Abstract
During HIV infection, the timing of opportunistic infections is not always associated with severity of CD4 T cell depletion and different opportunistic pathogens reactivate at different CD4 T cell thresholds. Here we review how differences in the phenotype and function of pathogen-specific CD4 T cells influence susceptibility to HIV infection. By focusing on three common opportunistic infections (Mycobacterium tuberculosis, human papillomavirus, and cytomegalovirus) we examine how differential depletion of pathogen-specific CD4 T cells impacts the natural history of these pathogens in HIV infection. A broader understanding of this relationship can better inform treatment strategies against co-pathogens.
Introduction
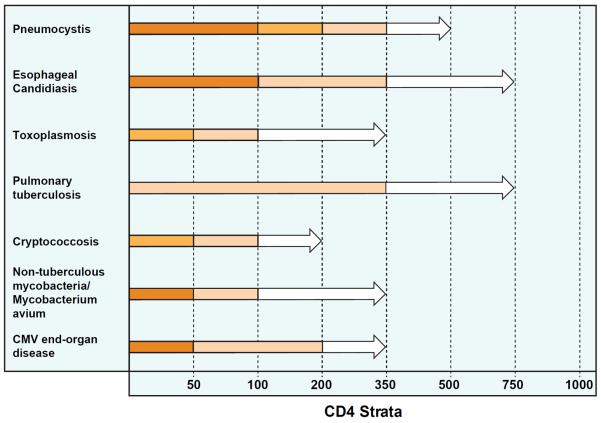
Chronic, untreated HIV infection is characterized by progressive depletion of CD4 T cells increasing the risk of co-infection with pathogens typically controlled by innate and adaptive immune responses. While the mechanisms leading to CD4 T cell depletion are complex, susceptibility to opportunistic infections has generally been associated with progressive declines in CD4 T cell counts. However, the timing of opportunistic infections is not always associated with severity of CD4 depletion. Tuberculosis (TB) infection can reactivate when CD4 T cell counts are high and infection with Candida species can occur at any CD4 T cell count (Figure 1).
Figure 1. Incidence of select opportunistic infections stratified by CD4 count.
Data is adapted from several cohort studies of HIV positive individuals.(Anglaret et al., 2012; Minga et al., 2008; Mocroft et al., 2013; Mocroft et al., 1998; Munoz et al., 1993; Yazdanpanah et al., 2001) The bar color indicates the approximate incidence rate (IR) per 100 person-years for each respective opportunistic pathogen. Dark orange represents IR >10, orange = IR between 5 and 10, light orange = IR between 1 and 5, and white = IR <1. As CD4 counts increase, differences in the incidence rates of opportunistic infections emerge. The incidence of CMV EOD is rare when CD4 counts are >200, while the incidence of esophageal candidiasis remains moderately elevated at CD4 counts between 200 and 350, and cases of pulmonary tuberculosis continue to occur at CD4 counts >500.
Initiation of anti-retroviral therapy (ART) halts HIV replication and raises CD4 T cell counts, but does not always restore pathogen-specific immunity to normal levels. For example, HIV positive individuals on ART with CD4 > 700/mm3 have 4-fold higher rates of TB disease than HIV uninfected individuals in the same community.(Gupta et al., 2012) The impact of ART on human papillomavirus (HPV) infection has been controversial, with some studies demonstrating reduced HPV prevalence and regression of HPV-associated squamous intraepithelial lesions (SIL), while others fail to show any impact on HPV-associated disease.(Adler, 2010)
It is thought that functional defects and depletion of pathogen-specific CD4 T cells by HIV occur at varying rates accounting for differences in pathogenesis of specific opportunistic infections.(Geldmacher and Koup, 2012) However, it remains unclear whether differences in pathogenesis are due to differences in pathogen-specific CD4 T cell susceptibility to HIV infection or other factors. Here, we review the immunopathogenesis of three infections causing substantial morbidity and mortality in HIV-infected individuals: TB, HPV, and cytomegalovirus (CMV). Understanding the complex interplay between HIV and these pathogens provides insight into differences in disease prevalence and impact of ART on the natural history of infection.
HIV-TB
Epidemiology and burden of disease
HIV and TB co-infection remains a serious global health problem. According to the World Health Organization, there were 8.7 million new cases of TB and 1.4 million deaths due to TB disease in 2011.(World Health Organization. and Global Tuberculosis Programme.) TB is a leading cause of death among HIV infected individuals, especially in Africa where over 50% of deaths in persons with HIV are due to TB disease.(Bates et al., 2013) HIV is a leading risk factor for TB disease with rates of active TB doubling within one year of HIV seroconversion and increasing more than 4-fold in chronic HIV infection.(Lodi et al., 2013; Sonnenberg et al., 2005) Although ART reduces the incidence of TB disease, rates of TB in individuals with reconstituted immune systems remain higher than the general population.(Gupta et al., 2012) This suggests that HIV infection induces functional defects in the immune response to TB that persist despite immune reconstitution.
Cell-mediated immune response to TB
Interactions between the host's innate and adaptive immune system and the organism dictate the outcome of infection with Mycobacterium tuberculosis (Mtb). Although innate immune cells are an important component of the immune response to TB infection (van Crevel et al., 2003) it is clear that T cells are essential for containing Mtb. Mice deficient in CD4 T cells have reduced survival and greater bacterial burden following aerosol exposure to Mtb than their wild type counterparts.(Caruso et al., 1999) Antibody mediated depletion of CD4 T cells in mice results in rapid reactivation of persistent TB infection and reduced survival.(Mogues et al., 2001) Similarly, non-human primates (NHP) depleted of CD4 T cells have an increased incidence of active TB disease following Mtb exposure and a higher rate of reactivation TB compared to non-CD4 depleted monkeys.(Lin et al., 2012) SIV infection of NHP with latent TB infection results in reactivation of TB in all infected monkeys, albeit at different rates.(Diedrich et al., 2010) Monkeys reactivating earlier exhibited a greater initial decline in CD4/8 T cells following SIV infection and had fewer CD4 T cells within their airways. Taken together, these animal studies support the critical role CD4 T cells play in controlling TB infection.
