Abstract
The major conjugated linoleic acid (CLA) isomers, c9,t11-CLA and t10,c12-CLA, have anticancer effects; however, the exact mechanisms underlying these effects are unknown. Evidence suggests that reversal of reduced gap junctional intercellular communication (GJIC) in cancer cells inhibits cell growth and induces cell death. Hence, we determined that CLA isomers enhance GJIC in human MCF-7 breast cancer cells and investigated the underlying molecular mechanisms. The CLA isomers significantly enhanced GJIC of MCF-7 cells at 40 μM concentration, whereas CLA inhibited cell growth and induced caspase-dependent apoptosis. CLA increased connexin43 (Cx43) expression both at the transcriptional and translational levels. CLA inhibited nuclear factor-κB (NF-κB) activity and enhanced reactive oxygen species (ROS) generation. No significant difference was observed in the efficacy of c9,t11-CLA and t10,c12-CLA. These results suggest that the anticancer effect of CLA is associated with upregulation of GJIC mediated by enhanced Cx43 expression through inactivation of NF-κB and generation of ROS in MCF-7 cells.
1. Introduction
Conjugated linoleic acid (CLA) is comprised of positional and geometric isomers of octadecadienoic acid (C18:2) with a conjugated double bond [1]. CLA has several health benefits in vivo, including anticarcinogenesis, antiatherogenesis, antiobesity, and modulation of immune function [1–5]. Numerous anticancer and anticarcinogenic effects of CLA and individual CLA isomers have been reported. CLA inhibits 7,12-dimethylbenz[a]anthracene-induced mouse skin carcinogenesis, benzo(a)pyrene-induced mouse forestomach tumorigenesis, 2-amino-3,4-dimethylimidazo[4,5-f] quinoline-induced rat colonic carcinogenesis, and N-methyl-N-nitrosourea-induced rat mammary carcinogenesis [1, 2, 6, 7]. Individual CLA isomers also show anticancer effects by inducing apoptosis in animals and cancer cell lines [7–9], but their mechanisms of action are still unknown.
Gap junctions are transmembrane channels that connect the cytoplasm of neighboring cells composed of two hemichannels and each consists of six connexin proteins [10]. Intercellular communication through gap junctions plays an important role in maintaining tissue homeostasis by allowing the passage of small cytoplasmic molecules and ions (<1 kD), such as cyclic AMP, diacylglycerol, inositol triphosphate, and Ca+2 by the process known as gap junctional intercellular communication (GJIC) [11]. GJIC has been postulated to regulate cell proliferation, differentiation, apoptosis, and adaptive responses of differentiated cells [11, 12]. Tumor promoters downregulate GJIC in carcinogenesis [13, 14], and GJIC is suppressed in most cancer cells compared to normal cells [15, 16]. In addition, growth factors and several oncogenes inhibit GJIC [17], and cancer drugs reverse downregulation of GJIC [18–21]. However, nothing is known regarding whether CLA isomers can reverse suppressed GJIC in cancer cells.
Up to now, three connexin proteins have been identified in human breast tissue, including Cx43, Cx26, and Cx32 [22–24]. Cx43 is the most widely studied of these connexin proteins because of its high expression in most cells [22]. Mutations or deficiencies in connexin genes are related to several diseases including cancer; thus, transfection of connexin genes into GJIC-deficient tumor cells restores the reduced GJIC and suppresses tumor growth [25–28]; therefore, connexin genes are related to restoration of GJIC in cancer cells. We previously demonstrated that CLA isomers prevent 12-O-tetradecanoylphorbol-13-acetate (TPA) induced downregulation of GJIC in non-tumorigenic mammary epithelial MCF-10A cells by inhibiting Cx43 phosphorylation [13, 29].
Therefore, we hypothesized that CLA isomers enhance GJIC in cancer cells by inducing Cx43 gene expression and that the enhancement of GJIC is related to anticancer effects. Here, we investigated the anticancer effects of major CLA isomers, c9,t11-CLA and t10,c12-CLA, on human MCF-7 breast cancer cells and the underlying molecular mechanisms.
2. Materials and Methods
2.1. Reagents
Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), and penicillin-streptomycin were obtained from Gibco BRL (Rockville, MD, USA). Bovine serum albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and phosphate-buffered saline (PBS) were purchased from Amresco (Solon, OH, USA). α-Tocopherol phosphate, L-ascorbic acid, Lucifer yellow dilithium salt (LY), 2′,7′-dihydrodichlorofluorescein diacetate (DCFH-DA), linoleic acid (LA, 99%), sodium selenite, transferrin, phenylmethanesulfonyl fluoride (PMSF), ribonuclease A, propidium iodide (PI), 0.25% trypsin containing 2 mM EDTA, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Trizol reagent was purchased from Invitrogen (Carlsbad, CA, USA). An Omniscript Reverse Transcriptase kit was purchased from Qiagen Inc. (Valencia, CA, USA). Super-Therm DNA polymerase was obtained from JMR (Side Cup, Kent, UK). Radio immune precipitation assay (RIPA) buffer was purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-Cx43 IgM, goat-anti-mouse IgM-FITC, goat-anti-mouse IgM, and mouse monoclonal anti-NF-κB IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal caspase-3 antibody was purchased from Delta Biolabs (Campbell, CA, USA). Mouse monoclonal anti-β-actin was purchased from Sigma-Aldrich. Cx43 gene of primers for 5′-ACATCAGGTGGACTGTTTCCT-3′ (sense) and 5′-ACGACTGCTGGCTCTGCTT-3′ (antisense) and β-actin primers 5′-CGTGACATCAAGGAGAAGCT-3′ (sense) and 5′-ATCCACATCTGCTGGAAGGT-3′ (antisense) were obtained from ABgene (Epsom, UK). All other reagents used in this study were of analytical grade.
2.2. Preparations of CLA Isomers
The c9,t11-CLA and t10,c12-CLA isomers were isolated from synthetic CLA methyl ester by low-temperature crystallization at −68 and −71°C in conjunction with urea treatment [30]. The purity of the CLA isomers was 94.5% for c9,t11-CLA and 98.5% for t10,c12-CLA when analyzed by gas chromatography [30]. Each CLA isomer or LA was complexed with fatty acid-free BSA according to the method described by van Greevenbroek et al. [31].
2.3. Cell Culture and Sample Treatments
The MCF-7 human breast cancer cells (Korean Cell Line Bank, Seoul, Republic of Korea) were cultured in RPMI 1640 medium supplemented with 10% FBS and penicillin-streptomycin in a humidified atmosphere with 5% CO2 at 37°C as described previously [8]. The cells were grown in Nunc culture dishes (35 mm, i.d., Rochester, NY, USA) to a confluency of 80% and trypsinized with 0.25% trypsin containing 2 mM EDTA to disperse the cells. The cells were subsequently collected by centrifugation (1000 g for 10 min) and resuspended in fresh culture media.
The cells were seeded either into a 96-well culture plate (Nunc) at 1 × 104 cells per well for the cell proliferation test using the MTT assay or Nunc culture dish (10 mm, i.d.) at 1 × 105 cells for other assays. After 24 h incubation, the cells were serum starved by an additional 24 h incubation in RPMI 1640 medium supplemented with 5 μg/mL transferrin, 5 ng/mL sodium selenite, and 0.1 mg/mL BSA.
