Abstract
Investment in signalling is subject to multiple trade-offs that vary with life-stage, leading to a complex relationship between survival and trait expression. We show a negative relationship between survival and song rate in response to simulated territorial intrusion in male banded wrens (Thryophilus pleurostictus), and test several explanations for this association. (1) Male age failed to explain the association: though age affected song rate in a cross-sectional analysis, longitudinal analysis showed that individuals did not increase their song rate as they got older. Reconciling these results suggests differential selection against young males that respond to intrusion with low song rates. (2) Mortality costs of high song rates did not appear to explain the negative relationship between song rate and survival because, though song rate in response to playback was condition-dependent, high song rates in a different context did not appear to impose mortality costs. (3) High levels of territorial pressure may have increased mortality, but were not associated with high song rates in response to playback. (4) Since song rates did not increase with age, but tended to increase only in the last year of life, we tentatively suggest that the negative relationship between song rate and survival could represent a terminal investment in territorial defence by males in their final breeding season, though further work is needed to confirm this conclusion.
Keywords: birdsong, life-history, sexual selection, terminal investment
Introduction
Sexually selected traits may signal aspects of individual quality such as longevity, but in the context of intra- and inter-sexual conflicts of interest, signal honesty is maintained by costs that could negatively influence survival (Jennions et al. 2001). The expression and cost of a trait are thus subject to a trade-off which should be borne better by high-quality individuals, and depend on future reproductive prospects (Grafen 1990). When survival prospects are low, individuals may invest terminally in greater expression of a trait (Candolin 1999; Sadd et al. 2006). Survival and the intensity of expression of sexually selected traits are most commonly positively correlated, consistent with trait expression signalling quality (reviewed in Jennions et al. 2001). However, negative relationships are also observed (Hunt et al. 2004), and theory predicts that the relationship may be either positive, negative, or neutral, depending on life-history trade-offs and mating skew (Kokko et al. 2002).
In many territorial songbirds, song rate functions in both inter- and intra-sexual selection (Catchpole & Slater 1995). Song rate is a flexible signal that can indicate motivation, body condition, parasite load, reproductive success, parental care and territory quality in a range of bird species (reviewed in: Vehrencamp 2000; Collins 2004; Searcy & Nowicki 2005). Song rate is positively correlated with male body condition (Nystrom 1997; Marchetti 1998), suggesting that high song rates are costly. Females prefer males that sing at high rates (Collins 2004), and benefit from earlier mating (Hofstad et al. 2002), higher quality territories (Hoileitner et al. 1995), higher offspring feeding rates (Welling et al. 1997), and higher nesting success (Hoileitner et al. 1993). The role of song rate in male-male interactions is less clear, though the flexibility of the signal likely allows it to signal aggressive motivation (Collins 2004). As a signal of body condition, it may play a role in determining the outcome of aggressive interactions. In song sparrows (Melodia melospiza), territorial challengers increased their song rate to twice that of defenders in the minutes before a physical fight, and winners had higher song rates than losers (Bower 2000; Bower 2005). In black-capped chickadees (Parus atricapillus), high ranking males sang at higher rates than low ranking males (Otter et al. 1997). There is no consistent relationship between song rate and age in birds: yearling collared flycatchers (Ficedula albicollis) sang at higher rates than older males (Garamszegi et al. 2007), while element rate increased with age in willow warblers (Phylloscopus trochilus), though high element rates appeared to be associated with higher mortality among young males (Gil et al. 2001). In contrast, male willow tits that sang at high rates had higher survival, suggesting that song rate was an honest indicator of male quality in this species (Welling et al. 1997).
We examine the relationship between survival and song rate in the context of territorial defence in male banded wrens (Thryophilus pleurostictus). Banded wrens are long-lived, sedentary songbirds and males sing vigorously to advertise and defend all-purpose territories (Molles & Vehrencamp 1999). Various aspects of intra-sexual vocal interactions have been investigated using playback experiments over the course of a long-term study (Molles & Vehrencamp 2001a,b; Hall et al. 2006; Illes et al. 2006; Molles 2006; Vehrencamp et al. 2007). Following an experiment testing the signal value of overlapping songs (Hall et al. 2006), we noted that males that did not survive to the start of the following breeding season had sung at higher rates in response to playback than males that did survive. Here we re-examine data from all playback experiments to determine whether there is a consistent relationship between survival and song rate in response to playback in banded wrens. We then use additional data on male age, dawn song rates, and body condition available in some years to test the signal value of song rate in banded wrens, and explore four explanations for the negative relationship between song rate and survival that are not mutually exclusive: (1) Age: song rate increases with age, and reduced survival probability among old males leads to a negative association between song rate and survival. Predictions: a) song rate increases with age in both cross-sectional and longitudinal samples (differential survival may confound cross-sectional analyses, Gil et al. 2001), and b) older males have higher mortality than younger males. (2) Costly signal: males that sing at high rates suffer costs of trait expression that increase their mortality. Predictions: a) there is individual consistency in song rate, so song rate in different contexts is positively correlated, and b) high song rate in any context is associated with higher mortality. (3) Territory pressure: males suffering high levels of boundary conflict with neighbours have higher mortality and are more aggressive towards simulated intruders, leading to an association between playback song rate and mortality. Predictions: a) males with more closely singing neighbours have higher mortality, and b) males with more closely singing neighbours respond to playback with higher song rates. (4) Terminal investment: males in their last breeding season invest terminally in high song rate. Predictions: a) males sing more in response to playback in their last year than in previous years, regardless of age.