Why are CD4 T cells important? For one, they are a major source of IFNγ, which is necessary for the production of reactive nitrogen intermediates and killing of intracellular mycobacteria by macrophages. In fact, IFNγ specifically from CD4 T cells is required for a robust CD8 T cell response and for inhibiting intracellular replication of tubercle bacilli within macrophages.(Green et al., 2013) The critical role of IFNγ in controlling Mtb is best demonstrated in mice devoid of IFNγ or with impaired IFNγ signaling, which rapidly succumb to TB disease.(Flynn et al., 1993; Kamijo et al., 1993) In the same vein, humans with mutations in genes encoding IFNγ receptor 1 or with polymorphisms in the IFNγ gene are at increased risk of mycobacterial disease.(Lopez-Maderuelo et al., 2003; Newport et al., 1996) CD4 T cells are also crucial in maintaining the integrity and architecture of granulomas in TB infection.(Heuts et al., 2013; Saunders et al., 2002) Expression of CXCR5, a chemokine receptor important in B and T cell homing to lymphoid tissue, appears to be an important mediator of CD4 T cell localization within granulomas, facilitating lymphoid follicle formation and better protective outcomes.(Slight et al., 2013)
Studies of chronic viral infections in animals and humans suggest that with viral control the ability of CD4/8 T cells to produce multiple cytokines (polyfunctional T cells) increases.(Harari et al., 2005; Pantaleo and Harari, 2006) Whether this paradigm holds true for TB infection remains debatable. TB vaccine studies in animals have equated increased frequencies of polyfunctional T cells to protection from aerosol Mtb challenge.(Aagaard et al., 2009; Forbes et al., 2008) Polyfunctional T cells are induced in humans following the administration of novel TB vaccine formulations (Abel et al., 2010; Scriba et al., 2012) but whether this results in enhanced protection remains to be seen. Individuals with smear positive TB disease or untreated TB disease have reduced frequencies of polyfunctional CD4 T cells compared to individuals with smear negative TB disease or with latent or treated TB infection.(Day et al., 2011; Harari et al., 2011) These data suggest that as mycobacterial burden is reduced or controlled, T cell responses are characterized by the ability to produce multiple cytokines. However, not all authors have reached similar conclusions.(Caccamo et al., 2010; Sutherland et al., 2009) It is possible that differences in methodology or patient populations may account for the discordant results.
Impact of HIV infection on M. tuberculosis cell-mediated immunity
HIV co-infection adversely affects T cell responses to Mtb. Peripheral T cells from HIV-TB co-infected individuals have impaired proliferation and reduced IFNγ production in response to Mtb antigen stimulation compared to TB mono-infected patients.(Geldmacher et al., 2008; Zhang et al., 1994) Similar defects have been observed in Mtb-specific CD4 T cells isolated from lung lavage samples of HIV infected individuals.(Bonecini-Almeida Mda et al., 1998; Kalsdorf et al., 2009) In addition, HIV co-infection alters the quality of the T cell response, resulting in reduced frequencies of polyfunctional T cells. (Day et al., 2008; Jambo et al., 2011; Kalsdorf et al., 2009)
How soon after HIV infection are impairments in T cell responses seen? Dramatic decreases in Mtb-specific memory CD4 T cells (CD27+CD45RO+) were observed within one year of HIV seroconversion in 4 out of 5 individuals with latent TB infection.(Geldmacher et al., 2008) The early depletion of Mtb-specific CD4 T cells can be explained by distinct functional and phenotypic characteristics of these cells. First, Mtb-specific CD4 T cells have increased expression of the chemokine receptor CCR5, which also serves as a HIV co-receptor, leading to increased HIV entry into CD4 T cells.(Geldmacher et al., 2010) More importantly, Mtb-specific CD4 T cells preferentially produce IL-2 upon stimulation. IL-2 production not only supports proliferation of antigen-specific T cells but promotes HIV replication.(Geldmacher et al., 2010) In contrast, CMV-specific CD4 T cells tend to secrete beta-chemokines, such as MIP-1β, which can reduce susceptibility to HIV infection by hindering HIV binding to CCR5. Lastly, most Mtb-specific CD4 T cells express CD27, a cell surface maker associated with an early differentiated phenotype and replicative potential. CMV-specific CD4 T cells, on the other hand, rarely express CD27 and more often express CD57. (Geldmacher and Koup, 2012) Antigen-specific CD4 T cells expressing CD57 are more terminally differentiated, having reduced proliferative capacity and thus harboring lower cellular HIV loads. (Brenchley et al., 2004)
While we believe the early depletion of Mtb-specific CD4 T cells following HIV infection is an important contributor to the increased risk of TB disease, other mechanisms increasing TB reactivation risk in HIV-TB co-infected individuals should not be overlooked. HIV infection of macrophages reduces the apoptotic response to Mtb and down-regulates macrophage autophagy. (Patel et al., 2009; Zhou and Spector, 2008) This not only results in bacterial persistence, but could limit antigen presentation to T cells. Observational studies have noted a high frequency of vitamin D deficiency among HIV infected individuals and vitamin D deficiency has been associated with active TB disease. (Viard et al., 2011; Wilkinson et al., 2000) The bioactive form of vitamin D, 1,25 dihydroxyvitamin D3, restricts Mtb growth by inducing the expression of cathelicidin antimicrobial peptide which is important in the induction of macrophage autophagy. (Campbell and Spector, 2012; Liu et al., 2006) Finding ways to manipulate this pathway to modulate the host immune response to TB is of great interest. One additional factor contributing to increased TB risk is the genetic diversity of the pathogen itself. Strains of Mtb vary in their inhibition of innate immune responses and in virulence. A recent study suggests that strains of the modern lineage induce a lower inflammatory response, selecting for more rapid progression to active TB than strains of the ancient lineage. (Portevin et al., 2011) Further research on the immunobiology and clinical outcomes of various Mtb strains is needed to more fully understand the consequences of strain diversity in Mtb.
