Abstract
Cannabinoids suppress fertility via reducing hypothalamic GnRH output. γ-Aminobutyric acid (GABA)A receptor (GABAA-R)-mediated transmission is a major input to GnRH cells that can be excitatory. We hypothesized that cannabinoids act via inhibiting GABAergic input. We performed loose-patch electrophysiological studies of acute slices from adult male GnRH-green fluorescent protein transgenic mice. Bath application of type 1 cannabinoid receptor (CB1) agonist WIN55,212 decreased GnRH neuron firing rate. This action was detectable in presence of the glutamate receptor antagonist kynurenic acid but disappeared when bicuculline was also present, indicating GABAA-R involvement. In immunocytochemical experiments, CB1-immunoreactive axons formed contacts with GnRH neurons and a subset established symmetric synapses characteristic of GABAergic neurotransmission. Functional studies were continued with whole-cell patch-clamp electrophysiology in presence of tetrodotoxin. WIN55,212 decreased the frequency of GABAA-R-mediated miniature postsynaptic currents (mPSCs) (reflecting spontaneous vesicle fusion), which was prevented with the CB1 antagonist AM251, indicating collectively that activation of presynaptic CB1 inhibits GABA release. AM251 alone increased mPSC frequency, providing evidence that endocannabinoids tonically inhibit GABAA-R drive onto GnRH neurons. Increased mPSC frequency was absent when diacylglycerol lipase was blocked intracellularly with tetrahydrolipstatin, showing that tonic inhibition is caused by 2-arachidonoylglycerol production of GnRH neurons. CdCl2 in extracellular solution can maintain both action potentials and spontaneous vesicle fusion. Under these conditions, when endocannabinoid-mediated blockade of spontaneous vesicle fusion was blocked with AM251, GnRH neuron firing increased, revealing an endogenous endocannabinoid brake on GnRH neuron firing. Retrograde endocannabinoid signaling may represent an important mechanism under physiological and pathological conditions whereby GnRH neurons regulate their excitatory GABAergic inputs.
Summary
Endocannabinoids synthesized in GnRH neurons decrease GnRH neuron firing rate via inhibition of GABAergic afferents.
The main psychoactive substance of the cannabis sativa plant, Δ-9-tetrahydro-cannabinol (THC), modulates a wide array of endocrine functions that operate under hypothalamic control (1). THC influences various aspects of reproductive physiology by inhibiting LH secretion from the adenohypophysis (2,3,4,5). A consensus opinion exists that cannabinoids suppress gonadotropin release via reducing the neurosecretory output from hypothalamic GnRH neurons. Accordingly, 1) THC does not inhibit adenohypophysial LH secretion in vitro (6,7), 2) GnRH prevents the inhibitory effects of cannabinoids on ovulation and LH secretion (3,6,8), and 3) the decreased serum LH after intracerebroventricular injection of THC coincides with an increased mediobasal hypothalamic GnRH content (6).
In the central nervous system the actions of THC are primarily exerted via the type 1 cannabinoid receptor (CB1), which has been localized to axon terminals (1,9). CB1 activation by either exogenous THC or the main endogenous cannabinoid ligands (endocannabinoids) 2-arachidonoylglycerol (2-AG) and anandamide inhibits the presynaptic release of neurotransmitters, including γ-aminobutyric acid (GABA) (9,10,11,12) and glutamate (13,14). The importance of CB1 receptors in central inhibitory regulation of the reproductive axis has been demonstrated by the ability of 2-AG to suppress LH secretion in wild-type (WT) but not in CB1 receptor knockout (KO) mice (15).
The pulsatile secretory activity of GnRH neurons is influenced by physiological or pathological alterations in the synaptic input (16). The amino acid neurotransmitter GABA plays a pivotal role in this afferent regulation. GnRH neurons receive GABAergic synapses (17) and express functional GABAA receptors (GABAA-Rs) (18,19,20,21,22) and GABAB (23) receptors. Whereas GABA acts as the major inhibitory neurotransmitter in the adult hypothalamus (24), mature GnRH neurons maintain high intracellular chloride concentrations, which can result in excitatory responses to GABAA-R activation in adult mice (18,21) and rats (25,26). Modulation of GABAergic drive onto GnRH neurons has been commonly implicated in metabolic (27), sex steroid (28), and circadian (29) signaling to GnRH neurons.
In the present studies, we hypothesized that cannabinoids inhibit GABAergic neurotransmission to GnRH neurons. The decreased GABAA-R mediated drive, in turn, decreases GnRH neuron activity. To test this hypothesis, we have carried out a series of electrophysiological and neuroanatomical studies.
Materials and Methods
Animals
Adult gonadally intact male mice were used from local colonies bred at the Medical Gene Technology Unit of the Institute of Experimental Medicine (IEM). They were housed in light-controlled (12-h light, 12-h dark cycle, lights on at 0700 h) and temperature-controlled (22 ± 2 C) environment, with free access to standard food and tap water. All studies were carried out with permissions from the Animal Welfare Committee of the IEM (No. A5769-01) and in accordance with legal requirements of the European Community (Decree 86/609/EEC). GnRH-green fluorescent protein (GFP) transgenic mice (n = 70) bred on a C57BL/6J genetic background were used for electrophysiological experiments. In this animal model, a GnRH promoter segment drives selective GFP expression in the majority of GnRH neurons (30). For immunohistochemistry, GnRH-GFP mice (n = 5) and homozygous CB1-KO mice (n = 12) and their WT littermates (CB1-WT; n = 12) were used. The parent stock of the CB1-KO animals was obtained from Institut de Recherche Interdisciplinaire en Biologie Humaine et Moleculaire, Université Libre de Bruxelles (Brussels, Belgium) (31), and transferred earlier onto a CD1 (Charles River, L’Arbreole, France) genetic background at IEM.
Brain slice preparation and recording
Mice were killed by cervical dislocation between 1100 and 1200 h. The brain was removed rapidly and immersed in ice-cold artificial cerebrospinal fluid (aCSF), which had been bubbled with a mixture of 95% O2 and 5% CO2. The solution contained the following (in mm): NaCl 135, KCl 3.5, NaHCO3 26, MgSO4 1.2, NaH2PO4 1.25, CaCl2 2.5, and glucose 10. Hypothalamic blocks were dissected, and 250-μm-thick coronal slices were prepared from the medial septum/preoptic area (POA) with a VT-1000S vibratome (Leica GmBH, Wetzlar, Germany) in ice-cold oxygenated aCSF. The slices containing the POA were bisected along the midline and equilibrated in aCSF saturated with O2/CO2 at room temperature for 1 h. During recording (between 1400 and 1800 h at 33 C), the brain slices were oxygenated by bubbling the aCSF with O2/CO2 gas. Axopatch 200B patch-clamp amplifier, Digidata-1322A data acquisition system, and pCLAMP 9.2 software (Molecular Devices Co., Sunnyvale, CA) were used for recording. Cells were visualized with a BX51WI IR-DIC microscope (Olympus Co., Tokyo, Japan). The patch electrodes (OD = 1.5 mm, thin wall; Garner Co., Claremont, CA) were pulled with a Flaming-Brown P-97 puller (Sutter Instrument Co., Novato, CA) and polished with an MF-830 microforge (Narishige, Tokyo, Japan).
GnRH-GFP neurons were identified by brief illumination at 470 nm using an epifluorescent filter set, based on their green fluorescence, typical fusiform shape, and topographic location in the POA (30). After control recording, the slices were treated with various drugs for 10 min and the recording repeated for 250 sec. Each neuron served as its own control when drug effects were evaluated.
