Abstract
Mounting evidence supports that cadmium, a toxic metal found in tobacco, air and food, is a cardiovascular risk factor. Our objective was to conduct a systematic review of epidemiologic studies evaluating the association between cadmium exposure and cardiovascular disease. Twelve studies were identified. Overall, the pooled relative risks (95% confidence interval) for cardiovascular disease, coronary heart disease, stroke, and peripheral arterial disease were: 1.36 (95%CI: 1.11, 1.66), 1.30 (95%CI: 1.12, 1.52), 1.18 (95%CI: 0.86, 1.59), and 1.49 (95%CI: 1.15, 1.92), respectively. The pooled relative risks for cardiovascular disease in men, women and never smokers were 1.29 (1.12, 1.48), 1.20 (0.92, 1.56) and 1.27 (0.97, 1.67), respectively. Together with experimental evidence, our review supports the association between cadmium exposure and cardiovascular disease, especially for coronary heart disease. The number of studies with stroke, HF and PAD endpoints was small. More studies, especially studies evaluating incident endpoints, are needed.
Keywords: Cadmium, Cardiovascular disease, Meta-analysis, Systematic Review
Introduction
Cadmium is a non-essential carcinogenic metal widely distributed in the environment [1, 2]. A byproduct of mining, smelting and refining zinc, lead and copper ores, cadmium production and use has substantially increased, particularly in nickel-cadmium batteries, fertilizers, coatings and plastic stabilizers [1, 3]. The impact of cadmium-containing products (nickel-cadmium batteries, electronic devices, jewelry and toys) [3] and cadmium-containing fertilizers on human exposure through soil and diet [4, 5] is a major concern. Indeed, leafy and root vegetables and grains bioconcentrate cadmium from the soil, especially in acidic soils, resulting in a major exposure pathway through the diet and smoking [4, 6, 7]. Ambient air and dust can also contribute to cadmium exposure, particularly in urban areas, in the vicinity of occupational and industrial sources [8, 9] and in certain occupational groups (metal and mining industry, transportation and repairing services) [10].
Experimental evidence [11] suggests that cadmium could contribute to the initiation of atherosclerosis and promote progression. In vitro, cadmium induces endothelial dysfunction, and in vivo, it accelerates atherosclerotic plaque formation [11]. Several mechanisms have been suggested to explain the role of cadmium in promoting atherosclerosis. Cadmium may increase reactive oxygen species formation [12] and interfere with anti-oxidative stress responses by binding metallothionein [13], a low molecular weight protein that regulates zinc homeostasis and acts as a free radical scavenger [12, 14]. Cadmium may also contribute to atherosclerosis by increasing blood pressure [15–17], or through kidney damage [18, 19] cadmium-related estrogenic activity [20–22] or epigenetic changes [23]. The relevance of these mechanisms to cadmium-induced atherogenesis is uncertain.
In epidemiologic studies, cadmium concentrations in blood and urine are established biomarkers of cadmium exposure and internal dose [1, 9, 24]. Both biomarkers reflect cumulative exposure, although blood cadmium also reflects short-term fluctuations in exposure [1, 9, 24]. Prospective studies [25–27, 28••, 29••] investigating the association of cadmium concentrations with cardiovascular outcomes have mostly supported an association with cardiovascular risk, but the evidence has not been appraised systematically. Our objective was to systematically review and synthesize results from epidemiologic studies on the association between cadmium biomarkers and cardiovascular disease.
Methods
Search Strategy, Study Selection and Data Abstraction
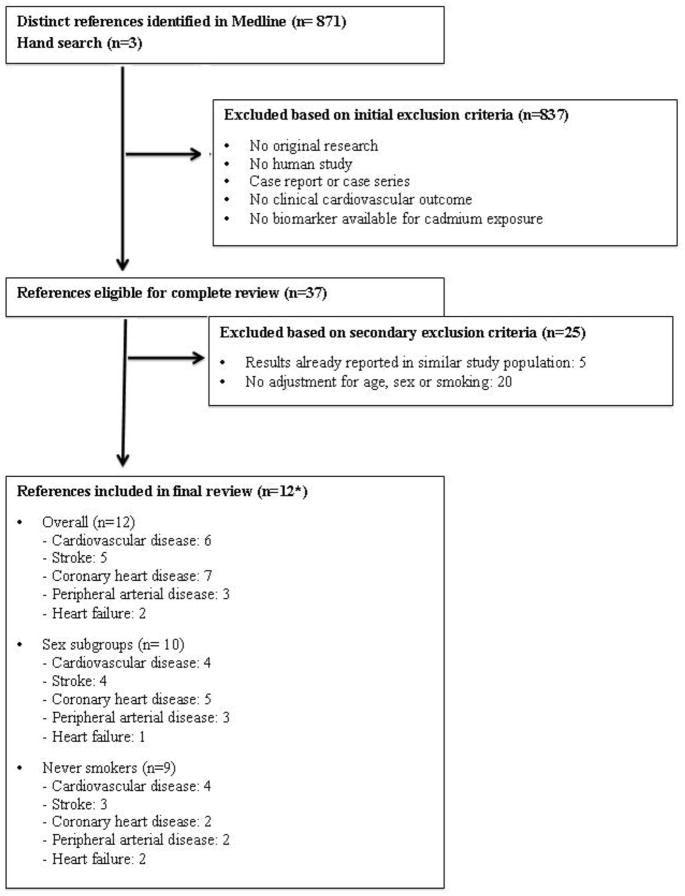
We searched PubMed for relevant published studies through April 15, 2013 using the following combination of Medical Subject Heading (MeSH) terms and text words: ((“cadmium”[Mesh] OR “cadmium poisoning”[Mesh]) OR “cadmium “[Substance Name]) AND (“cardiovascular diseases” [Mesh] OR “mortality” [Mesh] OR “atherosclerosis”[all fields] OR “coronary artery disease” [all fields] OR “cardiovascular diseases” [all fields] OR “myocardial infarction” [all fields] OR “stroke” [all fields] OR “mortality” [all fields]). The search strategy retrieved 871 citations (Figure 1). We included all articles assessing cadmium exposure using biomarkers. We limited the search to clinical CVD, defined a priori as coronary heart disease (CHD) (including myocardial infarction and ischemic heart disease), stroke (cerebrovascular disease, ischemic and hemorrhagic stroke) and peripheral arterial disease (PAD) (lower-extremity peripheral arterial disease, diseases of the peripheral arteries, and blackfoot disease), as well as overall CVD. The search had no language restrictions. We also included 3 relevant studies published after April 15, 2013 [29••, 30••, 31••].
Figure 1.
Summary of inclusion and exclusion criteria used in this systematic review of studies investigating the association between cadmium and clinical cardiovascular disease, April 15th 2013.
*12 references correspond to 7 unique study populations: Everett et al. (2008) [58] and Menke et al. 2009 [27] examined NHANES III subjects but assessed different cardiovascular endpoints; Peters et al. 2010 [60•], Tellez-Plaza et al 2010 [62•], Agarwal et al. 2011 [59] and Tellez-Plaza et al. 2012 [28••] examined NHANES 1999+ subjects but assessed different cardiovascular endpoints. Tellez-Plaza et al. 2013 [29••] and Tellez-Plaza et al. Submitted [31••] examined Strong Heart Study participants but assessed different incident cardiovascular disease endpoints.
Two investigators (M.T-P and M.R.J.) independently reviewed each of 874 papers and selected 37 papers applying the following study exclusion criteria (Figure 1): a) No original research (i.e. reviews, editorials, non-research letters); b) No human study; c) Case report or case series; d) No clinical cardiovascular outcomes (e.g. subclinical atherosclerosis); e) No cadmium exposure levels from biological tissues (e.g. environmental measures such as water or air, or distance from a cadmium source). Age, sex and smoking are major determinants of cadmium levels in the human body and major risk factors for cardiovascular disease. We thus excluded 20 studies not adjusting for age, sex or smoking [32–51]. One large prospective study from Japan [52••] did not adjust for smoking. Smoking was very rare among women in this rural population [52••] and we included the results for women as part of our systematic review and meta-analysis. For studies analyzing the same cardiovascular endpoints in the same study population we selected the most recent publication or the publication with the largest sample size, resulting in the exclusion of 5 papers [25, 53–56] and leaving 12 studies. Any discrepancies were resolved by consensus. A native speaker reviewed the full-text of any non-English article that could not be included or excluded based on the initial abstract review. After retrieval of articles from the search, the reference lists of selected articles were checked for other potentially relevant articles, identifying no additional studies. We assessed study quality according to the criteria adapted from Longnecker and colleagues [57] (Online Resource 1).
