Abstract
Obstructive sleep apnoea (OSA) is a highly prevalent condition with proven neurocognitive and cardiovascular consequences. OSA patients experience repetitive narrowing or collapse of the pharyngeal airway during sleep. Multiple factors likely underlie the pathophysiology of this condition with considerable inter-individual variation. Important risk factors for OSA include obesity, male gender, and ageing. However, the mechanisms underlying these major risk factors are not well understood. We briefly review the state-of-the-art knowledge regarding OSA pathogenesis in adults and highlight the potential role of genetics in influencing key OSA pathophysiological traits.
Keywords: Arousal, genioglossus, lung, obstructive sleep apnoea, upper airway, ventilatory control stability
The obesity pandemic is affecting global health in a variety of ways. One of the major respiratory manifestations of obesity is in the form of obstructive sleep apnoea (OSA). Sleep apnoea is defined by recurrent reductions (hypopnoeas) or stoppages (apnoeas) in breathing during sleep as result of pharyngeal airway narrowing or collapse1. OSA is defined by reduction in airfow in the presence of ongoing respiratory efforts2–4. In contrast, central sleep apnoea is characterized by the absence of respiratory effort during airfow attenuation5. Obstructive apnoea is considerably more common than central apnoea and is the focus of the present manuscript. In OSA, hypoxemia and hypercapnia result from these breathing disruptions with the ultimate result being catecholamine surges and associated hemodynamic consequences6,7. Loud snoring, caused by vibration of pharyngeal tissues, is a classic symptom of OSA8. In most but not all cases the termination of respiratory events is associated with electrocortical arousal from sleep9,10. These repetitive events result in a cyclical breathing pattern and sleep fragmentation as the patient fluctuates between wake and sleep11,12. Severe sleep apnoea patients can experience respiratory events in excess of 100 times per hour with each event, by definition, lasting at least 10 sec.
Symptomatic OSA affects at least 2–4 per cent of the US population13. Prevalence estimates from around the world support similar values14, and numbers are likely to increase with the obesity pandemic15. Sensitive indices of airflow (nasal pressure)16 and the realization that asymptomatic patients may have complications of OSA17 have both contributed to higher estimated prevalence. While non obese individuals may suffer from OSA, obesity is the main epidemiological risk factor. Indeed, increases in body mass index, central accumulation of adipose tissue, and neck circumference are strong predictors of this disease18. Although obesity is a major risk factor for OSA, roughly 30 per cent of patients with obstructive sleep apnoea syndrome are not obese, emphasizing the need for a high index of suspicion in clinical practice. Further, the prevalence of OSA is 2–3 times greater in men than in women and in older compared to middle aged individuals19. Menopause is a well established risk factor for OSA in women20.
OSA can yield major neurocognitive manifestations including excessive daytime sleepiness/fatigue, impaired cognition, reduced quality of life, and an up to seven fold increased risk of road traffic accidents21–24. Treatment of OSA leads to improvements in many of these outcome measures23,25. There is evolving evidence to support the role of OSA as an independent risk factor for adverse cardiovascular sequelae. Although some argue that OSA was simply a marker of an unfit patient group26, rigorous recent studies have shown that OSA is causally linked to a number of important sequelae. OSA is now a well established risk factor for hypertension (both incident and prevalent), stroke and probably myocardial infarction, congestive heart failure and death27–31. OSA has been causally linked to the development of hypertension based on large rigorous cross-sectional and longitudinal epidemiological studies, mechanistic animal studies and most recently interventional trials32–37.
The underlying causes of OSA vary considerably between afflicted individuals. Important components likely include pharyngeal anatomy38,39, pharyngeal dilator muscle responsiveness to respiratory challenges during sleep40–43, the arousal threshold (propensity to wake up from sleep)44,45, the instability of the negative feedback control system regulating ventilation (loop gain)46–48, and upper airway tethering via caudal traction from changes in end-expiratory lung volume (EELV)49–52. These various physiological traits and the potential for each to influence OSA have been described in detail elsewhere53. The focus of the current article will be to review the key pathophysiological factors and their interactions, to highlight recent innovations in our understanding of OSA pathogenesis, and to summarize the existing literature regarding the genetics of sleep apnoea3.
Pathophysiology
Anatomical and biomechanical factors
The evolution of speech in man, which demanded considerable laryngeal motility, yielded the human upper airway vulnerable to collapse based on its reliance on muscles and soft tissues to maintain pharyngeal patency. The anatomy and neural control of the upper airway has evolved to facilitate multiple functions including speech, swallowing and ventilation. Most notably, the upper airway is vulnerable to collapse throughout its length from the hard palate to the larynx54.
People with sleep apnoea have a smaller pharyngeal airway than do matched controls55–57. Multiple imaging and physiological studies have shown compromise of the pharyngeal luminal cross-sectional area in OSA as compared with controls39,57,58. Further, the soft tissue and bony structure surrounding the lumen appears to be altered in OSA patients which may place it at risk for collapse. Imaging studies during wakefulness, however, are complicated to interpret since ongoing pharyngeal dilator muscle activity (greater in OSA than controls59) may lead to observed differences between groups based on non anatomical (i.e., neuromuscular) factors. The critical closing pressure (Pcrit) is commonly used to quantify pharyngeal collapsibility60, with very negative values suggesting a stable upper airway and very positive values suggesting an unstable pharynx. The Pcrit can be measured both passively (as an index of anatomy) or actively (indicative of both anatomical and neuromuscular control)61,62. Passive Pcrit studies support increased propensity of the OSA airway to collapse on a purely biomechanical basis63. Perhaps the most persuasive data come from a study by Isono et al63 who observed increased closing pressure (more collapsible) in OSA as compared to controls under conditions of general anesthesia and muscle paralysis. Thus, in aggregate, multiple methodologies have shown that OSA patients have anatomical compromise leaving these individuals vulnerable to pharyngeal collapse during periods of susceptibility such as sleep and anaesthesia64.