Loose-patch-clamp experiments
Recording of action current firing of GnRH neurons was carried out at 33 C. Pipette potential was 0 mV, pipette resistance 1–2 MΩ, and resistance of loose-patch seal 7–40 MΩ. The pipette solution contained (in mm): NaCl 150, KCl 3.5, CaCl2 2.5, MgCl2 1.3, HEPES 10, and glucose 10 (pH 7.3 with NaOH).
After recording basal action currents, the CB1 agonist WIN55,212 was added at 1 μm for 10 min, and the recording was repeated. In experiments to investigate involvement of GABAA-R activation in WIN55,212 actions, ionotropic glutamate receptors were first blocked with 2 mm kynurenic acid (Kyn) for 10 min and then GABAA-Rs with 20 μm bicuculline (Bic)-methiodide for 10 min. Subsequently, WIN55,212 was added to the extracellular solution. Use of the ionotropic glutamate receptor blocker together with Bic was an important part of the strategy to prevent an unbalanced excitatory tone in the slices (21,32,33).
The putative effect of tonic endocannabinoid release on GnRH neuron firing was addressed in the presence of CdCl2 (200 μm; 10 min). CdCl2 in the aCSF allows actions potentials and neurotransmitter release due to spontaneous vesicle fusion to continue but blocks action potential-dependent neurotransmitter release (34). A putative endocannabinoid blockade of spontaneous vesicle fusion was addressed by studying changes in GnRH neuron firing after the addition of the CB1 antagonist AM251 (1 μm; Tocris, Ellisville, MO) to the aCSF for 10 min, in the presence of CdCl2. NaH2PO4 was omitted from the aCSF in this experiment, to avoid precipitation.
Whole-cell patch-clamp experiments
The cells were voltage clamped at −70 mV holding potential. Pipette offset potential, series resistance (Rs), and capacitance were compensated before recording. Only cells with low holding current (<50 pA) and stable baseline were used. Input resistance (Rin), Rs, and membrane capacity (Cm) were also measured before each recording by using 5-mV hyperpolarizing pulses. To ensure consistent recording qualities, only cells with Rs < 20 MΩ, Rin > 500 MΩ, and Cm > 10 pF were accepted. Further, if these values changed by more than 20% during measurements, the recordings were discarded (35). The pipette solution contained (in mm): HEPES 10, KCl 140, EGTA 5, CaCl2 0.1, Mg-ATP 4, and Na-GTP 0.4 (pH 7.3 with NaOH). The resistance of the patch electrodes was 2–3 MΩ. Spike-mediated transmitter release was blocked in all experiments by adding the voltage-sensitive Na-channel inhibitor tetrodotoxin (TTX) (750 nm; Tocris) to the aCSF 10 min before control miniature postsynaptic currents (mPSCs) were recorded. Picrotoxin (100 μm; Sigma, St. Louis, MO) was used in the aCSF to verify that mPSCs were related to GABAA-R activation. In subsequent experiments, modulation of mPSCs by CB1 was addressed by treating slices with the CB1 agonist WIN55,212 (1 μm; Tocris) for 10 min. In other experiments, slices were incubated with the CB1 antagonist AM251 (1 μm; Tocris) for 10 min and recorded. Then WIN55,212 (1 μm) was added and recording repeated after 10 min. Finally, the source of endogenous cannabinoids that regulate GABAergic afferents to GnRH neurons was investigated: the diacylglycerol (DAG) lipase inhibitor tetrahydrolipstatin (THL) was added to the intracellular solution at 10 μm to block 2-AG synthesis. To minimize THL spill, the GnRH cells were approached rapidly (<1 min), and the flow rate of aCSF was increased from 5–6 to 8–9 ml/min. Just before release of the positive pressure in the pipette, the flow rate was restored to 5–6 ml/min to avoid any mechanical movement of the slice. The pipette solution containing THL was allowed to equilibrate with the intracellular milieu of the cell for 25 min before control recording. Then AM251 was added for 10 min and recording repeated.
Statistical analysis
Each experimental group contained 8–10 recorded cells from six to seven animals. Recordings (250 sec) were stored and analyzed off-line. Baseline correction of the action current and mPSC recordings was carried out using the Corrector software developed in our laboratory (L. Tatai, G. Lőcsei, B. Wittner, and Judit Bálintné Farkas). In brief, background current fluctuations were smoothed by the running averages of a 300-point window, which was shifted point-by-point. At the sampling rate applied (4 kHz), this width caused no distortion of the mPSCs and action currents. Event detection was performed using the Clampfit module of the PClamp 9.2 software (Molecular Devices Co.). Group data were expressed as mean ± sem. Statistical significance was analyzed using the Student’s t test or ANOVA followed by Newman-Keuls (NK) test (GraphPad Software, Inc., GraphPad, San Diego, CA) and considered at P < 0.05.
Preparation of sections for immunohistochemistry
Mice were deeply anesthetized with a cocktail of ketamine (25 mg/kg), xylavet (5 mg/kg), and pipolphen (2.5 mg/kg) in saline and perfused through the ascending aorta first with 10 ml PBS [0.1 m (pH 7.4); PBS] and then with 40 ml of fixative in PBS. The fixative contained 4% paraformaldehyde (PFA) for brightfield and fluorescent microscopy and 4% PFA and 0.2% glutaraldehyde (GA) for electron microscopy. The solution containing GA was rinsed out from the vasculature immediately with 10 ml 4% PFA to prevent excessive cross-linking of tissues and antigens. The perfused brains were postfixed overnight in 2% PFA at 4 C. Serial 30-μm-thick coronal sections were cut on a Vibratome from the rostro-caudal extent of the POA/anterior hypothalamus (AH).
Confocal microscopic analysis of CB1-immunoreactive (IR) fibers apposed to GnRH neurons
Every third section of the POA and AH was pretreated with 0.5% Triton X-100 (20 min), 0.5% hydrogen peroxide (H2O2, 10 min), and 2% normal horse serum (20 min) before antibody incubations. All treatments and rinses with PBS (3 × 5 min) in between were carried out at room temperature, except for primary antibody and fluorochrom incubations performed at 4 C. The primary antibodies were applied in cocktails for 72 h; a goat anti-CB1 (1:100, raised against the C-terminal 31 amino acids of mouse CB1) (36) antiserum was mixed either with a rabbit antiserum to GnRH (1:10,000; LR1; from R. Benoit, McGill University, Montreal, Canada) or, as an alternative, with a rabbit antiserum to GFP (1:5,000, ab3080; Millipore, Bedford, MA) in studies of transgenic mice. The rabbit primary antibodies were visualized by sequential applications of biotinylated donkey antirabbit Igs (IgG, 1:500, 2 h; Jackson ImmunoResearch, West Grove, PA), peroxidase-conjugated avidin-biotin complex (ABC) (1:1000, 1 h; Vector Laboratories, Burlingame, CA), biotinylated tyramide (1:200, 0.5 h), and Alexa Fluor 488-conjugated streptavidin (1:500, 24 h; Molecular Probes, Eugene, OR). The CB1-IR sites were reacted with carbocyanine 3-conjugated donkey antigoat IgG (1:500, 12 h; Jackson ImmunoResearch). Finally, sections were mounted onto glass slides and coverslipped with Vectashield mounting medium (Vector Laboratories).
