Abstract
Circulatory antigens transit through the small intestine via the fenestrated capillaries in the lamina propria prior to entering into the draining lymphatics. But whether or how this process controls mucosal immune responses remains unknown. Here we demonstrate that dendritic cells (DCs) of the lamina propria can sample and process both circulatory and luminal antigens. Surprisingly, antigen cross-presentation by resident CX3CR1+ DCs induced differentiation of precursor cells into CD8+ T cells that expressed interleukin-10 (IL-10), IL-13 and IL-9 and could migrate into adjacent compartments. We conclude that lamina propria CX3CR1+ DCs facilitate the surveillance of circulatory antigens and act as a conduit for the processing of self- and intestinally-absorbed-antigens, leading to the induction of CD8+ T cells, that partake in the control of T cell activation during mucosal immune responses.
Introduction
The intestinal immune system is continuously exposed to foreign antigens during the absorption of nutrients and macromolecules by intestinal epithelial cells (Colony and Neutra, 1985; Heyman et al., 1982; Hooper and Macpherson, 2010; Kimm et al., 1997). It is widely accepted that the fenestrated capillaries in the small intestine not only function in nutrient absorption, but also allow for transport of macromolecules from the circulation into the lamina propria. In an adult human these processes establish a fluid gradient throughout the 200-400m2 of the small intestinal surface, which is requisite for the transports of lipids and peptide antigens from the lamina propria through the central lacteal (CL) into the mesenteric lymph nodes (MLNs) (Clementi and Palade, 1969; Dobbins and Rollins, 1970; Granger and Taylor, 1980a, b; Milici and Bankston, 1982; Simionescu et al., 1972).
Antigens are sampled and processed in the intestine by heterogeneous subsets of mononuclear phagocytes (MPs) that can acquire functional phenotypes corresponding to macrophages and dendritic cells (DCs) (Hashimoto et al., 2011; Satpathy et al., 2012). In the lamina propria of the small intestine, the majority of MPs are derived from precursors expressing CX3CR1, that develop characteristics of DCs with high CD11c and MHC class II expression (Niess et al., 2005), but also express F4/80 (Vallon-Eberhard et al., 2006) and CSF1R (Hashimoto et al., 2011). CX3CR1+ DCs are derived from monocytes through a GM-CSF-dependent pathway, and are distinct from CD103+ DCs derived from a common DC progenitor in an Flt3L-dependent pathway (Varol et al., 2009).
CX3CR1+ DCs are uniquely positioned for the recognition of environmental and circulatory antigens in the lamina propria, as they interact with both the epithelium and the capillary vessel system in the lamina propria (Niess et al., 2005). CX3CR1+ DCs are antigen-sampling cells in the intestine that have been found to remain restricted to the lamina propria under homeostatic conditions (Schulz et al., 2009) and to maintain their non-inflammatory phenotype during colonic inflammation (Weber et al., 2011).
While antigen processing in the gut associated immune system has been linked to the control of systemic immune responses (Mayer and Shao, 2004; Strobel and Mowat, 2006) it remains unclear whether DCs in the intestines are able to recognize antigens in the blood stream to coordinate peripheral and mucosal immune regulation.
We propose a mechanism whereby the mucosal immune system is integrated into immune surveillance of the circulation based upon antigen uptake by lamina propria CX3CR1+ DCs. This subset of DCs collected blood-derived antigens during their transition through the lamina propria from fenestrated capillaries to the lymphatics of the intestine. Processing of circulatory antigen by CX3CR1+ DCs induced CD8+ T cells with a specific cytokine expression profile in the small intestine, which in turn became intraepithelial lymphocytes (IELs) and controlled activation of CD4+ T cells in the small intestine.
Results
CX3CR1+ phagocytes acquire circulatory and luminal antigens in the lamina propria
To assess the uptake of antigens that reach the intestinal lamina propria through permeable fenestrated capillaries, Cx3cr1GFP/+ mice were injected intravenously (i.v.) with fluorescently labeled ovalbumin (OVA). CX3CR1+ phagocytes located in the lamina propria of the small intestine of Cx3cr1GFP/+ mice collected injected OVA within 10 minutes (Figure 1A-Panels a-c). The aggregation of OVA was first observed in CX3CR1+ phagocytes in the apical regions of villi with fenestrated capillaries (Figure 1A-Panel d). One hour after a single i.v. injection, OVA was detected in CX3CR1+ phagocytes throughout the lamina propria (Figure 1B-Panel a-c) and began to transition into the central lacteal (CL), where the antigen enriched over a period of 18 hours (Figure 1B-Panel d-f). In vivo imaging and electron microscopy revealed a close interaction of DCs with the fenestrated capillary endothelium. Thus, uptake of OVA from the circulation appeared to be associated with the retention of antigen in DCs during the transition of antigen from the lamina propria into intestinal lymphatics.
Figure 1. Uptake of circulatory antigen by DCs lining fenestrated capillaries in the lamina propria of the small intestine.
Alexa 647-labeled OVA was intravenously injected into Cx3cr1GFP/+ mice. To visualize the capillary vessel system in the lamina propria, Texas-Red-conjugated Wheat germ agglutinin (WGA) was intravenously injected into mice 7 minutes before imaging. (A) (a-c) 3D image reconstruction of confocal microscopic image series of a small intestinal villus of a Cx3cr1GFP/+ mouse 10 minutes after intravenous injection of Alexa 647-labeled OVA. (d) Transmission electron microscopy of lamina propria showing fenestrated capillary (F), Erythrocyte (E), 25,000× magnification. (B) 3D reconstruction of a small intestinal villus of a Cx3cr1GFP/+ mouse 1 hour (a-c) and 18 hours (d-f) after injection of Alexa 647-conjugated OVA. Villus (V), Crypt (Cr), central lacteal (CL). (C) OVA uptake from the circulation and transport to the CL in the presence (a-c) or after depletion by diphteria toxin (DT) (d-f) of lamina propria DCs in Itgax-DTR-EGFP mice. Basement (dotted line), apical surface of the epithelium (solid line).
