Abstract
Face recognition by normal subjects depends in roughly equal proportions on shape and surface reflectance cues, while object recognition depends predominantly on shape cues. It is possible that developmental prosopagnosics are deficient not in their ability to recognize faces per se, but rather in their ability to use reflectance cues. Similarly, super-recognizers’ exceptional ability with face recognition may be a result of superior surface reflectance perception and memory. We tested this possibility by administering tests of face perception and face recognition in which only shape or reflectance cues are available to developmental prosopagnosics, super-recognizers, and control subjects. Face recognition ability and the relative use of shape and pigmentation were unrelated in all the tests. Subjects who were better at using shape or reflectance cues were also better at using the other type of cue. These results do not support the proposal that variation in surface reflectance perception ability is the underlying cause of variation in face recognition ability. Instead, these findings support the idea that face recognition ability is related to neural circuits using representations that integrate shape and pigmentation information.
Keywords: Face recognition, face perception, reflectance, pigmentation, shape, super-recognizers
1. Introduction
Recognizing conspecifics is of critical importance to social species such as humans, and is foundational for social behavior and social cognition. Though there are many sources of information about the identity of another person, including voice, clothing, patterns of gait, and context (e.g. I expect to see my dentist when I go to his office), the primary cue for identifying other people is the face. Given the behavioral importance of face recognition, it is perhaps not surprising that there are entire cortical networks involved in face recognition in humans and other primates (Tsao, Moeller, & Freiwald, 2008). Further evidence for the notion that face recognition is an important and distinct ability is the discovery of neuropsychological cases presenting impaired recognition of faces with otherwise normal visual perception—in some cases with normal or relatively normal visual recognition of other kinds of objects—a condition called prosopagnosia (Farah, Wilson, Drain, & Tanaka, 1995; Henke, Schwinbuerger, Grigo, Klos, & Sommer, 1998).
Until a decade ago, most cases in the literature were of acquired prosopagnosia, where the deficits are a result of trauma, stroke, or other brain damage. During the past decade there have been numerous reports of people with very poor face recognition ability where the deficits cannot be tied to specific brain damage, a condition called developmental prosopagnosia (sometimes called congenital prosopagnosia or hereditary prosopagnosia) (Behrmann & Avidan, 2005; Duchaine & Nakayama, 2005). The discovery of many cases of developmental prosopagnosia is part of a larger discovery that the common range of face recognition ability is much wider than previously assumed. On the low end of face recognition ability lie the developmental prosopagnosics who have been estimated to comprise to comprise around 2% of the general population (Kennerknecht, et al., 2006). On the other end of the face recognition spectrum are super-recognizers (Russell, Duchaine, & Nakayama, 2009), who are far better than average at recognizing faces. Super-recognizers have been proposed to represent the high end of a unitary distribution of ability with developmental prosopagnosia at the low end, which would mean that both groups are quantitatively rather than qualitatively different from average (Russell, et al., 2009). Aside from the knowledge that face recognition ability is highly heritable (Wilmer, et al., 2010; Zhu, et al., 2010), little is known about the specific causes of the range of ability.
Prosopagnosia, and now super-recognition, have been a focus of research in part because of the debate over whether face recognition is separable from the recognition of other classes of object, a debate which is itself part of a larger question in cognitive neuroscience about modularity—whether the brain is organized along domain-specific or domain-general lines. According to the domain-specific account, the mind is divided according to the content of the information processed, while in the domain-general account, the mind is divided according to the kinds of processes it carries out (Kanwisher, 2000; Tarr & Gauthier, 2000). This debate has played out with respect to prosopagnosia with researchers debating whether the deficits seen in prosopagnosia are truly specific to the domain of faces or are instead specific to the process of expert recognition. Some researchers have argued that prosopagnosia is a deficit specific to the recognition of faces (Duchaine & Nakayama, 2005), while others have argued that prosopagnosia is a deficit not in face recognition per se, but rather in the recognition of any object class for which the observer has expertise (Gauthier, Behrmann, & Tarr, 1999). In this latter account all normal adults could be considered face experts, and some are also experts at recognizing other classes of stimuli, such as birds, dogs, or cars.