The fluorescent signals were studied with a Radiance 2100 confocal microscope (Bio-Rad Laboratories, Hemel Hempstead, UK) using the following laser lines and filters: 488 nm for Alexa Fluor 488 and enhanced GFP, 543 nm Cy3 and dichroic/emission filters, 560 nm/500–540 nm for Alexa Fluor 488 and enhanced GFP, and 650 nm/560–610 nm for Cy3. A series of optical sections was prepared using a ×60 oil immersion objective. The sequentially scanned red and green channels were merged and displayed with the Laser Vox software (Bio-Rad Laboratories) and an IBM-compatible personal computer (Dell Co., Austin, TX). Appositions were only considered if a gap could not be recognized between the CB1 and GnRH-IR profiles in optical slices less than 0.7 μm. The specificity of CB1 receptor immunolabeling was verified by the absence of immunoreactivity in sections of CB1-KO mice processed in parallel with tissues of the CB1-WT animals.
Light- and electron-microscopic analysis of CB1-IR input to GnRH neurons
A set of sections from brains fixed with the PFA-GA mixture was pretreated with 1% sodium borohydride for 30 min and with 0.5% H2O2 for 15 min. Then, they were cryoprotected with 15% (15 min), followed by 30% sucrose (12 h) in PBS. Sequential freeze-thaw cycles were carried out three times on liquid nitrogen to permeabilize sections. Finally, 2% normal horse serum was applied (20 min) to prevent nonspecific antibody binding. The pretreated sections were double-immunostained for CB1 and GnRH in two consecutive steps. First, the sections were incubated in goat anti-CB1 (1:900, 4 d), biotinylated donkey antigoat IgG (1:500, 1 d; Jackson ImmunoResearch), and ABC Elite solution (1:1000, 1.5 h). The signal was visualized with silver-gold intensified nickel-diaminobenzidine with modifications detailed elsewhere (37). Next, the sections were transferred into rabbit anti-GnRH antibodies (1:5000, 2 d), biotinylated donkey antirabbit IgG (1:500, 1 d), and ABC Elite solution (1:1000, 1.5 h). The GnRH signal was detected with diaminobenzidine. The double-labeled sections were treated with 1% osmium tetroxide for 60 min and 2% uranyl acetate (prepared in 70% ethanol) for 40 min, then dehydrated in an ascending series of ethanol and propylene oxide. Flat-embedding in TAAB 812 medium epoxy resin was carried out between a pair of glass microscope slides precoated with liquid release agent (Electron Microscopy Sciences, Hatfield, PA). The resin was allowed to polymerize at 56 C for 2 d. Ultrathin sections (50–60 nm) were collected with a Leica Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany) onto Formvar-coated single-slot grids, contrasted with 2% lead citrate and examined with a Jeol-100C transmission electron microscope.
Results
Activation of CB1 receptors decreases the activity of GnRH neurons
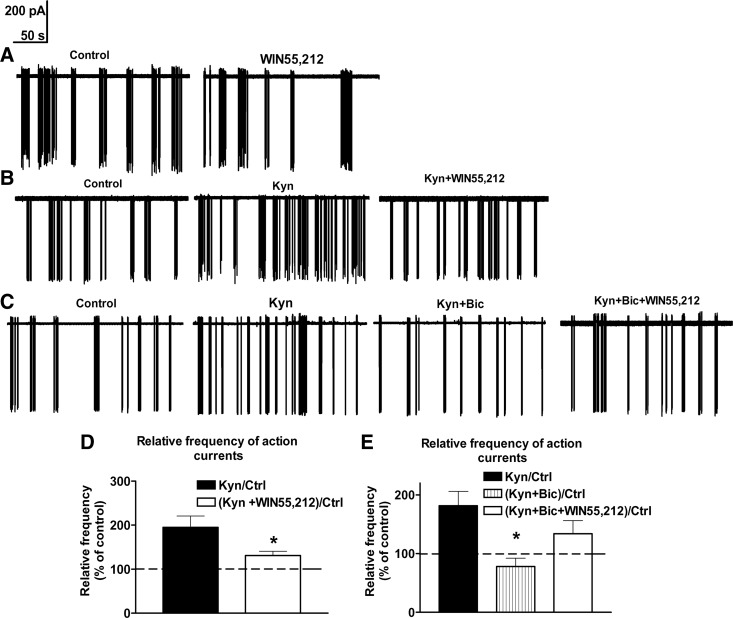
To test the hypothesis that cannabinoid signaling inhibits the electric activity of GnRH neurons, action currents were recorded from GnRH neurons of adult male GnRH-GFP transgenic mice using the loose-patch approach (38). Administration of the CB1 agonist WIN55,212 (1 μm) reduced the frequency of action currents to 49 ± 15% of the control (control, 0.6 ± 0.07 Hz; WIN55,212, 0.3 ± 0.08 Hz) (P = 0.0136 by paired Student’s t test) (Fig. 1A). This finding served as support for the hypothesis that CB1 signaling in the POA results in a net decrease in the electric activity of GnRH neurons. The amplitude did not change (107 ± 6.5% of control).
Figure 1.
The CB1 receptor agonist WIN55,212 inhibits GnRH neuron firing via a GABAA-R-dependent mechanism. When applied to acute slice preparations of the POA, WIN55,212 (1 μm) reduces the action current frequency of a representative GnRH neuron, compared with its control firing rate before treatment (A). The control firing rate of a representative GnRH neuron is increased in the presence of the ionotropic glutamate receptor blocker Kyn (2 mm). The subsequent application of WIN55,212 results in a decrease of this firing rate, indicating that WIN55,212 can act via nonglutamatergic mechanisms (B). Histograms show the relative percentages of action current frequencies from eight recorded cells (D). When the GABAA-R blocker Bic (20 μm) is applied in the presence of Kyn, the firing rate of GnRH neurons decrease. In this paradigm, WIN55,212 is not capable of reducing the action current frequency of GnRH neurons (C). This finding provides evidence that WIN55,212 reduces the firing of GnRH neurons via a GABAA-R-dependent mechanisms. Histograms of loose-patch recordings show the relative percentages of action current frequencies from eight recorded cells (mean ± sem) (E). *, P < 0.05 vs. Kyn. Ctrl, Control.
The average number of spikes within a burst (S/B) (4.2 ± 0.2 in control cells) decreased by 8 ± 2.5% (P = 0.043, Student’s paired t test) in response to WIN55,212 treatment. The interburst interval (IBI) (34 ± 11.2 sec in control cells) increased by 47 ± 11% (P = 0.022), whereas the interspike interval (time between S/B, 0.2 ± 0.03 sec in control cells) did not change.
WIN55,212 decreases GnRH neuron activity via modulating GABAA-R signaling
In their classic mode of action, endocannabinoids bind to presynaptic CB1 receptors to reduce neurotransmitter release (9). Because GABA acting through GABAA-R is the major neurotransmitter in the afferent regulation of GnRH neurons (16), we analyzed the effect of WIN55,212 in the presence of the GABAA-R blocker Bic (20 μm). Because correct interpretation of experimental results would not be possible in this paradigm due to an unbalanced excitatory tone (21,32,33), the extracellular solution also contained the ionotropic glutamate receptor blocker Kyn (2 mm).