To determine that the uptake of circulatory antigen indeed depended on either CD11c+ DCs or CD11c- macrophages, we followed the transport of OVA in Itgax-DTR-EGFP mice before and after depletion of CD11c+ cells with diphtheria toxin (DT). These experiments demonstrated that CD11c+ DCs were enriched with OVA (Figure 1C-Panels a-c) and depletion of this cell population reduced cellular aggregation of circulatory antigen within the lamina propria (Figure 1C-Panels d-f). However, the depletion of CD11c+ DCs did not prevent the transition of antigens from the circulation into the CL, suggesting that the transition of peptide antigens through the lamina propria is not only dependent upon the presence of DCs (Figure 1C-Panels d-f).
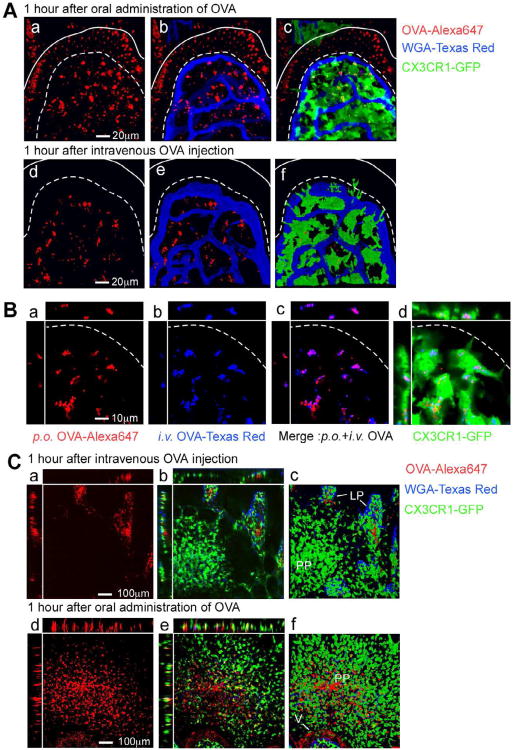
Upon oral administration of Alexa 647 conjugated OVA, confocal microscopic images of live small intestinal tissues and 3D reconstructions demonstrated that OVA was present in both the intestinal epithelium and lamina propria CX3CR1+ DCs (Figure 2A-Panels a-c, Movie S1). By contrast, upon i.v injection of OVA this fluorescent antigen was only detected in DCs of the lamina propria (Figure 2A-Panels d-f, Movie S2). To determine whether the same phagocyte subset collected antigen from the intestinal lumen as well as from the circulation, we administered Texas Red conjugated OVA by oral gavage and Alexa 647 conjugated OVA by i.v. injection into the same mouse. Subsequent confocal microscopy demonstrated that OVA indeed ended up in the same vesicular compartment of CX3CR1+ DCs (Figure 2B) regardless of the mode of administration. Surprisingly, the CX3CR1+ DC subsets in Peyer's patches (PPs) only collected antigen transported from the intestinal lumen, but not from the vasculature indicating that capillaries in PPs lack fenestrae (Figure 2C-Panels a-f). Taken together, these data clearly show that CX3CR1+ CD11c+ phagocytes sample both circulatory and luminal antigen specifically in the lamina propria of the small intestine.
Figure 2. Circulatory antigen uptake occurs specifically in the lamina propria of the small intestine.
(A) 3D reconstruction of a small intestinal villus of a Cx3cr1GFP/+ mouse 1 hour after oral gavage (a-c) or i.v. injection (d-f) of Alexa 647-conjugated OVA. (B) Alexa 647-labeled OVA and Texas Red-labeled OVA were orally gavaged or intravenously injected into Cx3cr1GFP/+ mice, respectively. Shown is a 3D reconstruction of a small intestinal villus of a Cx3cr1GFP/+ mouse 1 hour after OVA transfer. (C) 3D reconstruction of Peyer's patches (PP) in Cx3cr1GFP/+ mice 1 hour after i.v. injection (a-c) or oral gavage (d-f) of Alexa 647-conjugated OVA, Lamina propria (LP).
Processing of circulatory antigen by CX3CR1+ DCs in the small intestine
To determine which subset(s) of lamina propria DCs internalizes fluorescent-OVA we employed flow cytometric analyses of purified phagocytes. As shown in Figure 3A, the major lamina propria cells that were enriched in OVA after i.v injection consisted of CD11chi CD11b+CX3CR1hi DCs which also expressed F4/80 but not expressed Siglec-F (Figure 3A).
Figure 3. CX3CR1+ DCs process circulatory antigen in the lamina propria.
(A) Characterization of lamina propria phagocytes isolated from the small intestine of Cx3cr1GFP/+ mice 18 hours after i.v. injection of Alexa 647-conjugated OVA. (B). CX3CR1+ DCs (R1) and CD103+ DCs (R2) were analyzed with imaging flowcytometer to distinguish morphology and subcellular localization of GFP, CD103 and OVA derived signals. The frequency of antigen uptake in each cell population is shown as mean fluorescence intensity. (C) Analysis of colocalization of DQ-OVA (red) enriched in the endosomal system of small intestinal CX3CR1+DCs and the cathepsin-B-activatable near-infrared fluorescent (NIRF) probe ProSense 680 (PS-680, blue) 4 hours after injection of antigen and 1 hour after injection of PS-680. (D) Analysis of the Alexa 647-conjugated OVA content in CX3CR1+ DCs isolated from the small intestine at different timepoints after a single i.v. dose of conjugated OVA (gray histogram). Bar graphs represent the mean fluorescence intensity of conjugated OVA peptide in intestinal lamina propria CX3CR1+ DCs or CX3CR1- DCs at indicated time point.
To distinguish between antigen uptake by CX3CR1+ phagocytes and CD103+ DCs, which are associated with antigen transport in the intestine (Schulz et al., 2009), we also analyzed antigen uptake in lamina propria phagocytes using an imaging flow cytometer. Among CD11c+ phagocytes, intracellular enrichment of antigen from the circulatory system was primarily observed in CD11chi CX3CR1+ DCs, but not CD11c+CD103+ (αE integrin) DCs (Figure 3B). Our analysis of lamina propria DCs also demonstrated that CX3CR1+ and CD103+ DCs are distinct subsets, because CX3CR1-GFP and CD103 double positive events were either CX3CR1+ DCs that had phagocytosed CD103 positive material or CD103+ DCs with GFP positive aggregates on their surface (Figure S1A). In vivo imaging also demonstrated that CD103+ DCs were not able to aggregate circulatory antigen in the small intestinal lamina propria in vivo in contrast to CX3CR1+ DCs (Figure S1B). We conclude that CX3CR1+CD11chi DCs are capable of collecting antigen that reached the lamina propria through the blood circulation, and stored intracellular antigen in contrast to CD103+ DCs.