However, there is also a third possibility for why developmental prosopagnosics have face recognition deficits. Rather than being a deficit in face specific processes or expert specific processes, developmental prosopagnosia could be due to a deficit in a visual competency that is more relevant for recognizing faces than for recognizing other kinds of objects. In such an account, developmental prosopagnosia results not from abnormal development of face-specific neural circuits or expertise-specific neural circuits, but rather from abnormal development of a perceptual ability that is useful only or mostly for recognizing faces. An analogous situation has been found in auditory perception with a disorder that affects a specific component of high level perception and recognition—congenital amusia.
Congenital amusia is marked by impairments in music memory and recognition, as well as singing, and appears specific to the domain of music, without impairment to language comprehension (Zatorre, Belin, & Penhune, 2002). Like prosopagnosia, amusia has been a focus for speculation about the modularity of cognition (Peretz & Coltheart, 2003). While the most apparent deficit in this disorder is the inability to perceive music, it is now known that amusia is caused by severe deficiencies in the perception of changes in pitch (Ayotte, Peretz, & Hyde, 2002; Peretz, et al., 2002). The specificity of the disorder is not caused by damage to neural circuits specific for music. Instead it is a result of music perception and production being the only cognitive function that requires fine discrimination of pitch. While pitch plays a role in language (especially tonal languages such as Mandarin Chinese), the meaningful pitch variations are coarse compared to those of music, and can be comprehended by people with amusia. Thus, the origin of the disorder appears to be acoustic and music-relevant, not music-specific (Peretz, 2008; Peretz & Hyde, 2003). Prosopagnosia may similarly have origins that are perceptual and face-relevant, but not face-specific. For this to be the case, the impaired perceptual ability would need to be one that is required much more for the recognition of faces than for the recognition of other object classes.
The appearance of any three-dimensional object can be explained in terms of three variables: the shape of the object, the way that the surface of the object reflects and transmits light, and the illumination of the object. Considering the face as an object, we can explain the difference in appearance between two faces under the same conditions of illumination as arising from differences in their shape and differences in their surface reflectance properties. The reflectance properties of facial skin are quite complex, with a great deal of sub-surface scattering of light (Debevec, et al., 2000). Here we also use the term ‘pigmentation’ interchangeably with ‘reflectance’ to refer to all of the reflectance properties of the face, including the proportion of incident light that the surface reflects, the proportion of light it reflects as a function of wavelength, surface and sub-surface scattering of light, as well as variation across the surface of these properties (i.e. color and visual texture are included in our definition, among other properties). Work from several laboratories using a variety of methods has evaluated the use of shape and pigmentation information for face recognition, arriving at the general conclusion that the two kinds of cues are about equally useful for face recognition by normal observers (Caharel, Jiang, Blanz, & Rossion, 2009; Jiang, Blanz, & O’Toole, 2006; O’Toole, Vetter, & Blanz, 1999; Russell, Biederman, Nederhouser, & Sinha, 2007; Russell & Sinha, 2007; Russell, Sinha, Biederman, & Nederhouser, 2006; Siemionow & Agaoglu, 2006).
While the literature supports the idea that shape and reflectance are equally useful for recognizing faces, the situation with most other classes of objects is different. Most basic level categories of objects are recognized predominantly on the basis of shape cues (Biederman & Ju, 1988; Tanaka, Weiskopf, & Williams, 2001; Ullman, 1996). This is not to say that reflectance properties are irrelevant for recognizing objects, but rather that shape is clearly more important than surface reflectance properties. Thus one way that recognition of faces differs from recognition of most other classes of objects is in the utility of surface reflectance properties. Reflectance properties are much more important for recognizing faces than recognizing other kinds of objects.