Upper airway dilator muscle activity and recruitability
Patients with OSA have increased pharyngeal dilator muscle activity (as a percentage of maximum) versus matched controls59 that has been interpreted as evidence for neuromuscular protective compensatory reflexes in response to anatomical compromise in OSA. Through these protective reflexes, the increased muscle activity protects pharyngeal patency during wakefulness42,65. Although some data have suggested that increased pharyngeal dilator muscle activity is a result of denervation, these data are controversial. For example, if denervation was the critical factor underlying increased muscle activity, the mechanisms important in maintaining pharyngeal patency during wakefulness in OSA would remain unknown. One important pathophysiological mechanism relates to the ability of the upper airway dilator muscles to maintain a patent airway during sleep. In support of this concept, standard multi-unit genioglossal electromyogram activity is reduced at sleep onset in healthy individuals and OSA patients66. Thus, while healthy individuals experience a loss of upper airway muscle tone at sleep onset (alpha-theta transition in the electroencephalogram) and experience some degree of breathing instability, an individual reliant on muscle tone due to an anatomical vulnerability will be particularly susceptible to apnoea. As one might predict, hypopnoeas and apnoeas frequently occur at the transition from wakefulness to sleep in OSA12,67. Each respiratory event is typically associated with an electro-encephalographic arousal such that the OSA patient cycles between wakefulness and sleep leading to minimal deep sleep. Unlike the transition to sleep, slow wave sleep is associated with increased, not decreased, upper airway dilator muscle activity in the majority of studies68,69. Some have argued that deep sleep is a state of intrinsic stability with associated increases in upper airway dilator muscle activity being one important factor contributing to the improvement in apnoea severity68,69. The high arousal threshold (low propensity to wake up) and neurochemical milieu seen in slow wave sleep may also be important factors stabilizing breathing. On the other hand, slow wave sleep could simply be a marker of breathing stability such that delta sleep (N3) may only occur when respiratory arousals are not occurring68,69. This controversy is difficult to resolve since the direction of causation is unclear. That is, whether deep sleep stabilizes breathing or breathing stability permits deep sleep to occur is uncertain. However, pharmacological studies using agents to promote slow wave sleep may be one method to test this hypothesis.
Multiple factors can influence output from the hypoglossal motor nucleus to the major upper airway dilator muscle (the genioglossus)70–76. Respiratory drive from the central pattern generator in the brainstem is a major determinant of genioglossus activity77,78. In addition, local upper airway mechanoreceptors respond to subatmospheric (negative or suction) pressure and modulate genioglossus activity79–82. The negative pressure reflex describes the phenomenon whereby the genioglossus (and other upper airway dilator muscles such as the tensor palatini) is activated in response to negative pressure (i.e., suction pressures which promote pharyngeal collapse)83,84. Thus, pharyngeal patency can be protected by a robust activation of the dilator muscles in the face of a collapsing perturbation. This negative pressure reflex has been shown to be attenuated during stable non rapid eye movement (NREM) sleep in healthy individuals83,84. That is, the ability of the upper airway to maintain patency in the face of a collapsing stimulus is impaired during sleep. However, recent data have shown a maintained reflex during NREM sleep, particularly in the supine posture when the upper airway is most vulnerable to collapse40,85–88. Indeed, our understanding of the neuroanatomy of the genioglossus negative pressure reflex and hypoglossal motor nucleus inputs from rat studies has recently evolved89. Although pharyngeal dilator muscle responsiveness is likely impaired during NREM and REM sleep90, the genioglossus can respond to both sustained mechanoreceptive (negative pressure) and chemoreceptive stimuli, particularly when combined stimuli are present91. The implication of this finding is that, given sufficient time and magnitude of stimuli, the upper airway dilator muscles will eventually respond to respiratory stimuli, e.g., CO2 plus negative pressure92–95. Thus, in the setting of pharyngeal collapse, airway patency may be restored if upper airway muscles respond sufficiently before arousal occurs. Because intrathoracic pressure appears to be both the stimulus for arousal from sleep and closely related to the stimulus for genioglossal activation96–98, ventilatory drive can yield two competing mechanisms (i.e., arousal vs. restoration of airway patency)9,99. Not surprisingly, there is considerable inter-individual variability in the effectiveness of these compensatory mechanisms to restore airflow during sleep92, which may in part be due to high variablily in the respiratory arousal threshold.
Although traditional electromyogram (EMG) studies have been informative, such studies have relied on multiunit recordings which obscure the activity of individual motor units contributing to overall activity. High frequency sampling techniques have recently been used to define single motor units (SMUs) within the genioglossus with illuminating results100–103. These SMU techniques allow sorting of individual motor units within the muscle of interest to gain insight into muscle characteristics and regulation using electrophysiology. These SMU techniques have been used extensively in muscle physiology, but have only recently been rigorously applied to human upper airway muscles with a view towards understanding sleep apnoea pathogenesis. These SMU techniques when applied to the genioglossus electromyogram allow insights into cellular activity within the hypoglossal motor nucleus. Although research is ongoing, studies have already shown considerable complexity within the genioglossus muscle100. By combining neuroanatomical and neurochemical experiments in rodents with sensitive neurophysiological techniques in humans, major insights into motor control are likely to occur yielding the possibility of novel therapeutic targets for some OSA patients3. While, such targeted approaches may lead to improvements in OSA, given the heterogeneity of OSA pathogenesis, such an approach is unlikely to resolve respiratory events for all patients.