Methods
Playback experiments
We re-examine male song rate in six playback experiments conducted between 1998 and 2005 during the breeding season, between May and September (Molles & Vehrencamp 2001a,b; Hall et al. 2006; Illes et al. 2006; Molles 2006; Vehrencamp et al. 2007). All aspects of playback methods relevant to this study are described here and in Table 1; further details can be found in the relevant publications. In all except the Sender experiment, subjects were attracted to the area with a lure prior to presentation of the experimental stimuli. The rate at which playback songs were presented varied between experiments: songs were usually broadcast at intervals of 10 to 12 sec, but presentation rate was higher in the Trill experiment (mean 8.7 songs/min), and in the Overlap experiment stimulus song rate matched that of the subject with each playback song broadcast after a subject song. Experimental stimuli were presented from a single speaker in all except the Share and Trill experiments, where two speakers simulated a moving intruder and two intruders respectively.
Table 1.
Summary of playback experiments.
| Experiment | Year | Age range; # known-age | Song rate period (playback duration) | Treatments (trials/male) | Source |
|---|---|---|---|---|---|
| 1. Mimic | 1998 | 1 to 3 y; 0 | 3 min |
|
(Molles & Vehrencamp 2001a) |
| 2. Share | 1998/9 | 1 to 4 y; 0 | 4.6 min (range 3.7 - 4.4) |
|
(Molles & Vehrencamp 2001b)* |
| 3. Switch | 1998 | 1 to 3 y; 0 | 3 min |
|
(Molles 2006) |
| 4. Overlap | 2003 | 1 to 8 y; 4 | 4.6 min (range 1.1 - 19.6) |
|
(Hall et al. 2006) |
| 5. Trill | 2004 | 1 to 9 y; 7 | 1.4 min (range 0.8 - 1.9) |
|
(Illes et al. 2006) |
| 6. Sender | 2005 | 1 to 5 y; 12 | 5 min |
|
(Vehrencamp et al. 2007) |
A second experiment described in this publication is not included in this analysis because only two subjects died.
Song rate and survival
We searched intensively in the year after each experiment to determine whether males survived to the start of the following breeding season. Within the core study area, all males were colour-banded and resighted weekly on their territories throughout the field season, so all disappearances and movements within this area were known. The surrounding area (at least 200m up to more than 1km from the edge of the core area) was searched systematically at the start of each field season in 2003-2006, using playback on a 100 × 100 m grid to attract the wrens and determine whether or not they were colour-banded. Failure to resight males provided a reasonably good indicator that they had died, as males seldom dispersed after acquiring a territory – in 8 years of intensive study on a population encompassing 20 to 40 territories, we documented only 3 cases in which established males moved one to two territories.
We pooled data from all experiments to examine the relationship between survival and song rate during the playback period. Playback song rate in each trial was computed as the number of songs sung divided by playback duration (Table 1 gives playback duration for each experiment), and rate values were transformed (log(x +1)) to normalise model residuals. Playback treatments did not influence song rate (Molles & Vehrencamp 2001a; Hall et al. 2006; Molles 2006), but experiment was included as a fixed effect to control for differences between experiments (or years), and male identity was included as a random effect to control for non-independence associated with individuals that were subjects in more than one experiment (n = 38 males) or trial (Table 1 indicates number of trials per experiment). Since song rate during the playback period depended on how long subjects took to start singing in all except the Overlap experiment (because song rate was computed from the start of the playback period rather than from the time the subject sang his first song in response to playback), we also assessed the effect of survival on song latency for the remaining experiments. The distribution of song latencies was highly skewed, so we used the median song latency (35 sec) to classify males as fast or slow to start singing, and then used logistic regression for analysis. All statistical analyses were carried out using the statistical package JMP, and means are presented with standard errors throughout.
Male characteristics
We assigned male age by assuming that males banded as adults in possession of a territory were at least 1 year old; actual age was known for males banded as nestlings. We limited assessment of age effects to experiments conducted in 2003 to 2005 because there was a larger range of minimum ages and more known-age males (Table 1). We assessed the effect of minimum male age on song rate, transforming age (log(x + 1)) to normalise model residuals and using the same approach described above to combine data from 2003 to 2005 for analysis (Overlap, Trill, and Sender experiments). The relationship between male age and survival was examined using males in their last year only (n = 55). In this sample, transformation did not normalise age, so non-parametric statistics were used. For comparison, males were divided into ‘young’ (1 or 2 years old, mean = 1.5, n = 28) and ‘old’ (3 to 9 years old, mean = 4.4, n = 27).
We used focal recordings of dawn song by subjects of the Sender experiment to examine consistency between dawn and playback song rates and to estimate the level of territorial pressure being experienced by subjects. We used the first 15 minutes of a focal recording within 9.4 ± 1.7 days of the subject’s playback trial to compute male song rate in the dawn chorus. As a crude estimate of territorial pressure on males, we counted the number of neighbours’ songs audible during this 15-minute focal recording, that is, neighbouring males singing closely enough to the focal male to be picked up in the recording.
In 2005, we quantified song rate over longer playback and post-playback periods (5 min + 5 min) in the Sender experiment, and captured males to obtain measures of body condition so that we could examine flexibility of song rate over the course of playback and relate differences in song rate to body condition. We captured 28 of the 34 subjects (within 28.4 ± 3.8 days of their playback trial) to measure mass and head-bill length. Data from a larger sample of adult males (all captures in 2003 to 2005, n = 101) were used to correct for seasonal effects on mass, and the adjusted values used to estimate male condition (100 * residual mass/head-bill length). We tested predictors of both initial song rate (first five songs) and sustained song rate (ten-minute period during and after playback) in the Sender experiment, using standard least squares models in JMP and controlling for trial covariates where necessary (date, start time, and pre-playback song rate). The effects of survival, age, and body condition were assessed simultaneously. Results for each significant effect are given from the final model including all other significant effects only. Results for each non-significant effect are given from a model including all significant effects.