The equivalent importance of shape and pigmentation for face recognition but greater importance of shape for recognition of most other objects is consistent with recent neuroimaging work (Cant, Arnott, & Goodale, 2009; Cant & Goodale, 2007, 2011) showing a gradient in ventral cortex between regions involved in perception of shape and regions involved in perception of texture (reflectance). Specifically, lateral occipital complex (LO), a cortical region involved in object recognition (Malach, et al., 1995), lies at the end of the gradient dominated by shape, while the collateral sulcus (CoS) and parahippocampal place area (PPA) (Epstein & Kanwisher, 1998) lie at the end dominated by surface properties. The fusiform face area (FFA), a cortical region involved in the perception of faces (Kanwisher, McDermott, & Chun, 1997), sits midway between the shape and reflectance ends of the gradient and is sensitive to both shape and surface properties.
Developmental prosopagnosics are generally more impaired at recognizing faces than recognizing other classes of objects. Though developmental prosopagnosics report difficulties with face recognition in their day to day lives, few report difficulties with recognizing other kinds of objects. Indeed, it is possible for the same individual to have extreme face recognition impairments but be perfectly normal at recognizing other objects in standardized tests—i.e. have “pure” prosopagnosia (Duchaine, Dingle, Butterworth, & Nakayama, 2004), though a recent study with a large number of developmental prosopagnosics found that most are impaired at recognizing other object classes (Chatterjee, Russell, Duchaine, & Nakayama, In preparation). However, even in that study, the developmental prosopagnosics were more impaired with faces than with the other object classes.
Given that recognition of faces requires the ability to perceive and remember surface reflectance properties more than does recognition of other kinds of objects, and that prosopagnosics are much worse at recognizing faces than other kinds of objects, it is possible that prosopagnosia is a deficit of reflectance (pigmentation) perception. In other words, developmental prosopagnosia could be a selective deficit not of face recognition but rather of perception and memory for surface reflectance properties. Similarly, super-recognizers may simply be exceptionally good at perceiving reflectance properties. Here we describe efforts to test the idea that variation in the ability to perceive and remember facial pigmentation causes variation in face recognition ability. Unless pigmentation perception is completely irrelevant for face recognition, it should be related to some extent with face recognition ability. The critical question is whether pigmentation perception is more strongly related to face recognition than is the other perceptual component of recognition—shape perception.
To determine whether ability to use both shape and pigmentation information in face perception and face recognition varies as a function of face recognition ability, we identified three groups of subjects: developmental prosopagnosics (who have very poor face recognition ability), control subjects, and super-recognizers (who have very good face recognition ability). All the subjects were tested for their ability to perceive subtle differences between faces using either shape or pigmentation information, and to recognize faces using either shape or pigmentation information. In this way we sought to determine whether these groups show differences in their relative use of shape and pigmentation information to perceive and recognize faces. While we expected to find main effects of the subject group (with super-recognizers generally performing the best on the face processing tasks, the controls in the middle, and the developmental prosopagnosics performing the worst), the critical test of the hypothesis is the existence of a meaningful interaction between the subject groups and the cue type (i.e. the use of shape or pigmentation information).
2. Subjects
Participants in the study were identified and recruited into one of three groups: developmental prosopagnosics, controls, and super-recognizers. These three groups were each recruited by distinct procedures, as described below.
Ten developmental prosopagnosic subjects were recruited through the Prosopagnosia Research Centers website, www.faceblind.org, which exists to provide information about prosopagnosia and related disorders, and also to allow individuals to self-identify as having face recognition difficulties and interest in research participation.
Twenty six control subjects were recruited through the Harvard Psychology department study pool. These subjects were not asked whether they have difficulty recognizing faces. Thus, self-perception of face recognition ability was neither an inclusionary nor an exclusionary factor for this group.