Arousal from sleep
Arousal from sleep at the termination of a respiratory event is an important protective mechanism to restore pharyngeal patency9. In fact, most, but not all, respiratory events are associated with cortical arousal104. However, Younes9 has emphasized the notion that arousal is not essential for restoration of airflow. By applying intermittent continuous positive airway pressure (CPAP) reductions in OSA patients, Younes observed that inspiratory flow increased in 22 per cent of instances prior to arousal and was restored in 17 per cent of trials in the absence of EEG arousal. Subsequently, findings of a study by Jordan and colleagues92 suggest that these restorations in airflow without arousal may be mediated by genioglossus activation. In this study, challenges to pharyngeal patency (through CPAP drops) for up to 5 min resulted in genioglossus activation and changes in respiratory duty cycle (i.e., inspiratory time prolonged relative to expiratory time). These compensatory responses were similar between OSA patients and healthy individuals, although OSA patients were less able to restore ventilation without cortical arousal than controls. During a subsequent study (unpublished observations), physiological variables were recorded during periods of spontaneously occurring stable and unstable breathing. Jordan et al92 observed that genioglossus activity was likely responsible for these stable breathing periods. That is, during periods of stable breathing, genioglossal activity was high relative to unstable periods, whereas tensor palatini activity and end-expiratory lung volume were essentially unchanged. However, the therapeutic implications of this observation are unclear. Questions remain as to how these stable breathing periods can be induced e.g., by giving a hypnotic agent to raise the arousal threshold in patients with recruitable upper airway muscles105,106. For example, a hypnotic agent provided to OSA patients with a low arousal threshold but recruitable upper airway muscles may allow enough time for CO2 and negative pressure to accumulate sufficiently to augment dilator muscle activity yielding improvements in pharyngeal patency. On the other hand, a hypnotic agent may be deleterious if prolonged apnoeas occur with marked hypoxemia and insufficient muscle recruitment to restore pharyngeal patency (Figs 1 and 2). Further work is clearly needed in this area to define the subgroup of OSA patients who may or may not respond to manipulations in the arousal threshold.
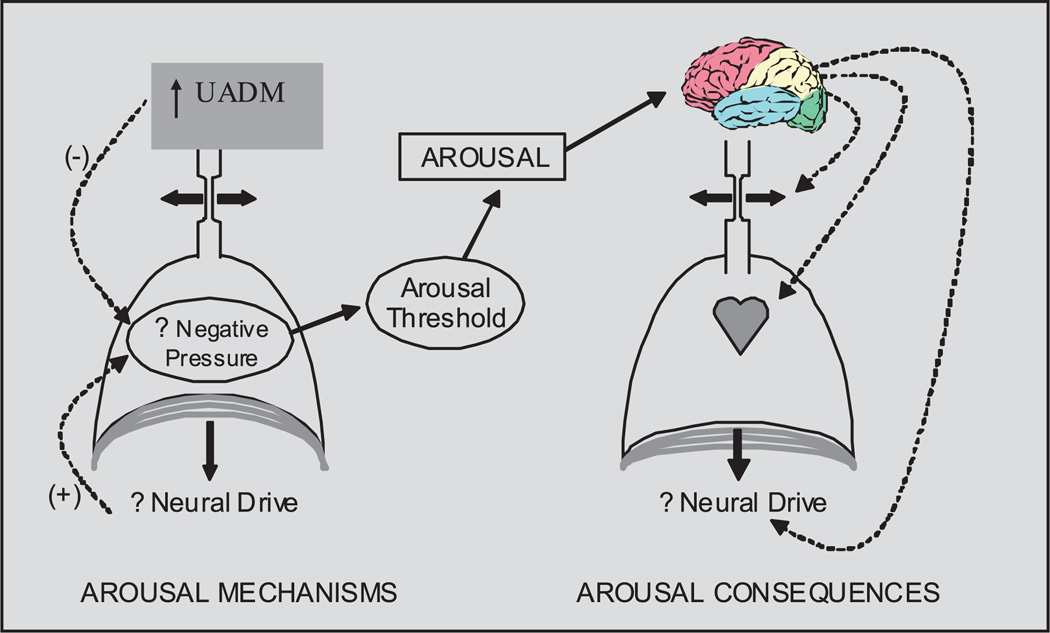
Fig. 1.
The role of UADM (upper airway dilator muscles) in stabilizing breathing. Negative intrathoracic pressure plus CO2 can activate UADM, but negative pressure can also trigger arousal. A hypnotic could potentially change the arousal threshold to stabilize breathing and potentially prevent the consequences of repetitive arousal.
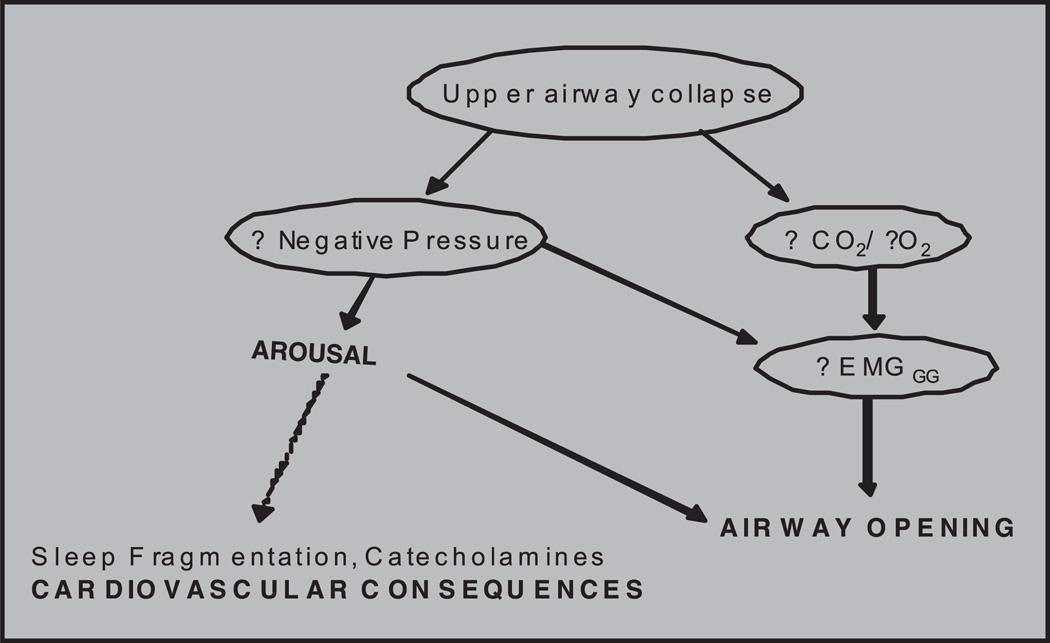
Fig. 2.
The double edged sword of arousal leading to both airway opening and possible cardiovascular consequences. Airway opening can be achieved without arousal by activating genioglossus (EMGGG) through negative pressure plus CO2.