Two experiments were conducted for the cytotoxicity assay. In the first experiment, the serum-starved MCF-7 cells were incubated with c9,t11-CLA (0–40 μM) in RPMI 1640 medium, supplemented with ascorbic acid (50 ng/mL) and α-tocopherol phosphate (20 ng/mL) for 72 h to determine the optimal concentration and incubation time. In the second experiment, the cytotoxicity of c9,t11-CLA was compared to that of t10,c12-CLA and LA for 48 h using a 40 μM concentration. For the other experiments, the serum-starved cells were incubated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. After incubation with the CLA isomers, the cells were trypsinized with 0.25% trypsin containing 2 mM EDTA and collected by centrifugation (1000 g for 10 min). These cells were washed with PBS and collected again by centrifugation before assay. Fresh medium was replaced in every 48 h. The exponentially growing cells were used throughout the experiments.
2.4. Cell Viability Assay
MCF-7 cell proliferation was measured using the MTT assay as described previously [29]. Briefly, the cells treated with the CLA isomers were exposed to MTT solution (5 mg/mL of PBS) for 4 h. The MTT solution was removed, and 200 μL DMSO was added to each well and mixed to dissolve the MTT formazan crystals formed by the viable cells. The absorbance of each well was measured at 570 nm using an Anthos 2020 microplate reader (Wals, Austria). Data are represented as relative cell viability against the cells at time 0.
2.5. Flow Cytometry Analysis
The cell cycle was analyzed as described previously [32]. Briefly, the cells treated with the CLA isomers were collected in 1 mL ice-cold PBS and fixed with 4 mL 70% ethanol for 30 min at 4°C. The fixed cells were washed with PBS and then incubated with 500 μL PI solution (1 mg/mL of RNAase, 50 μg/mL of PI, and 0.1% TritonX-100) for 20 min at 37°C. Flow cytometric analysis was performed using a BD FACSCalibur Flow Cytometer (BD Science, San Jose, CA, USA) equipped with CellQuest Pro Software (BD Science).
2.6. Hoechst 33258 Staining
The cells treated with the CLA isomers were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilized in PBS containing 0.1% Triton X-100 for 10 min, after which the nuclei were stained with Hoechst dye (10 μg/mL) for 10 min as described previously [8, 9]. Finally, the cells were observed under an Olympus IX70 inverted fluorescent microscope (Okaya, Japan).
2.7. GJIC Assay
GJIC of cells treated with CLA isomers was measured using the scrape loading/dye transfer (SL/DT) technique as previously described [29]. Briefly, cells were treated with 0.05% LY solution and scraped with a surgical steel scalpel blade at low light intensity. After 3 min of incubation (5% CO2, 37°C), the cells were washed four times with 2 mL PBS and fixed with 4% paraformaldehyde. The number of dye-communicating cells perpendicular to the scraped-line was counted under an Olympus IX70 inverted fluorescent microscope.
2.8. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR) for Cx43 Gene Expression
Total RNA of cells treated with the CLA isomers was isolated using Trizol reagent according to the method described by the manufacturer. RT-PCR analysis was performed as described previously [33]. cDNA was synthesized from total RNA using an Omniscript Reverse Transcriptase kit according to the manufacturer's instructions. The Cx43 gene was amplified by RT-PCR using 5′-ACATCAGGTGGACTGTTTCCT-3′ (sense) and 5′-ACGACTGCTGGCTCTGCTT-3′ (antisense) as primers in a 50 μL final volume containing Super-Therm DNA polymerase, 1.5 mM MgCl2, and 2 mM dNTP for 30 cycles (denaturation at 94°C for 30 seconds, annealing at 50°C for 1 min, and extension at 72°C for 1 min, followed by final incubation at 72°C for 7 min). β-actin primers 5′-CGTGACATCAAGGAGAAGCT-3′ (sense) and 5′-ATCCACATCTGCTGGAAGGT-3′ (antisense) were used as the control under the same PCR conditions. PCR products were analyzed by electrophoresis on 1% agarose gels.
2.9. Western Blot Analysis
Western blot analysis was performed as described previously [29]. Cells treated with CLA isomers were lysed at 4°C by shaking for 15 min followed by homogenization with RIPA buffer. After centrifugation at 13,000 g for 15 min, the supernatant was collected and stored at −70°C until use. Protein concentration was determined using the Bradford reagent (Hercules, CA, USA). Proteins were separated by 12.5% SDS-polyacrylamide gel electrophoresis, and Western blot analysis of Cx43, caspase-3, and NF-κB was performed according to the manufacturer's instructions. Protein bands were detected using an Enhanced Chemiluminescence Detection kit (Thermo Scientific, Rockford, IL, USA).
2.10. Preparation of Nuclear Protein for Western Blotting of NF-κB
Proteins in the nuclear fraction of cells were isolated as described previously [34]. Cells treated with CLA isomers were incubated for 20 min at 48°C in a buffer containing 20 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid) (HEPES) KOH (pH 7.9), 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dichlorodiphenyltrichloroethane (DTT), and 0.2 mM PMSF. Cell nuclei were collected as a pellet by centrifugation at 14,000 g for 5 min at 48°C. Nuclear proteins were extracted at 4°C by gently resuspending the nuclear pellets in a buffer solution containing 20 mM Tris (pH 7.5), 20% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, and 0.1% Triton X-100, followed by a 1 h incubation at 4°C with occasional vortexing. After centrifugation at 14,000 g for 15 min at 4°C, the supernatant, which contained the nuclear protein fraction, was collected. Protein concentration was determined using the Bradford reagent.
2.11. Immunocytochemistry Assay of Cx43 Protein
The cells treated with CLA isomers were seeded and cultured on chamber slides for immunocytochemistry analysis as described previously [35]. Cells were fixed with 4% paraformaldehyde on chamber slides. The fixed cells were permeabilized with 0.5% Triton X-100 in PBS and incubated with a blocking solution (PBS containing 2% BSA) at room temperature for 30 min. The cells were then incubated with anti-connexin 43 IgM as a primary antibody and FITC-conjugated goat-anti-mouse IgM as the secondary antibody. Fluorescent staining of Cx43 was imaged using an Olympus IX70 inverted fluorescent microscope.
2.12. Intracellular Reactive Oxygen Species (ROS) Assay
Intracellular ROS positive cells were determined as described previously [36]. Cells treated with CLA isomers were incubated with 50 μM DCFH-DA for 30 min at 37°C in the dark. After the incubation, the cells were washed with Locke's buffer (154 mM NaCl, 25 mM KCl, 2.3 mM CaCl2, 3.6 mM NaHCO3, 8.6 mM HEPES, and 5.6 mM glucose, pH 7.4). The number of ROS positive cells was measured using an Olympus FV-1000 confocal microscope equipped with an argon laser at 485 nm excitation and 530 nm emission wavelengths.
2.13. Statistical Analysis
Data are presented as means ± standard deviations and analyzed using one-way analysis of variance followed by Duncan's multiple range test. Differences were considered significant at P < 0.05.