Longitudinal analyses
To test for changes in song rate within males associated with aging and survival, we compared playback song rates of individuals tested in more than one year. We standardised song rate by experiment ((male song rate - experiment mean)/(experiment standard deviation)), and used a mean of standardised rates for males subject to multiple within-year trials. Other variables, such as body condition, collected in 2005 for the Sender experiment, were unavailable in earlier years and could not therefore be included in the longitudinal analysis. We compared standardised song rates of males in the first and last years that they were subjects in experiments.
Results
In five of six playback experiments, mean song rates during playback by males that died before the following breeding season were higher than those by males that survived (Fig. 1). The relationship between song rate and survival tended to differ between experiments (or years), but overall, males that died sang at significantly higher rates in response to simulated intrusion than males that survived (experiment*survival interaction F5,151 = 2.19, P = 0.06; survival β =0.05 ± 0.02, F1,151 = 5.45, P = 0.02; data from 6 experiments, 238 trials on 76 males). There was no difference between males that died and males that did not die in how quickly they started singing in response to playback (β = -0.69 ± 1.04, F1 = 0.44, P = 0.51; data from 5 experiments, 199 trials on 68 males).
Figure 1.
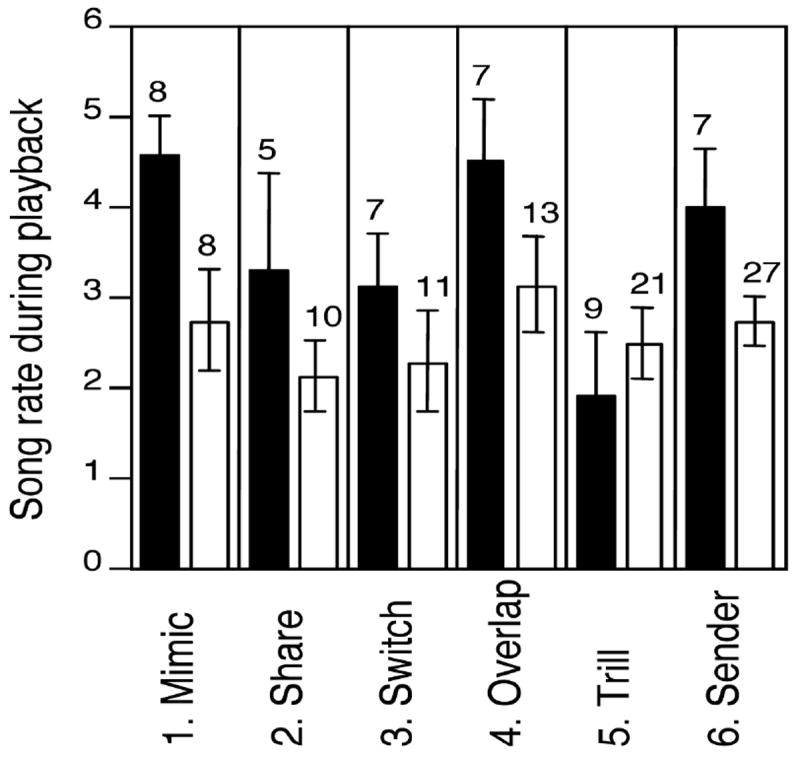
Mean song rates of males that died before the following breeding season (black bars) and males that survived (white bars), in six different playback experiments (songs/min, n males shown above SE bars – each represented by a single mean for experiments involving multiple trials).
In cross-sectional analysis, older males sang at higher rates than younger males (Fig. 2; log(age +1) β = 0.27 ± 0.12, F1,40 = 4.82, P = 0.03) and when age was controlled for, males that died sang at higher rates in some experiments, but not overall (experiment*survival interaction term F2,40 = 4.38, P = 0.02; survival effect β = 0.003 ± 0.03, F1,40 = 0.02, P = 0.90; data from Overlap, Trill, and Sender experiments, 98 trials on 52 males). Mortality was not significantly higher among old than young males (14 of 27 old males died, 9 of 28 young males died; Likelihood ratio χ2 = 2.2, P = 0.14). Furthermore, males that died were not significantly older than males that survived (3.4 ± 0.43 versus 2.6 ± 0.28 years old, Wilcoxon z = 1.37, P = 0.17, n = 55 males in their last year as test subjects - 23 died and 32 survived) and male age did not predict survival (nominal logistic regression controlling for between-year differences in survival: age effect β = 1.23 ± 1.8, Wald χ2 = 0.47, P = 0.49; year effect Wald χ2 = 7.55, P = 0.02).
Figure 2.