The six super-recognizer subjects were not actively recruited. Most of the super-recognizer subjects independently contacted the researchers in order to indicate that they believed they had superior face recognition ability, and were interested in participating in research. One subject was identified to the researchers by his friend who was tested in our laboratory for prosopagnosia.
Following recruitment, all subjects were given the same two standardized tests: the Cambridge Face Perception Test (CFPT) (Duchaine, Germine, & Nakayama, 2007) and the Cambridge Face Memory Test (CFMT) (Duchaine & Nakayama, 2006). Controls and super-recognizers were given the long form of the CFMT, which has better discrimination at the high end of performance (Russell, et al., 2009). Because the long form of the CFMT contains the short form, a subject who has completed the long form has also completed the short form. Performance by the different subject groups on the two standardized tests are presented in Table 1.
Table 1.
Mean age and performance on standardized tests by different subject groups
| N | Age (y) | CFMT short | CFMT long | CFPT upright | CFPT inverted | ||
|---|---|---|---|---|---|---|---|
| Developmental prosopagnosics | 10 | M | 49.5 | 39.1 | — | 67.8 | 75.3 |
| SD | 8.4 | 5.0 | 17.6 | 15.4 | |||
| Control subjects | 26 | M | 42.2 | 60.1 | 75.2 | 35.4 | 70.5 |
| SD | 14.1 | 8.5 | 11.6 | 12.9 | 15.7 | ||
| Super-recognizers | 6 | M | 40.7 | 71.3 | 95.0 | 24.7 | 56.7 |
| SD | 9.9 | 0.8 | 1.9 | 10.3 | 11.7 |
3. Experiment 1: Face perception
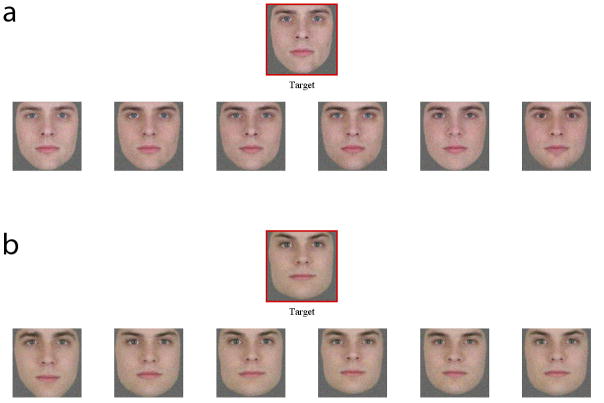
In this study, we sought to characterize the relative utility of shape and pigmentation cues for discriminating between simultaneously presented faces. In this way, we sought to investigate the ability of developmental prosopagnosics, controls, and super-recognizers to perceive shape and pigmentation differences in the face. To do this, we modified the design of the Cambridge Face Perception Test to include only upright faces and by introducing a shape condition and a pigmentation condition. The test consists of 16 trials, and in each trial the subject is presented a target face and six other faces that have been created using morphing software so that they vary subtly but systematically from the target. The task of the subject is to sort these six other faces in order of similarity to the target face. The dependent measure is the number of errors that the subject makes in placing the faces in the correct order.
Critically, the target face and the faces to be sorted differ from one another only in terms of their shape or their pigmentation. In some trials all the faces differed only in terms of shape and in other trials all the faces differed only in terms of pigmentation. Thus there were two different kinds of trials—shape trials, in which the subjects had to use shape cues to distinguish among the faces, and pigmentation trials in which the subjects had to use pigmentation cues to distinguish among the faces. Examples of two of the trials are shown in Figure 1.
Figure 1.
Example trials from Experiment 1. a) Trial from the pigmentation condition in which shape remains constant. The six faces have been put into the correct order, with the face that is most similar to the highlighted target face at the far left, the least similar face at the far right, and so forth in between. b) Trial from the shape condition in which pigmentation remains constant. The six faces are shown in the order that they appeared initially to the subjects, and hence have not yet been placed in the correct order. Notice that shape here refers not only to the outline of the face, but also to the shapes and locations of the internal features (eyes, eyebrows, nose, mouth).