To determine whether circulatory antigens are taken up by endosomes in CX3CR1+ phagocytes, the protease-sensitive fluorescent probe ProSense 680 (PS-680) (Weissleder et al., 1999) was co-injected into Cx3cr1GFP/+ mice together with DQ-OVA, which is fluorescent at 540 nm when enriched in endosomes. Confocal microscopic image analyses demonstrated that aggregated DQ-OVA was enriched in the late endosomal and lysosomal compartments of CX3CR1+ DCs, where proteases activated PS-680 resulting in enhanced emission at 680 nm (Figure 3C). CX3CR1+ phagocytes in the lamina propria of the small intestine indeed process circulatory peptide antigens, because one hour after a single injection of Alexa 647-conjugated OVA 90% of isolated lamina propria CD11+ CX3CR1+ DCs contained OVA. Furthermore, more than 18 hours after the injection circulatory antigen was still detectable in 40% of the CX3CR1+ DCs although the amount of detectable OVA reduced to 10% of the amount detected 1 hour after injection (Figure 3D). Taken together, these data show that CX3CR1+ DCs have the unique ability to locally collect and process antigens of circulatory origin, establishing a filter system to survey antigens during the transition from the fenestrated endothelium through the lamina propria to the CL.
Antigen cross-presentation by CX3CR1+ DCs induces the differentiation of CD8+ T cells
To assess whether antigen processing by CX3CR1+ DCs in the lamina propria can lead to T cell activation, DC subsets were isolated from the small intestine of mice that had been injected with Alexa 647 conjugated antigens. OVA containing CX3CR1+ DCs were sorted and ex vivo co-cultured with OVA-specific T cell receptor (TCR) transgenic CD8+ T (OT-I) cells. Surprisingly, only intestinal DCs that had acquired and processed antigen in the lamina propria were able to cross-present antigen. DCs isolated from the lamina propria required in situ processing in the intestinal lamina propria since DC isolated 1 hour or 4 hours after OVA injection or which received OVA after isolation in vitro were unable to activated OT-I CD8+ T cells (Figure S2A). However, CX3CR1+ DCs that were isolated 18 hours after antigen injection cross-presented antigen to OT-I cells, inducing CD25 expression and proliferation of OVA-specific CD8+ T cells (Figure 4A). In contrast, CD103+ DCs were able to cross-present antigen provided in vitro and induced CD25 expression and proliferation of CD8+ T cells. By contrast, CX3CR1+ DCs failed to induce proliferation and activation of OT-II CD4+ T cells in contrast to CD103+ DCs, regardless whether antigen was provided in vitro or in vivo (Figure S2A, B and C). Together these experiments indicate that antigen processing in the lamina propria is required to induce cross-presentation by CX3CR1+ DCs.
Figure 4. Lamina propria CX3CR1+DCs cross-present after antigen uptake in vivo.
(A) OT-I CD8+ T cell activation by lamina propria DCs isolated from small intestines of Cx3cr1GFP/+ mice 18 hours after i.v. injection of OVA, n=4. (B) Flowcytometric analysis of costimulatory molecule and granzyme B expression by OT-I CD8+ T cells activated by OVA presentation by small intestinal CX3CR1+ and CD103+ DCs in vitro, n=3. Not significant (ns), **p<0.001 and ***p<0.0001 in all panels.
Activation of CD8+ T cells by CX3CR1+ DCs and CD103+ DCs induced CD8αβ T cells that expressed programmed death (PD)-1, but only CD103+ DCs enhanced Granzyme B expression in CD8+ T cells (Figure 4B). Further, CX3CR1+ DCs induced CD8+ T cells that expressed the high affinity IL-2Rβ (CD 122) and glucocorticoid-induced TNF receptor-related protein (GITR) or GITR alone while CD103 + DCs induced CD122 and GITR double positive CD8+ (Figure 4B). Neither subset of DCs induced FoxP3 in CD8+ T cells (Figure S3).
We next determined cytokine secretion by CD8+ T cells differentiating in the presence of CX3CR1+ or CD103+ DCs. Whereas CX3CR1+ DCs induced CD8+ T cells that expressed IL10, IL-13, and IL-9, CD103+ DCs preferentially induced IL-17-secreting CD8+ T cells (Figure 5A, Figure S4). However, IL-2 and IFN-γ secretion by CD8+ T cells was caused by both DC subsets (Figure 5A). The ability of DCs to induce IL-10, IL-13 and IL-9 expression in CD8+ T cells in the mucosal immune system after the uptake of cognate antigen from the circulation was restricted to the lamina propria. CD11c+ DCs that acquired injected antigen in mesenteric lymph nodes were unable to induce the secretion of these cytokines during CD8+ T cell activation (Figure S5A and B), although various distinct subsets of CX3CR1+ and CX3CR1- phagocytes collected i.v. injected antigen in MLNs, (Figure S5C).
Figure 5. Lamina propria CX3CR1+DCs induce CD8+T cell found in the IEL compartment of the small intestine.
(A) Cytokine secretion by OT-I CD8+ T cells induced by cross-presentation of circulatory OVA processed by intestinal DCs, (B) Analysis of cytokine secretion, granzyme expression, and phenotypic characterization of CD8αβ and CD8αα IELs isolated from the small intestine of wildtype C57BL/6 mice, n=3 mice per group. n=3. Not significant (ns), *p<0.05, and **p<0.001 in all panels.