Statistical Analysis
Measures of association for a change in cadmium levels and their standard errors or 95% confidence intervals (CI) were abstracted or derived using the data reported in the publication. For some studies that reported only the association for cadmium categories, we reported the estimated relative risk comparing the highest to the lowest categories [27, 30••, 52••, 58]. Results presented separately for males and females were combined within each study. Pooled relative risk estimates for CVD were calculated overall, and in men, women and never smokers from individual studies using an inverse variance weighted random effects model. For descriptive purposes, we also estimated pooled relative risk for specific cardiovascular outcomes (coronary heart disease, stroke or peripheral arterial disease). Two study populations had information on both prevalence and mortality for cardiovascular disease and coronary heart disease [27, 28••, 58–60•]. We considered prevalence and mortality endpoints as qualitatively different endpoints and pooled them together in the main analyses. As a sensitivity analysis, we conducted the pooling after excluding prevalent cardiovascular endpoints in the aforementioned study populations (Online Resource 2). We evaluated heterogeneity between studies using the I2 statistic, which describes the total variability across all studies due to heterogeneity [61]. Additionally, we tested for influential studies by omitting each study sequentially and assessed publication bias using funnel plots.
RESULTS
Twelve studies met the primary and secondary exclusion criteria (Table 1; Figure 1). Eight studies were conducted in the US [27, 28••, 29••, 31••, 58–60•, 62•], 1 in Belgium [26], 1 in Korea [63•], 1 in Japan [52••] and 1 in Sweden [30••]. All the studies were conducted in general populations with no clear sources of environmental cadmium contamination, except the studies in Belgium [26], with a study population living near a cadmium-polluted area and including 42 smelter-workers, and in Japan [52••]. Seven studies were prospective cohorts [26–28••, 29••, 30••, 31••, 52••] and five were cross-sectional [58–60•, 62•, 63•]. All studies except one [63•], used urine as a biomarker of cadmium exposures, although some studies used also blood [26, 28••, 30••, 59, 60•, 62•, 63•]. One study used blood cadmium as the only biomarker of exposure [63•].
Table 1.
Studies of cadmium biomarkers and clinical cardiovascular disease outcomes (12 studies available)
| Study, year | Population | Men (%) | Age Range (yrs) | Cadmium Biomarker | Exposed vs. Reference | Endpoint Ascertainment | Outcome (s) | No. of cases/non-cases | Relative Risk estimate (95 % CI) | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||||
| Nawrot 2008 [26] | Inhabitants from northeastern Belgium, including a cadmium polluted area (Cadmibel study) | 44.0 | ≥ 20 | Blood | Doubling of the dose | Death certificates | CVD mortality Cardiac mortality Stroke mortality |
98/1125 64/1159 22/1201 |
1.29 (0.99–1.67) 1.31 (0.95–1.81) 0.85 (0.49–1.47) |
Sex, age, body mass index, smoking, γ-glutamyltransferase as index of alcohol intake, and SES |
| 24h urine | CVD mortality Cardiac mortality Stroke mortality |
1.11 (0.89–1.38) 1.09 (0.83–1.43) 0.61 (0.37–0.99) |
||||||||
| Menke 2009 [27] | General population NHANES III | 47.0 | ≥20 | Urine | Doubling of the dose | Death certificates | CVD mortality | Men: 449/6109 Women: 320/7078 Never smk men: 120 Never smk women: 206 |
Men: 1.21 (1.07–1.36) Women:0.93 (0.84–1.04) Never smk men: 1.30 (1.06–1.60) Never smk women: 0.93 (0.80–1.08) |
Age, race/ethnicity, postmenopausal status, urban residence, annual household income, high school education, smoking status, pack-years, physical activity, diabetes, BMI, alcohol consumption, C-reactive protein, total cholesterol, systolic blood pressure, blood pressure-lowering medication, blood lead, and eGFR. |
| Men: ≥ 0.48 μg/g vs. < 0.21 μg/g Women: ≥ 0.68 μg/g vs. < 0.29 μg/g |
CHD mortality | Men: 241/6317 Women: 126/7272 |
Men: 2.48 (0.85–7.27) Women: 0.45 (0.24–0.83) |
|||||||
| Li 2011 [52••] | Cd-polluted region in Japan (Kakehashi River) | 45.0 | ≥ 50 | Urine | ≥10.0 μg/g vs. < 3.0 μg/g | Death certificates | CVD mortality Stroke mortality |
Never smk women: 156/1537 Never smk women: 115/1578 |
Never smk women: 2.4 (1.1, 5.1) Never smk women: 3.6 (1.1–11.9) |
Age |
| Tellez-Plaza 2012 [28••] | General population NHANES 1999–2004 | 52.2 | ≥20 | Blood | 0.80 μg/L to 0.22 μg/Lc (Per 0.58 μg/L increase) | Death certificates | CVD mortality | 187/8802 Men:106/4386 Women:81/4416 Never smk:77/4826 |
1.69 (1.03, 2.77) Men: 1.50 (0.84, 2.68) Women: 1.90 (0.97, 3.71) Never smk: 1.17 (0.53, 2.55) |
Sex, race-ethnicity, education, annual household income, post- menopausal status for women, body mass index, blood lead, C-reactive protein, total cholesterol, HDL cholesterol, cholesterol lowering medication use, hypertension, diabetes, estimated glomerular filtration rate, pack-years, smoking, and serum cotinine. |
| CHD mortality | 88/8901 | 1.73 (0.88, 3.40) | ||||||||
| Urine | 0.57 μg/g to 0.14 μg/gc (Per 0.43 increase) | CVD mortality, | 1.74 (1.07, 2.83) Men: 1.87 (0.96, 3.66) Women: 1.62 (0.87, 3.01) Never smk: 1.98 (0.90, 4.35) |
|||||||
| CHD mortality | 2.09 (1.06, 4.13) | |||||||||
| Fagerberg 2013 [30••] | Women from Gothenburg (Sweden) participating in a study of diabetes | 0.0 | 64 | Blood Urine |
> 0.44μg/L vs. ≤0.25 μg/L >2.06 μg/g vs. ≤ 0.28 μg/g |
ABI | PAD | 55/403 | 2.4 (0.9 to 6.3) 2.5 (1.1 to 5.8) |
Pack-years of smoking, current smoking, systolic blood pressure, HbA1c, apolipoprotein B/A-I, statin treatment, stratification group at baseline (normal glucose tolerance, impaired glucose tolerance, diabetes). HbA1c, glycated haemoglobin |
| Tellez-Plaza 2013 [29••] | 13 native American communities from the US (Strong Heart Study) | 40% | 45–75 | Urine | 1.62 μg/g to 0.55 μg/gc (Per 1.07 μg/g increase) | Annual mortality and morbidity surveillance reviews of hospitalization and death records and at two research clinic visits conducted in 1993–1995 and 1998–1999 | CVD incidence | Overall: 1084/2264 Men: 455/884 Women: 629/1380 Never smokers: 350/822 |
1.24 (1.11–1.38) 1.30 (1.09–1.55) 1.20 (1.05–1.36) 1.12 (0.95–1.32) |
Sex, postmenopausal status for women, education, body mass index, total cholesterol, estimated LDL cholesterol, hypertension, diabetes, estimated glomerular filtration rate, smoking status and cumulative smoking dose (pack- years). Models were intrinsically adjusted for age as age was considered as time scale. |
| CHD incidence | Overall: 766/2582 Men: 358/981 Women: 408/1601 Never smokers: 243/929 |
1.22 (1.08–1.38) 1.11 (0.92–1.34) 1.29 (1.10–1.51) 1.16 (0.96–1.39) |
||||||||
| Stroke incidence | Overall: 244/3104 Men: 93/1246 Women: 151/1858 Never smokers: 68/1104 |
1.75 (1.17–2.59) 1.89 (1.03–3.45) 1.49 (0.94–2.36) 0.90 (0.49–1.65) |
||||||||
| Heart Failure incidence | Overall: 328/3020 Men: 100/1239 Women: 228/1781 Never smokers: 104/1068 |
1.39 (1.01–1.94) 1.75 (1.00–3.05) 1.23 (0.83–1.81) 1.18 (0.68–2.05) |
||||||||
