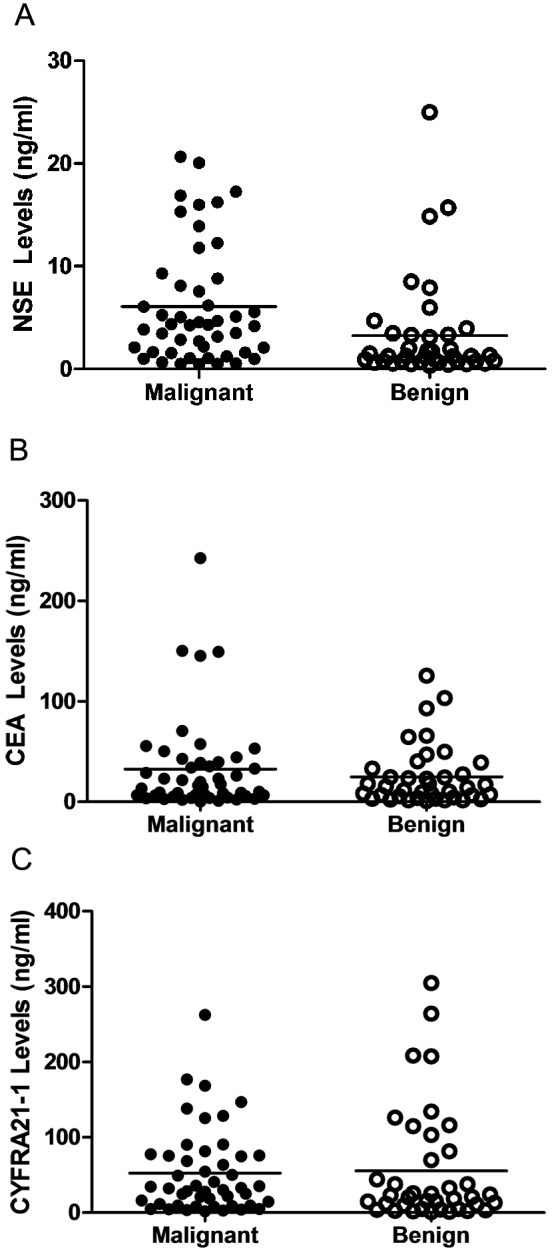
The level of NSE was significantly higher in lung cancer patients than those in benign diseases (P = 0.018) (A); no significant difference of NSE (P = 0.375) (B) or CYFRA21-1 (P = 0.838) (C) levels was found between malignant and nonmalignant groups. Horizontal lines represent the mean values.
Receiver Operating Characteristic (ROC) analysis and cut-off value of BALF NSE
Using logistic regression models, we calculated sensitivity and specificity of NSE in BALF to predict the possible threshold value for lung cancer. The diagnostic threshold afforded by the ROC analysis for NSE was 2.06 ng/ml. The area under the ROC was 0.711 (Fig. 3). With a threshold value of 2.06 ng/ml, NSE had a sensitivity of 72.9%, a specificity of 69.2%, a positive predictive value of 74.5%, and a negative predictive value of 67.5%, in predicting the malignant nature of pulmonary mass.
Figure 3. Receiver operating characteristic (ROC) curve was performed to evaluate the threshold value of NSE in differentiating malignant from benign pulmonary diseases.