Antigen sampling from the circulation by CX3CR1+ DCs may provide a supply of CD8+ T cells for the intraepithelial lymphocyte (IEL) compartment since activated CD8+ T cells can efficiently migrate into the intestinal epithelium (Masopust et al., 2004). We therefore analyzed small intestinal IELs from wild-type mice to determine whether CD8+ T cells that arise in the interaction with CX3CR1+ DCs are present in the small intestine. We found CD8αβ but not CD8αα T cells among intraepithelial lymphocytes (IELs) that specifically expressed IL-10 and significantly more IL-9 and IL-2 but less granzyme B compared to CD8αα IELs. (Figure 5B). CD8αβ T cells were further distinguished from CD8αα IELs by the expression of PD-1 resembling the phenotype induced by CX3CR1+ DCs in vitro (Figure 5C). In aggregate, these findings suggested that local sampling of circulatory antigen in the small intestine resulted in the induction of CD8+ T cells with a regulatory function in the IEL compartment.
Circulatory antigen sampling generates a subset of CD8+ intraepithelial lymphocytes
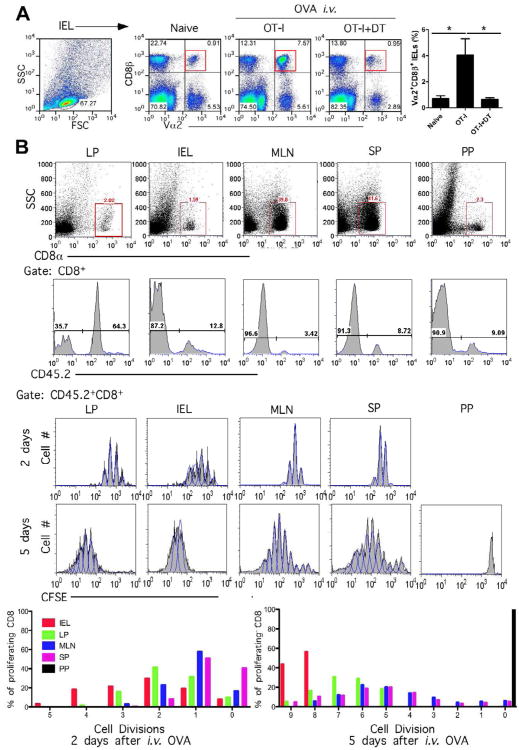
We transferred OT-I T cells into Itgax-DTR-EGFP mice to determine whether the induction of CD8+ T cells in the IEL compartment can be controlled by CD11c+ DCs. Since the recipient Itgax-DTR-EGFP mice also express CD45.2, we characterized the expansion of OT-I T cells based on their expression of the TCR Vα2 chain. In response to OVA injections, the expansion of OT-I T cells greatly enhanced the percentage of Vα2 TCR chain-positive CD8αβ T cells within the IEL compartment (Figure 6A). Deletion of DCs by DT injection into Itgax-DTR-EGFP mice before the injection of OVA prevented the expansion of antigen-specific Vα2 TCR-expressing T cells in the mucosa without affecting other CD8+ T cell subsets (Figure 6A). To determine whether circulatory antigen can generate cognate antigen-specific CD8+ T cells in the small intestine, CD45.1 congenic mice received OVA i.v., subsequent to the adoptive transfer of CFSE labeled CD45.2 CD8+ OT-I T cells. When we analyzed CD8+ T cells from the IEL compartment, LP, PPs, MLNs and spleens we found that 5 days after OVA injection, CD45.2 CD8+ OT-I T cells represented 64% of CD8+ T cells in the LP and 12% in the IEL compartment, while OVA specific CD8+ T cells represented between 3-10% of CD8+ T cells in PPs, MLNs and spleens (Figure 6B). CFSE staining of CD45.2 CD8+ T cells revealed that in vivo proliferation of CD8+ T cells occurred more rapidly in the intestinal compartments in which OT-I cells underwent 4-5 cell divisions in two days compared to three divisions in spleens and MLNs (Figure 6B). Five days after antigen injections CD45.2+ CD8+ T cells among IELs had undergone 8-9 divisions and 5-9 division in the lamina propria while most CD8+ T cells in spleen and MLN underwent 4-6 divisions in the same time frame. CD8+ T cell proliferation was detected in both the lamina propria and the intraepithelial compartment of the small intestine, but not in PPs, although OT-1 cells were able to enter this compartment (Figure 6B). These results are in agreement with an antigen uptake and presentation of circulatory antigen in the lamina propria leading to the proliferation of CD8+ T cells.
Figure 6. Induction of CD8+IELs in the small intestine.
(A) The generation of antigen-specific CD8+ T cells in the IEL compartment is dependent on lamina propria DCs. Naïve OT-I CD8+ T cells were adoptively transferred to Itgax-DTR-EGFP mice and OT-I CD8β+ Vα2+ IELs were analyzed 6 days after i.v. injection of OVA before and after depletion of DCs by diphtheria toxin (DT), n=3 mice per group. *p<0.05. (B) CFSE-labeled CD45.2+ OT-I CD8+ T cells were adoptively transferred into CD45.1+ B6 mice and CD8+ T cell migration and proliferation was assessed 2 and 5 days after OVA injection in different immune compartments. Dilution of CFSE staining of CD45.2 CD8+ T cells isolated from different tissues is shown as percentages of cells in each generation.
When we replaced the cognate antigen by anti-CD3 to trigger the TCR in the lamina propria of IL-10-GFP reporter mice directly (Kamanaka et al., 2006), the number of CD8αβ T cells expressing IL-10 in the IEL compartment of the small intestine was greatly enhanced (Figure S6). Analysis of 3D reconstructions of small intestinal villi of IL-10-GFP reporter mice revealed that TCR activation in the lamina propria resulted in the upregulation of IL-10 expression in CD8αβ T cells in the lamina propria and the IEL compartment (Figure S6).
Together these findings suggested that a subset of CD8+ T cells could expand in the lamina propria and migrate into the intraepithelial compartment. Consistent with this model, we found that CD8+ T cells activated by CX3CR1+ DCs expressed the αE integrin (CD103) implicated in T cell recruitment into the epithelium (Lin et al., 1994) (Figure S7A). Furthermore, CD25-expressing CD8+ T cells arising during antigen presentation by CX3CR1+ DCs expressed the chemokine receptor CCR6 that could mediate the recruitment into epithelial cell layers that express the corresponding ligand CCL20 (Figure S7A). In vivo, the number of CD8αβ T cells in the IEL compartment was indeed dependent on the expression of CCR6 but not CCR7, a chemokine receptor required for the migration of lymphocytes from the lamina propria to the MLNs (Figure S7B).