Most of the available evidence suggests that the level of intrapleural pressure, generated by respiratory effort is a major stimulus triggering arousal from sleep (Fig. 3)44,96. Experimentally, the respiratory arousal threshold is measured as the nadir oesophageal pressure (or minimum epiglottic pressure which is similar to oesophageal during airway occlusion) generated on the breath preceding arousal from a respiratory event or perturbation106. Although the arousal threshold values are highly variable between individuals, patients with OSA tend to have an impaired arousal response to airway occlusion (more negative pressure required for respiratory arousal) than controls99. However, the question remains as to whether the higher arousal threshold observed in OSA is a cause or a consequence of the disease. Studies examining the impact on arousal threshold in OSA with nasal CPAP therapy suggest some improvement (lowering) of arousal threshold as compared with untreated OSA44,107,108. These data suggest that an elevated arousal threshold in OSA may be at least in part acquired from the disease.
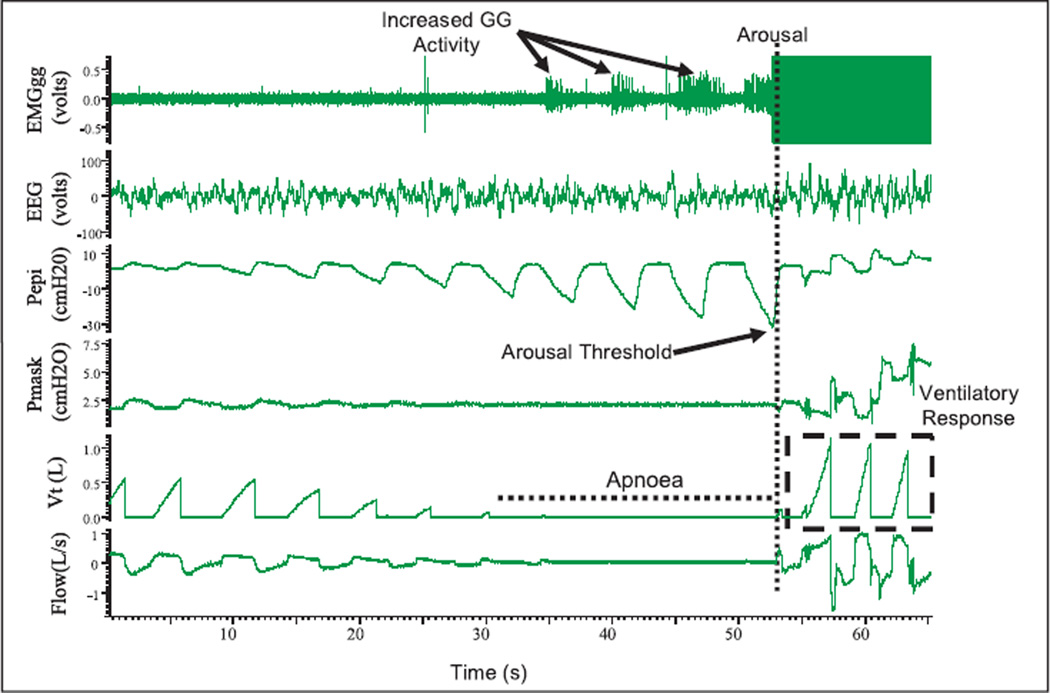
Fig. 3.
Example polysomnographic tracings of an obstructive sleep apnoea event induced by reducing continuous positive airway pressure (from therapeutic to 2cmH2O) in a 52 yr old male patient with severe OSA (apnoea/hypopnea index= 34.5 events per hour). EMGgg= Electromyogram of the genioglossus muscle (intramuscular), EEG= electroencephalogram (C3-A2), Pepi= pressure at the level of the epiglottis, Pmask= pressure measured via nasal mask, Vt = tidal volume and flow measured via nasal mask and pneumotachograph. There was increased EMGgg activity during the apnoeic event, though it was not significant enough to restore flow without arousal. The arousal threshold is characterized using Pepi, and after arousal there is a large ventilatory response.
Following arousal from sleep, augmented pharyngeal dilator muscle activity and a robust ventilatory response to arousal generally occur (Fig. 3)109. While these events are beneficial in restoring airflow and improving gas exchange, these changes can also be destabilizing and may perpetuate apnoea. That is, the ventilatory response to arousal can drive down carbon dioxide levels below the apnoea threshold such that apnoea can occur during subsequent sleep5,110–112.
Loop gain (ventilatory control stability/instability)
Another characteristic feature of OSA is the cyclical breathing pattern that develops whereby the patient oscillates between obstructive breathing events (sleep) and arousal (wakefulness). Further, obstructive events tend to occur during periods of low respiratory drive. Thus, instability in ventilatory control is likely a critical contributor to OSA.
Loop gain is an engineering term that is used to describe stability or instability in a negative feedback control system113–115. The regulation of room temperature provides a useful analogy whereby temperature will be prone to oscillation when there is a sensitive thermostat and an overly powerful heater (i.e., high loop gain). The room temperature analogy can be used to understand the impact of sensors and effectors on temperature stability12. In the context of ventilatory control, loop gain refers to the stability of the ventilatory control system and how responsive the system is to a perturbation to breathing47,95,116–120. That is, loop gain is the propensity for the ventilatory control system to develop fluctuations in ventilatory output (as seen in periodic breathing). There are three major components to loop gain: controller gain, plant gain and mixing gain12. In control of breathing, controller gain refers to the chemoresponsiveness of the system [i.e., chemosensitivity (hypoxic and hypercapnic ventilatory responses) plus responsiveness]121. Plant gain primarily reflects the efficiency of CO2 excretion (i.e., the ability of a given level of ventilation to excrete CO2). Mixing gain appears to be less crucial, but is a function of circulatory delay as well as hemoglobin binding of O2 and CO2. Mixing gain tends to be fairly constant, although circulatory delays may make mixing gain more clinically relevant in patients with congestive heart failure122,123. A high loop gain system is present if periodic breathing develops in the setting of minimal perturbation whereas a low loop gain system remains stable despite major perturbation. Younes has developed techniques to measure loop gain, including one using proportional assist ventilation (PAV)116,124,125. PAV studies have shown that OSA patients have an elevated loop gain compared to controls and suggest that ventilatory instability is an important mechanism underlying OSA. However, the PAV technique uses expiratory positive airway pressure (EPAP) which stabilizes the upper airway and relies on stable sleep without arousals119. Thus, transient events which may be important in perpetuating cyclical breathing may be neglected by this technique.