Kyn increased the firing rate of GnRH neurons by 95 ± 20.8% (control, 0.48 ± 0.07 Hz; P = 0.0022 by paired Student’s t test). The S/B increased by 14 ± 3.1% (P = 0.021; control, 4.2 ± 0.2) and IBI decreased by 48 ± 9% (P = 0.017; control, 34 ± 11.2 sec). In response to the subsequent administration of 1 μm WIN55,212, the frequency was reduced by 33 ± 2.4% (P = 0.015 by paired Student’s t test) (Fig. 1, B and D). The S/B parameter decreased by 13 ± 4% (P = 0.037) and IBI increased by 57 ± 13% (P = 0.029). The amplitude of the action currents did not change throughout the treatments. Results of the above study established that 1 μm WIN55,212 can decrease the firing activity of GnRH neurons independently of ionotropic glutamate receptors. To show that the underlying mechanisms include GABAA-R-dependent neurotransmission, we tested whether WIN55,212 can also reduce the firing activity of GnRH neurons in the presence of the GABAA-R antagonist Bic (20 μm). Similarly to findings in the previous study, the firing rate of GnRH neurons (0.55 ± 0.07 Hz, 100%) was increased in response to 2 mm Kyn, reaching 181 ± 24.4% of the control frequency (P = 0.037 by paired Student’s t test) (Fig. 1, C and E). S/B increased by 14 ± 3.1% (control, 4.2 ± 0.2; P = 0.037) and IBI decreased by 48 ± 9% (control, 34 ± 11.2 sec; P = 0.025). After Bic was also added for 10 min, firing rate decreased by 57 ± 10.3% (ANOVA, P = 0.0143 and NK test, P = 0.012; Kyn+Bic vs. Kyn) (Fig. 1, C and E). S/B decreased by 23 ± 4.2% (P = 0.033) and IBI increased by 63 ± 18% (P = 0.021). Subsequent addition of 1 μm WIN55,212 was unable to decrease further the firing rate, providing evidence that CB1 receptor activation reduces the activity of GnRH neurons via interfering with GABAA-R-mediated signaling. Interestingly, the firing rate of GnRH neurons tended to increase rather than decrease (by 70.5 ± 21.9%), although this effect did not reach statistical significance (NK test, P = 0.071, Kyn+Bic+WIN vs. Kyn+Bic) (Fig. 1, C and E). S/B and IBI did not change further. The amplitude of the action currents and interspike interval remained unchanged throughout the treatments.
GABAergic afferents to GnRH neurons express CB1 receptors
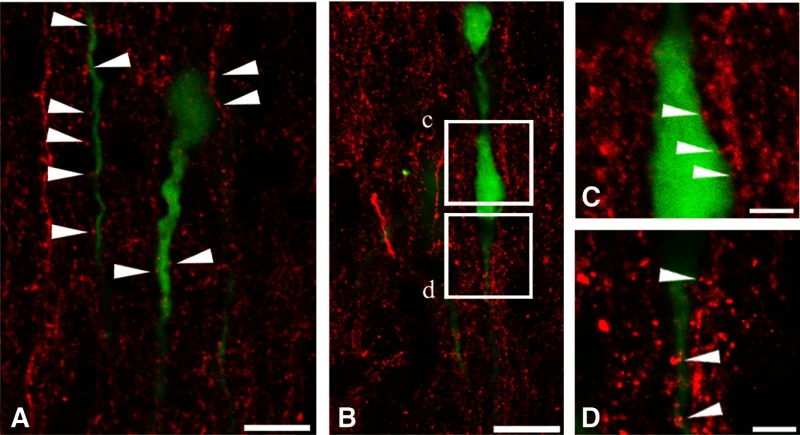
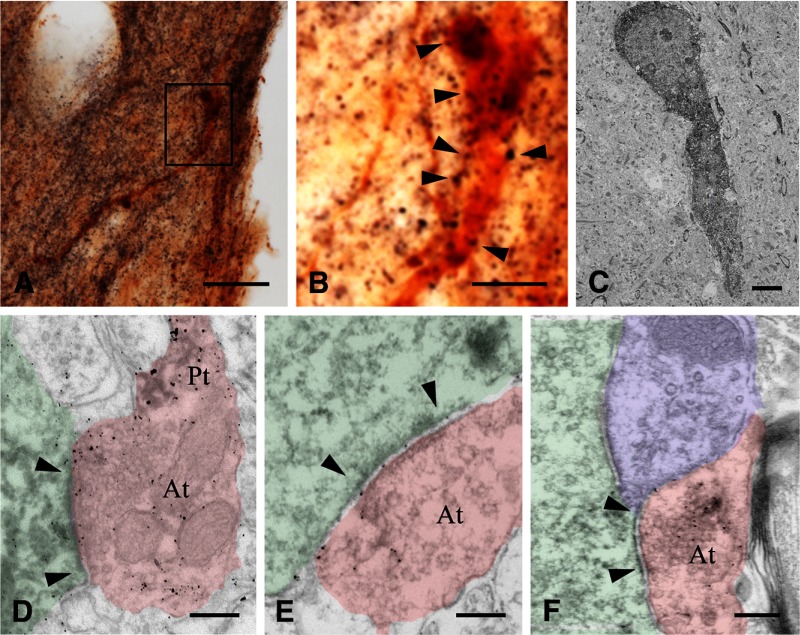
The above findings established that the inhibitory effect of WIN55,212 on GnRH cell activity is mediated by a neuronal circuitry that includes GABAergic interneurons and uses GABAA-Rs. The complexity of this circuitry remained unclear. Because GABA is the major neurotransmitter in the afferent regulation of GnRH neurons (16), further, GABAA-R activation excites GnRH neurons (18,21,25,26,39), we hypothesized that WIN55,212 directly inhibits the GABAergic input to GnRH neurons in its action to reduce GnRH neuron activity. To begin to examine this mechanism, we addressed the presence of CB1 receptors on synaptic afferents to GnRH neurons. CB1-IR axons were first examined with confocal microscopy. These studies revealed the frequent apposition of CB1-IR fibers to the perikaryon and dendrites of GnRH neurons (Fig. 2, A–D). Neuronal contacts were studied further with dual-label preembedding immunohistochemistry. When studied at light microscopic level, individual CB1-IR fibers stained with silver-gold intensified nickel-diaminobenzidine chromogen showed moderate labeling intensity and formed a dense network in the POA/AH where GnRH neurons (stained with brown DAB chromogen) reside (Fig. 3, A and B) . At the electron microscopic level, GnRH neurons exhibited medium electron density (Fig. 3C). CB1 immunoreactivity, detected by the highly electron dense silver-gold grains, was observed in axonal profiles (Fig. 3, D and E). The CB1-IR axon terminals formed synaptic contacts with GnRH-IR dendrites (Fig. 3D) and perikarya (Fig. 3, E and F). The synapses belonged to both asymmetric (Fig. 3, D and E) and symmetric (Fig. 3F) categories, suggesting a functional diversity of the CB1-IR input to GnRH neurons. Photographic panels illustrate immunocytochemical results obtained from five mice in each of the light-, confocal-, and electron-microscopic experiments.
Figure 2.
Confocal analysis of CB1-IR fibers and GnRH-GFP neurons provides evidence for CB1-IR neuronal input to GnRH neurons. GnRH neurons (green) occur embedded in a dense field of CB1-IR axons (red) in the POA. Arrowheads in A, C, and D indicate CB1 containing axons that form contacts with the perikaryon and dendrites of GnRH neurons. Boxed areas in B correspond to C and D at higher power. Scale bars, 15 μm (A and B) and 5 μm (C and D).
Figure 3.