3. Results
3.1. Growth Inhibition of MCF-7 Cells
First, we determined the optimal concentration of c9,t11-CLA that showed an anticancer effect on MCF-7 cells. The MTT test was performed on cells grown at various concentrations (5–40 μM) of c9,t11-CLA for 72 h (Figure 1(a)). The growth inhibitory effect of c9,t11-CLA was dose and time dependent. At 40 μM, cell viability was 70.6 and 65.9% at 48 and 72 h, respectively (P < 0.05).
Figure 1.
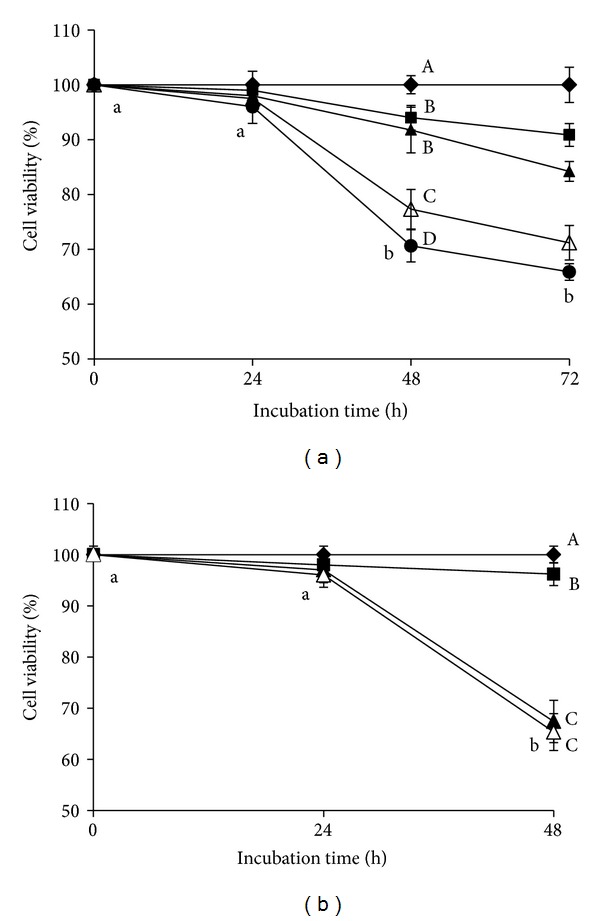
Viability of MCF-7 cells treated with CLA isomers. (a) Cells treated with 5 μM (■), 10 μM (▲), 20 μM (∆), and 40 μM (●) concentrations of c9,t11-CLA and control (♦) for 72 h. (b) Cells treated with 40 μM c9,t11-CLA (▲), t10,c12 -CLA (∆), and LA (■) and control cells (♦) for 48 h. Values are expressed as means ± standard deviations (n = 3). Means with different lower case letters or with different upper case letters on different concentration lines at the same incubation time are significantly different at P < 0.05 by Duncan's multiple-range test.
The inhibitory efficacy of c9,t11-CLA was compared to that of either t10,c12-CLA or LA at a concentration of 40 μM for 48 h (Figure 1(b)). t10,c12-CLA and LA significantly inhibited cell growth by 32.6% and 3.8%, respectively, as compared to that in the control. The potency of c9,t11-CLA and t10,c12-CLA was similar. Hence, 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h incubation were selected as the optimal conditions for subsequent experiments.
3.2. Induction of Apoptosis in MCF-7 Cells
We evaluated whether growth inhibition was related to apoptosis of the cells. The effect of c9,t11-CLA and t10,c12-CLA isomers on the cell cycle and apoptotic parameters was evaluated in MCF-7 cells (Figure 2(a)). The cell cycle analysis revealed that c9,t11-CLA and t10,c12-CLA significantly increased the percentage of cells in the sub-G1 phase to 35% and 33.6%, respectively, which was an indicator of DNA fragmentation due to cell death (Figure 2(b)). No significant difference in the sub-G1 phase cell population was found between cells treated with the c9,t11-CLA and t10,c12-CLA isomers.
Figure 2.
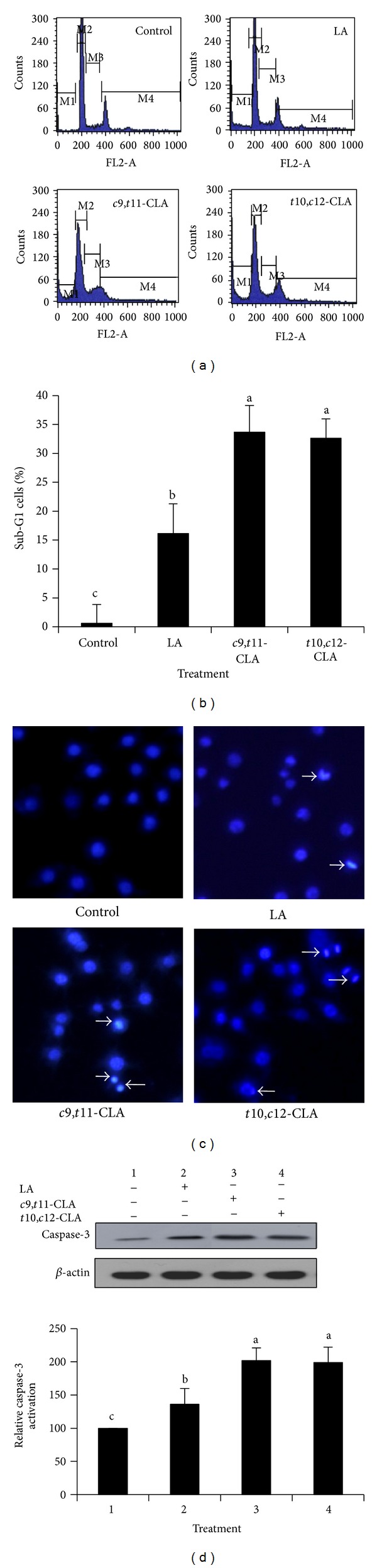
Induction of apoptosis in MCF-7 cells treated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. (a) Histograms of cells stained with propidium iodide and analyzed by flow cytometry. The peak area of M1, M2, M3, and M4 represents the cell population of Sub-G1, G0/G1, S, and G2/M phases, respectively. (b) Quantification of Sub-G1 cell population of (a), which is indicative of apoptosis. (c) Hoechst 33258 staining followed by fluorescence microscopic analysis of cells; arrows indicate fragmented or condensed nuclei. (d) Western blot analyses of the key executor enzyme of apoptotic cell death, activated caspase-3. The band intensities relative to control cells were quantified. Values are expressed as means ± standard deviations (n = 3). Means with different lowercase letters are significantly different at P < 0.05 by Duncan's multiple-range test.
To determine whether cell death was related to apoptosis, we further performed Hoechst 33258 staining (Figure 2(c)). As a result, cell shrinkage and nuclear condensation were visible in cells treated with c9,t11-CLA and t10,c12-CLA, which indicated apoptosis. To verify whether the apoptosis was caspase dependent, we assessed the level of caspase-3 protein, a key executionary protease, in the process. Western blotting revealed that the c9,t11-CLA and t10,c12-CLA isomers significantly increased caspase-3 levels (Figure 2(d)). Taken together, these results indicate that c9,t11-CLA and t10,c12-CLA induced cell death by inducing apoptosis.