Why high loop gain leads to sleep apnoea is unclear. There are two major possibilities. First, elevated loop gain may increase oscillations from the brain stem pattern generator. In theory, pharyngeal patency should be maintained during high output to the phrenic and hypoglossal nerves117. On the other hand, pharyngeal obstruction may occur when central motor output to the upper airway is at its nadir. Second, elevated loop gain may also augment the ventilatory response to arousal126,127, which would drive PaCO2 (partial pressure of CO2 in arterial blood) below the apnoea threshold. Obstructive or central apnoea would then occur depending on the prevailing upper airway mechanics. Thus, further work is clearly required in this area.
Functional residual capacity (End-expiratory lung volume)
Lung volume effects on pharyngeal patency are likely to be important in OSA pathogenesis. Clearly, upper airway mechanics can be affected by alterations in end-expiratory lung volume during wakefulness and sleep in healthy individuals. Hoffstein and colleagues128,129 demonstrated that pharyngeal cross-sectional area changes from residual volume (RV) to total lung capacity (TLC). This lung volume dependence on the upper airway appears to be more pronounced in OSA patients versus controls. However, studies during wakefulness are confounded by behavioural influences since a maximal inhalation to TLC is likely to activate upper airway muscles volitionally. During sleep, upper airway resistance increases as lung volume falls. Increasing end expiratory lung volume decreases airway collapsibility in healthy controls and improves respiratory mechanics in OSA patients49,52,130–132.
While studies have demonstrated that changes in lung volume may modulate upper airway patency in OSA, the underlying mechanisms are poorly defined130–132. A loss of caudal traction on upper airway structures may occur with reduced lung volume. When lung volume falls, the diaphragm migrates upward (cephalad), potentially resulting in a loss of caudal traction forces on the upper airway, and yielding a more collapsible upper airway. Conversely, elevated end-expiratory lung volume may lead to increased caudal traction and a more stable upper airway. Whether lung volume per se can be targeted therapeutically remains unclear, although part of the benefit of CPAP may be from augmentation of end-expiratory lung volume.
Influence of genetics on key OSA pathophysiological traits
A familial predisposition for OSA has been recognized for nearly 40 years since Strohl et al133 described a family with multiple affected relatives. Since that time, multiple studies have demonstrated that individuals who have a family member with OSA are at increased risk of having apnoea themselves134,135. Redline et al136 reported a dose-response curve such that compared to an individual with no affected relatives, having 1, 2, or 3 or more relatives with OSA increases one’s own risk by 1.5,2, and 3-fold respectively. Studies in both Caucasians and African-Americans as well as in the elderly have consistently found that approximately 1/3 of the total variance in apnoea / hypoapnoea index (AHI) can be explained by familial factors137–139.
Since obesity has a strong genetic basis with 60–80 per cent of the variance in BMI explained by familial factors140,141, some have postulated that perhaps the genetic basis for OSA is simply secondary to genes influencing levels of adiposity. Several lines of work suggest that this is not the case. Mathur & Douglas135 found that relatives of lean OSA patients were at increased risk of sleep apnoea themselves. Bivariate modeling in the Cleveland Family Study has found that approximately 40 per cent of the genetic basis defining AHI is explained by obesity leaving the majority of genetic variance in AHI mediated through obesityindependent mechanisms142.
Upper airway anatomy represents one potential obesity-independent genetic pathway. Bony facial features relevant to OSA such as length of the cranial base and the nasion-sella-basion angle demonstrate substantial heritability143. Relatives of people with OSA are more likely to have retro-positioned maxillae and mandibles135. In addition, the cephalic index (ratio of head width to head length) is almost completely genetically determined144. MRI studies demonstrate that soft tissue structures such as tongue and lateral pharyngeal wall volume also have a genetic basis with over a third of the variability in these structures explained by familial factors145.
Ventilatory drive may represent another mechanism by which genes influence OSA susceptibility. Ventilatory responses to hypoxia and to inspiratory resistive loading have been found to have a familial basis and be impaired in relatives of OSA patients compared to relatives of controls146–148. Also supporting a role for ventilatory control genes in defining OSA risk has been the finding that cases of OSA and sudden infant death syndrome (SIDS) co-segregate within families149–151. Other potentially important pathways in OSA pathogenesis such as airway dilator muscle tone and responsiveness, upper airway neural reflexes, and arousal threshold may also have genetic underpinnings; however, no studies have yet been performed to assess the familial basis for these traits directly. Thus, the definition of phenotypic traits may facilitate genetic investigations of complex diseases by allowing more precision i.e., there is unlikely to be a single gene for sleep apnoea but there may well be a predominant gene defining variability in arousal threshold, for example.
In terms of identifying causal genes, several whole genome linkage analyses have been performed with identification of possible candidate regions but in general these results have not been consistent137,138,152. Many candidate gene association studies have been performed with a growing number of positive results reported153–156. However, similar to work on the genetics of other complex diseases, for the most part these studies have been underpowered and difficult to replicate suggesting a high likelihood of the positive results representing false positive findings. The most consistently demonstrated association has been between OSA and the ε4 allele of the apolipoprotein E (APOE) gene. This allele, known to predispose to Alzheimer’s dementia, has been found to predict a greater AHI in the Wisconsin Sleep Study and Framingham Study157,158. However, these findings have not been replicated in other large cohorts159,160. The success of large scale genome wide association studies recruiting tens of thousands of patients to identify novel risk genes in diseases such as asthma, diabetes, and Crohn’s disease has raised interest in using such a study design for OSA. Efforts are underway to begin such large scale collaborative studies in the sleep-disordered breathing field.
Table.