| Tellez-Plaza, submitted [31••] | 13 native American communities from the US (Strong Heart Study) | 40% | 45–75 | Urine | 1.62 μg/g to 0.55 μg/gc (Per 1.07 μg/g increase) | ABI measured at three research clinic visits conducted in 1989–1991, 1993–1995 and 1998–1999 | PAD incidence | Overall: 470/2394 Men: 181/934 Women: 289/1460 Never smokers: 150/822 |
1.41 (1.05, 1.81) 1.32 (0.84, 1.90) 1.44 (1.01, 1.99) 1.25 (0.83, 1.80) |
Age, sex, postmenopausal status for women, education, body mass index, total cholesterol, estimated LDL cholesterol, hypertension, diabetes, estimated glomerular filtration rate, smoking status and cumulative smoking dose (pack- years) and study center location. |
| Cross-sectional studies | ||||||||||
| Everett 2008 [58] | General population (NHANES III) | NA | 45–79 | Urine | ≥0.88 μg/g creatinine vs < 0.43 μg/g creatinine | Myocardial infarction was determined by electro- cardiogram (ECG) using the Cardiac Infarction Injury Score. | MI prevalence | NA | 1.86 (1.26–2.75) Men: 1.26 (0.71–2.26) Women: 1.80 (1.06–3.04) Never smk: 1.85 (1.10–3.14) |
Framingham risk score, pack-years of smoking, race-ethnicity, family history of heart attack, and diabetes. |
| Peters 2010 [60•] | General population NHANES 1999–2006 | 50.8 | ≥ 30 | Blood | Per 1.5 μg/L increase | Self-reported | Stroke prevalence | 492/12,049 | 1.38 (1.14–1.67) women: 1.38 (1.11–1.72) men: 1.30 (0.93–1.79) never smk: 1.19 (1.02–1.37) |
Age, sex, race/ethnicity, education, body mass index, poverty income ratio, alcohol consumption, smoking status, diabetes, hypertension, hypercholesterolemia, chronic kidney disease and urine creatinine. Stroke models were further adjusted for prevalent CHD. |
| Heart failure prevalence | 471/12,005 | 1.48 (1.17–1.87) never smk: 1.10 (0.96, 1.27) |
||||||||
| MI prevalence | NA | 1.32 (1.13–1.54) | ||||||||
| Urine | Stroke prevalence | 171/3,909 | 1.10 (1.00–1.20) never smk: 1.06 (0.93–1.21) |
|||||||
| Heart failure prevalence | 135/3,898 | 1.12 (1.04–1.21) never smk: 1.02 (0.88, 1.18) |
||||||||
| MI prevalence | NA | 1.12 (1.03–1.21) | ||||||||
| Tellez-Plaza 2010 [62•] | General population NHANES 1999–2004 | 51.6 | ≥40 | Blood | 0.80 μg/L to 0.26 μg/L (Per 0.54 μg/L increase) | ABI | PAD prevalence | Men: 245/3,088 Women: 223/2,900 |
Men: 1.95 (1.28–2.96) Never smk men: 3.04 (0.62–14.9) Women: 1.47 (0.82–2.66) Never smk women: 0.95 (0.49–1.86) |
Age, race/ethnicity, survey year, education, postmenopausal status for women, body mass index, blood lead, C-reactive protein, total cholesterol, high density lipoprotein cholesterol, cholesterol-lowering medication, systolic blood pressure, blood pressure- lowering medication, diabetes, estimated glomerular filtration rate, smoking and serum cotinine. |
| Urine | 0.69 μg/g to 0.20μg/g (Per 0.49 μg/g increase) | Men: 3.28 (1.82–5.91) Never smk men: 1.67 (0.56–4.96) Women: 0.65 (0.35–1.21) Never smk women: 0.47 (0.21–1.05) |
||||||||
| Lee 2011 [63•] | General Population from Korea (KNHANES), without known source of cadmium | 47.9 | ≥20 | Blood | Per 0.91 μg/L increaseb | Self-reported | IHD prevalence | 36/1870 Men: 19/928 Women: 17/942 |
2.10 (1.29–3.43) Men: 1.88 (0.96–3.69) Women: 2.28 (1.26–4.15) |
Age, age2, education level, income, family hypertension history, systolic blood pressure, alcohol, smoking status, BMI, waist circumference and blood lead |
| Stroke prevalence | 44/18621 Men: 29/918 Women: 15/944 |
1.10 (0.79–1.54) Men: 1.26 (0.79–1.98) Women: 0.94 (0.50–1.75) |
||||||||
| Agarwal 2011 [59] | General population NHANES 1999–2006 | 48.3 | 46.5 (Mean) | Blood | ≥ 0.61 μg/g vs. < 0.22 μg/g | Self-report | CVD prevalence | 1601/13566 | 1.50 (1.10–2.04) | Age, sex, race, education, hypertension, diabetes, hypercholesterolemia, chronic kidney disease, body mass index, C-reactive protein, smoking status, serum cotinine. |
| Urine | Per 2.71 μg/g increase | 1/3 random subsample: 573/4464 | 2.35 (1.47–3.75) | |||||||
ABI: ankle-brachial blood pressure index; BMI: body mass index; CC: case-control; CI: confidence interval; CO: cohort; CS: cross-sectional; CVD: cardiovascular disease; CHD: coronary heart disease; IHD, ischemic heart disease; MI: myocardial infarction; PAD: peripheral arterial disease; p: percentile; RR: relative risk; SES: socioeconomic status; smk. smoker
RR and/or 95 % CI derived using the results reported in the original study;
Increase in interquartile range;
Increase in interquintile range
The CVD outcomes and methods of ascertainment varied across studies. Most of the prospective studies [26–28••, 29••, 52••], but not all [30••, 31••], used mortality endpoints. Other studies ascertained prevalent cases [58, 59, 62•, 63•], one prospective study measured PAD at the end of follow-up although prevalent PAD at baseline was unknown [30••] and 2 studies used incident cases [29••, 31••]. The studies with mortality endpoints used death certificates from national or local registers [26–28••, 29••, 52••]. The only prospective study with incident cases of CVD, CHD, stroke and HF used annual mortality and morbidity surveillance reviews of hospitalization and death records and adjudication of events by a panel of physicians [29••]. With respect to studies using prevalent endpoints, CVD, stroke and HF were solely based on self-report [59, 60•, 63•] and CHD was assessed by self-report [60•, 63•] or by applying established criteria based on ECG measurements [58]. PAD was assessed with standard criteria based on the ankle-blood pressure index [30••, 31••, 62•].
Overall, all the studies fulfilled most quality criteria (Online Resource 1). Five studies used objective diagnostic criteria [29••, 30••, 31••, 58, 62•]. Most studies collected information on cardiovascular risk factors, and all, except one [52••], adjusted for established cardiovascular risk factors in addition to age, sex and smoking. Cross-sectional studies were based on prevalent cases and interviewers were blinded to exposure status. All cross-sectional studies reported an overall response rate of at least 70% [64, 65].
In prospective studies, cadmium was associated with CVD, CHD, stroke, HF and PAD in 5 (out of 5, although the association in one study [26] was borderline statistically significant), 2 (out of 4), 2 (out of 3), 1 (out of 1) and 2 (out of 2) studies, respectively (Table 1). In cross-sectional studies, cadmium was associated with CVD, CHD, stroke, HF and PAD in 1 (out of 1), 3 (out of 3), 1 (out of 1), 1 (out of 1) and 1 (out of 1) studies, respectively (Table 1).
The pooled relative risk estimates comparing the highest to lowest cadmium exposure categories were 1.36 for CVD (95 % CI: 1.11, 1.66; p-heterogeneity 0.01; I2 65.0 %), 1.30 for CHD (95 % CI: 1.12, 1.52; p-heterogeneity 0.02; I2 61.4 %), 1.18 for stroke (95%CI; 0.86, 1.59; p-heterogeneity 0.006; I2 72.5 %), and 1.49 for PAD (95 % CI: 1.16, 1.93; p-heterogeneity 0.43; I2 0.0 %) (Table 2). In meta-analysis pooling by study design, the corresponding relative risks were 1.23 (95%CI 1.05, 1.44; p-heterogeneity 0.14; I2 42.8%) and 1.21 (95%CI 1.07, 1.37; p-heterogeneity 0.37; I2 3.7 %), for CVD and CHD, respectively, in prospective studies and 1.56 (95%CI 1.00, 2.45; p-heterogeneity 0.02; I2 83.4 %), for CHD in cross-sectional studies.
Table 2.