3.3. Enhancement of GJIC in MCF-7 Cells
Next, we determined whether the CLA isomers could reverse the reduced GJIC of MCF-7 cells. We evaluated the status of GJIC in MCF-7 cells treated with c9,t11-CLA, t10,c12-CLA, and LA (Figures 3(a) and 3(b)). CLA isomers and LA increased GJIC, relative to control, but the efficacy of the c9,t11-CLA and t10,c12-CLA isomers was similar and greater than that of LA. This finding suggests that the inhibition of cell growth might be associated with increased GJIC.
Figure 3.
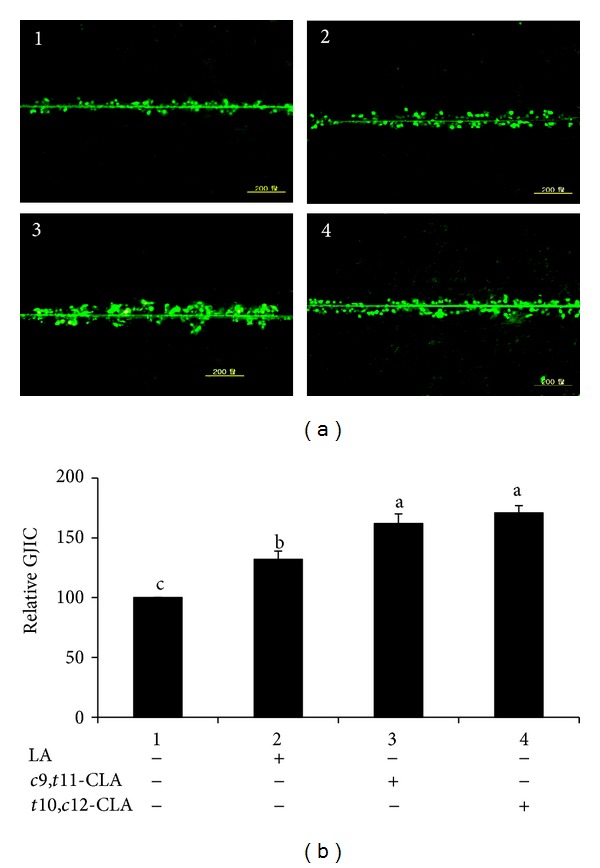
Gap junctional intercellular communication (GJIC) in MCF-7 cells measured by scrape-loading/dye-transfer (SL/DT) technique. MCF-7 cells treated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. (a) Representative images of GJIC. (b) Quantification of data (a). Values are expressed as means ± standard deviations (n = 3). Means with different lowercase letters are significantly different at P < 0.05 by Duncan's multiple-range test.
3.4. Increased Cx43 Gene Expression in MCF-7 Cells
Cx43 is a major protein in the gap junction channel that regulates GJIC. Previous results have shown that some chemical compounds upregulate GJIC by upregulating Cx43 expression in human cancer cells [32, 37, 38]. Thus, we assessed the expression levels of the Cx43 gene at the transcriptional and translational levels to investigate the mechanism of GJIC restoration by the CLA isomers (Figure 4). The CLA isomers upregulated the expression of the Cx43 gene at the transcriptional (Figure 4(a)) and translational levels (Figures 4(b) and 4(c)). Both c9,t11-CLA and t10,c12-CLA significantly enhanced the level of Cx43 mRNA in MCF-7 cells. No significant difference was observed in the upregulated expression of Cx43 mRNA between c9,t11-CLA and t10,c12-CLA. A similar pattern was observed for Cx43 protein expression. These results indicate that both the c9,t11-CLA and t10,c12-CLA isomers equally enhanced Cx43 expression.
Figure 4.
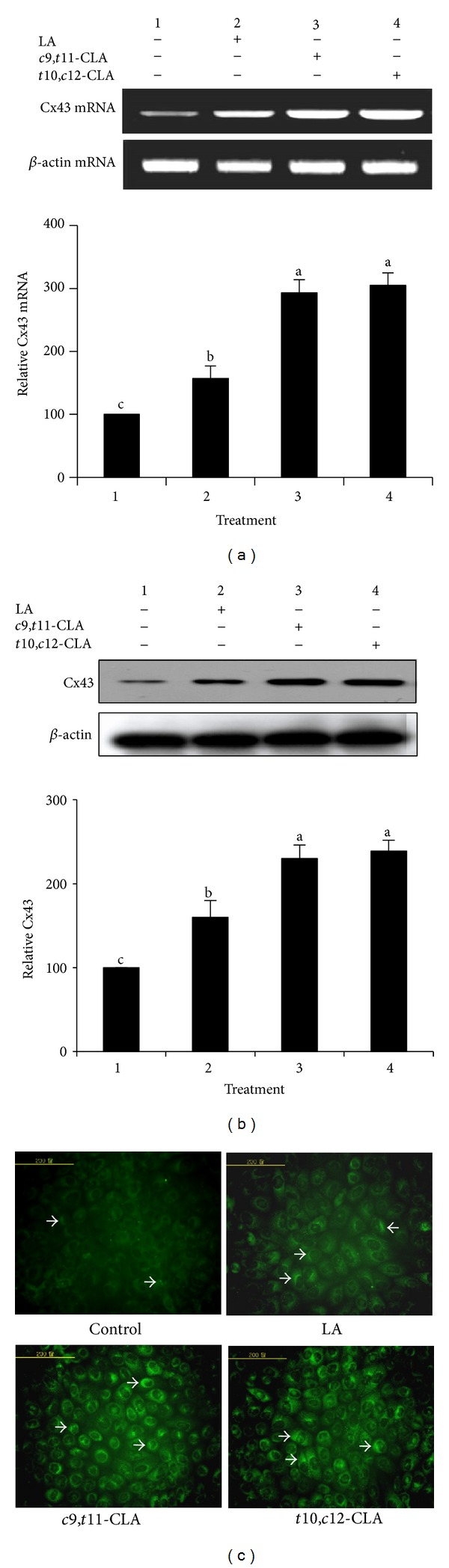
Expression of Cx43 gene in MCF-7 cells treated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. (a) Reverse transcription polymerase chain reaction of Cx43 mRNA. (b) Western blot analyses of the Cx43 protein. (c) Representative images of Cx43 expression and its distribution in the plasma membrane of cells, and arrows indicate Cx43 protein expression. Band intensities relative to control cells were quantified. Values are expressed as means ± standard deviations (n = 3). Means with different lowercase letters are significantly different at P < 0.05 by Duncan's multiple-range test.
Immunofluorescence staining was performed to determine the distribution and expression levels of Cx43 in the cell membranes and to further validate the influence of the CLA isomers on Cx43 expression (Figure 4(c)). Compared to the control and LA-treated cells exhibiting only limited expression of Cx43, the cells treated with c9,t11-CLA and t10,c12-CLA showed an increased level and wider distribution of the Cx43 protein. The increased immunostaining involved both the extent and size of fluorescent zones, suggesting a possible up-regulation in the number and size of gap junctions.