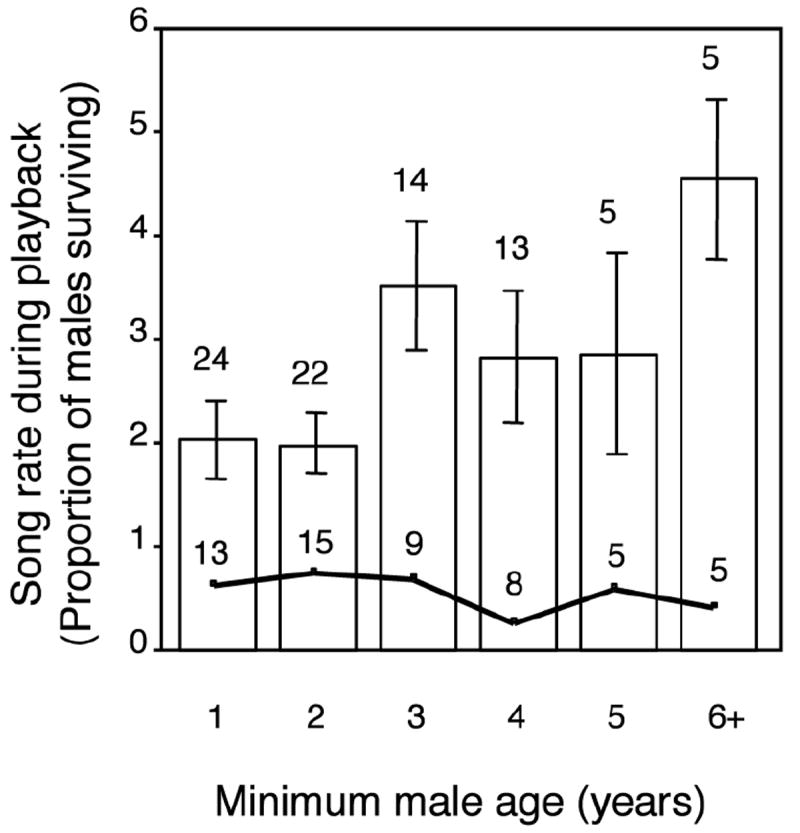
Relationships between male age and song rate and survival (bars show mean ± SE songs/min, line shows proportion of males surviving, total n = 55 males in their last year with the number in each age category shown above the line, numbers above SE bars indicate sample size for song rate – larger since some males were tested in multiple years).
Contrary to the age effect apparent in the cross-sectional analysis above, longitudinal analysis showed no effect of age on song rate, but survival tended to affect song rate (Fig. 3). Song rate clearly did not increase with age within males: individuals that survived after the last year they were tested did not sing at higher rates in their last year (paired t = -0.13, df = 19, P = 0.90). Males that died after the last year they were tested did tend to sing at higher rates in their last year than in the first year they were tested (paired t = 2.12, df = 9, P = 0.06). This effect was not confounded by an age difference between the two groups (age at first testing of males that survived: 2.1 ± 0.3 years old; died: 2.3 ± 0.3 years old; Wilcoxon rank test S = 174.5, P = 0.38). A trend for a longer gap between tests among males that died (survived: 1.2 ± 0.9 years; died: 2.2 ± 0.6 years; Wilcoxon rank test S = 189, P = 0.07) was due to two males in the died group with 5 and 6 year gaps between tests, respectively (all other males were tested one or two years apart). When these two males were excluded, the trend remained for males that died to sing at higher rates in their last year than in the first year they were tested (paired t = 2.09, df = 7, P = 0.08).
Figure 3.
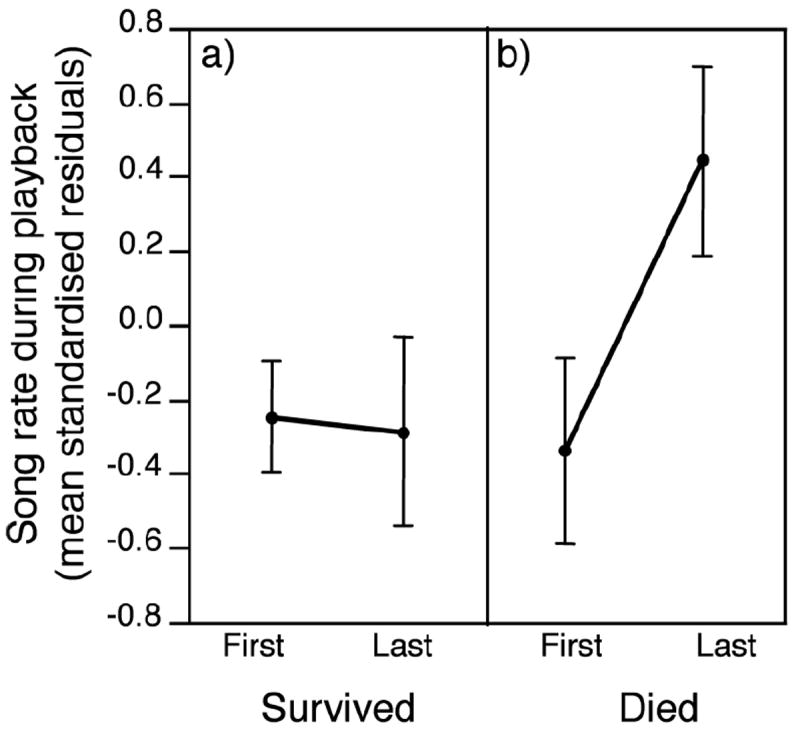
Longitudinal comparison of standardised residual song rates by males in the first and last years they were tested, split by whether they survived after the last year (a) or not (b).
We examined song during the dawn chorus to see if mortality was associated with consistently high song rates or with high levels of territorial pressure. Individuals that sang at high rates during playback did not sing at high rates at dawn (correlation r = 0.10, P = 0.58, n = 34 males recorded at dawn in 2005), and dawn song rates of males that died did not differ from those of males that survived (songs/min by males that died = 5.46 ± 0.55, n = 7; survived = 5.31 ± 0.20, n = 27; t = 0.26, df = 7.8, P = 0.80). Males that died may have experienced more songs by neighbours singing closely during their dawn chorus than males that survived (neighbour songs/min for males that died = 2.16 ± 0.52, n = 7; survived = 1.18 ± 0.21, n = 27; Wilcoxon z = 1.60, P = 0.11). However, individuals experiencing more close neighbour songs at dawn did not respond to playback with high song rates (Spearman’s r = −0.19, P = 0.27, n = 34 males).