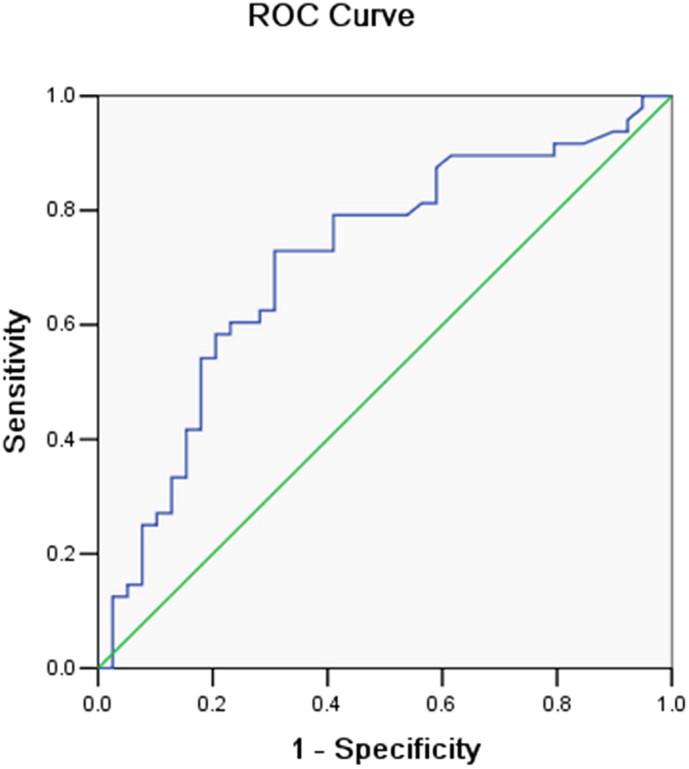
NSE reached a sensitivity of 72.9%, a specificity of 69.2%, a positive predictive value of 74.5%, and a negative predictive value of 67.5%. (cut-off value: 2.06 ng/ml; area under the curve: 0.711).
Disscusion
VEGF-C, a member of the VEGF family, is an important regulator of angiogenesis in the process of tumor growth and metastasis. There is evidence that the level of VEGF-C is significantly increased in peripheral blood among patients with lung cancer14,15. Based on this background, we performed the present study to investigate the potential of VEGF-C in BALF as a possible biomarker in differentiating diagnosis of lung cancer. The results showed that the level of VEGF-C was higher among patients with lung cancer than in patients with benign diseases, but the difference did not reach statistical significance. When the lung cancer cases were categorized by tumor histology, a statistically significant difference was found between patients with squamous cell carcinoma and nonmalignant controls, which were consistent with previous studies19,20.
Yamashita and colleague have analyzed VEGF-C expression in 117 lung cancer patients by immunohistochemical staining19. They observed that the positive expression of VEGF-C was detected in 48.7% subjects and VEGF-C expression was more frequently in squamous cell carcinoma19. In another report, Saintigny et al.20 identified that the percentage of VEGF-C expression was 50%, 40%, and 10% for squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, respectively (P = 0.04). All of these studies provided evidence that VEGF-C might be a risk factor and biomarker for squamous cell carcinoma, but not for cancers of other cell types in the lung owing to the varying mechanisms of carcinogenesis in different cell types of lung cancer. Such evidence on the function of VEGF-C may contribute to a better understanding of lung cancer biology. More importantly, it would be of great help for doctors to choose therapies in an individual manner.
Considering that tumor markers are produced directly by the tumor or by non-tumor cells as a response to the presence of tumor cells, the elevation of tumor markers can be detected earlier than radiographic abnormalities18. Therefore, it is very important to assess the appropriate tumor markers in BALF for diagnosis or even in predicting the recurrence of lung cancer21. Nowadays, CEA, NSE, and CYFRA21-1 are the most widely used tumor markers in clinical practice for the differentiation of malignant and benign lung diseases16,17,18. In the present study, these tumor markers were investigated in BALF and this is hoped to be a useful method for distinguishing lung cancer from benign diseases.
In our study, we found the level of NSE was significantly higher in patients with malignance, indicating that NSE in BALF might be a good marker for lung cancer diagnosis. ROC curve was further performed to evaluate the ability of NSE in differentiating lung cancer patients from those with benign diseases. The diagnostic threshold afforded by the ROC analysis for NSE was 2.06 ng/ml. With this cut-off value, NSE had a sensitivity of 72.9% and a specificity of 69.2%. These results provided a new approach with a higher diagnostic value in patients with pulmonary mass discovered by chest radiograph or CT screening. In addition, the presence of low level of NSE in BALF indicated a low probability of malignancy, which might serve to avoid performing an invasive procedure.
However, there were no significant differences in CEA concentration between malignant and benign groups. CEA, synthesized by epithelial and tumor cells, is a tumor marker belonging to the immunoglobulin gene superfamily of adhesion molecules22. A bulk of studies has reported the importance of CEA level in peripheral blood of lung cancer patients23,24,25,26. In contrast, only a few studies have investigated CEA concentrations in BALF. Charalabopoulos et al.10 measured CEA in BALF among 50 patients with lung cancer, 20 benign lung lesions, and 20 healthy controls. In support of our findings, they demonstrated that CEA measurement in BALF alone has little value in the diagnosis of malignancy.