3.5. Generation of Intracellular ROS in MCF-7 Cells
There is evidence that elevated ROS depolarizes the cell membrane and opens connexin hemichannels to increase connexin gene expression, resulting in increased cell death [39]. Hence, we evaluated intracellular ROS generation in MCF-7 cells after CLA treatment. The effect of c9,t11-CLA and t10,c12-CLA isomers on ROS generation was measured by DCFH-DA dye method at 40 μM concentration for 48 h (Figures 5(a) and 5(b)). The levels of DCF fluorescence in the cells treated with c9,t11-CLA and t10,c12-CLA increased significantly relative to the control and LA-treated cells. No significant difference in ROS accumulation was found between cells treated with c9,t11-CLA and t10,c12-CLA. These results indicate that the CLA isomers induced the formation of ROS.
Figure 5.
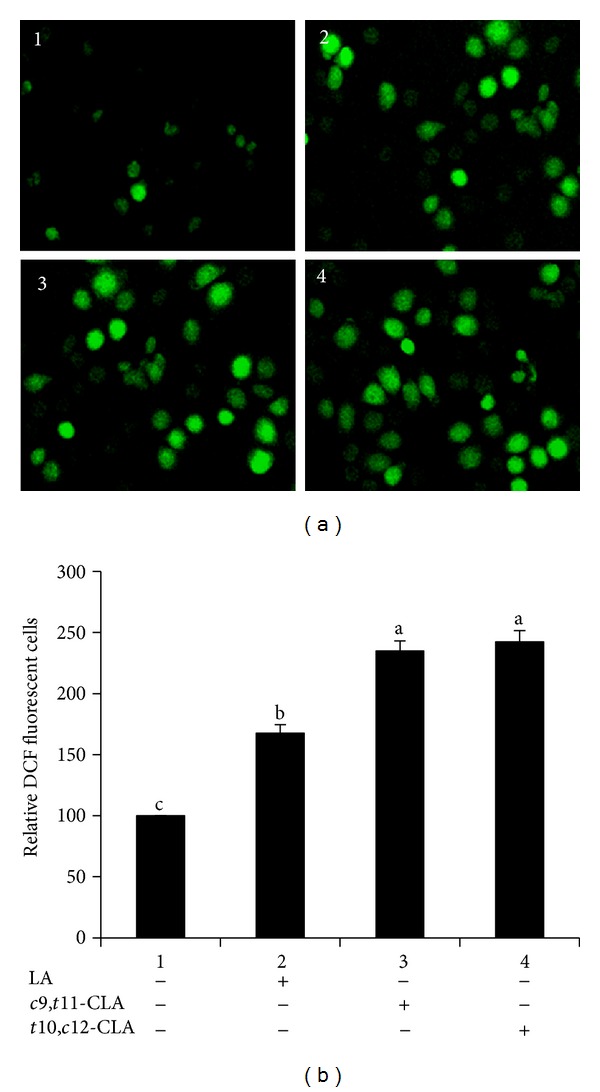
ROS generation in MCF-7 cells treated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. (a) Representative images of cells with ROS. (b) Relative DCF-fluorescent MCF-7 cells. Values are expressed as means ± standard deviations (n = 3). Means with different lowercase letters are significantly different at P < 0.05 by Duncan's multiple-range test.
3.6. Induction of NF-κB Inactivation in MCF-7 Cells
NF-κB is involved in cell proliferation and survival and in the induction of apoptosis, and Cx43 expression is regulated by NF-κB activity [40, 41]. To analyze if the effects of the CLA isomers on inhibited cell growth are related to NF-κB activity, the expression of NF-κB in MCF-7 cells treated with the c9,t11-CLA and t10,c12-CLA isomers was determined by Western blotting (Figures 6(a) and 6(b)). The c9,t11-CLA and t10,c12-CLA isomers significantly decreased the level of the NF-κB nuclear fraction relative to the control and LA-treated cells. These results indicate that inhibiting NF-κB activity might be involved in the inhibition of MCF-7 cell growth by these CLA isomers.
Figure 6.
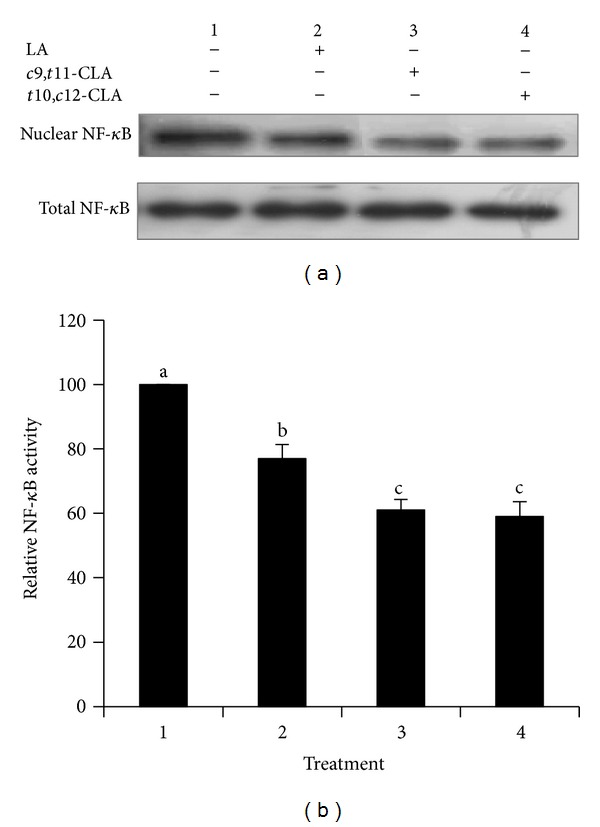
Inhibition of NF-κB activity in MCF-7 cells treated with 40 μM c9,t11-CLA, t10,c12-CLA, and LA for 48 h. (a) Western blot analyses of nuclear NF-κB. (b) Band intensities relative to untreated control cells. Values are expressed as means ± standard deviations (n = 3). Means with different lowercase letters are significantly different at P < 0.05 by Duncan's multiple-range test.
4. Discussion
We showed previously that both c9,t11-CLA and t10,c12-CLA isomers inhibit the growth of human MCF-7 breast cancer cells by inducing apoptosis [8]; however, the exact mechanism was not clearly elucidated. It is evident that enhancing GJIC through Cx43 upregulation in tumor cells is positively correlated with anticancer activities by inhibiting the growth of cells through the induction of apoptosis [32, 37, 38, 42, 43]. Hence, the main questions addressed by these studies are whether the growth inhibitory effect of c9,t11-CLA and t10,c12-CLA isomers on MCF-7 cells, which reduces GJIC, is mediated by enhanced GJIC to induce apoptosis and whether the effects are associated with upregulation of Cx43.
Apoptosis is one of the most common mechanistic actions of anticarcinogenic CLA isomers in animal and cancer cells [7–9]. The efficacy of individual CLA isomers on the induction of apoptosis is partly mediated by mitochondrial dysfunctional apoptosis, but the mechanism of action is completely unknown. Rakib et al. [13, 29] demonstrated an antipromotional action of c9,t11-CLA, t10,c12-CLA, and t,t-CLA isomers mediated by the inhibition of downregulation of GJIC in human MCF-10A cells, a nontumorigenic mammary epithelial cell line, treated with TPA, and they suggested that CLA isomers protected phosphorylation of Cx43 to maintain GJIC. These results suggest that CLA isomers could restore the down-regulated GJIC in cancer cells, similar to MCF-7 cells. This hypothesis was solved in the present study as the c9,t11-CLA and t10,c12-CLA isomers induced apoptosis by enhancing GJIC through Cx43 gene expression (Figures 3 and 4). Our results agree with reports that anticarcinogens, such as caffeic acid phenethyl ester, coleusin factor, tellimagrandin, and fucoxanthin, induce apoptosis via enhanced GJIC with elevated Cx expression [26, 32, 37, 38].