Thus, we conclude that CD8+ T cells are part of mucosal control of T cell activation in the small intestine. Furthermore, IL-10-expressing CD8+ T cells form a subset of IELs that are naturally induced during the processing of circulatory antigen by CX3CR1+ DCs.
CD8+ T cells inhibit antigen-specific CD4+ T cell activation and prevent T cell-mediated small intestinal inflammation
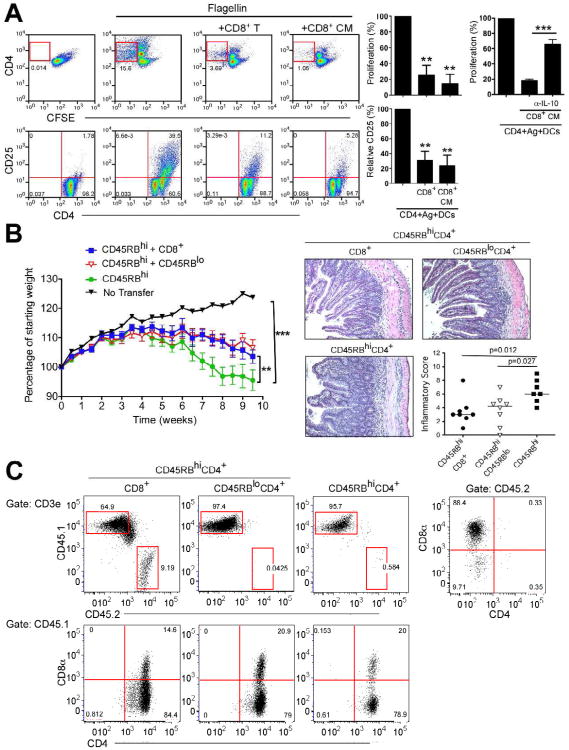
We next addressed the key question of whether CD8+ T cells induced during cross-presentation by CX3CR1+ DCs suppress antigen specific CD4+ T cell responses. We first activated OT-I CD8+ T cells with CX3CR1+ DCs which had collected OVA in the lamina propria and added the induced CD8+ T cells to flagellin-specific TCR transgenic (SM1) CD4+ T cells together with lamina propria CD103+ DCs in the presence of flagellin (McSorley et al., 2002). Cell sorted OT-I CD8+ T cells or their cell culture supernatant prevented the activation and proliferation of SM1 CD4+ T cells as evaluated by CD25 expression and CFSE dilution, respectively (Figure 7A). The inhibition of CD4+ T cell activation was partially dependent on IL-10 as anti-IL-10 blocking antibodies restored CD4 T cell activation (Figure 7A). These findings indicated that CD8+ T cells induced by CX3CR1+ DCs could inhibit CD4 T cell activation.
Figure 7. CD8+ T cells induced by CX3XR1+ DCs control CD4+ T cell-mediated intestinal inflammation.
(A) Salmonella flagellin-specific SM1 CD4+ T cells were activated with flagellin loaded-lamina propria CD103+ DCs and cultured in the absence or presence of (1:1) with OT-I CD8+ T cells generated in the presence of mucosal CX3CR1+ DCs isolated after OVA uptake in vivo, or their culture media (CD8 CM) and with or without anti IL-10 blocking antibodies. CD25 expression and CFSE dilution of SM1 CD4+ T cells were analyzed. n=3 mice per group, **p<0.001, ***p<0.0001 in all panels. (B) Weight development of Rag1-/- mice after the transfer of 5 × 105 CD45RBhi CD4+ T cells alone or in the presence of 2.5×105 of CD8αβ T cells generated in the presence of CX3CR1+ DCs or 2.5×105 CD45RBlo CD4+ T cells, n=8 in each group. Representative histology and inflammatory score of the small intestines from the experimental groups are shown. (C) Analysis of lamina propria lymphocytes isolated from Rag1-/-, mice that received CD45.1+CD45RBhi CD4+ T cells alone or together with CD45.2+CD8+ T cells or CD45RBlo T cells 10 weeks earlier. **p<0.001, ***p<0.0001 in all panels.
To determine whether CD8+ T cells induced by CX3CR1+ DCs are able to suppress intestinal inflammation in vivo we used the CD45RBhi CD4+ T cells transfer model of colitis. Rag1-/- recipient mice transferred with CD45RBhi CD4+ (CD45.1) showed a progressive weight loss and developed clinical disease after ten weeks compared to naïve Rag1-/- mice (Figure 7B and C). In contrast, Rag1-/- mice that received CX3CR1+ DCs induced CD8+ T cells (CD45.2) together with CD45RBhi CD4+ T cells showed substantially less weight loss and disease activity similar to mice that received CD45RBlo CD4+ T cells together with CD45RBhiCD4+ T cells (Figure 7B). Both CD8+ T cells and CD45RBlo CD4+ T cells reduced small intestinal inflammation induced by CD45RBhi CD4+ T cells resulting in an increased villus-to-crypt ratio, and reduced inflammatory cell infiltration of villi and basal lamina propria (Figure 7B) compared to mice that received CD45RBhi CD4+ T cells alone. To determine whether transferred CD8+ T cells migrate and colonize the small intestine, we co-transferred CX3CR1+ DCs induced CD45.2+CD8+ T cells and CD45.1 CD45RBhi CD4+ T cells into CD45.2 Rag1-/- mice. Remarkably, transferred CD45.2+CD8+ T cells were able to repopulate and expand in the lamina propria of the small intestine (Figure 7C). After transfer of CD45RBhi CD4+ and CD45RBlo CD4+ T cells, we were able to detect CD45.1+CD4 and CD8 double positive cell population in the lamina propria but no CD45.2+CD8+ T cells. Together, these results demonstrate that CD8+ T cells generated by lamina propria CX3CR1+ DCs presenting of circulatory antigen are able to control effector CD4+ T cell function in the small intestine.
Discussion
While it is widely accepted that the intestinal microenvironment plays a role in conditioning mucosal DCs to preferentially induce regulatory T cells that control innate and adaptive immune responses (Grainger et al., 2010), it has been unclear how antigenic information in the peripheral circulation can contribute to this process.