Preembedding immunocytochemical studies reveal CB1-IR synaptic afferents to GnRH neurons. Simultaneous detection of CB1-IR fibers with silver-gold grains (black color in light microscopy and electron dense metallic deposits in electron micrographs) and GnRH-IR neurons with diaminobenzidine (brown color in light microscopy and medium electron density in electron micrographs) enables the ultrastructural analysis of CB1-IR contacts on GnRH neurons. Boxed area in low-power light micrograph A is enlarged in B. Arrowheads indicate appositions of CB1-IR fibers to a GnRH-IR neuron. Low-power electron micrograph in C illustrates a GnRH-synthesizing neuron (electron dense profile) from the organum vasculosum of the lamina terminalis region. A CB1-IR axon terminal (At) (pink shading in D) establishes an asymmetric synapse (arrowheads) with a GnRH-IR dendrite (light green shading). Arrowheads point to an asymmetric axo-somatic synapse in E and a symmetric axo-somatic synapse in F. Note the accumulation of silver particles in the preterminal (Pt) segments of the CB1-IR axon (pink shading) in D–F. Blue shading in F labels a CB-negative synaptic terminal. Scale bars, 50 μm (A), 15 μm (B), 4 μm (C), and 200 nm (D–F).
Cannabinoid signaling via presynaptic CB1 receptors reduces GABAergic mPSCs in GnRH neurons
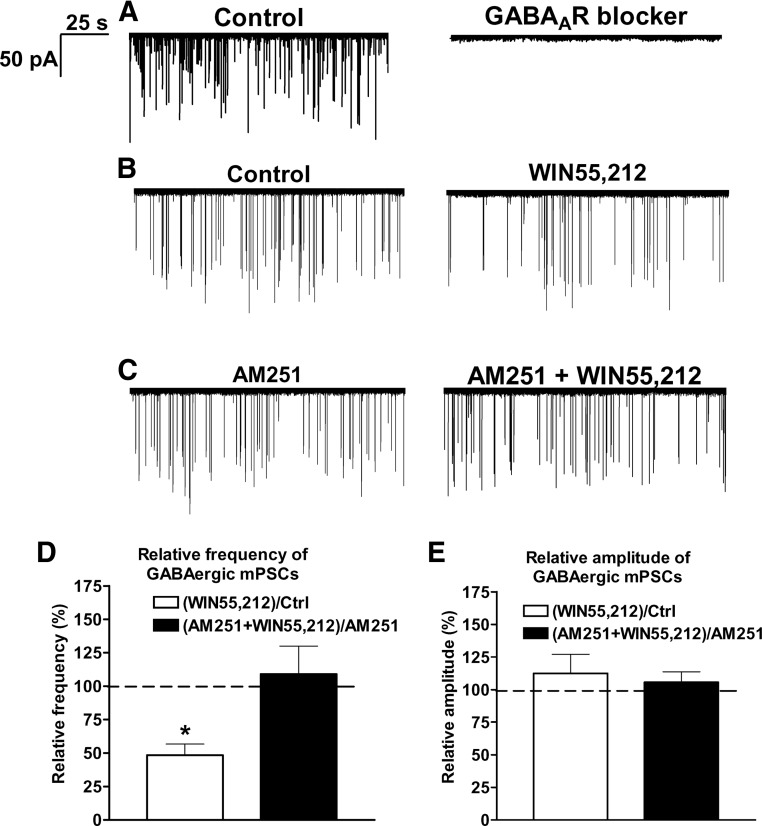
All inward mPSCs were abolished by the extracellular application of 100 μm picrotoxin (n = 8 cells) (Fig. 4A), demonstrating that they were evoked by GABAA-R activation.
Figure 4.
Whole-cell patch-clamp recordings of mPSCs reveal that CB1 receptor activation inhibits the GABAergic afferent drive onto GnRH neurons. All recorded mPSCs in a representative GnRH neuron can be eliminated with 100 μm picrotoxin showing that they are related to GABAA-R activation (A). The frequency of GABAergic mPSCs from a representative GnRH neuron is reduced by the synthetic endocannabinoid agonist WIN55,212 (1 μm) (B). GnRH neurons preincubated with the CB1 antagonist AM251 (1 μm) fail to respond with reduced mPSCs to the WIN55,212 challenge (C). Histograms of whole-cell patch-clamp recordings show the relative percentages of mPSC frequencies (D) and amplitudes (E). Bars correspond to the mean ± sem of 10 recorded cells for each treatment. *, P < 0.05 vs. Ctrl. Note that all changes detected in these experiments affect the frequency, but not the amplitude, of mPSCs. Ctrl, Control.
The CB1 receptor agonist WIN55,212 (1 μm) reduced the frequency of mPSCs (Fig. 4, B and D) in GnRH neurons by 52 ± 8% (control, 0.4 ± 0.03 Hz, Student’s t test, P = 0.0035). The amplitude of mPSCs showed no significant change (control, −49 ± 6 pA; WIN55,212, −54 ± 104 pA) (Fig. 4E). In the presence of AM251, WIN55,212 was not able to change either the frequency or the amplitude of mPSCs (AM251-control, 0.6 ± 0.08 Hz; AM251+WIN55,212, 0.7 ± 0.05 Hz; AM251-control, −50 ± 8 pA; AM251+WIN55,212, −50 ± 8 pA) (Fig. 4, C–E), confirming that CB1 receptors mediate presynaptic inhibition of spontaneous vesicle fusion in GABAergic afferents of GnRH neurons.
Vesicular GABA release onto GnRH neurons is inhibited by endocannabinoids
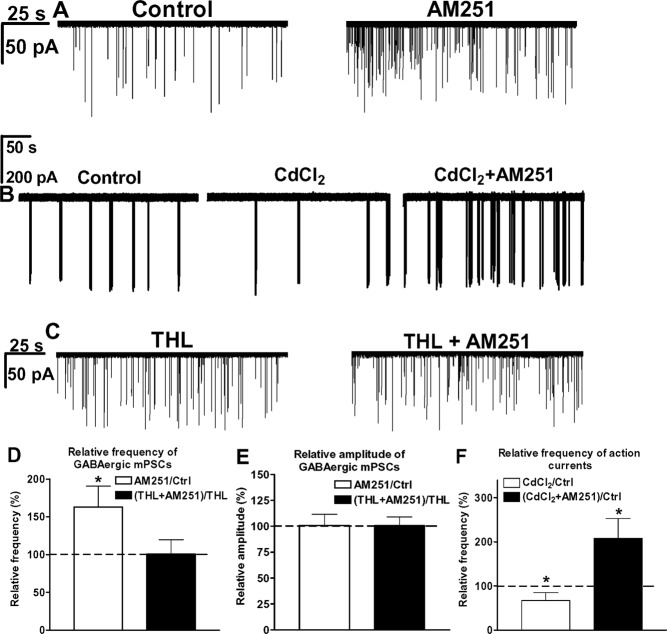
The application of the CB1 antagonist AM251 (1 μm) alone increased the frequency of mPSCs by 63 ± 15% (control, 0.4 ± 0.03 Hz; AM251, 0.6 ± 0.08 Hz) (Student’s paired t test, P = 0.0489) (Fig. 5, A and D). The increased mPSC frequency suggested that AM251 can suspend an endogenous inhibitory endocannabinoid action on GABAergic terminals. The amplitude of mPSCs remained unaltered (control, −49 ± 6 pA; AM251, −50 ± 8 pA) (Fig. 5E).
Figure 5.