ROS are elevated in apoptotic cancer cells induced by CLA isomers [44], whereas NF-κB activation is inhibited [45]. Hence, it is of significance to elucidate that the elevated GJIC through Cx43 protein expression in MCF-7 cells by c9,t11-CLA and t10,c12-CLA isomers (Figure 4) is associated with enhanced ROS levels (Figure 5) and/or reduced NF-κB activity (Figure 6). Many chemical compounds that exhibit anticarcinogenic activity can reduce NF-κB activity and elevates ROS level in cancer cells. For example, anticarcinogenic niclosamide reduces NF-κB activity and elevates ROS concentration in acute myelogenous leukemia stem cells [34]. Evidently, the loss of NF-κB results in the accumulation of ROS because NF-κB activity suppresses the expression of some antioxidant genes [46–48]. The elevated ROS, which decreases the fluidity of the biological membranes and increases membrane permeability, depolarizes the cell membrane, opens connexin hemichannels, and expresses connexin genes, resulting in increased cell death [39]. Moreover, the NF-κB inhibitor BAY11-7082 stimulates Cx43 gene expression and restores GJIC in the Toll-like receptor (TLR3) ligand polyinosinic cytidylic acid-induced downregulation of Cx43 gene expression and GJIC [49]. These results suggest that compounds exhibiting anticarcinogenic action produce ROS to induce Cx43 gene expression and enhance GJIC in cancer cells. Taken together, the apoptosis induced by the c9,t11-CLA and t10,c12-CLA isomers in MCF-7 cells was associated with upregulation of GJIC through Cx43 expression mediated by inactivation of NF-κB and production of ROS as shown in Figure 7.
Figure 7.
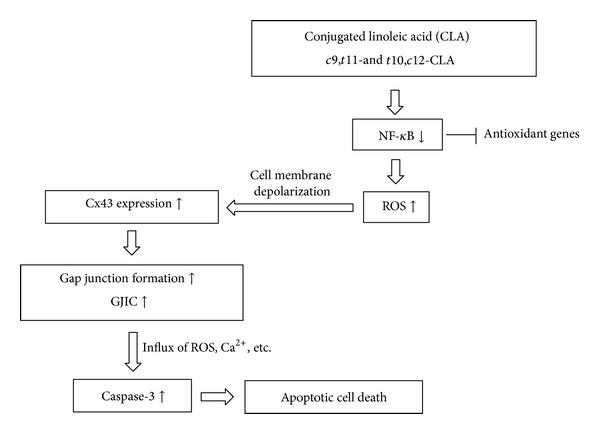
Schematic hypothetical diagram of growth inhibition of MCF-7 cells by apoptosis in relation to enhanced GJIC treated with c9,t11-CLA and t10,c12-CLA isomers. Oxidative stress induced by CLA isomers might depolarize the cell membrane and open gap junction channel by expressing Cx43. Open gap junction channels allow exchange of cell death signaling ions and molecules which accelerate cell death through apoptosis as discussed in this study.
One of the known mechanisms of restoring GJIC is the upregulation of connexin gene expression. Down-regulated connexin gene expression in many cancer cells is related to impaired Cx43 expression, including transcriptional silencing through DNA methylation at the connexin gene promoter sites [50]; lower concentrations of cAMP or estrogen [51]; and instability of mRNA and the Cx43 protein [52]. Although further studies are needed to clarify these mechanistic issues involved in the CLA isomers regarding expression of Cx43 in MCF-7 cells, we found that both the c9,t11-CLA and t10,c12-CLA isomers clearly reversed the reduced GJIC in MCF-7 cells by upregulating Cx43 genes at the transcriptional and translational levels (Figure 4). This finding was consistent with the result that fucoxanthin [32] and quinoline [53] compounds upregulate GJIC and Cx43 expression in human SK-HP-1 hepatoma cells and human T47D breast cancer cells in relation to growth inhibition. The open hemichannels allow direct entry of intracellular ROS in connexin-expressing cells leading to cancer cell death by altering cell survival mechanisms, including mitochondrial dysfunction [54]. In the present study, increased intracellular ROS were observed in the CLA isomer-treated MCF-7 cells along with increased DNA fragmentation, DNA condensation, and caspase-3 expression (Figure 2). Consequently, the influx of ROS into the cells through the open hemichannels might be involved in the caspase-dependent MCF-7 cell death.
It has been revealed that the antiproliferative activity of individual CLA isomers is dependent upon cancer cells. The anticarcinogenic efficacies of t10,c12-CLA and c9,t11-CLA isomers are nearly identical in MG-63 osteosarcoma cells and human MCF-7 breast cancer cells [8, 9], but different efficacies are observed in HT-29 colorectal cancer and PC-3 prostate cancer cells with the stronger potential of t10,c12-CLA [55, 56]. In the present study, we found no differences in efficacy of c9,t11-CLA and t10,c12-CLA to inhibit proliferation of MCF-7 cells with enhanced GJIC by inactivating NF-κB and reducing ROS. These results might be partly due to the similar structural configuration of the c9,t11-CLA and t10,c12-CLA isomers. Incorporation of these CLA isomers in the plasma membrane of MCF-7 cells is believed to be one of the factors dictating their similar anticancer activity, including Cx43 gene expression and inactivation of NF-κB, which affect the distribution and function of several membrane raft associated proteins [57, 58]. Furthermore, these CLA isomers may compete as ligands for the NF-κB transcription factor in cancer cells [34], and hence, the similar effects of c9,t11-CLA and t10,c12-CLA isomers on inhibiting MCF-7 cell proliferation might be due to similar binding affinities of the CLA isomers to NF-κB expressed in the cells. Further studies are needed to clarify these issues.