Serum CYFRA21-1 is not only a sensitive and specific tumor marker in pulmonary, but also a useful adjunctive marker for disease monitoring26,27,28,29. Nevertheless, we failed to find a significant difference in the level of CYFRA21-1 in BALF between benign and malignant groups. Our results agreed with a previous study, in which the researchers demonstrated that the measurement of CYFRA 21-1 in BALF has poor diagnostic value in lung cancer8.
One of the limitations of our study was that the serum concentrations of VEGF-C, CEA, NSE, and CYFRA21-1 were not investigated. A large number of published studies have investigated these cytokines in peripheral blood and suggested that they can serve as sensitive and specific markers for lung cancer14,15,16,17,18,23,24,25,26,27,28,29. However, in this study, we did not identify a significant difference in CEA or CYFRA21-1 concentration in BALF between lung cancer and non-cancer controls, suggesting that in this context CEA and CYFRA21-1 produced within airways is disproportionate to their systemic production.
The pulmonary mass is a common and challenging clinical problem. It is essential to distinguish malignancy from benign diseases, because malignant masses should be identified and resected promptly to improve patients' life quality and survival. To our knowledge, this study is the first study to date that has assessed the utility of VEGF-C in BALF for differential diagnosis of lung cancer. The findings from our study indicated that VEGF-C was a biomarker for lung squamous cell carcinoma. Moreover, we found that NSE was significantly higher in BALF of lung cancer patients than in that of patients with benign diseases. Measurement of NSE in BALF might be helpful for differential diagnosis of lung cancer. However, there was no significant difference in CEA or CYFRA21-1 concentration between malignant and benign groups. Future studies are wanted to validate these results and elucidate their benefit in clinics.
Methods
Subjects
From February 2011 to July 2013, 87 patients who were found pulmonary mass by chest radiograph or CT screening at the Affiliated Hospital of Ningbo University were enrolled in this study. Approval for this study was obtained from the local ethics committee, and informed consent was obtained from all participating subjects. All patients had histological confirmed and the subjects were excluded if they previously had received preoperative chemotherapy or radiotherapy. Table 1 summarizes the patients and tumor characteristics. The patients' ages ranged from 42–80 years with a median age of 60. There were 48 lung cancer patients (38 males and 10 females; age: 60.2 ± 1.3 years) and 39 patients with benign diseases (17 males and 22 females; age: 56.9 ± 1.4 years). The pathologic types included 19 squamous cell carcinomas, 15 adenocarcinomas, 11 small cell carcinomas, and 3 of other cell types.
Table 1. The characteristics of the patients.
| Malignant group | Benign group | |
|---|---|---|
| Age, years | 60.2 ± 1.3 | 56.9 ± 1.4 |
| Gender | ||
| Male | 38 | 17 |
| Female | 10 | 22 |
| Smoking status | ||
| Smokers | 25 | 14 |
| Non-smokers | 23 | 25 |
| Pack/years | 60.1 ± 8.0 | 38.9 ± 5.9 |
| Histological type | ||
| Squamous cell carcinoma | 19 | |
| Adenocarcinoma | 15 | |
| Small-cell lung cancer | 11 | |
| Others | 3 |
Bronchoalveolar lavage (BAL)
BAL was performed through a fiberoptic bronchoscope before brushing or biopsies. After the local upper airways were anesthetized with 5 mL of 2% lidocaine, the bronchus on the disease side was washed with 0.9% saline solution and the fluid was gently withdrawn into a siliconized container. The lavage fluid was filtered through a nylon filter to remove mucus and centrifuged at 3,000 revolutions per minute for 10 min, and supernatant aliquots were frozen at −80°C for later analysis.
Measurements of VEGF-C, CEA, NSE, and CYFRA21-1
The level of VEGF-C (pg/ml) was measured using enzyme-linked immunosorbent assay kits (ELISA; R&D systems, Minneapolis, MN, USA). The assays were conducted according to the manufacturer's guidelines. Levels of CEA, NSE, and CYFRA21-1 (ng/ml) were determined by commercially available test kits (electrochemiluminescence immunoassay, ECLIA; Roche Diagnostics, Mannhein, Germany).