We propose a cellular circuitry in which lamina propria DCs, together with the fenestrated capillary system of the small intestine, form a central component of immune regulation by providing a surveillance system for self and foreign antigens. In this model CX3CR1+ DCs, process circulatory antigens and initiate differentiation of CD8+ αE+ PD1+CCR6+ T cells which migrate from the lamina propria to the intestinal epithelial compartment. The local induction of CD8+ T cells is possible since recent thymic emigrant CD8+ T cells express CCR9 and α4β7 for direct migration into the small intestinal lamina propria without cycling through secondary lymphoid tissues (Staton et al., 2006). The availability of naïve CD8+ T cells in the small intestine allows CD8+ T cell to be differentiated locally and constituted circuitries that require specific priming in MLNs for recruitment into mucosal surfaces during host defense activation (Iwata et al., 2004). Furthermore, small intestinal CD8αβ IEL generation is independent of sphingosine 1-phosphate signaling (Kunisawa et al., 2007) further supporting a local differentiation process leading to the generation of CD8+ IELs.
Although both CX3CR1+ and CD103+ DCs are exposed in the lamina propria to circulatory antigens, we only observed antigen retention in CX3CR1+ DCs of the small intestine. This may be due to the close interaction of CX3CR1+ DCs with the fenestrated capillaries. Alternatively, differential endosomal uptake and processing may allow circulatory antigen to accumulate in CX3CR1+ DCs but not in CD103+ DCs. Since the rate of lysosomal processing in antigen presenting cells determines the availability of antigen for presentation (Delamarre et al., 2005), it will be important to determine whether specific lysosomal processing in CX3CR1+ DCs favors MHC class I versus class II presentation.
The processing of circulatory antigens in the mucosal immune system is not limited to the lamina propria, as our studies show the antigen is further transferred via the CL and could reach the MLNs together with intestinally absorbed compounds or through migrating CD103+ DCs (Schulz et al., 2009). However, our data indicate that specifically lamina propria DC of the small intestine, but not MLN DCs, are able to induce IL-10, IL-13 and IL-9 expressing CD8+ T cells. In contrast, antigen reaching the MLN directly or through migrating DCs has been linked to previously recognized mechanisms of mucosal CD4+Foxp3+ Treg differentiation (Coombes and Maloy, 2007; Sun et al., 2007). Indeed, CD4+Foxp3+ Tregs can be regulated with various mononuclear phagocytes including CD103+ DCs, CD11b+F4/80+CD11c- and CX3CR1+ DCs in the intestine (Coombes and Maloy, 2007; Denning et al., 2007; Hadis et al., 2011; Matteoli et al., 2010).
Here we show that antigen sampling by CX3CR1+ DCs in the absence of mucosal inflammation, induce the differentiation of CD8+ T cells, which are able to inhibit pro-inflammatory CD4+ T cell activation. The regulatory phenotypes of CD8+ T cells induced by cross presentation by either CX3CR1+ or CD103+ DCs were found to very distinct. We demonstrate that CD8+ T cells induced by CX3CR1+ DCs express IL-10 and can be part of the mucosal control mechanisms that inhibit inflammation specifically in the small intestine. Such a region-specific adaptation of T cell function may be required for mucosal homeostasis, as suggested by experiments demonstrating that mucosal T cell activation by super-antigens or anti- CD3 injections leads to IL-10 expression in CD8+ T cells in the small intestine, while IL-10 expression was induced in CD4+ T cells in the colon (Kamanaka et al., 2006). Our study further uncovered CX3CR1+ DCs induced CD8αβ+ T cells as a source of IL-13 and IL-9 expression in the intestinal immune system. IL-13 and IL-9 are involved in the induction of Th2 type immune responses. Th2 cytokines can have a protective effect through the inhibition of Th1 and Th17 inflammatory responses in the intestine (Danese, 2011) but also have been implicated in fibrosis and allergies and including celiac disease (DePaolo et al., 2011).
The activation of CD8+ T cells by CX3CR1+ DCs is further associated with the induction of PD-1, GITR and CD122 expression, all of which have been recognized in the differentiation of regulatory T cells. Inhibition of PD-L1 signaling can lead to expansion of OVA-specific CD8+ T cells and their differentiation into effector cells capable of producing pro-inflammatory cytokines (Reynoso et al., 2009). Both CD122 (Lee et al., 2008; Rifa'i et al., 2004) and GITR (Mayer et al., 2011) expression has been linked to the control of CD8+ T cells, although GITR is also required for viral defenses and CD8+ T cell expansion (Snell et al., 2010).
Naturally and adaptively induced regulatory CD8+ T cell subtypes have been recognized as essential regulators of the immune system that prevent autoimmunity through the inhibition of effector lymphocyte function (Menager-Marcq et al., 2006; Suzuki et al., 2008; Zhang and Bevan, 2011). Regulatory CD8+ T cells can develop on oral administration of self or non-self antigen, leading to tolerance in a variety of experimental models (Faria and Weiner, 2005), although it had been unclear where and how these CD8+ T cells acquired their inhibitory function.
Understanding this mechanism for immune regulation through circulatory antigen sampling in the intestine will have important implications for patients with inflammatory bowel disease whose CD8+ T cell function in the intestine has been reduced (Brimnes et al., 2005). Our findings also have relevance for the mechanism leading to food allergies such as celiac disease, in which CD8+ T cells in the IEL compartment become pathogenic (DePaolo et al., 2011). CX3CR1+ DCs have an unexpected function that allows the mucosal immune system of the small intestine to survey circulatory antigens for the control of mucosal T cell activation. It needs to be determined whether the intersection of environmental and self-antigen recognition in the intestine has a further role in peripheral tolerance and autoimmune diseases.