Impact of ART on TB disease
Suppression of HIV replication by ART leads to increases in CD4 T cell count and demonstrable mortality and morbidity benefits in patients co-infected with TB. This is especially true in individuals with CD4 counts less than 50 cells/mm3 if ART is started after 2 weeks of TB treatment. (Havlir et al., 2011) ART reduces the risk of TB disease by 67%. (Lawn et al., 2011) This may be due to increases in naïve and central memory CD4 T cell pools along with increases in IFNγ secretion or polyfunctional T cell responses following ART. (Sutherland et al., 2010; Wilkinson et al., 2009) However, despite HIV viral load suppression and reconstitution of CD4 T cell counts, ART-treated patients continue to have risks of TB disease that exceed that of the HIV-uninfected population. (Girardi et al., 2005) One possible explanation for this is that ART does not restore Mtb-specific immune responses to levels observed in HIV-uninfected individuals. (Schluger et al., 2002; Sutherland et al., 2006)
HIV-HPV
Epidemiology and burden of disease
HPV is the most common sexually transmitted infection (STI) globally. Persistent infection with oncogenic HPV types is linked to development of dysplastic changes in the cervix, vulva, and anorectal regions. If persistent, these dysplastic changes can progress to invasive squamous cell carcinoma.
Infection with HIV is associated with an increased prevalence of HPV infection, infection with multiple HPV types, and increased incidence of cervical cancer and other anogenital malignancies. (Luchters et al., 2010; Singh et al., 2009) Lower CD4 T cell counts and higher HIV viral loads are independent risk factors for HPV infection and infection with multiple HPV types. (Palefsky et al., 1999)
Natural history studies demonstrate that 70–80% of sexually active young women who develop incident cervical HPV infection clear their infection within 12 to 30 months. (Evander et al., 1995; Ho et al., 1998) Young women with low grade cervical lesions (CIN I) show spontaneous regression rates ranging from 45% to 90%, while women with pre-cancerous lesions (CIN III) show only an 11% regression rate. (Insinga et al., 2009) Although factors influencing the natural history of these different lesions are not completely known, cell-mediated immune responses are thought to play role.
Cell-mediated immune response to HPV infection
Immunohistopathological studies reveal that at the time of regression, HPV-infected genital lesions are infiltrated with CD8 cytotoxic T cells (CTL), CD4 T cells, and macrophages, suggesting that a cell-mediated immune response is integral to the control of HPV infection. (Coleman et al., 1994) Further evidence implicating T cells in the control of HPV infection comes from studies evaluating functional responses of T cells to HPV early antigens. Cellular immune responses directed against HPV16 E6 and E7 are associated with regression of HPV16-associated cervical lesions. (Kadish et al., 2002; Sarkar et al., 2005) HPV16-specific T cell responses are more frequently detected in women with resolved cervical HPV16 infection than with persistent infection. (Farhat et al., 2009; Nakagawa et al., 2000) Finally, HPV16-specific responses are largely absent or severely impaired in women with cervical cancer. (de Jong et al., 2004) These data imply that control of HPV infection requires a robust systemic T cell response. This observation is further supported by a recent study in which vaccine-induced regression of HPV16 high-grade vaginal intraepithelial neoplasia (VIN) was more common in women exhibiting strong and broad IFNγ-associated CD4 T cell responses to HPV 16 E6 and E7 proteins. (Welters et al., 2010)
Impact of HIV infection on HPV cell-mediated immunity
Evidence of direct biological effects of HIV on HPV infection comes from two studies of HPV reactivation. Strickler et al. evaluated the rate of new HPV DNA detection in sexually inactive (18 months of sexual abstinence) HIV positive and HIV negative women. HPV DNA was detected in 5% of sexually inactive HIV negative women compared to 22% of HIV positive women with advanced disease (CD4 T cell count <200 cells/mm3). (Strickler et al., 2005) Higher HPV reactivation rates in HIV infected women were also reported by Thieler and colleagues, who noted an association between HPV reactivation and declining CD4 T cell counts. (Theiler et al., 2010) These studies not only support the concept of HPV reactivation, but show that progressive CD4 depletion increases the risk of HPV reactivation.
If the risk of HPV infection is related to CD4 depletion, the risk should increase soon after HIV seroconversion. Why would this be the case? It is well known that during acute HIV and SIV infection there is rapid depletion of memory CD4 T cells within the mucosa, including the endocervical compartment. (Mattapallil et al., 2005; Veazey et al., 1998; Zhang et al., 1999) If CD4 T cells are critical to the control of HPV infection, the profound depletion of CD4 T cells within the genital tract soon after HIV infection should increase the risk of HPV infection.
Recently, two groups evaluated the impact of incident HIV on the detection of HPV infection. Relative to HIV uninfected women, HIV infected women had higher rates of HPV detection at their first visit following HIV seroconversion. (Nowak et al., 2011; Wang et al., 2011) HIV seroconverters had a 5-fold increased risk of HPV infection with multiple HPV types and a 2.5-fold increased risk of infection with any single HPV type. (Nowak et al., 2011) Moreover, HIV seroconversion was associated with a 2.5-fold increased risk of low-grade cervical cytological abnormalities. (Wang et al., 2011) These studies provide evidence of increased rates of HPV infection following HIV seroconversion which may be driven in part by the rapid HIV-associated CD4 T cell depletion within the cervical mucosa.
Why would CD4 T cells in the genital tract be subject to rapid depletion? A recent study of cervical CD4 T cells from female sex workers in Kenya characterized a subset of activated cervical CD4 T cells that produce IL-22 and IL-17a, identifying a Th17 cell population within the cervix. (McKinnon et al., 2011) Th17 cells are consistently depleted from the gut mucosa in HIV positive individuals. (Brenchley et al., 2008; Raffatellu et al., 2008) The authors showed that cervical Th17 cells express HIV susceptibility markers CCR5 and integrin α4β7. Integrin α4β7 is a lymphocyte mucosal homing marker which also serves as a cellular receptor for HIV-1 gp120. (Cicala et al., 2011) While integrin α4β7 is not required for HIV infection, it can enhance CD4 T cell susceptibility to HIV infection. McKinnon and colleagues showed that co-expression of these receptors by cervical CD4 T cells increased susceptibility to HIV infection and targeted depletion. (McKinnon et al., 2011) Although the rapid depletion of cervical CD4 T cells is driven by productive HIV infection of these cells, increased immune activation within genital mucosa may also contribute to CD4 T cell loss. (Jaspan et al., 2011) While CD4 T cell depletion within the genital mucosa may contribute to the increased risk of HPV infection, it remains unclear whether HPV-specific T cells are being preferentially targeted and depleted. Further research assessing the effects of HIV on the kinetics of HPV-specific T cells within the genital mucosa is needed.