Summary of OSA pathophysiological traits. The mechanisms that may contribute to these traits are highlighted as well as potential targeted treatments approaches. Possible genetic links to these traits are also included
| OSA pathophysiology | Possible mechanism | Possible targeted treatment option | Genetic link |
|---|---|---|---|
| Compromised upper airway anatomy |
Anatomically smaller airway, altered soft tissue |
Surgery in selected cases | Bony facial features, postion of maxillae and madible are heritable |
| Decreased dilator muscle activity during sleep |
Neurochemical alterations at sleep onset; UA trauma/oedema leading to neuoropathy/myopathy |
Hypoglossal activation/ stimulation; pharmacotherapy in the future |
Unknown |
| Decreased arousal threshold | Cannot recruit dilator muscle to open airway before arousal occurs |
Hypnotic agents to subjects with low arousal thresholds and recruitable muscles |
Unknown |
| Unstable ventilatory control (increased loop gain) |
Increases oscillations from brain stem pattern generator, augment ventilatory response to arousal driving PaCO2 below apnoea threshold |
Oxygen; acetazolamide | Similar response to hypoxia and inspiratory resistance among relatives. SIDS and OSA co- segregate in families |
| Decreased lung volume | Increasing EELV improves respiratory mechanics possibly by increasing caudal traction on upper airway |
Lung volume Augmentation; Cuirass; Negative extrathoracic pressure |
Indirectly, obesity may cause decreased lung volume |
EELV, End-expiratory lung volume; SIDS, sudden infant death syndrome; UA, upper airway
Acknowledgment
Dr Malhotra has received consulting and/or research funding from Respironics, NMT Medical, Itamar Medical, Cephalon, Pfizer, Apnex Medical, Restore Medical, Inspiration Medical, Medtronics, and Sepracor. Ms. Campana, Drs. Eckert and Patel report no conflicts. Dr Eckert is supported by an Overseas Based Biomedical Fellowship from the National Health and Medical Research Council of Australia (510392). Dr Patel is PI on NIH HL081385 and ATS Research Program Award. Dr Malhotra is PI on NIH P50 K24HL093218, RO1-HL73146, RO1-HL085188-01, RO1HL090897 and AHA Established Investigator Award.
References
- 1.Malhotra A, White DP. Obstructive sleep apnea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Badr MS. Pathogenesis of obstructive sleep apnea. Prog Cardiovasc Dis. 1999;41:323–330. doi: 10.1053/pcad.1999.0410323. [DOI] [PubMed] [Google Scholar]
- 3.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pack AI. Obstructive sleep apnea. Adv Intern Med. 1994;39:517–567. [PubMed] [Google Scholar]
- 5.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 8.Rahangdale S, Campana L, Malhotra A. Not so good vibrations. Sleep. 2008;31:1204–1205. [PMC free article] [PubMed] [Google Scholar]
- 9.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 10.Douglas NJ, Martin SE. Arousals and the sleep apnea/hypopnea syndrome. Sleep. 1996;19(10 Suppl):S196–S197. [PubMed] [Google Scholar]
- 11.Phillipson EA, Bowes G, Sullivan CE, Woolf GM. The influence of sleep fragmentation on arousal and ventilatory responses to respiratory stimuli. Sleep. 1980;3:281–288. doi: 10.1093/sleep/3.3-4.281. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement? Sleep. 2006;29:16–18. [PubMed] [Google Scholar]
- 13.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;32:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 14.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 15.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer. Am J Respir Crit Care Med. 1998;157:1461–1467. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 17.Barbe F, Mayoralas LR, Duran J, Masa JF. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134:1065–1067. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 18.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnea syndrome. Thorax. 1992;47:101–105. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan A, McEvoy D. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–389. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 20.Young T. Menopause, hormone replacement therapy, and sleep-disordered breathing: are we ready for the heat. Am J Respir Crit Care Med. 2001;163:597–598. doi: 10.1164/ajrccm.163.3.ed09-01a. [DOI] [PubMed] [Google Scholar]
- 21.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 24.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Long-term benefits in self-reported health status of nasal continuous positive airway pressure therapy for obstructive sleep apnea. QJM. 2001;94:95–99. doi: 10.1093/qjmed/94.2.95. [DOI] [PubMed] [Google Scholar]
- 26.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of contnous positive airways pressure: a systematic review of the research evidence. BMJ. 1997;314:851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 28.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 29.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 30.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 32.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieto F, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 34.Lavie P, Here P, Hoffstein V. Obstructive sleep apnea syndrome as a risk factor for hypertension. Br Med J. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 36.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 37.Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, et al. Refractory hypertension and sleep apnea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 38.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 39.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–1112. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 43.Malhotra A, Pillar G, Fogel R, Beauregard J, Edwards J, White DP. Upper-airway collapsibility: measurements and sleep effects. Chest. 2001;120:156–161. doi: 10.1378/chest.120.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 45.Berry RB, Light RW. Effect of hyperoxia on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:330–334. doi: 10.1164/ajrccm/146.2.330. [DOI] [PubMed] [Google Scholar]
- 46.Khoo M. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 47.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 48.Cherniack NS. Respiratory dysrhythmias during sleep. N Engl J Med. 1981;305:325–330. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 49.Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis. 1990;141:854–860. doi: 10.1164/ajrccm/141.4_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 50.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 51.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol. 1991;70:1328–1363. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 52.Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 53.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 54.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 55.Haponik E, Smith P, Bohlman M, Allan R, Goldman S, Bleecker E. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 56.Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1983;127:487–492. doi: 10.1164/arrd.1983.127.4.487. [DOI] [PubMed] [Google Scholar]