Materials and Methods
Mice
C57BL/6, Rag1-/-, CD45.1+ C57BL/6, Itgax-DTR-EGFP and OT-I mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Cx3cr1GFP/+ have been previously described (Jung et al., 2000). Tg(TcraCN.B1,TcrbCN.B1)SM1Jen (SM1 TCR transgenic) C57BL/6 mice (McSorley et al., 2002) have been reported previously. Il10tm1.1karp (IL-10 GFP) mice have been reported previously (Madan et al., 2009). Mice were maintained in H. hepaticus- and Pasteurella-free or specific pathogen-free animal facilities according to institutional guidelines. All experiments were carried out on mice at 6 to 14 weeks of age according to protocols approved by the Subcommittee on Research Animal Care at the Massachusetts General Hospital and Harvard Medical School.
Isolation of lamina propria DCs and IELs
To isolate lamina propria DCs from small intestine, the intestine was cut into 3-4 pieces and then inverted onto polyethylene tubes (Becton Dickinson, Franklin Lakes, NJ), washed three times with calcium- and magnesium-free phosphate buffered saline (PBS; Lonza, Walkersville, MD) and mucus was removed with 1mM dithiothreitol (DTT; Sigma-Aldrich, St. Louis, MO). The intestinal epithelium was eluted with 30mM EDTA at room temperature, followed by digestion of the tissue with 36U/ml type IV collagenase (Sigma-Aldrich) in Dulbecco's Modified Eagle's Medium (DMEM; Lonza, Basel, Switzerland) containing 5% fetal bovine serum (FBS) for 90 minutes at 37°C in a 5% CO2 humidified incubator. The digested tissue was gently shaken for 10 minutes at room temperature and then passed through a 70μm nylon cell strainer and washed with DMEM. A final OptiPrep density centrifugation at ρ=1.055 g/ml (Axis Shield, Oslo, Norway) yielded lamina propria DCs. To purify CX3CR1-expressing DCs, cells were isolated from CxScrlGFP/+ mice and stained with PE or APC-conjugated anti-CD11c (HL3; BD Pharmingen, San Jose, CA) or anti CD103 (M290; BD Pharmingen, San Jose, CA) and sorted using a Becton-Dickinson FACSVantage SE/DiVa cell sorter.
To isolate IELs, small intestines were cut open longitudinally and washed three times with ice-cold HBSS containing 2% FBS. The intestine was cut into pieces and transferred to 15ml of warm HBSS containing 10% FBS and 0.1mM EDTA followed by 20 minutes of shaking at 37°C. Collected supernatants were passed through a 70μm cell strainer and spun at 1500rpm for 5 minutes. After resuspension in HBSS containing 5% FBS, the cell suspension was passed through a nylon wool column and spun at 1500rpm for 5 minutes. Resuspended cells in 3ml of 44% Percoll were overlaid upon 3ml of 67% Percoll, spun at 1800rpm for 20 minutes without brake and the interface layer collected and washed with HBSS containing 5% FBS.
Flow cytometry analysis
Isolated cells were incubated in 10% serum and Fc block (BD Pharmingen) for 20 minutes at 4°C and then stained with fluorescent-conjugated antibodies. Biotin- or PE-conjugated anti-mouse CD11c (HL3; BD Pharmingen), APC-conjugated anti CCR6 (BD Pharmingen), biotin-conjugated anti CCR7 (BD Pharmingen), PE-conjugated anti PD1 (BioLegend, San Diego, CA), APC-conjugated anti CD25 (eBioscience, San Diego, CA), PE- or APC-conjugated anti CD8a (BD Pharmingen), FITC- or PE-conjugated anti CD8β (BD Pharmingen), PE-conjugated anti CD4 (BD Pharmingen), APC eFlour 780- conjugated anti CD45.1 (eBioscience), PerCP/Cy5.5-conjugated anti CD45.2 (eBioscience), PE-conjugated anti-anti CD103 (M290; BD Pharmingen), or Pacific Blue-conjugated anti-CD103 (2E7; BioLegend) were used for DC or T cell staining. Cells were analyzed on a FACSCalibur flow cytometer or LSRII flow cytometer (BD Bioscience) and then analyzed by FlowJo software (Tree Star, Ashland, OR). The stained cells were also analyzed on Image Stream X (Amnis, Seattle, WA) and then analyzed by IDEAS Application v4.0 (Amnis).
Confocal microscopy of antigen uptake
Cx3cr1GFP/+ mice were injected with 100μg Alexa Fluor 647-conjugated OVA (Molecular Probes, Invitrogen, Carlsbad, CA) via tail vein. To visualize the vascular system, 100μg Alexa Fluor 647- or Texas Red-conjugated wheat germ agglutinin (Molecular Probes) was intravenously injected into tail veins 7 minutes before imaging. The Cathepsin-B (CatB) sensitive near-infrared fluorescent (NIRF) probe (ProSense 680) was purchased from Visen Medical, Woburn, MA. After 16-18 hours, mice were sacrificed and tissues were immediately removed, opened by longitudinal incision and rinsed with PBS. Living tissue specimens were imaged with an A1R-A1 confocal microscope (Nikon, Melville, NY). 3D reconstructions were completed with Volocity software (PerkinElmer, Waltham, MA).
T cell activation assay
CD8+ T cells were prepared from the spleen and lymph nodes of OT-I mice and isolated by CD8+ T cell isolation kit (Miltenyi Biotec, Auburn, CA). 3 × 105 T cells labeled with 1μM CFSE (Molecular Probes) were cultured with sorted 2 × 104 DCs and 200μg/ml chicken ovalbumin (Sigma-Aldrich) for 5 days. To analyze cytokines from culture supernatants, the cells were further stimulated with plate-bound anti-CD3 antibody (10μg/ml) and soluble anti-CD28 antibody (1μg/ml) for 3 days. The supernatants were analyzed with the Mouse Cytokine Plex assay system (Panomics/Affymetrix, Santa Clara, CA).
In vitro T cell inhibition assay
CD4+ T cells from lymph node of SM1 (CD45.1 congenic) TCR transgenic mice were isolated using CD4+ T cell isolation kit (Miltenyi Biotec) and labeled with 1μM CFSE (Molecular Probes). Sorted CD103+CD11c+ DCs from lamina propria of Cx3cr1GFP/+ mice were used for antigen-presenting cells. 3 × 105 SM1 transgenic CD4+ T cells and 2 × 104 DCs were cocultured with 2μg/ml flagellin (Invivogen, San Diego, CA) together with OT-I CD8+ T cells generated by lamina propria CX3CR1+ DCs for 5 days.