Males in better condition sang at significantly higher rates during the playback period in the Sender experiment (β = 0.24 ± 0.09, F1,26 = 6.85, P = 0.01). Neither male age nor survival affected playback song rate in this experiment when male condition was controlled for (log(age +1) β = 0.97 ± 1.15, F1,24 = 0.71, P = 0.41; survival β = -0.05 ± 0.33, F1,24 = 0.02, P = 0.88). Condition was not correlated with age (Spearman r = 0.05, n = 28, p = 0.80), but males that died tended to be in better condition than males that survived (died = 2.0 ± 1.1, survived = -0.6 ± 0.5; t = 2.1, d.f. = 8.7, P = 0.07).
We took advantage of the long playback and postplayback periods in the Sender experiment to examine factors influencing song rate over the course of the trial and to evaluate song rate as a flexible signal. The rate at which males sang their first five songs was positively correlated with their song rate over the entire ten-minute period during and after playback (correlation r = 0.34, P = 0.05, n = 34). Initial song rates were higher than sustained song rates, declining from 6.7 ± 0.7 to 4.0 ± 0.6 songs/min by males that died, and from 4.7 ± 0.4 to 2.8 ± 0.3 songs/min by males that survived. Initial song rates by males in their last year were significantly higher than by males that survived (β = 0.99 ± 0.43, F1,32 = 5.28, P = 0.03), and were unaffected by male age (β log(age +1) = 0.08 ± 1.65, F1,31 = 0.002, P = 0.96) or condition (Fig. 4a; β = -0.13 ± 0.16, F1,25 = 0.65, P = 0.43). In contrast, sustained song rate depended on body condition (Fig. 4b; β = 0.28 ± 0.09, F1,25 = 10.79, P = 0.003; controlling for Julian day), tended to increase with age (β for log(age +1) = 1.79 ± 1.03, F1,24 = 3.02, P = 0.09), but was unaffected by survival (β = 0.41 ± 0.29, F1,24 = 1.99, P = 0.17). The relationship between song rate and condition did not differ between males that survived and those that died (condition*survival interaction effect on initial song rate F1,23 = 0.16, p = 0.69; on sustained song rate F1,23 = 0.96, P = 0.34).
Figure 4.
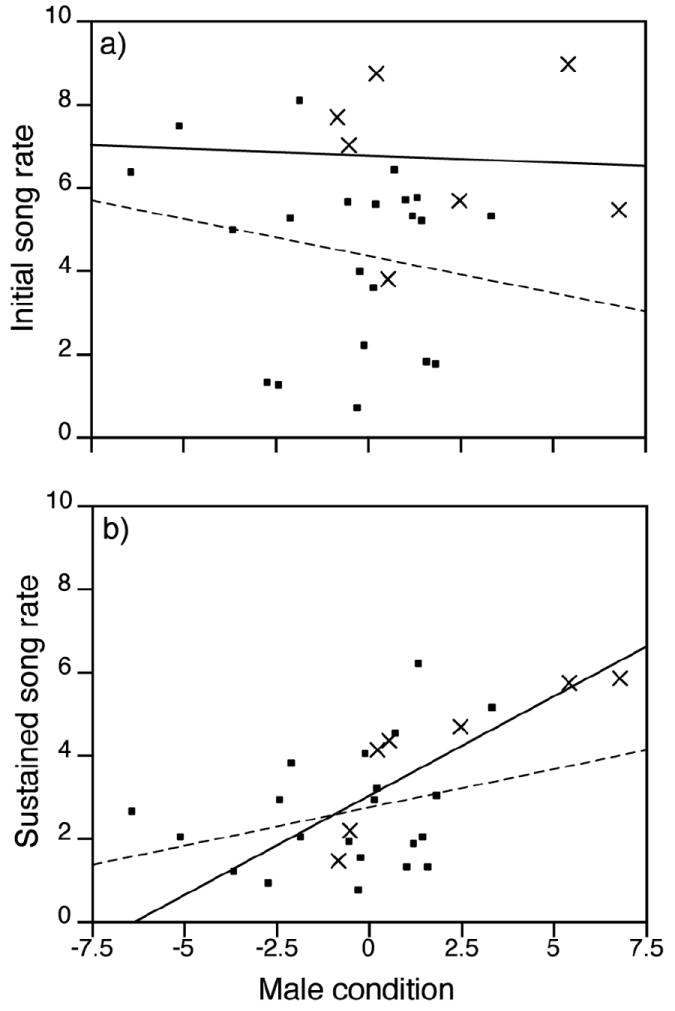
Relationship between male condition and (a) the rate at which they sang their first five songs (initial song rate), and (b) their song rate in the ten minutes during and after playback (sustained song rate) in the Sender experiment (songs/min; males that died represented as x’s and with unbroken regression lines).
Discussion
Male banded wrens in their last breeding season responded to simulated intrusion with higher song rates than males that survived. This relationship between song rate and survival was not a consequence of individuals increasing their song rate as they aged. Song rate was condition-dependent, but did not seem to impose mortality costs. High levels of territorial pressure may increase mortality, but did not seem to be associated with high playback song rates. High song rates in response to playback by males in their last year may represent a terminal investment, signalling strong motivation to defend a resource necessary for their last reproductive opportunity. Song rate is a complex, flexible signal that seems to convey multiple messages in banded wrens. Initial song rates in response to playback were 40% higher by males that did not survive than by males that did survive, regardless of body condition, and may signal motivation. However, sustained song rates were unrelated to survival but depended on body condition, suggesting that high song rate is an honest indicator of this component of male quality. Furthermore, combining cross-sectional and longitudinal analyses of age suggested that there was differential selection against males that sang at low rates, consistent with the idea that responding to simulated intrusion with high song rates is important for maintaining territory ownership.