Pooled Estimated Relative Risk and 95 % confidence interval
| CVD | CHD | Stroke | PAD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| No. Studies | RR (95 % CI) | I2 (p-value) | No. Studies | RR (95 % CI) | I2 (p-value) | No. Studies | RR (95 % CI) | I2 (p-value) | No. Studies | RR (95 % CI) | I2 (p-value) | |
| Study design | ||||||||||||
| Prospective | 5 [26–28••, 29••, 52••] | 1.23 (1.05, 1.44) | 42.8 (0.14) | 4 [26–28••, 29••] | 1.21 (1.07, 1.37) | 3.7 (0.37) | 3 [26, 29••, 52••] | 1.41 (0.57, 3.50) | 85.7 (0.001) | 2 [30••, 31••] | 1.63 (1.00, 2.66) | 39.3 (0.06) |
| Cross-sectional | 1 [59] | NA | NA | 3 [58, 60•, 63•] | 1.56 (1.00, 2.45) | 83.4 (0.02) | 2 [60•, 63•] | 1.10 (1.01, 1.20) | 0.0 (1.00) | 1 [62•] | NA | NA |
| Biomarker | ||||||||||||
| Blood cadmium | 3 [26, 28••, 59] | 1.41 (1.18, 1.70) | 0.0 (0.57) | 4 [26, 28••, 60•, 63•] | 1.41 (1.19, 1.68) | 18.4 (0.30) | 3 [26, 60•, 63•] | 1.19 (0.93, 1.52) | 42.5 (0.18) | 2 [29••, 30••] | 1.83 (1.33, 2.53) | 0.0 (0.56) |
| Urine cadmium | 6 [26–28••, 29••, 52••, 59] | 1.36 (1.11, 1.66) | 65.0 (0.01) | 6 [26–28••, 29••, 58, 60•] | 1.23 (1.07, 1.40) | 50.4 (0.07) | 4 [26, 29••, 52••, 60•] | 1.22 (0.78, 1.91) | 79.3 (0.002) | 3 [30••, 31••, 62•] | 1.49 (1.15, 1.92) | 0.0 (0.44) |
| Sex | ||||||||||||
| Men | 3 [27, 28••, 29••] | 1.29 (1.12, 1.48) | 35.3 (0.21) | 4 [27, 29••, 58, 63•] | 1.30 (0.97, 1.74) | 27.4 (0.25) | 3 [29••, 60•, 63•] | 1.37 (1.07, 1.75) | 0.0 (0.52) | 2 [31••, 62•] | 2.06 (0.82, 5.17) | 84.9 (0.01) |
| Women | 4 [27, 28••, 29••, 52••] | 1.20 (0.92, 1.56) | 80.5 (0.002) | 4 [27, 29••, 58, 63•] | 1.26 (0.75, 2.12) | 81.4 (0.001) | 4 [29••, 52••, 60•, 63•] | 1.39 (1.06, 1.82) | 25.8 (0.26) | 3 [30••, 31••, 62•] | 1.29 (0.67, 2.46) | 73.4 (0.02) |
| Never smokers | 4 [27, 28••, 29••, 52••] | 1.27 (0.97, 1.67) | 45.7 (0.14) | 2 [29••, 58] | 1.37 (0.88, 2.12) | 63.0 (0.10) | 3 [29••, 52••, 60•] | 1.17 (0.74, 1.87) | 53.7 (0.11) | 2 [31••, 62•] | 1.21 (0.83, 1.75) | 0.0 (0.56) |
| Overall a | 6 [26–28••, 29••, 52••, 59] | 1.36 (1.11, 1.66) | 65.0 (0.01) | 7 [26–28••, 29••, 58, 60•, 63•] | 1.30 (1.12, 1.52) | 61.4 (0.02) | 5 [26, 29••, 52••, 60•, 63•] | 1.18 (0.86, 1.59) | 72.5 (0.006) | 3 [30••, 31••, 62•] | 1.49 (1.15, 1.92) | 0.0 (0.44) |
CI, confidence interval, CVD, cardiovascular disease; CHD, coronary heart disease; PAD, peripheral arterial disease; RR, relative risk; NA, not available
For the overall we kept only one result for one specimen (urine preferred) but for the individual biomarker pooling we used the biomarker if available even if not included in the overall
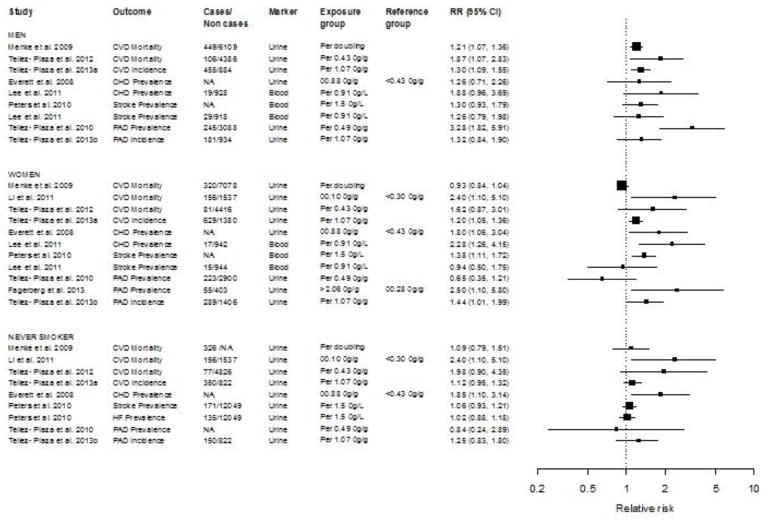
The pooled relative risk for CVD by cadmium biomarker was 1.36 for CVD (95% CI: 1.11, 1.66) for urine cadmium and 1.41 [95% CI: 1.18, 1.70] for blood cadmium. The pooled relative risk for CVD in men, women and never smokers (including both men and women) were 1.29 (95 % CI: 1.12, 1.48; p-heterogeneity 0.21; I2 35.3 %), 1.20 (95 % CI: 0.92, 1.56; p-heterogeneity 0.002; I2 89.5 %) and 1.27 (95 % CI: 0.97, 1.67; p-heterogeneity 0.14; I2 45.7 %), respectively. The relative risk estimates for CVD endpoints ranged from 1.21 [27] to 3.28 [62•] in men, from 0.65 [62•] to 2.50 [30••] in women, and from 0.84 [62•] to 2.40 [52••] in never smokers (Figure 2). In sensitivity analysis excluding prevalent CVD and CHD in NHANES populations with available CVD and CHD endpoints, the results were similar (Online Resource 2). The evaluation of publication bias was limited due to the small number of studies.
Figure 2.
Relative risks for cardiovascular disease endpoints per change in cadmium levels. The area of each black square (individual study) is proportional to the inverse of the variance of the estimated log relative risk. Horizontal lines represent 95 % confidence intervals. CI, confidence interval; CVD, cardiovascular; CHD, coronary heart disease; HF, heart failure; PAD, peripheral arterial disease; RR, relative risk.
DISCUSSION
Cadmium is an established carcinogen [2] also known to cause respiratory, kidney and bone disease in highly polluted environments, including occupational settings [1, 9]. The findings from this systematic review support that cadmium is associated with cardiovascular disease in general populations exposed to low-to-moderate levels of cadmium exposure. The association was similar for both men and women, although it was not significant for women. The association for never smokers was suggestive but not significant. The number of studies with stroke, HF and PAD endpoints was small and more studies are needed, especially prospective studies ascertaining incident outcomes.
Cadmium metabolism and biomarkers
Cadmium is incorporated into the body through the respiratory and digestive tracts using transporters for essential divalent metals (e.g., zinc, iron, manganese, and calcium) such as the zinc transporter Zrt, Irt-like member 8 protein (ZIP-8) and divalent metal transporter (DMT-1) [9, 66]. In blood, cadmium is transported bound mainly to metallothioneins (MTs), which are proteins with a high heavy-metal-binding capacity [13] that have been associated with protection against toxic metals [13]. In the kidney, filtered cadmium-MT compounds are transported into the proximal tubule cells, where they bioaccumulate [9]. Urine cadmium reflects kidney cadmium [67] and, consequently, has been considered a biomarker of cumulative body burden. Recent studies, however, have shown that urine cadmium levels are also related to physiological changes [68] and there are some concerns about the usefulness of urine cadmium as a biomarker of long-term cadmium exposure in populations exposed to low-moderate cadmium levels [69]. Blood cadmium is more dependent on daily fluctuations in exposure and has been considered a marker of ongoing exposure. However, in cadmium-exposed workers, the half-life of blood cadmium showed a fast component (3–4 months) and a slow component (10 years) supporting the hypothesis that, after long-term exposure, blood cadmium levels may also reflect the body’s burden of cadmium [24]. In our meta-analysis, we separately combined studies using blood and urine cadmium biomarkers, with similar results. Altogether, these results support that both biomarkers can reflect cumulative exposure.