Results of whole-cell patch-clamp and loose-patch recordings provide evidence for tonic 2-AG release from GnRH neurons that inhibits GnRH neuron excitability. Bath application of the CB1 antagonist AM251 alone enhances the control frequency of mPSCs in a representative GnRH neuron (A). Firing rate decreases in response to CdCl2 treatment and increases when AM251 is subsequently applied to suspend a tonic endocannabinoid blockade of spontaneous vesicle fusion-related neurotransmitter release (B). Application of the 2-AG synthesis inhibitor THL (10 μm) in the patch electrode prevents any subsequent effect of AM251 on the mPSC frequency of a representative GnRH cell (C). Histograms of whole-cell patch-clamp and loose-patch recordings show the relative percentages of mPSC frequencies (D), amplitudes (E), and firing rate (F). The bars correspond to the mean ± sem of 10 recorded cells for each treatment, except for loose-patch experiments (n = 8). *, P < 0.05.
Endocannabinoids act as an endogenous brake on GnRH neuron spiking
AM251 increased the frequency of mPSCs by suspending an endogenous blockade of random GABA vesicle fusion. To test whether this blockade also acts as a brake on GnRH neuron spiking, loose-patch measurements were carried out in the presence of CdCl2 (200 μm). CdCl2 maintains actions potentials and blocks the action potential-dependent neurotransmitter release. Because it spares neurotransmitter release due to random, spontaneous vesicle fusion, it has been used successfully in earlier studies of GABAergic mPSCs in cerebellar Purkinje cells and their suppression by the CB1 agonist WIN55,212 (34).
CdCl2 application for 10 min decreased the firing rate of GnRH cells by 33 ± 12% (control, 0.6 ± 0.04 Hz; P = 0.030, Student’s t test), which could reflect the absence of action potential-dependent neurotransmitter release from GABA inputs or loss of low-voltage-activated calcium channel initiated spiking (Fig. 5, B and F) (40,41). After AM251 was also added, the firing rate increased by 210 ± 22% (P = 0.012) (Fig. 5, B and F). CdCl2 decreased S/B by 10 ± 2% (control, 4.2 ± 0.2; P = 0.043), whereas AM251 increased S/B to 17 ± 5.2% (P = 0.046). CdCl2 increased IBI by 82 ± 23% (control, 34 ± 11.2 sec; P = 0.028), whereas AM251 decreased it by 63 ± 17% (P = 0.034). These data suggest that GnRH neuron excitability is under an inhibitory endocannabinoid tone.
2-AG production by GnRH neurons tonically inhibits GABAergic drive to GnRH neurons
In a classic mode of retrograde endocannabinoid signaling, presynaptic neurotransmitter release is inhibited by endocannabinoids from postsynaptic sources (9). The most abundant endocannabinoid in the brain is 2-AG (11). Therefore, we hypothesized that GnRH neurons generate 2-AG, which then accounts for the presynaptic inhibition of GABA release. The DAG lipase inhibitor THL was added to the intracellular solution at 10 μm to block 2-AG synthesis in GnRH cells. THL significantly increased the frequency of mPSCs in comparison with controls [control, 0.4 ± 0.03 Hz; THL, 148 ± 12% of the control (Fig. 5, C in comparison with A); intergroup analyses/ANOVA followed by post hoc NK test/ANOVA, P = 0.00008, F = 8.481; NK, P = 0.0235]. In addition, THL prevented the effect of AM251 on the frequency of mPSCs (THL-control, 0.6 ± 0.07 Hz; THL+AM251, 0.6 ± 0.05 Hz) (Fig. 5, C and D). The amplitude of mPSCs was unaffected (THL-control, −48 ± 5 pA; THL+AM251, −49 ± 2 pA) (Fig. 5, C and E). These data provided evidence that tonic inhibition is caused by 2-AG production in GnRH neurons.
Discussion
This study provides electrophysiological and morphological evidence that retrograde endocannabinoid signaling reduces GABAergic afferent drive onto GnRH neurons via the activation of presynaptic CB1 receptors. The reduced GABAA-R signaling, in turn, inhibits GnRH neuron firing activity. This mechanism is represented schematically in Fig. 6.
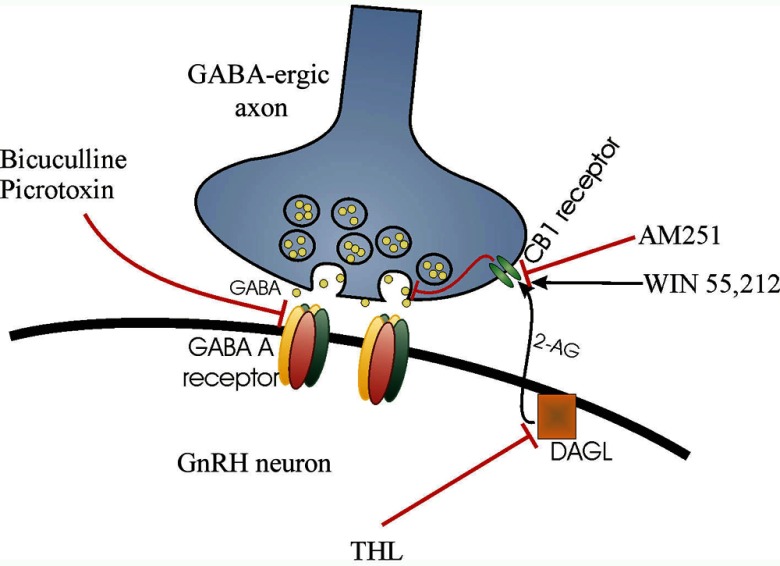
Figure 6.
Schematic illustration of the retrograde endocannabinoid signaling mechanism that regulates GABAA-R-mediated drive onto GnRH neurons. 2-AG is synthesized by the GnRH neuron via the DAG-lipase (DAGL) pathway. This process can be blocked by the intracellular application of the DAGL inhibitor THL. 2-AG is released tonically and binds to CB1 receptors located on GABAergic synaptic afferents. The activation of CB1 inhibits spontaneous GABA release, an effect mimicked by the synthetic CB1 agonist WIN55,212. The CB1 antagonist AM251 can block agonist binding to CB1, which results in increased GABA release. GABA binds preferentially to postsynaptic GABAA-Rs, which can be antagonized by Bic or picrotoxin.
Although somewhat controversial (19,20), a growing body of evidence suggests that GABA can act as an excitatory neurotransmitter on postsynaptic GABAA-R channels of adult GnRH neurons of rodents and fish (18,21,25,26,39). The findings of the present study that WIN55,212 reduces the firing rate of GnRH neurons via GABAA-R-dependent mechanisms and CB1 receptor activation on GABAergic afferents decreases the GABAA-R-mediated mPSCs in GnRH neurons provide circumstantial evidence to support an excitatory role of GABA on GnRH neuron activity.
Although reduced GnRH neuron electric activity in the presence of WIN55,212 could result from cannabinoid actions on a complex multineuronal circuit impinging on GnRH neurons, results of morphological experiments showed that GnRH neurons receive a direct cannabinoid-sensitive neuronal input. Symmetric morphology of a subset of CB1-IR synapses was also indicative of GABAergic neurotransmission (17). GABA is the major neurotransmitter in the afferent regulation of GnRH neurons (16).
To study further the cannabinoid-dependent modulation of GABA release from these synapses, mPSCs were recorded from GnRH neurons in the presence of TTX to exclude activity-dependent effects. We found that the CB1 agonist WIN55,212 decreased GABA release onto GnRH neurons, as revealed by the reduced frequency of GABAA-R-mediated mPSCs. This finding was in accordance with the concept that cannabinoids can modulate neurotransmitter release via action potential-independent mechanisms (1,9). The observation that the frequency, but not the amplitude, of mPSCs was altered was in keeping with the presynaptic effect of WIN55,212 treatment on GABA release (42).