- 57.Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnea and weight matched controls. Eur Respir J. 1989;2:613–622. [PubMed] [Google Scholar]
- 58.Ciscar MA, Juan G, Martínez V, Ramón M, Lloret T, Mínguez J, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 59.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 61.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 62.Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 63.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 64.Eastwood P, Szollosi I, Platt P, Hillman D. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 65.Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 66.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 67.Skatrud JB, Henke KG, Dempsey J. A sleep-induced apneic threshold. Prog Clin Biol Res. 1990;345:191–199. [PubMed] [Google Scholar]
- 68.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 69.Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, et al. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J Appl Physiol. 2000;89:1275–1282. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- 70.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnea. Respir Res. 2001;2:286–294. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 72.Horner RL, Bradley TD. Update in sleep and control of ventilation 2006. Am J Respir Crit Care Med. 2007;175:426–431. doi: 10.1164/rccm.200701-043UP. [DOI] [PubMed] [Google Scholar]
- 73.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- 74.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 75.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneuron in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 76.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 77.Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- 78.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathew OP. Upper airway negative pressure effects on respiratory activity of upper airway muscles. J Appl Physiol. 1984;56:500–505. doi: 10.1152/jappl.1984.56.2.500. [DOI] [PubMed] [Google Scholar]
- 80.Mathew OP. Maintenance of upper airway patency. J Pediatr. 1985;106:863–869. doi: 10.1016/s0022-3476(85)80227-4. [DOI] [PubMed] [Google Scholar]
- 81.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle response to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 82.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wheatley J, Mezzanotte W, Tangel D, White D. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 84.Wheatley J, Tangel D, Mezzanotte W, White D. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol. 1993;75:2117–2124. doi: 10.1152/jappl.1993.75.5.2117. [DOI] [PubMed] [Google Scholar]
- 85.Ryan S, Nolan P. Superior laryngeal and hypoglossal afferents tonically influence upper airway motor excitability in anesthetized rats. J Appl Physiol. 2005;99:1019–1028. doi: 10.1152/japplphysiol.00776.2004. [DOI] [PubMed] [Google Scholar]
- 86.Doherty LS, Nolan P, McNicholas WT. Effects of topical anesthesia on upper airway resistance during wake-sleep transitions. J Appl Physiol. 2005;99:549–555. doi: 10.1152/japplphysiol.01221.2004. [DOI] [PubMed] [Google Scholar]
- 87.Ryan S, McNicholas WT, O’Regan RG, Nolan P. Upper airway muscle paralysis reduces reflex upper airway motor response to negative transmural pressure in rat. J Appl Physiol. 2003;94:1307–1316. doi: 10.1152/japplphysiol.00052.2002. [DOI] [PubMed] [Google Scholar]
- 88.Malhotra A, Pillar G, Fogel R, Beauregard J, White D. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 89.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shea S, Edwards J, White D. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 92.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 94.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 95.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 96.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 97.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berry RB, McNellis MI, Kouchi K, Light RW. Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. Am J Respir Crit Care Med. 1997;156:127–132. doi: 10.1164/ajrccm.156.1.9608037. [DOI] [PubMed] [Google Scholar]
- 99.Eckert D, Jordan A, Wellman A, et al. The respiratory arousal threshold in obstructive sleep apnea. Am J Respir Crit Care Med. 2008;177:A594. doi: 10.1164/rccm.201312-2115LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 101.Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, et al. Neural drive to human genioglossus in obstructive sleep apnea. J Physiol. 2007;585:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97:933–936. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- 104.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- 105.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–488. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 106.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, et al. Trazodone increases arousal threshold in obstructive sleep apnea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berry RB, Kouchi KG, Der DE, Dickel MJ, Light RW. Sleep apnea impairs the arousal response to airway occlusion. Chest. 1996;109:1490–1496. doi: 10.1378/chest.109.6.1490. [DOI] [PubMed] [Google Scholar]
- 108.Haba-Rubio J, Sforza E, Weiss T, Schroder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath. 2005;9:12–19. doi: 10.1007/s11325-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 109.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 110.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 111.Dempsey J. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2004;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 112.Dempsey J, Skatrud J, Smith C. Powerful stabilizing effects of CO2 during CPAP treatment. Sleep. 2005;28:12–13. [PubMed] [Google Scholar]
- 113.Khoo MC. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1044–1045. doi: 10.1164/ajrccm.163.5.ed1101c. [DOI] [PubMed] [Google Scholar]
- 114.Khoo M. Theoretical models of periodic breathing. New York: Marcel Dekker; 2000. [Google Scholar]
- 115.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 116.Younes M. Proportional assist ventilation. In: Mancebo J, Net A, Brochard L, editors. Update in intensive care and emergency medicine. New York: Springer; 2002. pp. 39–73. [Google Scholar]
- 117.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wellman A, Malhotra A, Fogel RB, Edwards JK, Schory K, White DP. Respiratory system loop gain in normal men and women measured with proportional-assist ventilation. J Appl Physiol. 2003;94:205–212. doi: 10.1152/japplphysiol.00585.2002. [DOI] [PubMed] [Google Scholar]
- 119.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol. 2007;581:291–298. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: Role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dempsey JA. CO2 response: stimulus definition and limitations. Chest. 1976;70(1 Suppl):114–118. doi: 10.1378/chest.70.1.114. [DOI] [PubMed] [Google Scholar]