Cadmium and clinical CVD endpoints
The findings from our meta-analysis indicate statistically significantly higher risk with higher cadmium levels for all clinical cardiovascular endpoints, except for stroke. Overall, this is supportive evidence that cadmium is a cardiovascular risk factor. Half of the studies included in the meta-analysis were conducted in the National Health and Nutrition Examination Survey (NHANES). NHANES is a major program of the U.S. National Center for Health Statistics. A complex, multistage, probability sampling design was used to select participants that are representative of the civilian non-institutionalized U.S. population [64]. The geometric means of urine cadmium were 0.28 and 0.40 μ/g in men and women, respectively, in NHANES III (1988 – 1994) and 0.22 and 0.34 μ/g in men and women, respectively, in NHANES 1999 – 2004. In NHANES III, Everett et al. evaluated prevalent CHD [58], whereas Menke et al. evaluated CHD mortality [27]. Similarly, in NHANES 1999+, Awargal et al. and Peters et al. evaluated self-reported CVD [59] and CHD [60•] prevalence, respectively, whereas Tellez-Plaza et al. evaluated CVD and CHD mortality [28••]. The pooled relative risk was similar with and without inclusion of NHANES prevalent outcomes, although heterogeneity was substantially lower when only mortality outcomes were considered.
Studies conducted in Korea [63•] (blood cadmium geometric mean 1.53 μg/L), Japan [52••] (urine cadmium geometric mean in women 7.2 μg/g), Sweden [30••] (urine cadmium geometric mean in women 0.36 μg/g creatinine), Belgium [26] (urine cadmium geometric mean at baseline 0.98 μg/24hr) and 13 American Indian communities in the US [29••, 31••] (urine cadmium geometric mean 0.92 μg/g), also supported cadmium-related cardiovascular effects. In the study from Belgium [26], however, blood cadmium was borderline associated with increased cardiovascular deaths (HR= 1.29 [95%CI 0.99–1.67]), but the association with urine cadmium was not statistically significant (HR=1.11 [95%CI 0.89–1.38]).
For descriptive purposes we estimated combined relative risks for stroke and PAD, although the small number of studies and the heterogeneity regarding study design and outcome definition limits the interpretation of these associations across studies. In our meta-analysis we found no association with stroke although only 5 studies met the inclusion criteria [26, 29••, 52••, 60•, 63•]. For PAD, our meta-analysis relied only on 3 studies [30••, 31••, 62•]. The association, however, was relatively strong and significant. One study from a Belgium population reported an association of blood, but not urine, cadmium with PAD [70]. The point estimates and associated confidence intervals, however, were not reported and we were thus unable to include this study in our meta-analysis.
Only two studies reported associations with HF (Peters et al. [60•] and Tellez-Plaza et al. [29••]). Moreover, the only studies that prospectively evaluated incident cases of stroke and HF [29••] and PAD [31••] found statistically significant positive associations with urine cadmium. More studies evaluating not only fatal but also non-fatal events of stroke, HF and PAD are needed.
Finally, our search strategy retrieved some occupational studies evaluating clinical CVD endpoints [71–74], although they did not meet inclusion criteria because they did not use biomarkers of cadmium exposure and did not account for potential confounding introduced by age, sex or smoking. In most of the studies, cadmium exposure was not associated with CVD [72–74]. One study from England, however, reported a borderline statistically significant excess of cerebrovascular disease mortality [71]. The healthy worker effect, uncertainties in exposure and outcome assessment, the lack of adjustment for relevant confounders and likely exposures to multiple toxicants, limit the interpretability of those results.
Cadmium and CVD: differences by sex
We evaluated pooled associations separately in men and women because women have higher cadmium levels than men and some [25, 27, 58, 62•, 63•, 75–77], but not all [18, 26, 60•, 78], epidemiologic studies have found differences in health outcomes by sex. Based on data from cadmium-polluted areas from Japan, where women showed increased mortality and incidence of kidney and bone disease [25, 76, 79–82], it has been hypothesized that women are more susceptible to cadmium health effects [77]. Ten articles in our systematic review reported results separately for men and women [27, 28••, 29••, 30••, 31••, 52••, 58, 60•, 62•, 63•]. In men, all the studies consistently reported positive association of cadmium and CVD endpoints [27, 28••, 29••, 31••, 58, 60•, 62•, 63•]. In women 3 studies reported inverse associations [27, 62•, 63•]. In NHANES III (1988–1994), the HR (95%CI) of CVD and CHD mortality in women were 0.93 (0.84, 1.04) and 0.45 (0.24, 0.83), respectively, per doubling of urine cadmium [27]. In NHANES 1999–2004, there were no sex differences for cadmium-related CVD or CHD mortality [28••] but there were differences for PAD among never smoking women [62•]. The prevalence of stroke was associated with cadmium exposure in men, but not women, in participants of the Korean NHANES [63•].
It is possible that different cardiovascular endpoints show sex differences at different concentrations of exposure, different sources, routes and patterns of cadmium exposure and differential residual confounding for smokers versus non-smokers. It is also possible that published sex-differences across studies and cardiovascular endpoints are due to random sampling variability. In our meta-analysis, we obtained similar pooled relative risks for men and women supporting that there is no effect modification by sex in cadmium-related cardiovascular effects. Findings of sex differences on cadmium-related cardiovascular end-points must be interpreted carefully given the conflicting literature.
Cadmium, smoking and CVD
Smoking is a major cardiovascular risk factor [83] and an important determinant of cadmium exposure [9, 84]. In the US, indeed, changes in the prevalence of smoking status, cumulative dose, and recent dose have played an important role in the decline of urine cadmium concentrations in the U.S. population, benefiting both smokers and never smokers [84]. In our systematic review, it was important to include adjustment for smoking as inclusion criteria because residual confounding by smoking is a typical concern in epidemiologic studies assessing cadmium-related cardiovascular effects. Smoking status and pack-years are usually defined by self-report and information on serum cotinine levels (an objective biomarker of recent exposure to tobacco smoke) is rarely available. In 3 studies [27, 28••, 62•] that adjusted for pack-years and serum cotinine in addition to smoking status, the associations between cadmium and cardiovascular outcomes remained after adjustment. Nonetheless, positive residual confounding due to misreport of cumulative smoking and smoking status is still possible, among both never and ever smokers.
An additional strategy to evaluate confounding by smoking is to perform separate analyses in never smokers. We estimated combined relative risks from 8 studies from the US [27, 28••, 29••, 31••, 58, 60•, 62•] and 1 study from Japan [52••] reporting cadmium-related cardiovascular associations in never smokers. Only 2 studies reported associations of cadmium and CHD in never smokers. Overall, the magnitude of the combined relative risk for cadmium-related CVD in never smokers was similar compared to the overall combined relative risk, but only borderline statistically significant. These findings are consistent with a potential cardiovascular effect of cadmium independent of exposure to tobacco smoke, but need confirmation in larger studies among non-smokers. Among the retrieved papers 7 [27, 28••, 29••, 31••, 52••, 58, 60•] of 8, showed positive associations between cadmium and clinical cardiovascular disease, although only 2 [52••, 58] of them were statistically significant. The association between cadmium and CVD among never smokers could also be affected by negative confounding since intake of contaminated vegetables is a major source of cadmium exposure among never smokers [9]. Altogether, there is supportive evidence that cadmium is a cardiovascular risk factor among never smokers, but this evidence is not conclusive. More studies reporting results among never smokers are needed.
Cadmium and CVD risk factors
In addition to clinical cardiovascular outcomes, chronic exposure to cadmium has also been associated with CVD risk factors and with subclinical endpoints. Increasing evidence supports that cadmium may play a role in the development of a number of traditional CVD risk factors, including hypertension [16, 63•, 78, 85] and chronic kidney disease [18, 79, 86], which could mediate in part the cardiovascular effects of cadmium. The association with diabetes is inconsistent across studies [86–88]. In 195 young women from Austria, cumulative cadmium exposure was associated with increased prevalence of elevated intima media thickness (OR 1.6 [95% CI: 1.1, 2.3] per standard deviation unit increase in serum cadmium levels) [11]. In 465 women older than 65 years from Sweden, urine cadmium >0.76 μg/g versus <0.18 μg/g was associated with elevated carotid plaque (OR 2.7 [95% CI: 1.2, 6.1] [89••]. In the same Swedish population, urine but not blood cadmium was related to markers of endothelial dysfunction and vascular inflammation such as intercellular adhesion molecule-1 (ICAM-1). [30••] The relevance of cadmium-related changes in markers of endothelial dysfunction and inflammation to CVD endpoints remains unclear.