Impact of ART on HPV Infection
The impact of ART on the natural history of HPV infection and its associated complications remains uncertain. The partial immune reconstitution observed with ART has decreased rates of some AIDS-defining malignancies, namely Kaposi's sarcoma and non-Hodgkin lymphoma; however, the same benefits have not been observed with respect to HPV-associated cervical cancer (Table 1). One theoretical explanation is that by prolonging survival of HIV infected women ART increases the cumulative exposure to oncogenic HPV types allowing for progression of HPV-related disease.
Table1.
Incidence of AIDS and non-AIDS defining malignancies in HIV positive individuals and the influence of ART.
| Cancer Type | Mean Standardized Incidence Ratio (Range) | Incidence Rate per 100 000 Person-years | |||
|---|---|---|---|---|---|
| Pre ART Era | Early ART Era | Late-ART Era | 0–6 mos post-ART initiation | 6mo-1 Ovr post-ART intiation | |
| AIDS Defining Cancers | |||||
| Kaposi Sarcoma | 1555.5 (246 – 2628.5) | 448.3 (47.8 – 848.8) | 317.1 (22.9 – 572) | 1342 | 164 |
| Non-hodgkin lymphoma | 537.3 (103 – 1011.8) | 260.4 (26.7 – 494.4) | 107.3 (16.2 – 212.2) | 357 | 134 |
| Non-hodgkin lymphoma (CNS) | 160 | 24 | |||
| Cervical | 69.8 (8.4 – 149.9) | 99.2 (3.7 – 194.6) | 88 (41.5 – 134.5) | n/a | n/a |
| Non-AIDS Defining Cancers | |||||
| Anal | 44.5 (19 – 97.9) | 90.1 (48.3 – 112.0) | 78.4 (44 – 141.4) | 72 | 69 |
| Hodgkin lymphoma | 16.5 (4.5 – 34.3) | 28.9 (11.1 – 54.7) | 36.4 (20.7 – 64.4) | 144 | 47 |
| Liver | 9.1 (0 – 19.9) | 17.5 (5.9 – 35.9) | 13.7 (6.1 – 35.4) | 18 | 39 |
| Lung | 24.3 (0 – 91.9) | 33.2 (2.8 – 93.8) | 23.5 (2.4 – 84.9) | 54 | 56 |
| Prostrate | 5.3 (0 – 14.7) | 13.3 (0 – 38.0) | 9.9 (0 – 37.5) | 22 | 58 |
| Breast | 19.1 (0.6 – 56.0) | 35.5 (1.2 – 69.9) | 32.5 (0.6 – 96.0) | 177 | 122 |
| Head and neck | 9.2 (1.4 – 29.0) | 12.1 (2.2 – 31) | 10.6 (1.5 – 36.9) | n/a | n/a |
| Melanoma | 4.4 (0 – 15.6) | 8.5 (0 – 24.8) | 10.7 (0.6 – 37.5) | n/a | n/a |
| HPV-related cancers | 108 | 103 | |||
The mean standardized incidence ratios (SIR) were calculated by averaging SIR reported in several studies of cancer risk in persons with HIV.(Dal Maso et al., 2009; Franceschi et al., 2010; Patel et al., 2008; Powles et al., 2009) The pre ART era covers the years prior to 1996, the early ART era covers 1996–2001, and the late ART era covers 2002–2007, though there is some overlap between the studies. The incidence ratios of Kaposi sarcoma (KS) and non-hodgkin lymphoma (NHL), both AIDS defining cancers, decrease considerably from the pre ART era to the late ART era. This is not seen with cervical cancer. In a separate study, administration of ART for >6 months reduced the incidence of both KS and NHL while no difference in incidence of HPV-related malignancies was noted.(Yanik et al., 2013)
A number of clinical studies have evaluated the impact of ART on HPV infection and HPV-related cervical disease and are reviewed in detail elsewhere. (Adler, 2010) Importantly, two large, longitudinal cohort studies of HIV positive women offered conflicting data on the effects of ART on HPV disease prevalence and HPV-related cervical disease. (Adler, 2010) However, in a very recent publication, Blitz et al. found that ART increases the likelihood of cervical SIL regression (HR 3.3), while also increasing clearance rates of oncogenic HPV types, although this was type specific. (Blitz et al., 2013) The conflicting results may be due to differences in primary end-points, cohort design, or definitions of cytological progression/regression. One additional factor to consider is the assessment of ART efficacy. Minkoff and colleagues showed that women who were adherent to ART had a more significant decline in detection of oncogenic HPV and more rapid clearance of HPV SIL, compared to non-adherent women.(Minkoff et al., 2010) While there seems to be mounting evidence that ART may have a beneficial impact on the natural history of HPV the extent and magnitude of this effect is not clear and the influence of ART on the kinetics of HPV-specific immunity remains largely unknown.
Impact of HPV on HIV
Sexually transmitted infections such as herpes simplex virus-2 and syphilis have a profound impact on HIV acquisition. A recent review assessed the association of HPV infection with HIV acquisition. Women with prevalent HPV infection, irrespective of HPV type, had a 2-fold higher risk of HIV infection. An increased risk of HIV acquisition was also observed in men with HPV infection, though this data was generated from only two observational studies.(Houlihan et al., 2012).
Biologically, it is plausible that HPV infection could affect HIV acquisition and dissemination. As just described, T cells are an integral component of the host immune response to HPV. Given that CD4 T cells are primary targets for HIV, a robust T cell response to HPV infection could increase risk of HIV acquisition and dissemination. Langerhans cells are found in the mucosal layer of the genital tract and are generally refractory to HIV transmission.(de Witte et al., 2007) Depletion of Langerhans cells within the cervical epithelium in HPV infection could contribute to increased HIV acquisition.(Connor et al., 1999; Tay et al., 1987) Additionally, E-cadherin, an epithelial adhesion molecule, is down-regulated during HPV infection (Matthews et al., 2003; Vessey et al., 1995) which could potentially increase genital mucosa permeability to HIV.
Given that HPV is the most common STI worldwide, a more thorough understanding of the contribution of HPV infection to the HIV epidemic is required. Further research into the biological mechanisms underlying this increased risk should be pursued. With the implementation of routine HPV vaccination, studies evaluating the impact of HPV vaccination on the HIV epidemic should be considered.