Song rate and survival
In apparent contrast to the idea that song indicates viability, male banded wrens that died before the following breeding season responded to simulated intrusion with higher song rates than males that survived. The negative relationship between song rate and survival was statistically significant overall, despite differences in experimental treatments and designs and potential differences in conditions between years. High playback song rates by males that died were not a consequence of shorter latencies to start singing, but an actual elevation of song rates to levels similar to or above the high song rates usually heard during the dawn chorus. In contrast to banded wrens, song appears to indicate viability in willow tits (Parus montanus), where males with higher song output were more likely to survive (Welling et al. 1997). Likewise, survival was higher among male great tits (Parus major) with longer strophe lengths (Lambrechts & Dhondt 1986). However, high element rate in willow warblers appeared to be associated with higher mortality among young males (Gil et al. 2001), suggesting a mortality cost to trait expression.
Song rate and age
The negative relationship between song rate and survival did not appear to be caused by an age effect. Cross-sectional analysis showed an increase in song rate with age, but following individuals in successive years showed clearly that males did not increase their song rate as they aged, tending to increase song rate only in their terminal year. The results of this longitudinal analysis indicate that age effects apparent in cross-sectional analyses are a consequence of differential selection: males singing at high rates were over-represented in older age classes because there was higher mortality among young males that responded to simulated intrusion with low song rates. Evidence supporting the second prediction, that older males had higher mortality than younger males, was limited. While mean differences were in the expected direction, despite using several different statistical approaches we were unable to show statistically significant age-related differences in mortality in this sample of 55 males in their last year, though perhaps a larger sample might reveal an effect.
While song seems to be an honest indicator of age in some species, apparent age effects can be a consequence of differential mortality, as is the case in banded wrens, highlighting the importance of longitudinal studies. Repertoire size and element rate increased with age in willow warblers, but a cross-sectional approach did not show an increase in element rate with age, suggesting higher mortality among young males with high element rates (Gil et al. 2001). Cross-sectional analysis of strophe length in the great tit showed an increase in strophe length with age, while longitudinal analysis showed no change in strophe length with age, leading to the conclusion that survival was higher among males with longer strophe lengths (Lambrechts & Dhondt 1986). Young song sparrows with small repertoires did not retain their territories for as long as birds with larger repertoires, so repertoire size was larger among older than younger song sparrows (Hiebert et al. 1989).
Song rate as a costly signal
We found that song rate was condition-dependent, and therefore potentially costly. Body condition was a strong predictor of sustained playback song rates in the Sender experiment, suggesting that song rate could signal male quality in intra-sexual interactions. Initial playback song rates were unrelated to body condition and may have signalled motivation. In other species, males that are heavier or in better condition also often sing at higher rates (Marchetti 1998; Nystrom 1997) (but see Galeotti et al. 1997). Consistent with this, there is a negative relationship between song rate and temperature in cold weather, and food supplementation increases song rate (Gottlander 1987; Reid 1987; Strain & Mumme 1988). Signals of both condition and motivation are likely to be important in intra-sexual contexts, as both may predict the outcome of aggressive interactions (Brown et al. 2006).
Though sustained song rate was condition-dependent, we found no evidence to support either of the predictions of the hypothesis that mortality costs of high song rates caused the negative relationship between song rate and survival. Males showed no consistency in song rates in two different contexts, the dawn chorus and in response to playback. Condition-dependence and energetic costs of song in Thryothorus wrens (Eberhardt 1994) might suggest that males singing at high rates would suffer higher mortality, but there was no evidence that high song rates during the dawn chorus were associated with increased mortality. The condition-dependent sustained song rates of the Sender experiment were unrelated to survival, but initial song rate, which was unrelated to condition, did differ between males that survived and those that died. Furthermore, combining cross-sectional and longitudinal assessment of age effects suggested that it was in fact males responding to simulated intrusion with low song rates that were less likely to survive. Rather than imposing mortality costs, it seems high song rates in response to territorial intrusion are necessary for maintaining territories. In this sedentary species, territory ownership is an essential pre-requisite for reproduction and survival. High song rates may serve as a signal of male quality or motivation to other males, so males that fail to defend their territory with high song rates are unable to retain their territories and survive.
Song rate and territory pressure
It could be that high song rates are neither the cause (costs of song) nor consequence (aging or terminal investment) of individuals reaching the end of their lives, but rather that high song rate and mortality are each independently related to a third variable. For example, a high level of territorial pressure could be the underlying cause of the negative association between survival and high playback song rates if territorial conflict both increases mortality and causes males to respond to simulated intrusion with higher song rates. We found some support for the first prediction: there was a suggestion that males that died may have been experiencing more territorial pressure. However, our crude measure of territory pressure provided no evidence for the second prediction: males experiencing higher levels of territorial pressure did not respond to playback with higher song rates. Further work is required to determine cause and effect relationships between song rate, territorial conflict, and survival in banded wrens.
Song rate as a terminal investment
High song rates by males responding to playback in their last breeding season may represent terminal investment in the context of intra-sexual competition for resources. Longitudinal analysis showed that males tended to increase song rates only in their last breeding season, and not with age per se. Since breeding success depends on retaining ownership of a territory, males are likely to be highly motivated to defend this resource during their last opportunity for reproduction. The flexibility of song rate makes it particularly useful for signalling motivation, and high initial song rates in the Sender experiment were strongly dependent on survival, suggesting that terminally investing males used high song rates to signal motivation to defend their territories against rivals. The importance of high playback song rates for retaining territory ownership is highlighted by the implication of the age effect apparent in cross-sectional, but not longitudinal analysis: that there is differential selection against young males that respond to playback with low song rates.