Genetic studies and cadmium risk
A novel area of research is the evaluation of genetic loci related to cadmium levels in the human body and cadmium internal dose. For instance women could be genetically predisposed to have higher long-life cadmium levels for a given level of exposure. The heritability of blood cadmium was 65% in Swedish non-smoking same-sex female twins compared to only 13% among non-smoking male twins [90]. In 2,926 adult twins living in Australia, suggestive linkage peaks related to red blood cell cadmium levels were found in chromosomes 2, 18, 20, and X [91]. One polymorphism in the transferrin receptor gene was consistently associated to urine cadmium levels in non smoking women from Argentina (N=172) and Bangladesh (N=359)[92]. In 370 individuals from Thailand, polymorphisms of the glutathione S-transferases were associated to blood cadmium concentrations [93]. In 2 studies from Turkey, polymorphisms of the MT2A gene was associated to blood [94] and autopsy kidney cadmium levels [95].
A limited number of studies [96] have evaluated the role of genetic polymorphisms and susceptibility to cadmium health effects. As MTs bind metals and have been associated with protection against cadmium [9], MTs related polymorphisms could determine susceptibility to cadmium. Indeed, a polymorphism in metallothionein 1A (MT1A) was associated with cadmium-related excretion of urinary beta 2-microglobulin, a marker of cadmium-related renal damage [96]. Polymorphisms in other genes, in addition to MTs, may also modulate cadmium health effects. Co-exposure to other metals could also modify CVD risk in the presence of cadmium, but a systematic evaluation of these factors, including newly developed statistical methods for the evaluation and display of multiple join effects, is needed.
An emerging area of research is the potential mediating role of aberrant DNA methylation, histone modifications and changes in microRNA expression [23] and endocrine disruption pathways [20, 22] in the cardiovascular toxicity of cadmium. High quality experimental and epidemiological studies are needed to evaluate the contribution of cadmium exposure to epigenetic modifications and their potential mediation to CVD development. Correspondingly, appropriate causal inference statistical frameworks for assessing mediation need to be developed. Finally, DNA methylation alterations, which result in changes in gene expression, are mitotically and meiotically heritable [97]. Given that cadmium has been associated with DNA methylation alterations [23] and the fact that reproductive tissues are target organs for cadmium exposure [9], parental cadmium exposure burden may be heritable across generations. However, in order to evaluate trans-generational effects of cadmium exposure, large-scale family studies with complex pedigrees are needed.
Conclusions
This systematic review and meta-analysis strengthens the evidence in support of chronic cadmium exposure as an independent risk factor for CVD, especially CHD. Given the widespread exposure to cadmium and the few available prospective studies investigating incident outcomes at lower exposure levels, additional prospective studies with long follow-up, including individual-level exposure assessment and standardized CVD outcomes, and appropriately accounting for confounding by smoking, are needed to establish a causal effect of chronic cadmium exposure on cardiovascular disease. Understanding the role of cadmium in cardiovascular disease at the population level could substantially improve cardiovascular health as cadmium exposure can be monitored and controlled. In addition to preventive strategies in high-risk patients, which are common in clinical practice, population-based preventive strategies, such as promoting public and private smoke-free environments, reviewing food safety policies and cadmium safety standards, and limiting cadmium industrial releases into the environment, may reduce cadmium-related cardiovascular disease in the population.
Supplementary Material
Acknowledgments
This research is supported by grants from the US National Heart Lung and Blood Institute (R01HL090863) and the US National Institute of Environmental Health Sciences (R01ES021367). M.T.-P. and A.D.L. were supported by a Miguel Servet grant from the Funds for Research in Health Sciences, Ministry of Economy and Competitiveness, Spain (CP12/03080). M.R.J. was also supported by the Cardiovascular Epidemiology Institutional Training from the National Heart, Lung and Blood Institute (T32HL007024).
Abbreviations
- CI
confidence interval
- CHD
Coronary Heart Disease
- CVD
Cardiovascular Disease
- PAD
Peripheral Arterial Disease
- HF
Heart Failure
Footnotes
Conflict of Interest:
Maria Tellez-Plaza, Miranda R Jones, Alejandro Dominguez-Lucas, Eliseo Guallarm and Ana Navas-Acien declare that they have no conflicto of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Epidemiologic studies of particular interest published from 2010 have been highlighted as:
• Of importance
•• Of major importance
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed July 2013];Toxicological Profile for Cadmium. 2012 Available at: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15] [PubMed]
- 2.International Agency for Research on Cancer (IARC) [Accessed July 2013];Berillium, cadmium, mercury, and exposures in the glass manfactury industry. 1993 Available at: http://monographs.iarc.fr/ENG/Monographs/vol58/
- 3.U.S. Geological Survey. [Accessed July 2013];Cadmium statistics and information. 2013 Available at: http://minerals.usgs.gov/minerals/pubs/commodity/cadmium/index.html-myb.
- 4.Lalor GC. Review of cadmium transfers from soil to humans and its health effects and Jamaican environment. The Science of the total environment. 2008;400(1–3):162–172. doi: 10.1016/j.scitotenv.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Vyncke G, Lauwerys RR, et al. Transfer of cadmium from a sandy acidic soil to man: a population study. Environmental research. 1992;58(1):25–34. doi: 10.1016/s0013-9351(05)80202-6. [DOI] [PubMed] [Google Scholar]
- 6.DalCorso G, Farinati S, Maistri S, Furini A. How plants cope with cadmium: staking all on metabolism and gene expression. Journal of integrative plant biology. 2008;50(10):1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 7.Golia EE, Dimirkou A, Mitsios IK. Heavy-metal concentration in tobacco leaves in relation to their available soil fractions. Commun Soil Sci Plant Anal. 2009;40(1–6):106–120. [Google Scholar]
- 8.Hogervorst J, Plusquin M, Vangronsveld J, et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environmental research. 2007;103(1):30–37. doi: 10.1016/j.envres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Nordberg GF, Nogawa K, Nordberg M, Friberg L. Cadmium. In: Nordberg GF, Fowler BF, Nordberg M, Friberg L, editors. Handbook on the toxicology of metals. Amsterdam: Elsevier; 2007. pp. 445–486. [Google Scholar]
- 10.Yassin AS, Martonik JF. Urinary cadmium levels in the U S working population, 1988–1994. Journal of occupational and environmental hygiene. 2004;1(5):324–333. doi: 10.1080/15459620490445499. [DOI] [PubMed] [Google Scholar]
- 11.Messner B, Knoflach M, Seubert A, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(9):1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current medicinal chemistry. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 13.Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19(4–5):529–535. [PubMed] [Google Scholar]
- 14.Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem : a European journal of chemical biology. 2009;10(1):55–62. doi: 10.1002/cbic.200800511. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environmental health perspectives. 2010;118(12):1676–1684. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caciari T, Sancini A, Fioravanti M, et al. Cadmium and hypertension in exposed workers: A meta-analysis. International journal of occupational medicine and environmental health. 2013 doi: 10.2478/s13382-013-0111-5. [DOI] [PubMed] [Google Scholar]
- 17.Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: role for cadmium, p450 and heme oxygenases? The Tohoku journal of experimental medicine. 2006;208(3):179–202. doi: 10.1620/tjem.208.179. [DOI] [PubMed] [Google Scholar]
- 18.Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. American journal of epidemiology. 2009;170(9):1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwangbo Y, Weaver VM, Tellez-Plaza M, Guallar E, Lee BK, Navas-Acien A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environmental health perspectives. 2011;119(12):1800–1805. doi: 10.1289/ehp.1003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14(4):545–553. doi: 10.1210/mend.14.4.0441. [DOI] [PubMed] [Google Scholar]
- 21.Fechner P, Damdimopoulou P, Gauglitz G. Biosensors paving the way to understanding the interaction between cadmium and the estrogen receptor alpha. PloS one. 2011;6(8):e23048. doi: 10.1371/journal.pone.0023048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluxen FM, Hofer N, Kretzschmar G, Degen GH, Diel P. Cadmium modulates expression of aryl hydrocarbon receptor-associated genes in rat uterus by interaction with the estrogen receptor. Archives of toxicology. 2012;86(4):591–601. doi: 10.1007/s00204-011-0787-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Current medicinal chemistry. 2012;19(16):2611–2620. doi: 10.2174/092986712800492913. [DOI] [PubMed] [Google Scholar]