HIV-CMV
Epidemiology and burden of disease
CMV is a member of the human herpesvirus family. More than half of the adult human population is infected with CMV with higher rates of infection in HIV positive individuals.(Bate et al., 2010; Lang et al., 1989) Following primary infection, the virus is not eliminated but remains in a latent state within many tissues of the host. In healthy individuals, CMV can reactivate intermittently with little consequence; however, in immunocompromised hosts reactivation can lead to end-organ disease (EOD).
In HIV infection, CMV EOD typically occurs when CD4 cell counts decrease to <50 cells/mm3. CMV retinitis, the most common form of EOD in HIV infection, occurs in 40% of individuals with CD4 cell counts <50 cells/mm3.(Pertel et al., 1992) The incidence of CMV EOD has decreased substantially with the introduction of ART.(Ledergerber et al., 1999; Palella et al., 1998) The increased incidence of CMV disease in patients with AIDS and restoration of protective immunity to CMV with ART suggests that cellular immunity is critical to the control of CMV infection.
Cell-mediated immune response to CMV
The immune system utilizes tremendous resources to control CMV. It is estimated that approximately 10% of the total memory CD4 and CD8 T cell pool in CMV seropositive healthy donors is specific for CMV antigens, with higher frequencies in certain populations.(Khan et al., 2004; Sester et al., 2002; Sylwester et al., 2005) The role of CD8 T cells in controlling CMV viral replication has been well described in animal models. (Barry et al., 2007; Polic et al., 1998; Reddehase et al., 1985) In humans, seminal works by Riddell and Walter showed that infusion of donor-derived CMV-specific CD8 T cells not only restored CMV-specific cellular immunity but also provided protection against CMV clinical disease.(Riddell et al., 1992; Walter et al., 1995)
While CD8 T cells have a clear role in controlling CMV infection, CD4 T cells are also critical to immune control. During primary CMV infection, there is a major expansion of CMV-specific CD4 T cells and these responses appear before CD8 T cell responses.(Harari et al., 2004) Delays in CD4 T cell response during primary infection have been associated with symptomatic infection and prolonged viral shedding.(Gamadia et al., 2003; Tu et al., 2004) It has also been shown that recovery from primary infection requires CMV-specific CD4 T cell responses.(Gamadia et al., 2003) Why might this be the case? Komanduri et al. demonstrated that while CD4 and CD8 T cell responses to CMV are sustained over time, individuals maintaining robust CD4 T cell responses are more likely to exhibit higher frequencies of CMV-specific CD8 T cells.(Komanduri et al., 2001) Thus, CMV-specific CD4 T cells are important for maintaining and expanding the CMV-specific CD8 T cell pool.
Apart from providing help to CD8 T cells, CD4 T cells may control infection through direct killing of virus-infected cells. Casazza and colleagues demonstrated that certain populations of CMV-specific CD4 T cells contain granzyme A, granzyme B, and perforin, and that these cells are capable of degranulating and killing cells expressing their cognate antigen.(Casazza et al., 2006)
Impact of HIV on CMV immunobiology
Just as loss of Mtb-specific CD4 T cells contributes to the increased risk of TB disease in HIV-TB co-infection, loss of CMV-specific T cells contributes to the pathogenesis of CMV EOD in HIV infected individuals. Patients with active CMV disease have diminished CMV-specific T cell responses compared to individuals with immune recovery from CMV EOD.(Jacobson et al., 2001; Komanduri et al., 1998) In a study evaluating the dynamics of CMV-specific T cell responses, significant reductions in CMV-specific IFNγ-producing CD4 T cells were seen in the year prior to onset of CMV EOD.(Bronke et al., 2005) Coincident with the reduction in CMV-specific CD4 T cells was a decline in CMV-specific CD8 T cells producing IFNγ. This is not surprising as CD4 T cell help is required for maintaining and expanding the CD8 T cell pool.
Unlike Mtb-specific CD4 T cell responses, the frequency of CMV-specific CD4 T cell responses is generally maintained in chronic HIV infection and is similar to that observed in CMV seropositive, HIV negative individuals.(Komanduri et al., 1998) In a recent study evaluating CMV-specific T cell responses across various HIV disease states, CMV-specific CD4 and CD8 T cell responses were higher in chronic, untreated HIV infection than in acute HIV infection.(Naeger et al., 2010)
Why is it that following HIV seroconversion Mtb-specific CD4 T cells are lost whereas CMV-specific CD4 T cells are generally maintained? CMV-specific CD4 T cells produce MIP-1β and MIP-1α upon stimulation and bind to CCR5, which also serves as an HIV co-receptor. In addition, CMV-specific CD4 T cells producing MIP-1 β have lower surface expression of CCR5 and are less frequently infected with HIV than CMV-specific CD4 T cells that do not produce MIP-1β.(Casazza et al., 2009) We proposed that the autocrine production of beta-chemokines by CMV-specific CD4 T cells blocks R5-tropic HIV entry, either by direct competition or down-regulation of CCR5 surface expression. Recently, Hu and colleagues have extended this observation. In a novel study, they showed that neutralization of beta-chemokines led to enhanced HIV infection in tetanus toxoid- and C. albicans-specific CD4 T cells, while HIV infection of CMV-specific CD4 T cells remained low suggesting post entry restriction of HIV by CMV-specific CD4 T cells.(Hu et al., 2013) Microarray analysis revealed that a broad array of innate antiviral genes was activated in CMV-specific CD4 T cells, including several type-I IFN response genes and HIV/SIV restriction factors (TRIM22 and TRIM5). This implies that other mechanisms may be involved in determining differential susceptibility of pathogen-specific CD4 T cells to HIV infection.