In conclusion, both c9,t11-CLA and t10,c12-CLA isomers effectively inhibited growth of MCF-7 cells, and this effect was associated with enhanced GJIC through the upregulation of Cx43 expression in conjunction with inactivation of NF-κB. Our results support the hypothesis that CLA isomers upregulate reduced GJIC, which plays a role in propagating cell death as a result of upregulated caspase-3 activity.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
This study was partly supported by a scholarship from the BK21 Plus and a grant from Basic Research Program through the National Research Foundation of Korea (2013-R1A1A2011587) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
References
- 1.Ha YL, Grimm NK, Pariza MW. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8(12):1881–1887. doi: 10.1093/carcin/8.12.1881. [DOI] [PubMed] [Google Scholar]
- 2.Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Research. 1990;50(4):1097–1101. [PubMed] [Google Scholar]
- 3.Nicolosi RJ, Rogers EJ, Kritchevsky D, Scimeca JA, Huth PJ. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atharosclerosis in hypercholesterolemic hamsters. Artery. 1997;22(5):266–277. [PubMed] [Google Scholar]
- 4.Byeon JI, Oh TW, Kim YS, et al. Reduction of visceral and body fats in mice by supplementation of conjugated linoleic acid with gamma oryzanol. Journal of Life Science. 2008;18:1212–11218. [Google Scholar]
- 5.Hayek MG, Han SN, Wu D, et al. Dietary conjugated linoleic acid influences the immune response of young and old C57BL/6NCrlBR mice. Journal of Nutrition. 1999;129(1):32–38. doi: 10.1093/jn/129.1.32. [DOI] [PubMed] [Google Scholar]
- 6.Liew C, Schut HAJ, Chin SF, Pariza MW, Dashwood RH. Protection of conjugated linoleic acids against 2-amino-3-methylimidazo [4,5-f] quinoline-induced colon carcinogenesis in the F344 rat: a study of inhibitory mechanisms. Carcinogenesis. 1995;16(12):3037–3043. doi: 10.1093/carcin/16.12.3037. [DOI] [PubMed] [Google Scholar]
- 7.Islam MA, Kim YS, Oh TW, et al. Superior anticarcinogenic activity of trans, trans-conjugated linoleic acid in N-methyl-N-nitrosourea-induced rat mammary tumorigenesis. Journal of Agricultural and Food Chemistry. 2010;58(9):5670–5678. doi: 10.1021/jf100117a. [DOI] [PubMed] [Google Scholar]
- 8.Islam MA, Kim YS, Jang WJ, et al. A mixture of trans, trans conjugated linoleic acid induces apoptosis in MCF-7 human breast cancer cells with reciporocal expression of bax and bcl-2. Journal of Agricultural and Food Chemistry. 2008;56(14):5970–5976. doi: 10.1021/jf8004977. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Cerbo RM, Hah CK, Bahn KN, Kim JO, Ha YL. Growth inhibition of osteosarcoma cell MG-63 by a mixture of trans,trans conjugated linoleic acid isomers: possible mechanistic actions. Journal of Food Science. 2008;73(1):T7–T15. doi: 10.1111/j.1750-3841.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan R, Ruangvoravat C, Joo D, et al. Structure, sequence and expression of the mouse Cx43 gene encoding connexin 43. Gene. 1993;130(2):191–199. doi: 10.1016/0378-1119(93)90419-4. [DOI] [PubMed] [Google Scholar]
- 11.Trosko JE, Chang CC. Modulation of cell-cell communication in the cause and chemoprevention/chemotherapy of cancer. BioFactors. 2000;12(1–4):259–263. doi: 10.1002/biof.5520120139. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Nguyen TA. Gap junction enhancer potentiates cytotoxicity of cisplatin in breast cancer cells. Journal of Cancer Science and Therapy. 2012;4(11):371–3378. doi: 10.4172/1948-5956.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakib MA, Kim YS, Jang WJ, et al. Attenuation of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced gap junctional intercellular communication (GJIC) inhibition in MCF-10A cells by c9,t11-conjugated linoleic acid. Journal of Agricultural and Food Chemistry. 2010;58(22):12022–12030. doi: 10.1021/jf103205c. [DOI] [PubMed] [Google Scholar]
- 14.Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Frontiers in Bioscience. 1998;3:d208–d236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 15.Cesen-Cummings K, Fernstrom MJ, Malkinson AM, Ruch RJ. Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis. 1998;19(1):61–67. doi: 10.1093/carcin/19.1.61. [DOI] [PubMed] [Google Scholar]
- 16.Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature. 1966;209(5029):1248–1249. doi: 10.1038/2091248a0. [DOI] [PubMed] [Google Scholar]
- 17.Katoh F, Klein JL, Bignami N, Yamasaki H. Association of viral oncogene-induced changes in gap junctional intercellular communication and morphological transformation in BALB/c3T3 cells. Carcinogenesis. 1993;14(3):435–440. doi: 10.1093/carcin/14.3.435. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, Prasain K, Nguyen TD, Hua DH, Nguyen TA. The effect of the PQ1 anti-breast cancer agent on normal tissues. Anticancer Drugs. 2012;23(9):897–905. doi: 10.1097/CAD.0b013e328354ac71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trosko JE, Chang CC, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular signalling leading to altered gap junctional intercellular communication. Toxicology Letters. 1998;102-103:71–78. doi: 10.1016/s0378-4274(98)00288-4. [DOI] [PubMed] [Google Scholar]
- 20.Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Current Drug Targets. 2002;3(6):465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- 21.Leone A, Longo C, Trosko JE. The chemopreventive role of dietary phytochemicals through gap junctional intercellular communication. Phytochemistry Reviews. 2012;11(2-3):285–307. [Google Scholar]
- 22.Pozzi A, Risek B, Kiang DT, Gilula NB, Kumar NM. Analysis of multiple gap junction gene products in the rodent and human mammary gland. Experimental Cell Research. 1995;220(1):212–219. doi: 10.1006/excr.1995.1308. [DOI] [PubMed] [Google Scholar]
- 23.McLachlan E, Shao Q, Laird DW. Connexins and gap junctions in mammary gland development and breast cancer progression. Journal of Membrane Biology. 2007;218(1–3):107–121. doi: 10.1007/s00232-007-9052-x. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson S, Going JJ, D'Arcy R, George WD. Expression of gap junction proteins connexin26 and connexin43 in normal human breast and in breast tumors. Journal of Pathology. 1998;184(1):37–43. doi: 10.1002/(SICI)1096-9896(199801)184:1<37::AID-PATH966>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nature Reviews Cancer. 2010;10(6):435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 26.Na HK, Wilson MR, Kang KS, Chang CC, Grunberger D, Trosko JE. Restoration of gap junctional intercellular communication by caffeic acid phenethyl ester (CAPE) in a ras-transformed rat liver epithelial cell line. Cancer Letters. 2000;157(1):31–38. doi: 10.1016/s0304-3835(00)00470-5. [DOI] [PubMed] [Google Scholar]
- 27.de Feijter-Rupp HL, Hayashi T, Kalimi GH, et al. Restored gap junctional communication in non-tumorigenic HeLa-normal human fibroblast hybrids. Carcinogenesis. 1998;19(5):747–754. doi: 10.1093/carcin/19.5.747. [DOI] [PubMed] [Google Scholar]