- 122.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132:433–439. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sinha AM, Skobel EC, Breithardt OA, Norra C, Markus KU, Breuer C, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 124.Meza S, Giannouli E, Younes M. Control of breathing during sleep assessed by proportional assist ventilation. J Appl Physiol. 1998;84:3–12. doi: 10.1152/jappl.1998.84.1.3. [DOI] [PubMed] [Google Scholar]
- 125.Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992;145:114–120. doi: 10.1164/ajrccm/145.1.114. [DOI] [PubMed] [Google Scholar]
- 126.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffstein V, Fredberg JJ. The acoustic reflection technique for non-invasive assessment of upper airway area. Eur Respir J. 1991;4:602–611. [PubMed] [Google Scholar]
- 129.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–178. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 130.Heinzer R, White DP, Malhotra A, Lo YL, Dover L, Stevenson KE, et al. Effect of expiratory positive airway pressure on sleep disordered breathing. Sleep. 2008;31:429–432. doi: 10.1093/sleep/31.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Strohl KP, Saunders NA, Feldman NT, Hallett M. Obstructive sleep apnea in family members. N Engl J Med. 1978;299:969–973. doi: 10.1056/NEJM197811022991801. [DOI] [PubMed] [Google Scholar]
- 134.Pillar G, Lavie P. Assessment of the role of inheritance in sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:688–691. doi: 10.1164/ajrccm/151.3_Pt_1.688. [DOI] [PubMed] [Google Scholar]
- 135.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 1995;122:174–178. doi: 10.7326/0003-4819-122-3-199502010-00003. [DOI] [PubMed] [Google Scholar]
- 136.Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:682–687. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 137.Palmer LJ, Buxbaum SG, Larkin E, Patel SR, Elston RC, Tishler PV, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–350. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Palmer LJ, Buxbaum SG, Larkin E, Patel SR, Elston RC, Tishler PV, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169:1314–1321. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- 139.Carmelli D, Colrain IM, Swan GE, Bliwise DL. Genetic and environmental influences in sleep-disordered breathing in older male twins. Sleep. 2004;27:917–922. doi: 10.1093/sleep/27.5.917. [DOI] [PubMed] [Google Scholar]
- 140.Koeppen-Schomerus G, Wardle J, Plomin R. A genetic analysis of weight and overweight in 4-year-old twin pairs. Int J Obes Relat Metab Disord. 2001;25:838–844. doi: 10.1038/sj.ijo.0801589. [DOI] [PubMed] [Google Scholar]
- 141.Pietiläinen KH, Kaprio J, Rissanen A, Winter T, Rimpelä A, Viken RJ, et al. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: a study of 4884 twins and 2509 singletons. Int J Obes Relat Metab Disord. 1999;23:107–115. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- 142.Patel SR, Larkin EK, Redline S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int J Obes. 2008;32:795–800. doi: 10.1038/sj.ijo.0803803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nance WE, Nakata M, Paul TD, Yu PI. The use of twin studies in the analysis of phenotypic traits in man. In: Janerich DT, Skalko RG, Porter IH, editors. Congenital defects new directions in research. New York: Academic Press; 1974. pp. 23–49. [Google Scholar]
- 144.Osborne RH, De George FV. Genetic basis of morphologic variation: An evaluation and application of the twin study method. Cambridge: Harvard University Press; 1959. [Google Scholar]
- 145.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–463. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.el Bayadi S, Millman RP, Tishler PV, Rosenberg C, Saliski W, Boucher MA, et al. A family study of sleep apnea. Anatomic and physiologic interactions. Chest. 1990;98:554–559. doi: 10.1378/chest.98.3.554. [DOI] [PubMed] [Google Scholar]
- 147.Redline S, Leitner J, Arnold J, Tishler PV, Altose MD. Ventilatory-control abnormalities in familial sleep apnea. Am J Respir Crit Care Med. 1997;156:155–160. doi: 10.1164/ajrccm.156.1.9610016. [DOI] [PubMed] [Google Scholar]
- 148.Lavie P, Rubin AE. Effects of nasal occlusion on respiration in sleep. Evidence of inheritability of sleep apnea proneness. Acta Otolaryngol. 1984;97:127–130. doi: 10.3109/00016488409130972. [DOI] [PubMed] [Google Scholar]
- 149.Mathur R, Douglas NJ. Relation between sudden infant death syndrome and adult sleep apnea/hypopnoea syndrome. Lancet. 1994;344:819–820. doi: 10.1016/s0140-6736(94)92375-2. [DOI] [PubMed] [Google Scholar]
- 150.Gislason T, Johannsson JH, Haraldsson A, Olafsdottir BR, Jonsdottir H, Kong A, et al. Familial predisposition and cosegregation analysis of adult obstructive sleep apnea and the sudden infant death syndrome. Am J Respir Crit Care Med. 2002;166:833–838. doi: 10.1164/rccm.2107121. [DOI] [PubMed] [Google Scholar]
- 151.Tishler PV, Redline S, Ferrette V, Hans MG, Altose MD. The association of sudden unexpected infant death with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;153:1857–1863. doi: 10.1164/ajrccm.153.6.8665046. [DOI] [PubMed] [Google Scholar]
- 152.Gottlieb DJ, O’Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet. 2007;8(Suppl 1):S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riha RL, Brander P, Vennelle M, McArdle N, Kerr SM, Anderson NH, et al. Tumour necrosis factor-alpha (−308) gene polymorphism in obstructive sleep apnea-hypopnoea syndrome. Eur Respir J. 2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 154.Bayazit YA, Yilmaz M, Ciftci T, Erdal E, Kokturk O, Gokdogan T, et al. Association of the-1438G/A polymorphism of the 5-HT(2A) receptor gene with obstructive sleep apnea syndrome. ORL J Otorhinolaryngol Relat Spec. 2006;68:123–128. doi: 10.1159/000091216. [DOI] [PubMed] [Google Scholar]
- 155.Ylmaz M, Bayazit YA, Ciftci TU, Erdal ME, Urhan M, Kokturk O, et al. Association of serotonin transporter gene polymorphism with obstructive sleep apnea syndrome. Laryngoscope. 2005;115:832–836. doi: 10.1097/01.MLG.0000157334.88700.E6. [DOI] [PubMed] [Google Scholar]
- 156.Zhang LQ, Yao WZ, He QY, Wang YZ, Ren B, Lin YP. Polymorphisms in the beta2 and beta3 adrenergic receptor genes in obstructive sleep apnea/hypopnea syndrome. Zhonghua Nei Ke Za Zhi. 2005;44:333–336. [PubMed] [Google Scholar]
- 157.Kadotani H, Kadotani T, Young T, Peppard PE, Finn L, Colrain IM, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–2890. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 158.Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 159.Foley DJ, Masaki K, White L, Redline S. Relationship between apolipoprotein E epsilon4 and sleep-disordered breathing at different ages. JAMA. 2001;286:1447–1448. doi: 10.1001/jama.286.12.1447. [DOI] [PubMed] [Google Scholar]
- 160.Larkin EK, Patel SR, Redline S, Mignot E, Elston RC, Hallmayer J. Apolipoprotein E and obstructive sleep apnea: evaluating whether a candidate gene explains a linkage peak. Genet Epidemiol. 2006;30:101–110. doi: 10.1002/gepi.20127. [DOI] [PubMed] [Google Scholar]