Our study is correlative, and we cannot conclusively demonstrate terminal investment in song rate without experimental manipulation. Terminal investment in signalling has been demonstrated in male three-spined stickleback for a signal that functions in both inter- and intra-sexual selection (Candolin 1999). Other studies have used immune challenges to show terminal investment in signalling for mate attraction and in reproductive output (Sadd et al. 2006; Velando et al. 2006). Male collared flycatchers subjected to an immune challenge reduced their song rates (Garamszegi et al. 2004). However, that study did not control for life-history stage, and if subjects were not nearing the end of their lives, the immune challenge may have caused males to reallocate resources from advertisement to self-maintenance by reducing song rates rather than increasing advertisement as a terminal investment. To experimentally test whether an immune challenge could induce terminal investment in high song rate in a territorial songbird would require controlling for life-history stage. The puzzling trend in 2005 for males that died to be in better condition than males that survived (unfortunately we did not have data on male body condition for any other year to test whether this was a consistent pattern) suggests the possibility that males in good condition might maladaptively invest too much in song rate, hence causing their own death. This seems unlikely as it would contradict two other results: (1) selection appeared to act against low (rather than high) song rates in young males, and (2) initial song rates were related to survival but not to body condition. However, since we could not measure the fitness consequences of high investment in song rate, we cannot say conclusively that this represented an adaptive terminal investment.
Terminal investment, signal honesty, and motivation
High initial song rates in response to playback by terminally investing banded wrens might be considered dishonest because they are unrelated to body condition, but we argue they could be honest signals of quality or motivation. A condition of signal honesty is that signalling imposes greater costs on low quality than high quality individuals (Grafen 1990), so signals of terminally investing individuals may be dishonest if this condition is not satisfied when survival prospects are so poor that low quality individuals have little to lose (Kokko 1998). Consistent with this, a recent study suggested that production of more attractive pheromones by terminally investing male mealworm beetles represented dishonest signals with respect to body condition (Sadd et al. 2006). However, the honesty of a signal from the receiver’s perspective depends on what it receives for any given level of signal. With no future to consider, terminally investing individuals can allocate all resources to current investment, so terminal signalling remains honest with respect to body condition if the increase in signal intensity is no greater than the resources transferred from future to current investment. If ‘motivation’ reflects willingness to re-allocate resources, then terminal signalling may be an honest indicator of motivation. Terminally investing individuals could be honestly signalling their willingness to ‘fight to the death’.
Comparing terminally investing individuals and surviving individuals of the same condition may provide an avenue for quantifying resource allocation to future investment, something that is often unknown. Initial song rates were over 40% higher in terminally signalling banded wrens than in males that survived. Song output is a flexible signal that responds rapidly to changes in resources (Strain & Mumme 1988; Thomas et al. 2003), which may make large allocation to future investment unnecessary. By contrast, reproductive output of terminally investing males in the long-lived blue-footed booby (Sula nebouxii) increased by 98% compared to a decrease of 18% for non-terminal males after an immune challenge (Velando et al. 2006). This larger difference between surviving and terminally investing males suggests that where resources take longer to accumulate or replenish, greater allocation of resources to future investment is necessary.
Acknowledgments
The Area Conservación de Guanacaste gave permission to work in Santa Rosa National Park, and we would like to thank Roger Blanco and María Marta Chavarría for their consistent support of this long-term project. Erin Bohman, Andrew Conroy, Anastasia Dalziell, Kelly Doordan, Stephanie Lessard-Pilon, Simoneta Negrete-Yankelovich, Kate Neville, Corey Niles, Elizabeth Ochoa, Heidi Silvia, Alex Trillo, and Jennifer Willis helped conduct the field experiments. We thank Carlos Botero, Hanna Kokko and three anonymous reviewers for discussion and comments that significantly improved the manuscript. This research was funded by the National Institute of Mental Health (grant R01-MH60461), and adhered to the Animal Behavior Society Guidelines for the Use of Animals in Research, the legal requirements of Costa Rica, and all institutional guidelines.
Contributor Information
Michelle L Hall, Email: hall@orn.mpg.de.
Laura E Molles, Email: mollesl@lincoln.ac.nz.
Anya E Illes, Email: ailles@u.washington.edu.
Sandra L Vehrencamp, Email: slv8@cornell.edu.
References
- Bower JL. Acoustic interactions during naturally occurring territorial conflict in a song sparrow (Melospiza melodia) neighborhood. Ithaca: Cornell University; 2000. [Google Scholar]
- Bower JL. The occurrence and function of victory displays within communication networks. In: McGregor PK, editor. Animal Communication Networks. Cambridge: Cambridge University Press; 2005. pp. 114–126. [Google Scholar]
- Brown WD, Smith AT, Moskalik B, Gabriel J. Aggressive contests in house crickets: size, motivation and the information content of aggressive songs. Anim Behav. 2006;72:225–233. [Google Scholar]
- Candolin U. The relationship between signal quality and physical condition: is sexual signalling honest in the three-spined stickleback? Anim Behav. 1999;58:1261–1267. doi: 10.1006/anbe.1999.1259. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird song: biological themes and variations. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Collins S. Vocal fighting and flirting: the functions of birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s music. Oxford: Elsevier academic press; 2004. pp. 39–79. [Google Scholar]