- 24.Jarup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellstrom T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scandinavian journal of work, environment & health. 1983;9(4):327–331. doi: 10.5271/sjweh.2404. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa H, Nishijo M, Morikawa Y, et al. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environmental research. 2006;100(3):323–329. doi: 10.1016/j.envres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Nawrot TS, Van Hecke E, Thijs L, et al. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environmental health perspectives. 2008;116(12):1620–1628. doi: 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environmental health perspectives. 2009;117(2):190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environmental health perspectives. 2012;120(7):1017–1022. doi: 10.1289/ehp.1104352. This is a large prospective study representative of the general US population from 1999–2004 with 8 yr of follow-up. After adjusting for sociodemographic and CVD risk factors, including smoking status, recent smoking dose, and cumulative smoking dose increasing cadmium exposure was associated with cardiovascular disease, heart disease and coronary heart disease mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Tellez-Plaza M, Guallar E, Howard BV, et al. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology. 2013;24(3):421–429. doi: 10.1097/EDE.0b013e31828b0631. This is a large prospective study in American Indian communities from the US with 20 years of follow-up. Cadmium exposure was moderately to strongly associated with incident cardiovascular disease, coronary heart disease, stroke and heart failure. The associations were similar in never smokers. The end-points were ascertained by annual mortality and morbidity reviews of hospitalization and death records and at two research clinic visits. This is the first epidemiologic study evaluating incident cardiovascular outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Fagerberg B, Bergstrom G, Boren J, Barregard L. Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: a cohort and an experimental study. BMJ open. 2013;3(3) doi: 10.1136/bmjopen-2012-002489. This is a prospective study of 64 year-old women from Sweden, with 6 yr of follow-up. In this study baseline cadmium exposure was associated with peripheral arterial disease measured at the end of the follow-up. The association appeared to be non-lineal, although the number of cases at the lower range of cadmium levels was limited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Tellez-Plaza M, Guallar E, Fabsitz RR, et al. Cadmium Exposure and Incident Peripheral Arterial Disease. Submitted. This is a large prospective study in American Indian communities from the US followed up to 10 years through 3 examination visits. Cadmium exposure was associated with incident peripheral arterial disease after adjusting for cardiovascular risk factors, including smoking status, and cumulative smoking dose. The associations was also observed in never smokers, although it was not significant. [Google Scholar]
- 32.Afridi HI, Kazi TG, Kazi GH, Jamali MK, Shar GQ. Essential trace and toxic element distribution in the scalp hair of Pakistani myocardial infarction patients and controls. Biological trace element research. 2006;113(1):19–34. doi: 10.1385/BTER:113:3. [DOI] [PubMed] [Google Scholar]
- 33.Afridi HI, Kazi TG, Kazi N, et al. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biological trace element research. 2010;134(1):1–12. doi: 10.1007/s12011-009-8450-6. [DOI] [PubMed] [Google Scholar]
- 34.Afridi HI, Kazi TG, Kazi N, et al. Interactions between cadmium and zinc in the biological samples of Pakistani smokers and nonsmokers cardiovascular disease patients. Biological trace element research. 2011;139(3):257–268. doi: 10.1007/s12011-009-8607-3. [DOI] [PubMed] [Google Scholar]
- 35.Arslan C, Altan H, Akgun OO, et al. Trace elements and toxic heavy metals play a role in Buerger disease and atherosclerotic peripheral arterial occlusive disease. International angiology : a journal of the International Union of Angiology. 2010;29(6):489–495. [PubMed] [Google Scholar]
- 36.Liu JH. Case control study on relationship between serum lead, cadmium and coronary heart disease. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases. 2008;26(11):679. [PubMed] [Google Scholar]
- 37.Tang YR, Zhang SQ, Xiong Y, et al. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biological trace element research. 2003;92(2):97–104. doi: 10.1385/BTER:92:2:97. [DOI] [PubMed] [Google Scholar]
- 38.Tsai JL, Horng PH, Hwang TJ, Hsu JW, Horng CJ. Determination of urinary trace elements (arsenic, copper, cadmium, manganese, lead, zinc, selenium) in patients with Blackfoot disease. Archives of environmental health. 2004;59(12):686–692. doi: 10.1080/00039890409602954. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Xu Y, Pan S, et al. Long-term heavy metal pollution and mortality in a Chinese population: an ecologic study. Biological trace element research. 2011;142(3):362–379. doi: 10.1007/s12011-010-8802-2. [DOI] [PubMed] [Google Scholar]
- 40.Morgan JM. Tissue cadmium concentration in man. Archives of internal medicine. 1969;123(4):405–408. [PubMed] [Google Scholar]
- 41.Adamska-Dyniewska H, Bala T, Florczak H, Trojanowska B. Blood cadmium in healthy subjects and in patients with cardiovascular diseases. Cor et vasa. 1982;24(6):441–447. [PubMed] [Google Scholar]
- 42.Adamska-Dyniewska H, Kawecka M. Blood cadmium level in the acute phase of myocardial infarction. Polskie Archiwum Medycyny Wewnetrznej. 1983;69(1):9–14. [PubMed] [Google Scholar]
- 43.Ponteva M, Elomaa I, Backman H, Hansson L, Kilpio J. Blood cadmium and plasma zinc measurements in acute myocardial infarction. European journal of cardiology. 1979;9(5):379–391. [PubMed] [Google Scholar]
- 44.Rong YZ. Clinical significance of blood zinc, copper, cadmium and magnesium determinations in acute myocardial infarction. Zhonghua xin xue guan bing za zhi. 1983;11(4):256–259. [PubMed] [Google Scholar]
- 45.Scott R, Aughey E, Reilly M, Cunningham C, McClelland A, Fell GS. Renal cadmium content in the West of Scotland. Urological research. 1983;11(6):285–290. doi: 10.1007/BF00256348. [DOI] [PubMed] [Google Scholar]
- 46.Smetana R, Glogar D, Weidinger F, Meisinger V. Heavy metal and trace element deviations. A comparison of idiopathic dilated cardiomyopathy and coronary heart disease. Wien Med Wochenschr. 1987;137(23):553–557. [PubMed] [Google Scholar]
- 47.Sun RX, Su YX, Sun JH. Determination of trace elements in cerebrospinal fluid (CSF) of patients suffering cerebrovascular disease by atomic absorption spectrometry. Guang pu xue yu guang pu fen xi = Guang pu. 2006;26(4):720–722. [PubMed] [Google Scholar]
- 48.Voors AW, Shuman MS, Johnson WD. Additive statistical effects of cadmium and lead on heart-related disease in a North Carolina autopsy series. Archives of environmental health. 1982;37(2):98–102. doi: 10.1080/00039896.1982.10667544. [DOI] [PubMed] [Google Scholar]
- 49.Adamska-Dyniewska H, Bala T, Florczak H, Trojanowska B, Trzcinka M. Blood cadmium level in coronary disease depending on the risk factors. Kardiologia polska. 1980;23(6):467–473. [PubMed] [Google Scholar]
- 50.Adamska-Dyniewska H, Bala T, Trojanowska B, Trzcinka M. Blood cadmium level in chronic circulatory insufficiency. Pol Tyg Lek. 1980;35(31):1173–1175. [PubMed] [Google Scholar]
- 51.Voors AW, Shuman MS, Gallagher PN. Atherosclerosis and hypertension in relation to some trace elements in tissues-1. World review of nutrition and dietetics. 1975;20:299–326. doi: 10.1159/000396067. [DOI] [PubMed] [Google Scholar]
- 52••.Li Q, Nishijo M, Nakagawa H, et al. Relationship between urinary cadmium and mortality in habitants of a cadmium-polluted area: a 22-year follow-up study in Japan. Chinese medical journal. 2011;124(21):3504–3509. This is a large prospective study of Japanese inhabitants of a cadmium-polluted area, with 22 yr follow-up. In this study, cadmium exposure as measured in urine was associated with cardiovascular disease mortality in both men and women, and with stroke mortality only in women. A limitation of this study is the lack of adjustment for smoking and other relevant cardiovascular risk factors. An additional limitation is that the number of cardiovascular deaths is limited, specially for women. [PubMed] [Google Scholar]
- 53.Ferraro PM, Sturniolo A, Naticchia A, D’Alonzo S, Gambaro G. Temporal trend of cadmium exposure in the United States population suggests gender specificities. Internal medicine journal. 2012;42(6):691–697. doi: 10.1111/j.1445-5994.2011.02627.x. [DOI] [PubMed] [Google Scholar]
- 54.Guallar E, Silbergeld EK, Navas-Acien A, et al. Confounding of the relation between homocysteine and peripheral arterial disease by lead, cadmium, and renal function. American journal of epidemiology. 2006;163(8):700–708. doi: 10.1093/aje/kwj090. [DOI] [PubMed] [Google Scholar]
- 55.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 56.Ju YR, Chen WY, Liao CM. Assessing human exposure risk to cadmium through inhalation and seafood consumption. Journal of hazardous materials. 2012;227–228:353–361. doi: 10.1016/j.jhazmat.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 57.Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA : the journal of the American Medical Association. 1988;260(5):652–656. [PubMed] [Google Scholar]
- 58.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environmental research. 2008;106(2):284–286. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62(5):422–429. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 60•.Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environmental research. 2010;110(2):199–206. doi: 10.1016/j.envres.2009.12.004. This is a large crossectional study conducted in a representative sample of the US general population in 1999–2006. In this study cadmium exposure was associated with the prevalence of self-reported myocardial infarction, stroke and heart failure, after adjustment for cardiovascular risk factors including smoking status but not cumulative smoking dose and recent smoking dose. The associations were similar for men and women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 62•.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. American journal of epidemiology. 2010;172(6):671–681. doi: 10.1093/aje/kwq172. This is a large crossectional study conducted in a representative sample of the US general population in 1999–2004. In this study higher blood and urine cadmium levels were associated with increased prevalence of PAD, but women never smokers showed a U-shaped relation with increased prevalence of PAD at very low cadmium levels. The models adjusted for sociodemographic and CVD risk factors, including smoking status, recent smoking dose, and cumulative smoking dose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Lee MS, Park SK, Hu H, Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environmental research. 2011;111(1):171–176. doi: 10.1016/j.envres.2010.10.006. This is a crossectional study conducted in a representative sample of the Korean general population in 2005. Blood cadmium was associated with the prevalence of self-reported ischemic heart disease among men and women, and with the prevalence of self-reported stroke only in men. The models were adjusted for sociodemographic and CVD risk factors, including smoking status. The number of prevalent cardiovascular cases, however, was relatively low. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Center for Health Statistics. [Accessed July 2013];National Health and Nutrition Examination Survey. 2013 Available at: http://www.cdc.gov/nchs/nhanes.htm.