CD45RBhiCD4+ T cell-induced colitis
The CD45RBhi to Rag1-/-, model was used as previously described (De Jong et al., 2000). Briefly, CD45RBhiCD4+ T cells were isolated from spleens of wild-type B6 mice (CD45.1+ donors) and then sorted as a CD8-CD4+CD45RBhi population. 5×105 CD45RBhiCD4+ T cells were injected i.p. into Rag1-/- mice either alone or with 2.5×105 OT-I CD8+ T cells (CD45.2+ donors) generated by lamina propria CX3CR1+ DCs or CD45RBloCD4+ T cells (CD45.1+ donors).
Histology and IBD scoring
Intestinal samples were harvested, fixed in 10% formalin and embedded in paraffin for H&E staining. Histological scoring was performed with scores between 0= normal, 1 =mild, 2 =moderate, 3 = severe were attributed to changes in crypt architecture, basal lymphoplasmacytosis, expansion of lamina propria and epithelial hyperplasia. Scores were graphed in a total range of 0–12.
In vivo OT-I adoptive transfer
OT-I CD8+ T cells were isolated, labeled with 10μM CFSE and then adoptively transferred into CD45.1+ B6 mice. The next day, 100μg OVA was injected intravenously. After 6 days, CFSE dilution on OT-I CD8+ T cells was analyzed. For DC depletion, Itgax-DTR-EGFP bone marrow chimera mice were treated i.p. with 100ng diphtheria toxin (List Biological Laboratories, Inc., Campbell, CA) 1 day before and after circular antigen transfer. At day 6, CD8β+Vα2+ OT-I cells within the IEL were analyzed.
Statistics
GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis. Student's t-test or ANOVA were used for comparisons. All results are expressed as mean ± SD.
Supplementary Material
Acknowledgments
This work was supported by grants DK-068181 (HCR), DK-033506 (HCR), AI093588 (HCR), DK-043351 (HCR, CT), AI-057992 (CLK) and DK-52510 (CT) from the National Institutes of Health.
Footnotes
Author Contributions: SYC, JHS, BG, CAC, SA, YZ, HSC, MO and GL carried out experiments; MNK and AB provided pathology advice in the assessments of colitis; CLK, AHS and CT developed research tools and mice; HCR directed the research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- Clementi F, Palade GE. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969;41:33–58. doi: 10.1083/jcb.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colony PC, Neutra MR. Macromolecular transport in the fetal rat intestine. Gastroenterology. 1985;89:294–306. doi: 10.1016/0016-5085(85)90329-4. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19:116–126. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Danese S. IBD: of mice and men-shedding new light on IL-13 activity in IBD. Nat Rev Gastroenterol Hepatol. 2011;8:128–129. doi: 10.1038/nrgastro.2011.17. [DOI] [PubMed] [Google Scholar]
- De Jong YP, Comiskey M, Kalled SL, Mizoguchi E, Flavell RA, Bhan AK, Terhorst C. Chronic murine colitis is dependent on the CD154/CD40 pathway and can be attenuated by anti-CD154 administration. Gastroenterology. 2000;119:715–723. doi: 10.1053/gast.2000.16485. [DOI] [PubMed] [Google Scholar]
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins WO, 3rd, Rollins EL. Intestinal mucosal lymphatic permeability: an electron microscopic study of endothelial vesicles and cell junctions. J Ultrastruct Res. 1970;33:29–59. doi: 10.1016/s0022-5320(70)90117-6. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–316. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Taylor AE. Permeability of intestinal capillaries to endogenous macromolecules. Am J Physiol. 1980a;238:H457–464. doi: 10.1152/ajpheart.1980.238.4.H457. [DOI] [PubMed] [Google Scholar]
- Granger DN, Taylor AE. Permselectivity of intestinal capillaries. Physiologist. 1980b;23:47–52. [PubMed] [Google Scholar]
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman M, Ducroc R, Desjeux JF, Morgat JL. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am J Physiol. 1982;242:G558–564. doi: 10.1152/ajpgi.1982.242.6.G558. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kimm MH, Hardin JA, Gall DG. Transport of albumin into the intestinal lumen of the rat. Can J Physiol Pharmacol. 1997;75:193–198. [PubMed] [Google Scholar]
- Kunisawa J, Kurashima Y, Higuchi M, Gohda M, Ishikawa I, Ogahara I, Kim N, Shimizu M, Kiyono H. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- Lin T, Matsuzaki G, Yoshida H, Kobayashi N, Kenai H, Omoto K, Nomoto K. CD3-CD8+ intestinal intraepithelial lymphocytes (IEL) and the extrathymic development of IEL. Eur J Immunol. 1994;24:1080–1087. doi: 10.1002/eji.1830240511. [DOI] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- Mayer CT, Floess S, Baru AM, Lahl K, Huehn J, Sparwasser T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol. 2011;41:716–725. doi: 10.1002/eji.201040913. [DOI] [PubMed] [Google Scholar]
- Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28-regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–1785. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milici AJ, Bankston PW. Fetal and neonatal rat intestinal capillaries: permeability to carbon, ferritin, hemoglobin, and myoglobin. Am J Anat. 1982;165:165–186. doi: 10.1002/aja.1001650206. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, Freeman GJ, Turley SJ. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182:2102–2112. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N, Simionescu M, Palade GE. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972;53:365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell LM, McPherson AJ, Lin GH, Sakaguchi S, Pandolfi PP, Riccardi C, Watts TH. CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J Immunol. 2010;185:7223–7234. doi: 10.4049/jimmunol.1001912. [DOI] [PubMed] [Google Scholar]
- Staton TL, Habtezion A, Winslow MM, Sato T, Love PE, Butcher EC. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- Strobel S, Mowat AM. Oral tolerance and allergic responses to food proteins. Curr Opin Allergy Clin Immunol. 2006;6:207–213. doi: 10.1097/01.all.0000225162.98391.81. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–789. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol. 2006;176:2465–2469. doi: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Weber B, Saurer L, Schenk M, Dickgreber N, Mueller C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur J Immunol. 2011;41:773–779. doi: 10.1002/eji.201040965. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.