- Eberhardt LS. Oxygen consumption during singing by male Carolina wrens (Thryothorus ludovicianus) Auk. 1994;111:124–130. [Google Scholar]
- Galeotti P, Saino N, Sacchi R, Moller AP. Song correlates with social context, testosterone and body condition in male barn swallows. Anim Behav. 1997;53:687–700. [Google Scholar]
- Garamszegi LZ, Moller AP, Torok J, Michl G, Peczely P, Richard M. Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol. 2004;15:148–157. [Google Scholar]
- Garamszegi LZ, Torok J, Hegyi G, Szollosi E, Rosivall B, Eens M. Age-dependent expression of song in the collared flycatcher, Ficedula albicollis. Ethology. 2007;113:246–256. [Google Scholar]
- Gil D, Cobb JLS, Slater PJB. Song characteristics are age dependent in the willow warbler, Phylloscopus trochilus. Anim Behav. 2001;62:689–694. [Google Scholar]
- Gottlander K. Variation in the song rate of the male pied flycatcher Ficedula hypoleuca - causes and consequences. Anim Behav. 1987;35:1037–1043. [Google Scholar]
- Grafen A. Biological signals as handicaps. J Theor Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Hall ML, Illes A, Vehrencamp SL. Overlapping signals in banded wrens: long-term effects of prior experience on males and females. Behav Ecol. 2006;17:260–269. doi: 10.1093/beheco/arj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert SM, Stoddard PK, Arcese P. Repertoire size, territory acquisition and reproductive success in the song sparrow. Anim Behav. 1989;37:266–273. [Google Scholar]
- Hofstad E, Espmark Y, Moksnes A, Haugan T, Ingebrigtsen M. The relationship between song performance and male quality in snow buntings (Plectrophenax nivalis) Can J Zool-Rev Can Zool. 2002;80:524–531. [Google Scholar]
- Hoileitner M, Nechtelberger H, Dittami J. The relationship between individual differences in male song frequency and parental care in blackcaps. Behaviour. 1993;126:1–12. [Google Scholar]
- Hoileitner M, Nechtelberger H, Hoi H. Song rate as a signal for nest site quality in blackcaps (Sylvia atricapilla) Behav Ecol Sociobiol. 1995;37:399–405. [Google Scholar]
- Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiere LF. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. [DOI] [PubMed] [Google Scholar]
- Illes AE, Hall ML, Vehrencamp SL. Vocal performance influences male receiver response in the banded wren. Proc R Soc B. 2006;273:1907–1912. doi: 10.1098/rspb.2006.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions MD, Moller AP, Petrie M. Sexually selected traits and adult survival: A meta-analysis. Q Rev Biol. 2001;76:3–36. doi: 10.1086/393743. [DOI] [PubMed] [Google Scholar]
- Kokko H. Should advertising parental care be honest. Proc R Soc B. 1998;265:1871–1878. [Google Scholar]
- Kokko H, Brooks R, McNamara JM, Houston AI. The sexual selection continuum. Proc R Soc B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts M, Dhondt AA. Male quality, reproduction, and survival in the great tit (Parus major) Behav Ecol Sociobiol. 1986;19:57–63. [Google Scholar]
- Marchetti K. The evolution of multiple male traits in the yellow-browed leaf warbler. Anim Behav. 1998;55:361–376. doi: 10.1006/anbe.1997.0586. [DOI] [PubMed] [Google Scholar]
- Molles LE. Singing complexity of the banded wren (Thryothorus pleurostictus): do switching rate and song-type diversity send different messages? Auk. 2006;123:991–1003. [Google Scholar]
- Molles LE, Vehrencamp SL. Repertoire size, repertoire overlap, and singing modes in the banded wren (Thryothorus pleurostictus) Auk. 1999;116:677–689. [Google Scholar]
- Molles LE, Vehrencamp SL. Neighbour recognition by resident males in the banded wren, Thryothorus pleurostictus, a tropical songbird with high song type sharing. Anim Behav. 2001a;61:119–127. doi: 10.1006/anbe.2000.1561. [DOI] [PubMed] [Google Scholar]
- Molles LE, Vehrencamp SL. Songbird cheaters pay a retaliation cost: evidence for auditory conventional signals. Proc R Soc B. 2001b;268:2013–2019. doi: 10.1098/rspb.2001.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom KGK. Food density, song rate, and body condition in territory- establishing willow warblers (Phylloscopus trochilus) Can J Zool. 1997;75:47–58. [Google Scholar]
- Otter K, Chruszcz B, Ratcliffe L. Honest advertisement and song output during the dawn chorus of black-capped chickadees. Behav Ecol. 1997;8:167–173. [Google Scholar]
- Reid ML. Costliness and reliability in the singing vigour of Ipswich sparrows. Anim Behav. 1987;35:1735–1743. [Google Scholar]
- Sadd B, Holman L, Armitage H, Lock F, Marland R, Siva-Jothy MT. Modulation of sexual signalling by immune challenged male mealworm beetles (Tenebrio molitor, L.): evidence for terminal investment and dishonesty. J Evol Biol. 2006;19:321–325. doi: 10.1111/j.1420-9101.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- Searcy WA, Nowicki S. The evolution of animal communication: reliability and deception in signaling systems. Princeton: Princeton University Press; 2005. [Google Scholar]
- Strain JG, Mumme RL. Effects of food supplementation, song playback, and temperature on vocal territorial behavior of Carolina wrens. Auk. 1988;105:11–16. [Google Scholar]
- Thomas RJ, Cuthill IC, Goldsmith AR, Cosgrove DF, Lidgate HC, Proctor SLB. The trade-off between singing and mass gain in a daytime-singing bird, the European robin. Behaviour. 2003;140:387–404. [Google Scholar]
- Vehrencamp SL. Handicap, index, and conventional signal elements of bird song. In: Espmark YO, Amundsen T, Rosenqvist G, editors. Animal signals: signalling and signal design in animal communication. Trondheim: Tapir Academic Press; 2000. pp. 277–300. [Google Scholar]
- Vehrencamp SL, Hall ML, Bohman ER, Depeine CD, Dalziell AH. Song matching, overlapping, and switching in the banded wren: the sender’s perspective. Behav Ecol. 2007 doi: 10.1093/beheco/arm054. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc B. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling PP, Rytkonen SO, Koivula KT, Orell MI. Song rate correlates with paternal care and survival in willow tits - advertisement of male quality. Behaviour. 1997;134:891–904. [Google Scholar]