Conclusion
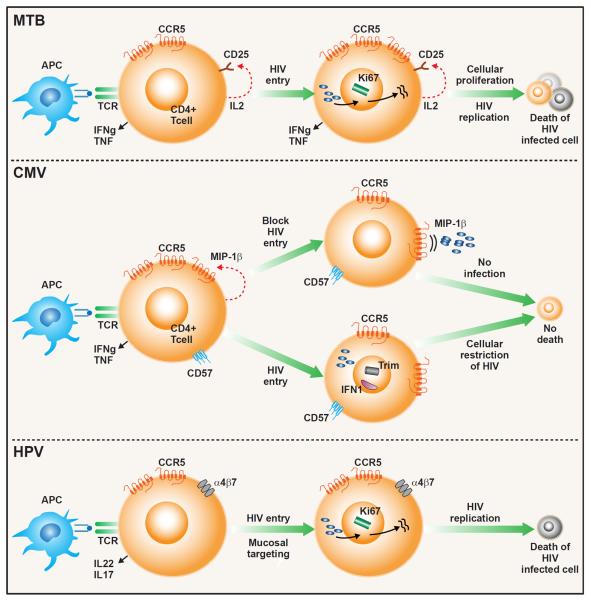
The timing of opportunistic infections in HIV infection is not always associated with severity of CD4 T cell depletion and pathogen-specific differences in the incidence of infection within CD4 strata exist. Recent data suggest that functional or phenotypic differences in pathogen-specific CD4 T cells may influence the timing of disease reactivation (figure 2). For example, Mtb-specific CD4 T cells have high expression of CCR5 and preferentially produce IL-2 leading to increased susceptibility to HIV infection. Cervical CD4 T cells also have increased CCR5 expression but also express integrin α4β7 increasing their susceptibility to HIV infection. In contrast, CMV-specific CD4 T cells preferentially produce beta-chemokines, and express type I interferon response genes and genes encoding HIV restriction factors all of which reduce their susceptibility to productive HIV infection and delay disease reactivation until there is a profound loss of CD4 T cells. While ART has reduced the burden of many opportunistic infections, pathogen-specific immunity is not always fully restored as the risk of infection from certain opportunistic pathogens in immune reconstituted HIV positive individuals continues to exceed that of the general population.
Figure 2. Proposed model for HIV-associated depletion of pathogen-specific CD4 T cells.
(Top panel) Mtb-specific CD4 T cell is activated upon binding to its cognate antigen on an HIV infected antigen-presenting cell (APC). T cell activation induces secretion of IL-2 and up-regulation of CD25 (IL-2 receptor) and CCR5 (HIV co-receptor). Binding of IL-2 to CD25 induces cellular proliferation (expression of proliferation marker Ki67) and promotes viral replication leading to productive HIV infection and cell death. (Middle Panel). Upon activation, CMV-specific CD4 T cells produce MIP-1β, which binds to its ligand, CCR5, an HIV co-receptor, blocking HIV entry into the cell. Binding of MIP-1β to CCR5 may also down-regulate CCR5 further impeding HIV entry. CMV-specific CD4 T cells express CD57, a marker to replicative senescence, and do not enter the cell cycle, limiting HIV viral replication. Expression of TRIM and Type I IFN may further restrict HIV replication. These cells are less susceptible to productive HIV infection and targeted depletion. (Bottom Panel). Activation of cervical CD4 cells leads to increased expression of both CCR5 and the mucosal homing receptor, integrin α4β7, which binds to HIV gp120. Co-expression of these receptors increases susceptibility to HIV infection. These cells are also highly activated (express Ki67), promoting HIV replication, leading to productive infection and cell death.
The immunobiology of HIV and co-pathogen infection remains quite complex and the mechanisms leading to differential susceptibility of pathogen-specific CD4 T cells to HIV infection are only now beginning to be understood. Powerful technologies such as mulitparameter flow cytometry and microarrays have advanced our understanding but novel platforms allowing analysis at the single cell level may provide us with a better appreciation of how these pathogen-specific differences are generated. As ART is initiated even earlier in HIV infection, it will be important to assess whether earlier implementation improves pathogen-specific immunity. Although mechanistic studies of HIV and co-pathogen infection are complex, a more thorough understanding of this relationship will better inform treatment strategies against co-pathogens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PloS one. 2009;4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. American journal of respiratory and critical care medicine. 2010;181:1407–1417. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler DH. The impact of HAART on HPV-related cervical disease. Current HIV research. 2010;8:493–497. doi: 10.2174/157016210793499240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, Traore M, Coulibaly A, Freedberg KA, Lewden C, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:714–723. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AP, Silvestri G, Safrit JT, Sumpter B, Kozyr N, McClure HM, Staprans SI, Feinberg MB. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J Immunol. 2007;178:8002–8012. doi: 10.4049/jimmunol.178.12.8002. [DOI] [PubMed] [Google Scholar]
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys. 2010. pp. 1988–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, Mudenda V, Mwaba P, Zumla A. Deaths due to respiratory tract infections in Africa: a review of autopsy studies. Current opinion in pulmonary medicine. 2013;19:229–237. doi: 10.1097/MCP.0b013e32835f4fe4. [DOI] [PubMed] [Google Scholar]
- Blitz S, Baxter J, Raboud J, Walmsley S, Rachlis A, Smaill F, Ferenczy A, Coutlee F, Hankins C, Money D. Evaluation of HIV and Highly Active Antiretroviral Therapy on the Natural History of Human Papillomavirus Infection and Cervical Cytopathologic Findings in HIV-Positive and High-Risk HIV-Negative Women. The Journal of infectious diseases. 2013 doi: 10.1093/infdis/jit181. [DOI] [PubMed] [Google Scholar]
- Bonecini-Almeida Mda G, Werneck-Barroso E, Carvalho PB, de Moura CP, Andrade EF, Hafner A, Carvalho CE, Ho JL, Kritski AL, Morgado MG. Functional activity of alveolar and peripheral cells in patients with human acquired immunodeficiency syndrome and pulmonary tuberculosis. Cellular immunology. 1998;190:112–120. doi: 10.1006/cimm.1998.1399. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. Journal of virology. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronke C, Palmer NM, Jansen CA, Westerlaken GH, Polstra AM, Reiss P, Bakker M, Miedema F, Tesselaar K, van Baarle D. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. The Journal of infectious diseases. 2005;191:873–880. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. European journal of immunology. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS pathogens. 2012;8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. The Journal of experimental medicine. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza JP, Brenchley JM, Hill BJ, Ayana R, Ambrozak D, Roederer M, Douek DC, Betts MR, Koup RA. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS pathogens. 2009;5:e1000646. doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. Journal of translational medicine. 2011;9(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, Taylor-Robinson D, Stanley MA. Immunological events in regressing genital warts. American journal of clinical pathology. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- Connor JP, Ferrer K, Kane JP, Goldberg JM. Evaluation of Langerhans' cells in the cervical epithelium of women with cervical intraepithelial neoplasia. Gynecologic oncology. 1999;75:130–135. doi: 10.1006/gyno.1999.5559. [DOI] [PubMed] [Google Scholar]