- 65.Korean Centers for Disease Control and Prevention. [Accessed July 2013];Korean National Health and Nutrition Examination Survey (KHANES) 2013 Available at: http://knhanes.cdc.go.kr/knhanes/index.do.
- 66.Himeno S, Yanagiya T, Fujishiro H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie. 2009;91(10):1218–1222. doi: 10.1016/j.biochi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Akerstrom M, Barregard L, Lundh T, Sallsten G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicology and applied pharmacology. 2013;268(3):286–293. doi: 10.1016/j.taap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Akerstrom M, Sallsten G, Lundh T, Barregard L. Associations between urinary excretion of cadmium and proteins in a nonsmoking population: renal toxicity or normal physiology? Environmental health perspectives. 2013;121(2):187–191. doi: 10.1289/ehp.1205418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akerstrom M, Lundh T, Barregard L, Sallsten G. Sampling of urinary cadmium: differences between 24-h urine and overnight spot urine sampling, and impact of adjustment for dilution. International archives of occupational and environmental health. 2012;85(2):189–196. doi: 10.1007/s00420-011-0658-z. [DOI] [PubMed] [Google Scholar]
- 70.Plusquin M, Nawrot TS, Staessen JA. Peripheral arterial disease and metals in urine and blood. Environmental health perspectives. 2005;113(8):A510–511. doi: 10.1289/ehp.113-a510b. author reply A511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elliott P, Arnold R, Cockings S, et al. Risk of mortality, cancer incidence, and stroke in a population potentially exposed to cadmium. Occupational and environmental medicine. 2000;57(2):94–97. doi: 10.1136/oem.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorahan T, Waterhouse JA. Mortality study of nickel-cadmium battery workers by the method of regression models in life tables. British journal of industrial medicine. 1983;40(3):293–300. doi: 10.1136/oem.40.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elinder CG, Kjellstrom T, Hogstedt C, Andersson K, Spang G. Cancer mortality of cadmium workers. British journal of industrial medicine. 1985;42(10):651–655. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kazantzis G, Lam TH, Sullivan KR. Mortality of cadmium-exposed workers. A five-year update Scandinavian journal of work, environment & health. 1988;14(4):220–223. doi: 10.5271/sjweh.1929. [DOI] [PubMed] [Google Scholar]
- 75.Ishihara T, Kobayashi E, Okubo Y, et al. Association between cadmium concentration in rice and mortality in the Jinzu River basin, Japan. Toxicology. 2001;163(1):23–28. doi: 10.1016/s0300-483x(01)00367-5. [DOI] [PubMed] [Google Scholar]
- 76.Nishijo M, Satarug S, Honda R, Tsuritani I, Aoshima K. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Molecular and cellular biochemistry. 2004;255(1–2):87–92. doi: 10.1023/b:mcbi.0000007264.37170.39. [DOI] [PubMed] [Google Scholar]
- 77.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environmental research. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environmental health perspectives. 2008;116(1):51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellstrom L, Elinder CG, Dahlberg B, et al. Cadmium exposure and end-stage renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;38(5):1001–1008. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- 80.Kasuya M. Recent epidemiologial studies on Itai-itai disease as a chronic cadmium poisoning in Japan. Water Science and Technology. 2000;42:147–155. [Google Scholar]
- 81.Staessen JA, Roels HA, Emelianov D, et al. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet. 1999;353(9159):1140–1144. doi: 10.1016/s0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- 82.Chen X, Zhu G, Jin T, et al. Changes in bone mineral density 10 years after marked reduction of cadmium exposure in a Chinese population. Environmental research. 2009;109(7):874–879. doi: 10.1016/j.envres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 83.US Department of Health and Human Services. A report of the surgeon general: how tobacco smoke causes disease. [Accessed July 2013];The biology and behavioral basis for smoking-attributable disease. 2010 Available at: http://www.surgeongeneral.gov/library/reports/tobaccosmoke/index.html. [PubMed]
- 84.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environmental health perspectives. 2012;120(2):204–209. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environmental research. 2010;110(6):612–616. doi: 10.1016/j.envres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Punta B, Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environmental research. 2012;112:194–198. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes care. 2003;26(2):468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 88.Barregard L, Bergstrom G, Fagerberg B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environmental research. 2013;121:104–109. doi: 10.1016/j.envres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 89••.Fagerberg B, Bergstrom G, Boren J, Barregard L. Cadmium exposure is accompanied by increased prevalence and future growth of atherosclerotic plaques in 64-year-old women. Journal of internal medicine. 2012;272(6):601–610. doi: 10.1111/j.1365-2796.2012.02578.x. In this study of 64 year old women from Sweden, with over 6 yr of follow-up, cadmium exposure was cross-sectionally and prospectively associated with the presence of carotide plaque, after adjusting for cardiovascular risk factors including cumulative smoking dose. Cadmium exposure at baseline was also positively associated with the change in carotid plaque area at the end of the follow-up. Cadmium-related subclinical atherosclerosis is especially relevant for clinical cardiovascular disease prevention. [DOI] [PubMed] [Google Scholar]
- 90.Bjorkman L, Vahter M, Pedersen NL. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environmental health perspectives. 2000;108(8):719–722. doi: 10.1289/ehp.108-1638287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitfield JB, Dy V, McQuilty R, et al. Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium, and zinc in erythrocytes. Environmental health perspectives. 2010;118(6):776–782. doi: 10.1289/ehp.0901541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rentschler G, Kippler M, Axmon A, et al. Polymorphisms in iron homeostasis genes and urinary cadmium concentrations among nonsmoking women in Argentina and Bangladesh. Environmental health perspectives. 2013;121(4):467–472. 472e461–467. doi: 10.1289/ehp.1205672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khansakorn N, Wongwit W, Tharnpoophasiam P, et al. Genetic variations of glutathione s-transferase influence on blood cadmium concentration. Journal of toxicology. 2012;2012:356126. doi: 10.1155/2012/356126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kayaalti Z, Aliyev V, Soylemezoglu T. The potential effect of metallothionein 2A -5A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels. Toxicology and applied pharmacology. 2011;256(1):1–7. doi: 10.1016/j.taap.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 95.Kayaalti Z, Mergen G, Soylemezoglu T. Effect of metallothionein core promoter region polymorphism on cadmium, zinc and copper levels in autopsy kidney tissues from a Turkish population. Toxicology and applied pharmacology. 2010;245(2):252–255. doi: 10.1016/j.taap.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Lei L, Chang X, Rentschler G, et al. A polymorphism in metallothionein 1A (MT1A) is associated with cadmium-related excretion of urinary beta 2-microglobulin. Toxicology and applied pharmacology. 2012;265(3):373–379. doi: 10.1016/j.taap.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Feinberg AP. Genome-scale approaches to the epigenetics of common human disease. Virchows Archiv : an international journal of pathology. 2010;456(1):13–21. doi: 10.1007/s00428-009-0847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.