Statistical analysis
Data are presented as means ± standard error of the mean (SEM). Comparison between different groups was done using the Student's t-test. The area under the ROC curve was reported to evaluate the ability of the potential tumor markers in discriminating cancer from benign diseases. The analyses were conducted using SPSS version 13.0 (SPSS, Chicago, IL, USA), and all tests were two-sided with a significance level of 0.05.
Author Contributions
C.C., Z.C.D., Z.B.C., S.F.S., Y.M.Y. and Q.L.D. designed the experiments. C.C., Z.C.D. and Z.B.C. carried out the experiments and calculations. C.C., Z.C.D., Z.B.C., S.F.S., Y.M.Y. and Q.L.D. wrote and edited the paper.
Acknowledgments
This work was supported by the grant of Social Development of Science and Technology Project of Ningbo (No. 2011C50025, C Cao) and grant of Natural Science Foundation of Ningbo (No. 2012A610257, ZC Deng).
References
- Mazzone P., Jain P., Arroliga A. C. & Matthay R. A. Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clin. Chest. Med. 23, 137–158 (2002). [DOI] [PubMed] [Google Scholar]
- Karahalli E., Yilmaz A., Türker H. & Ozvaran K. Usefulness of various diagnostic techniques during fiberoptic bronchoscopy of endoscopically visible lung cancer: should cytologic examinations be performed routinely. Respiration. 68, 611–614 (2001). [DOI] [PubMed] [Google Scholar]
- Poletti V., Romagna M., Allen K. A., Gasponi A. & Spiga L. Bronchoalveolar lavage in the diagnosis of disseminated lung tumors. Acta. Cytol. 39, 472–477 (1995). [PubMed] [Google Scholar]
- Weynants P., Marchandise F. X. & Sibille Y. Pulmonary perspective: immunology in diagnosis and treatment of lung cancer. Eur. Respir. J. 10, 1703–1719 (1997). [DOI] [PubMed] [Google Scholar]
- Bugdayci G. et al. Matrix metalloproteinase-9 in broncho-alveolar lavage fluid of patients with non-small cell lung cancer. Exp. Oncol. 28, 169–171 (2006). [PubMed] [Google Scholar]
- Domagała-Kulawik J., Hoser G., Safianowska A., Grubek-Jaworska H. & Chazan R. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch. Immunol. Ther. Exp. 54, 143–147 (2006). [DOI] [PubMed] [Google Scholar]
- Cremades M. J., Menéndez R., Rubio V. & Sanchis J. Fibronectin in bronchoalveolar lavage fluid in lung cancer: tumor or inflammatory marker? Respiration. 65, 178–182 (1998). [DOI] [PubMed] [Google Scholar]
- Cremades M. J., Menéndez R., Pastor A., Llopis R. & Aznar J. Diagnostic value of cytokeratin fragment 19 (CYFRA 21-1) in bronchoalveolar lavage fluid in lung cancer. Respir. Med. 92, 766–771 (1998). [DOI] [PubMed] [Google Scholar]
- Ohta Y. et al. Vascular endothelial growth factor expression in airways of patients with lung cancer: a possible diagnostic tool of responsive angiogenic status on the host side. Chest. 121, 1624–1627 (2002). [DOI] [PubMed] [Google Scholar]
- Charalabopoulos K. et al. CEA levels in serum and BAL in patients suffering from lung cancer: correlation with individuals presenting benign lung lesions and healthy volunteers. Med. Oncol. 24, 219–225 (2007). [DOI] [PubMed] [Google Scholar]
- Emad A. & Emad V. The value of BAL fluid LDH level in differentiating benign from malignant solitary pulmonary nodules. J. Cancer. Res. Clin. Oncol. 134, 489–493 (2008). [DOI] [PubMed] [Google Scholar]
- Cao C. et al. Utility of VEGF and sVEGFR-1 in bronchoalveolar lavage fluid for differential diagnosis of primary lung cancer. Asian. Pac. J. Cancer. Prev. 14, 2443–2446 (2013). [DOI] [PubMed] [Google Scholar]