We propose that inhibition of GABAergic afferent input to GnRH neurons by presynaptic CB1 may represents the primary mechanism whereby cannabis drugs act to inhibit LH release (2,3,4,5,6,8). In the physiological context, reduced mPSC frequency in GnRH neurons after the intracellular application of THL indicated that GnRH neurons produce 2-AG to tonically inhibit their GABAergic input. Because GABA plays a crucial role in the regulation of GnRH neuronal activity (29,43,44,45), any alteration of this retrograde signaling results in functional consequences on GnRH neuronal activity. Accordingly, modulation of GABAergic drive onto GnRH neurons has been commonly implicated in metabolic (27,45), estrogen (29), and circadian (29) signaling to GnRH neurons. It will require clarification if GABAergic pathways communicating these afferent modalities are modulated by retrograde endocannabinoid signaling from the GnRH neuron.
The observation that AM251 on its own increased the frequency of GABAergic mPSCs in GnRH neurons suggested the tonic production and retrograde action of endocannabinoids. Furthermore, results of the loose-patch experiments carried out in the presence of CdCl2 provide evidence that endocannabinoids act as an endogenous brake to inhibit GnRH neuron spiking, which could be suspended with AM251.
The absence of tonic inhibition after the intracellular blockade of DAG lipase in GnRH cells designated the GnRH neuron as the source of the acting endocannabinoid, 2-AG. This inhibitory tone may be particularly important in view of the excitatory role of GABA in the afferent regulation of GnRH neurons (18,21,25,26,39). As GnRH neuron activity is driven by two excitatory neurotransmitters, GABA and glutamate, other control systems are required to stabilize the GnRH network activity. There is evidence to show that activation of GnRH neurons represses the GABAergic afferent drive to these cells (46). We propose that retrograde endocannabinoid signaling may play a critically important role in this phenomenon. Similarly to other types of neurons (9), GnRH cells likely respond to activation with an enhanced endocannabinoid production, which, in turn, reduces or switches off GABA release onto GnRH neurons. A similarly strong control of GABA-driven activity by endocannabinoids has been proposed to act in developing cortical networks, in the same specific context of depolarizing GABA (47).
It remains to be established if cannabinoids also inhibit the glutamatergic input to GnRH neurons. The present immunoelectron microscopic studies found morphological evidence for CB1-IR asymmetric, in addition to symmetric, synapses on GnRH neurons, suggesting that some cannabinoid-sensitive afferents may be glutamatergic. Alternatively, such synapses can also belong to atypical GABAergic terminals that have been observed in the hypothalamus (48,49). It is worth noting that a group of neurons in the anteroventral periventricular nucleus of female rats exhibits a dual GABA/glutamate phenotype (50) and their synaptic morphology may be atypical. Nevertheless, although GABAergic PSCs were detectable in all GnRH neurons in the present and previous studies (19,20,27,28), optimized recording conditions failed to detect glutamatergic excitatory PSCs in 20–35% of GnRH neuron somata (51). This can only be partly attributed to the technical issue that excitatory input is often received by distal dendrites, which exert lower impact on the recordings from cell bodies (51). It is worth of note that in loose-patch experiments, WIN55,212 showed a tendency to increase the firing rate of GnRH neurons when ionotropic glutamate- and GABAA-R-mediated neurotransmissions were both suspended. Although this effect did not reach statistical significance (P = 0.07), the trend suggests that additional cannabinoid sensitive mechanisms also contribute to the regulation of the GnRH neuronal network. The increased activity of GnRH neurons in this experiment could be due to the reduced presynaptic release of GABA, which could withdraw the postsynaptic GABAB receptor-mediated inhibition of GnRH neurons (23). Alternatively, WIN55,212 could reduce the release of an unknown inhibitory neurotransmitter/neuromodulator directly upon GnRH neurons or influence a multisynaptic pathway impinging on GnRH neurons.
The present evidence for retrograde endocannabinoid signaling that inhibits the GABAergic afferent drive onto GnRH neurons does not exclude the possibility that cannabinoid ligands also influence GnRH release via additional mechanisms and sites of action. It has been demonstrated that anandamide reduces GnRH secretion from mediabasal hypothalamic explants that contain the GnRH neurosecretory terminals (52). This finding, together with the previous observations of CB1 immunoreactivity on unidentified neurosecretory axon terminals in the median eminence of mice (53) and on GnRH axon terminals of the frog (54), raise the possibility that cannabinoids also influence GnRH release directly from the GnRH neurosecretory terminals. In vitro, GT1-7 neurons are not only cannabinoid source but also target cells. They synthesize, transport, and degrade cannabinoids and possess cannabinoid receptors whose activation suppresses the pulsatile secretion of GnRH (55). Because mouse GnRH neurons did not contain detectable CB1 mRNA levels in dual-label in situ hybridization experiments (55), the physiological relevance of these data to the mouse hypothalamus will require clarification.
The retrograde endocannabinoid signaling may be used widely by other neuronal systems in the neuroendocrine hypothalamus. Our previous immunocytochemical experiments revealed a dense innervation of the POA and the hypothalamus by CB1-IR fibers (53,56) and demonstrated CB1-IR synaptic terminals in the arcuate, supraoptic, and paraventricular nuclei (53,56). Functional observations by others indicate that endocannabinoid signaling modulates GABAergic and/or glutamatergic synaptic transmission to proopiomelanocortin neurons of the arcuate nucleus (42,57) and to parvocellular (58,59) and magnocellular neurons (60) of the paraventricular nucleus. Further evidence indicates that endocannabinoid signaling strongly contributes to the mechanisms whereby ghrelin (59) and glucocorticoids (61) modulate the glutamatergic synaptic input to parvocellular neurons of the paraventricular nucleus.
Tonic production and retrograde action of endocannabinoids is not a unique phenomenon to GnRH neurons. Accordingly, an increased frequency of mPSCs after CB1 inhibition has also been demonstrated in hypothalamic proopiomelanocortin (42,57) and oxytocin neurons (62,63) and in hippocampal pyramidal cells (64). In the hippocampus, tonic release of endocannabinoids by CA3 pyramidal cells results in a persistent activation of presynaptic cannabinoid receptors and provides a state-dependent switch in cortical networks (64). A similar silencing of GABAergic inputs to GnRH cells by endocannabinoids may underlie an important gating mechanism for the transmission of metabolic, circadian, stress, emotional, and steroid signals to the GnRH network. The driving force behind the production of endocannabinoids by GnRH neurons, as well as the functional characteristics of the regulated cannabinoid-sensitive GABAergic afferents to GnRH cells, remain to be determined. Estrogen might be one critically important modulator of endocannabinoid synthesis, because higher levels of endocannabinoids were detected in the hypothalamus of the ovariectomized and estrogen-substituted female vs. the ovariectomized and vehicle-treated female or intact male rat (52). In our patch-clamp study, tonic 2-AG release was detected in the presence of TTX, suggesting that the phenomenon is, at least partly, activity independent. The underlying mechanism may be related to the function of currently unknown constitutively active receptors in GnRH neurons. For example, metabotropic glutamate receptors (mGluRs) can show such constitutive activity in the hippocampus (65), and mGluR activation can, indeed, result in elevated synthesis of 2-AG in hippocampal, cerebellar, and corticostriatal neurons (for a review see Ref. 11). mGluRs are also present in GnRH neurons (66). The putative involvement of mGluR and/or other constitutively active receptors in tonic endocannabinoid release from GnRH cells requires clarification.
We provide evidence in this study that retrograde endocannabinoid signaling represents an important regulatory mechanism whereby GnRH neurons decrease their excitatory GABAergic input under physiological and pathological conditions.