- 28.Rae RS, Mehta PP, Chang CC, Trosko JE, Ruch RJ. Neoplastic phenotype of gap-junctional intercellular communication-deficient WB rat liver epithelial cells and its reversal by forced expression of connexin 32. Molecular Carcinogenesis. 1998;22(2):120–127. doi: 10.1002/(sici)1098-2744(199806)22:2<120::aid-mc7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Rakib MA, Kim YS, Jang WJ, Jang JS, Kang SJ, Ha YL. Preventive effect of t,t-conjugated linoleic acid on 12-O- tetradecanoylphorbol-13-acetate-induced inhibition of gap junctional intercellular communication in human mammary epithelial MCF-10A cells. Journal of Agricultural and Food Chemistry. 2011;59(8):4164–4170. doi: 10.1021/jf1046909. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Park KA, Park HY, Kim JO, Ha YL. Preparation of a large quantity of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers from synthetic CLA. Journal of Food Science and Nutrition. 2000;5(2):86–92. [Google Scholar]
- 31.van Greevenbroek MMJ, Voorhout WF, Erkelens DW, van Meer G, de Bruin TWA. Palmitic acid and linoleic acid metabolism in Caco-2 cells: different triglyceride synthesis and lipoprotein secretion. Journal of Lipid Research. 1995;36(1):13–24. [PubMed] [Google Scholar]
- 32.Liu CL, Huang YS, Hosokawa M, Miyashita K, Hu ML. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chemico-Biological Interactions. 2009;182(2-3):165–172. doi: 10.1016/j.cbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of human breast cell line (MCF-7) Toxicology in Vitro. 2007;21(2):217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Lu Z, Ding K, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-κB pathway and generation of reactive oxygen species. Cancer Research. 2010;70(6):2516–2527. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 35.Quentmeier H, Osborn M, Reinhardt J, Zaborski M, Drexler HG. Immunocytochemical analysis of cell lines derived from solid tumors. The Journal of Histochemistry and Cytochemistry. 2001;49(11):1369–1378. doi: 10.1177/002215540104901105. [DOI] [PubMed] [Google Scholar]
- 36.Possel H, Noack H, Augustin W, Keilhoff G, Wolf G. 2,7-Dihydrodichlorofluorescein diacetate as a fluorescent marker for peroxynitrite formation. FEBS Letters. 1997;416(2):175–178. doi: 10.1016/s0014-5793(97)01197-6. [DOI] [PubMed] [Google Scholar]
- 37.Geng S, Sun B, Liu S, Wang J. Up-regulation of connexin 43 and gap junctional intercellular communication by Coleusin Factor is associated with growth inhibition in rat osteosarcoma UMR106 cells. Cell Biology International. 2007;31(11):1420–1427. doi: 10.1016/j.cellbi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Yi ZC, Liu YZ, Li HX, et al. Tellimagrandin I enhances gap junctional communication and attenuates the tumor phenotype of human cervical carcinoma HeLa cells in vitro. Cancer Letters. 2006;242(1):77–87. doi: 10.1016/j.canlet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PloS ONE. 2007;2(1):p. e712. doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer C, Sims AH, Morgan K, et al. Transcript and protein profiling identifies signaling, growth arrest, apoptosis, and NF-κB survival signatures following GNRH receptor activation. Endocrine-Related Cancer. 2013;20(1):123–136. doi: 10.1530/ERC-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso F, Krattinger N, Mazzolai L, et al. An angiotensin II-and NF-κB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovascular Research. 2010;87(1):166–176. doi: 10.1093/cvr/cvq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trosko JE, Goodman JI. Intercellular communication may facilitate apoptosis: implications for tumor promotion. Molecular Carcinogenesis. 1994;11(1):8–12. doi: 10.1002/mc.2940110103. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MR, Close TW, Trosko JE. Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Experimental Cell Research. 2000;254(2):257–268. doi: 10.1006/excr.1999.4771. [DOI] [PubMed] [Google Scholar]
- 44.Hsu YC, Ip MM. Conjugated linoleic acid-induced apoptosis in mouse mammary tumor cells is mediated by both G protein coupled receptor-dependent activation of the AMP-activated protein kinase pathway and by oxidative stress. Cellular Signalling. 2011;23(12):2013–2020. doi: 10.1016/j.cellsig.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song HJ, Sneddon AA, Heys SD, Wahle KWJ. Induction of apoptosis and inhibition of NF-κB activation in human prostate cancer cells by the cis-9, trans-11 but not the trans-10, cis-12 isomer of conjugated linoleic acid. The Prostate. 2006;66(8):839–846. doi: 10.1002/pros.20351. [DOI] [PubMed] [Google Scholar]
- 46.Lampiasi N, Azzolina A, D’Alessandro N, et al. Antitumor effects of dehydroxymethylepoxyquinomicin, a novel nuclear factor-κB inhibitor, in human liver cancer cells are mediated through a reactive oxygen species-dependent mechanism. Molecular Pharmacology. 2009;76(2):290–300. doi: 10.1124/mol.109.055418. [DOI] [PubMed] [Google Scholar]
- 47.Stein SJ, Baldwin AS. NF-κB suppresses ROS levels in BCR-ABL(+) cells to prevent activation of JNK and cell death. Oncogene. 2011;30(45):4557–4566. doi: 10.1038/onc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pozo-Guisado E, Merino JM, Mulero-Navarro S, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. International Journal of Cancer. 2005;115(1):74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Rivieccio MA, Lutz S, Scemes E, Brosnan CF. The TLR3 ligand polyI:C downregulates connexin 43 expression and function in astrocytes by a mechanism involving the NF-κB and PI3 kinase pathways. Glia. 2006;54(8):775–785. doi: 10.1002/glia.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piechocki MP, Burk RD, Ruch RJ. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20(3):401–406. doi: 10.1093/carcin/20.3.401. [DOI] [PubMed] [Google Scholar]
- 51.You S, Li W, Lin T. Expression and regulation of connexin43 in rat leydig cells. Journal of Endocrinology. 2000;166(2):447–453. doi: 10.1677/joe.0.1660447. [DOI] [PubMed] [Google Scholar]
- 52.Schiller PC, Roos BA, Howard GA. Parathyroid hormone up-regulation of connexin 43 gene expression in osteoblasts depends on cell phenotype. Journal of Bone and Mineral Research. 1997;12(12):2005–2013. doi: 10.1359/jbmr.1997.12.12.2005. [DOI] [PubMed] [Google Scholar]
- 53.Gakhar G, Ohira T, Shi A, Hua DH, Nguyen TA. Antitumor effect of substituted quinolines in breast cancer cells. Drug Development Research. 2008;69(8):526–534. [Google Scholar]
- 54.Ishikawa S, Kuno A, Tanno M, et al. Role of connexin-43 in protective PI3K-Akt-GSK-3β signaling in cardiomyocytes. American Journal of Physiology and Heart and Circulatory Physiology. 2012;302(12):H2536–H2544. doi: 10.1152/ajpheart.00940.2011. [DOI] [PubMed] [Google Scholar]
- 55.Cho HJ, Kim WK, Jung JI, et al. Trans-10, cis-12, not cis-9, trans-11, conjugated linoleic acid decreases ErbB3 expression in HT-29 human colon cancer cells. World Journal of Gastroenterology. 2005;11(33):5142–5150. doi: 10.3748/wjg.v11.i33.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochoa JJ, Farquharson AJ, Grant I, Moffat LE, Heys SD, Wahle KWJ. Conjugated linoleic acids (CLAs) decrease prostate cancer cell proliferation: different molecular mechanisms for cis-9, trans-11, and trans-10, cis-12 isomers. Carcinogenesis. 2004;25(7):1185–1191. doi: 10.1093/carcin/bgh116. [DOI] [PubMed] [Google Scholar]
- 57.Subbaiah PV, Gould IG, Lal S, Aizezi B. Incorporation profiles of conjugated linoleic acid isomers in cell membranes and their positional distribution in phospholipids. Biochimica et Biophysica Acta. 2011;1811(1):17–24. doi: 10.1016/j.bbalip.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stulnig TM, Huber J, Leitinger N, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. Journal of Biological Chemistry. 2001;276(40):37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]