- Cheng D., Liang B. & Li Y. Serum vascular endothelial growth factor (VEGF-C) as a diagnostic and prognostic marker in patients with ovarian cancer. PLoS. One. 8, e55309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Sun X. & Li X. L. Expression and clinical significance of STAT3, P-STAT3, and VEGF-C in small cell lung cancer. Asian. Pac. J. Cancer. Prev. 13, 2873–2877 (2012). [DOI] [PubMed] [Google Scholar]
- Nakajima T. et al. Endobronchial ultrasound doppler image features correlate with mRNA expression of HIF1-α and VEGF-C in patients with non-small-cell lung cancer. J. Thorac. Oncol. 7, 1661–1667 (2012). [DOI] [PubMed] [Google Scholar]
- Seemann M. D., Beinert T., Fürst H. & Fink U. An evaluation of the tumour markers, carcinoembryonic antigen (CEA), cytokeratin marker (CYFRA 21-1) and neuron-specific enolase (NSE) in the differentiation of malignant from benign solitary pulmonary lesions. Lung. Cancer. 26, 149–155 (1999). [DOI] [PubMed] [Google Scholar]
- Chu X. Y. et al. Diagnostic values of SCC, CEA, Cyfra21-1 and NSE for lung cancer in patients with suspicious pulmonary masses: a single center analysis. Cancer. Biol. Ther. 11, 995–1000 (2011). [DOI] [PubMed] [Google Scholar]
- Wang P., Piao Y., Zhang X., Li W. & Hao X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer. Biomark. 13, 123–130 (2013). [DOI] [PubMed] [Google Scholar]
- Yamashita T. et al. Association between lymphangiogenesis-/micrometastasis- and adhesion-related molecules in resected stage I NSCLC. Lung. Cancer. 70, 320–328 (2010). [DOI] [PubMed] [Google Scholar]
- Saintigny P. et al. Vascular endothelial growth factor-C and its receptor VEGFR-3 in non-small-cell lung cancer: concurrent expression in cancer cells from primary tumour and metastatic lymph node. Lung. Cancer. 58, 205–213 (2007). [DOI] [PubMed] [Google Scholar]
- Crohns M. et al. Cytokines in bronchoalveolar lavage fluid and serum of lung cancer patients during radiotherapy - Association of interleukin-8 and VEGF with survival. Cytokine. 50, 30–36 (2010). [DOI] [PubMed] [Google Scholar]
- Barnett T. R. et al. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J. Cell. Biol. 108, 267–276 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantapet P., Riantawan P., Lebnak P. & Getngern P. Utility of serum cytokeratin 19 fragment (CYFRA 21-1) and carcinoembryonic antigen (CEA) as tumour markers for non-small cell lung cancer. J. Med. Assoc. Thai. 83, 383–391 (2000). [PubMed] [Google Scholar]
- Tas F. et al. Utility of the serum tumor markers: CYFRA 21.1, carcinoembryonic antigen (CEA), and squamous cell carcinoma antigen (SCC) in squamous cell lung cancer. J. Exp. Clin. Cancer. Res. 19, 477–481 (2000). [PubMed] [Google Scholar]
- Arrieta Rodriguez O. G. et al. Usefulness of Serum Carcinoembryonic Antigen (CEA) in evaluating response to chemotherapy in patients with advanced non small-cell lung cancer: a prospective cohort study. BMC. Cancer. 13, 254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K. et al. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung. Cancer. 80, 45–49 (2013). [DOI] [PubMed] [Google Scholar]
- Rastel D., Ramaioli A., Cornillie F. & Thirion B. CYFRA 21-1, a sensitive and specific new tumour marker for squamous cell lung cancer. Report of the first European multicentre evaluation. CYFRA 21-1 Multicentre Study Group. Eur. J. Cancer. 30, 601–606 (1994). [DOI] [PubMed] [Google Scholar]
- Lai R. S., Hsu H. K., Lu J. Y., Ger L. P. & Lai N. S. CYFRA 21-1 enzyme-linked immunosorbent assay. Evaluation as a tumor marker in non-small cell lung cancer. Chest. 109, 995–1000 (1996). [DOI] [PubMed] [Google Scholar]
- Ono A. et al. Prognostic impact of serum CYFRA 21-1 in patients with advanced lung adenocarcinoma: a retrospective study. BMC. Cancer. 13, 354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]