Acknowledgments
We thank Dr. R. A. Benoit for the kind donation of the LR1 GnRH antibodies, Dr. Norbert Hájos and Dr. János Szabadics for their invaluable comments, and Hajni Bekó and Barna László for the technical assistance.
Footnotes
This work was supported by the National Science Foundation of Hungary (Országos Tudományos Kutatási Alapprogramok K69127 and T73002) and the Hungarian Health Research Council Fund (Egészségügyi Tudományos Tanács 122/2009). The research leading to these results has received funding from the European Community’s Seventh Framework Program (FP7/2007-2013) under Grant Agreement 245009.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 6, 2010
Abbreviations: ABC, Avidin-biotin complex; AH, anterior hypothalamus; aCSF, artificial cerebrospinal fluid; 2-AG, 2-arachidonoylglycerol; Bic, bicuculline; CB1, type 1 cannabinoid receptor; DAG, diacylglycerol; THC, Δ-9-tetrahydro-cannabinol; GA, glutaraldehyde; GABA, γ-aminobutyric acid; GABAA-R, GABAA receptor; GFP, green fluorescent protein; IBI, interburst interval; IEM, Institute of Experimental Medicine; IR, immunoreactive; KO, knockout; Kyn, kynurenic acid; mGluR, metabotropic glutamate receptor; mPSC, miniature postsynaptic current; NK, Newman-Keuls; PFA, paraformaldehyde; POA, preoptic area; S/B, spikes within a burst; THL, tetrahydrolipstatin; TTX, tetrodotoxin; WT, wild type.
References
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R 2006 The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27:73–100 [DOI] [PubMed] [Google Scholar]
- Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ 1981 Effects of Δ9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab 52:50–55 [DOI] [PubMed] [Google Scholar]
- Smith CG, Smith MT, Besch NF, Smith RG, Asch RH 1978 Effect of Δ9-tetrahydrocannabinol (THC) on female reproductive function. Adv Biosci 22- 23:449–467 [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Ellingboe J, Skupny AS, Lex BW, Griffin M 1986 Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther 237:862–866 [PubMed] [Google Scholar]
- Tyrey L 1978 δ-9-Tetrahydrocannabinol suppression of episodic luteinizing hormone secretion in the ovariectomized rat. Endocrinology 102:1808–1814 [DOI] [PubMed] [Google Scholar]
- Wenger T, Rettori V, Snyder GD, Dalterio S, McCann SM 1987 Effects of Δ-9-tetrahydrocannabinol on the hypothalamic-pituitary control of luteinizing hormone and follicle-stimulating hormone secretion in adult male rats. Neuroendocrinology 46:488–493 [DOI] [PubMed] [Google Scholar]
- Dalterio S, Steger R, Peluso J, de Paolo L 1987 Acute Δ9-tetrahydrocannabinol exposure: effects on hypothalamic-pituitary-testicular activity in mice. Pharmacol Biochem Behav 26:533–537 [DOI] [PubMed] [Google Scholar]
- Ayalon D, Nir I, Cordova T, Bauminger S, Puder M, Naor Z, Kashi R, Zor U, Harell A, Lindner HR 1977 Acute effect of δ1-tetrahydrocannabinol on the hypothalamo-pituitary-ovarian axis in the rat. Neuroendocrinology 23:31–42 [DOI] [PubMed] [Google Scholar]
- Piomelli D 2003 The molecular logic of endocannabinoid signallig. Nat Rev Neurosci 4:873–884 [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR 2000 Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 20:2470–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M 2009 Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89:309–380 [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF 2008 Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14:923–930 [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM 2001 CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol 85:468–471 [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS 2001 Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol 532:731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh M, Milloh H, Wenger T 2008 The role of endocannabinoids in the regulation of luteinizing hormone and prolactin release. Differences between the effects of AEA and 2AG. Mol Cell Endocrinol 286:S36–S40 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F 1985 Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE 2002 Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE 2004 Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Ronnekleiv OK, Kelly MJ 2009 γ-Aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology 150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE 1993 Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 469:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakuma Y, Kato M 2009 GABAA receptors mediate excitation in adult rat GnRH neurons. Biol Reprod 81:327–332 [DOI] [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M 2008 Activation of A-type γ-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 20:566–575 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, DeFazio RA, Moenter SM 2003 Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23:8578–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2005 GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 72:33–41 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M 1999 Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401–404 [DOI] [PubMed] [Google Scholar]
- Laschet JJ, Kurcewicz I, Minier F, Trottier S, Khallou-Laschet J, Louvel J, Gigout S, Turak B, Biraben A, Scarabin JM, Devaux B, Chauvel P, Pumain R 2007 Dysfunction of GABAA receptor glycolysis-dependent modulation in human partial epilepsy. Proc Natl Acad Sci USA 104:3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I 2008 Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res 78:50–59 [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Linden DJ 2000 Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J Neurophysiol 83:1167–1180 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Makara J, Katona I, Nyíri G, Németh B, Ledent C, Watanabe M, de Vente J, Freund T, Hájos N 2007 Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci 27:10211–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalló I, Butler JA, Barkovics-Kalló M, Goubillon ML, Coen CW 2001 Oestrogen receptor α-immunoreactivity in gonadotropin-releasing hormone-expressing neurons: regulation by oestrogen. J Neuroendocrinol 13:741–748 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2003 A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane R, Oka Y 2010 Excitatory action of GABA in the terminal nerve gonadotropin-releasing hormone neurons. J Neurophysiol 103:1375–1384 [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM 2010 Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 30:3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK 2009 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ 2006 Estrogen differentially modulates the cannabinoid- induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology 84:123–137 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2008 Critical roles for fast synaptic transmission in mediating estradiol negative and positive feedback in the neural control of ovulation. Endocrinology 149:5500–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM 2009 Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29:5616–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 γ-Aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology 145:1194–1202 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H 2005 Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA 102:9388–9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE 2008 Heterogeneous expression of γ-aminobutyric acid and γ-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol 506:708–732 [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ 2002 GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience 113:581–592 [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL 2004 Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Pielecka-Fortuna J, Moenter SM 2009 Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod 80:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorticati C, Fernández-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A, Billi S, McCann SM, Rettori V 2004 The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci USA 101:11891–11896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Deli L, Kallo I, Hrabovszky E, Watanabe M, Liposits Z, Fekete C 2007 Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurology 503:270–279 [DOI] [PubMed] [Google Scholar]
- Meccariello R, Franzoni MF, Chianese R, Cottone E, Scarpa D, Donna D, Cobellis G, Guastalla A, Pierantoni R, Fasano S 2008 Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology 149:2149–2158 [DOI] [PubMed] [Google Scholar]
- Gammon CM, Freeman Jr GM, Xie W, Petersen SL, Wetsel WC 2005 Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology 146:4491–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli L, Wittmann G, Kalló I, Lechan RM, Watanabe M, Liposits Z, Fekete C 2009 Type 1 cannabinoid receptor-containing axons innervate hypophysiotropic thyrotropin-releasing hormone-synthesizing neurons. Endocrinology 150:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT 2005 Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25:9746–9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG 2003 Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M 2008 The orexigenic effect of ghrelin Is mediated through central activation of the endogenous cannabinoid system. PLoS One 3:e1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG 2005 Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol 569:751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG 2009 Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci 29:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS 2007 Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci 27:1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG 2009 Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 145:4292–4301 [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biró AA, Nusser Z 2004 Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA 101:1362–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Somogyi P, Nusser Z 2003 Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol 89:1910–1919 [DOI] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M 2008 Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]