- Dal Maso L, Polesel J, Serraino D, Lise M, Piselli P, Falcini F, Russo A, Intrieri T, Vercelli M, Zambon P, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. British journal of cancer. 2009;100:840–847. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. The Journal of infectious diseases. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer research. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature medicine. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PloS one. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Human papillomavirus infection is transient in young women: a population-based cohort study. The Journal of infectious diseases. 1995;171:1026–1030. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009;19:508–512. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of experimental medicine. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, Bouchardy C, Dehler S, Jundt G, Ess S, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. British journal of cancer. 2010;103:416–422. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- Geldmacher C, Koup RA. Pathogen-specific T cell depletion and reactivation of opportunistic pathogens in HIV infection. Trends in immunology. 2012;33:207–214. doi: 10.1016/j.it.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. The Journal of experimental medicine. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. The Journal of infectious diseases. 2008;198:1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, Dabis F, Reiss P, Kirk O, Bernasconi E, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol. 2013;190:270–277. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PloS one. 2012;7:e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nature medicine. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- Harari A, Zimmerli SC, Pantaleo G. Cytomegalovirus (CMV)-specific cellular immune responses. Human immunology. 2004;65:500–506. doi: 10.1016/j.humimm.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. The New England journal of medicine. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuts F, Gavier-Widen D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6482–6487. doi: 10.1073/pnas.1219985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. The New England journal of medicine. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- Houlihan CF, Larke NL, Watson-Jones D, Smith-McCune KK, Shiboski S, Gravitt PE, Smith JS, Kuhn L, Wang C, Hayes R. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS. 2012;26:2211–2222. doi: 10.1097/QAD.0b013e328358d908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Nau M, Ehrenberg P, Chenine AL, Macedo C, Zhou Y, Daye ZJ, Wei Z, Vahey M, Michael NL, et al. Distinct gene-expression profiles associated with the susceptibility of pathogen-specific CD4 T cells to HIV-1 infection. Blood. 2013;121:1136–1144. doi: 10.1182/blood-2012-07-446278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of Human Papillomavirus (HPV) Disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC infectious diseases. 2009;9:119. doi: 10.1186/1471-2334-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MA, Schrier R, McCune JM, Torriani FJ, Holland GN, O'Donnell JJ, Freeman WR, Bredt BM. Cytomegalovirus (CMV)-specific CD4+ T lymphocyte immune function in long-term survivors of AIDS-related CMV end-organ disease who are receiving potent antiretroviral therapy. The Journal of infectious diseases. 2001;183:1399–1404. doi: 10.1086/319854. [DOI] [PubMed] [Google Scholar]
- Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, Heyderman RS, Gordon SB. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66:375–382. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspan HB, Liebenberg L, Hanekom W, Burgers W, Coetzee D, Williamson AL, Little F, Myer L, Coombs RW, Sodora D, et al. Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. The Journal of infectious diseases. 2011;204:1550–1556. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, He W, Romney SL, Johnson A, Angeletti R, et al. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:483–488. [PubMed] [Google Scholar]
- Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. American journal of respiratory and critical care medicine. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R, Le J, Shapiro D, Havell EA, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. The Journal of experimental medicine. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- Komanduri KV, Donahoe SM, Moretto WJ, Schmidt DK, Gillespie G, Ogg GS, Roederer M, Nixon DF, McCune JM. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- Komanduri KV, Viswanathan MN, Wieder ED, Schmidt DK, Bredt BM, Jacobson MA, McCune JM. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nature medicine. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kovacs AA, Zaia JA, Doelkin G, Niland JC, Aledort L, Azen SP, Fletcher MA, Gauderman J, Gjerset GJ, et al. Seroepidemiologic studies of cytomegalovirus and Epstein-Barr virus infections in relation to human immunodeficiency virus type 1 infection in selected recipient populations. Transfusion Safety Study Group. J Acquir Immune Defic Syndr. 1989;2:540–549. [PubMed] [Google Scholar]
- Lawn SD, Harries AD, Williams BG, Chaisson RE, Losina E, De Cock KM, Wood R. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2011;15:571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, Battegay M, Vernazza P, Bernasconi E, Opravil M, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA : the journal of the American Medical Association. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, Flynn JL. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS research and human retroviruses. 2012;28:1693–1702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Lodi S, del Amo J, d'Arminio Monforte A, Abgrall S, Sabin C, Morrison C, Furrer H, Muga R, Porter K, Girardi E. Risk of tuberculosis following HIV seroconversion in high-income countries. Thorax. 2013;68:207–213. doi: 10.1136/thoraxjnl-2012-201740. [DOI] [PubMed] [Google Scholar]
- Lopez-Maderuelo D, Arnalich F, Serantes R, Gonzalez A, Codoceo R, Madero R, Vazquez JJ, Montiel C. Interferon-gamma and interleukin-10 gene polymorphisms in pulmonary tuberculosis. American journal of respiratory and critical care medicine. 2003;167:970–975. doi: 10.1164/rccm.200205-438BC. [DOI] [PubMed] [Google Scholar]
- Luchters SM, Vanden Broeck D, Chersich MF, Nel A, Delva W, Mandaliya K, Depuydt CE, Claeys P, Bogers JP, Temmerman M. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC infectious diseases. 2010;10:18. doi: 10.1186/1471-2334-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, Doorbar J, Hibma M. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. Journal of virology. 2003;77:8378–8385. doi: 10.1128/JVI.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- Minga AK, Anglaret X, d' Aquin Toni T, Chaix ML, Dohoun L, Abo Y, Coulibaly A, Duvignac J, Gabillard D, Rouet F, et al. HIV-1 DNA in peripheral blood mononuclear cells is strongly associated with HIV-1 disease progression in recently infected West African adults. J Acquir Immune Defic Syndr. 2008;48:350–354. doi: 10.1097/QAI.0b013e3181775e55. [DOI] [PubMed] [Google Scholar]
- Minkoff H, Zhong Y, Burk RD, Palefsky JM, Xue X, Watts DH, Levine AM, Wright RL, Colie C, D'Souza G, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. The Journal of infectious diseases. 2010;201:681–690. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, Abgrall S, Ayayi S, Bartmeyer B, Braun D, et al. The Incidence of AIDS-Defining Illnesses at a Current CD4 Count >=200 Cells/muL in the Post-Combination Antiretroviral Therapy Era. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Youle M, Phillips AN, Halai R, Easterbrook P, Johnson MA, Gazzard B. The incidence of AIDS-defining illnesses in 4883 patients with human immunodeficiency virus infection. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. Archives of internal medicine. 1998;158:491–497. doi: 10.1001/archinte.158.5.491. [DOI] [PubMed] [Google Scholar]
- Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. The Journal of experimental medicine. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A, Schrager LK, Bacellar H, Speizer I, Vermund SH, Detels R, Saah AJ, Kingsley LA, Seminara D, Phair JP. Trends in the incidence of outcomes defining acquired immunodeficiency syndrome (AIDS) in the Multicenter AIDS Cohort Study: 1985–1991. American journal of epidemiology. 1993;137:423–438. doi: 10.1093/oxfordjournals.aje.a116691. [DOI] [PubMed] [Google Scholar]
- Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PloS one. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. The Journal of infectious diseases. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. The New England journal of medicine. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- Nowak RG, Gravitt PE, Morrison CS, Gange SJ, Kwok C, Oliver AE, Howard R, Van der Pol B, Salata RA, Padian NS, et al. Increases in human papillomavirus detection during early HIV infection among women in Zimbabwe. The Journal of infectious diseases. 2011;203:1182–1191. doi: 10.1093/infdis/jiq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, Young M, Melnick S, Miotti P, Burk R. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. Journal of the National Cancer Institute. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nature reviews Immunology. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- Patel NR, Swan K, Li X, Tachado SD, Koziel H. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. Journal of leukocyte biology. 2009;86:53–60. doi: 10.1189/JLB.0908574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, Holmberg SD, Brooks JT. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Annals of internal medicine. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- Pertel P, Hirschtick R, Phair J, Chmiel J, Poggensee L, Murphy R. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1992;5:1069–1074. [PubMed] [Google Scholar]
- Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski UH. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. The Journal of experimental medicine. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portevin D, Gagneux S, Comas I, Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS pathogens. 2011;7:e1001307. doi: 10.1371/journal.ppat.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, Mandelia S, Moller H, Bower M. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nature medicine. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. Journal of virology. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Sarkar AK, Tortolero-Luna G, Follen M, Sastry KJ. Inverse correlation of cellular immune responses specific to synthetic peptides from the E6 and E7 oncoproteins of HPV-16 with recurrence of cervical intraepithelial neoplasia in a cross-sectional study. Gynecologic oncology. 2005;99:S251–261. doi: 10.1016/j.ygyno.2005.07.099. [DOI] [PubMed] [Google Scholar]
- Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cellular immunology. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, Mauff K, Moyo S, Brittain N, Lawrie A, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV− and/or Mycobacterium tuberculosis-infected adults. American journal of respiratory and critical care medicine. 2012;185:769–778. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sester M, Sester U, Gartner B, Kubuschok B, Girndt M, Meyerhans A, Kohler H. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. Journal of virology. 2002;76:3748–3755. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Anastos K, Hoover DR, Burk RD, Shi Q, Ngendahayo L, Mutimura E, Cajigas A, Bigirimani V, Cai X, et al. Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women. The Journal of infectious diseases. 2009;199:1851–1861. doi: 10.1086/599123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. The Journal of clinical investigation. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. The Journal of infectious diseases. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, Hall C, Bacon M, Levine AM, Watts DH, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. Journal of the National Cancer Institute. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. European journal of immunology. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Young JM, Peterson KL, Sanneh B, Whittle HC, Rowland-Jones SL, Adegbola RA, Jaye A, Ota MO. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J Immunol. 2010;184:6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Yang H, Scriba TJ, Ondondo B, Robinson N, Conlon C, Suttill A, McShane H, Fidler S, McMichael A, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS. 2006;20:821–829. doi: 10.1097/01.aids.0000218545.31716.a4. [DOI] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of experimental medicine. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay SK, Jenkins D, Maddox P, Campion M, Singer A. Subpopulations of Langerhans' cells in cervical neoplasia. British journal of obstetrics and gynaecology. 1987;94:10–15. doi: 10.1111/j.1471-0528.1987.tb02244.x. [DOI] [PubMed] [Google Scholar]
- Theiler RN, Farr SL, Karon JM, Paramsothy P, Viscidi R, Duerr A, Cu-Uvin S, Sobel J, Shah K, Klein RS, et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women: risk factors for cervical viral shedding. Obstetrics and gynecology. 2010;115:1150–1158. doi: 10.1097/AOG.0b013e3181e00927. [DOI] [PubMed] [Google Scholar]
- Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Advances in experimental medicine and biology. 2003;531:241–247. doi: 10.1007/978-1-4615-0059-9_20. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Vessey CJ, Wilding J, Folarin N, Hirano S, Takeichi M, Soutter P, Stamp GW, Pignatelli M. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. The Journal of pathology. 1995;176:151–159. doi: 10.1002/path.1711760208. [DOI] [PubMed] [Google Scholar]
- Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, Gatell J, Pedersen C, Bogner JR, Lundgren JD, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England journal of medicine. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Wang C, Wright TC, Denny L, Kuhn L. Rapid rise in detection of human papillomavirus (HPV) infection soon after incident HIV infection among South African women. The Journal of infectious diseases. 2011;203:479–486. doi: 10.1093/infdis/jiq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Seldon R, Meintjes G, Rangaka MX, Hanekom WA, Maartens G, Wilkinson RJ. Dissection of regenerating T-Cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. American journal of respiratory and critical care medicine. 2009;180:674–683. doi: 10.1164/rccm.200904-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Tuberculosis Programme . Global tuberculosis control : WHO report. Global Tuberculosis Programme; Geneva: p. v. [Google Scholar]
- Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S, Olshan A, Dittmer DP, Kitahata MM, Mugavero MJ, Saag M, et al. Incidence and Timing of Cancer in HIV-Infected Individuals Following Initiation of Combination Antiretroviral Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 doi: 10.1093/cid/cit369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah Y, Chene G, Losina E, Goldie SJ, Merchadou LD, Alfandari S, Seage GR, 3rd, Sullivan L, Marimoutou C, Paltiel AD, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. International journal of epidemiology. 2001;30:864–871. doi: 10.1093/ije/30.4.864. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. The Journal of clinical investigation. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]