In comparing performance with two different kinds of cues, it is important to equate the amount of information available in the two cue types (i.e., the similarity of the stimuli in the two different conditions). The face images used in this task were modified from sets of faces differing in terms of either shape or pigmentation that were used in two previous studies (Russell, et al., 2007; Russell, et al., 2006). Those shape and pigmentation sets were equated for perceptual similarity, as measured by the Gabor-jet model developed by von der Malsburg and colleagues (Lades, et al., 1993). This process of equating the stimuli sets is described in detail in Russell et al. (2007). The stimuli for the current work, e.g. the shape set, were created by morphing together the stimuli from the shape set from Russell et al. (2007). Because of this, the degree of similarity between stimuli in the shape and pigmentation sets should have remained roughly matched. However, there was one relevant difference. The Gabor-jet model is designed to compare the similarity of grayscale but not color images. The shape and pigmentation sets in Russell et al. (2007) that were matched for Gabor-jet similarity were grayscale images. However the images used here were full-color versions of these same images. Because color variations are present in the pigmentation but not the shape sets, we would expect that the faces in the pigmentation set are somewhat less similar to one another than those in the shape set.
The results from Experiment 1 are presented in Figure 2. The super-recognizers performed better at this task than the controls, who in turn performed better than the developmental prosopagnosics. All subject groups performed better using shape cues than pigmentation cues. A repeated measures analysis of variance (ANOVA) of performance (number of errors) with cue type as a within subject factor and subject group as a between groups factor found significant main effects of cue type (F1,39 = 14.07, p <0.01, η2 = 0.265) and subject group (F2,39= 21.88, p <0.001, η2 = 0.529). There was a trend toward an interaction between cue type and subject group, though it was not significant (F2,39= 3.07, p = 0.058), with the developmental prosopagnosics and super-recognizers, but not the controls, performing relatively better in the shape condition than the pigmentation condition.
Figure 2.
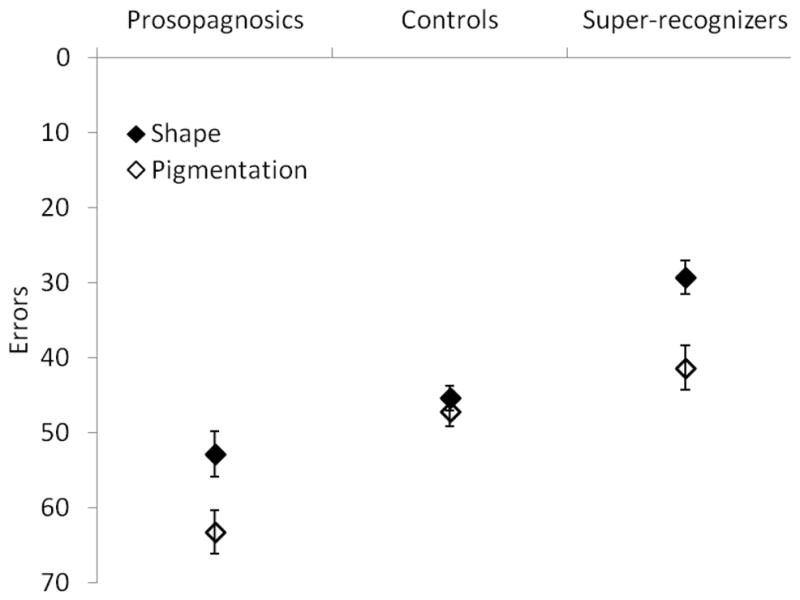
Performance in Experiment 1 by the different subject groups. Performance is measured in errors, thus a score higher on the y-axis represents better performance.
As would be expected based on previous research (Russell, et al., 2009), subject groups with better face recognition ability were better at performing this face discrimination task. Consistent with previous work with these same images (Russell, et al., 2007; Russell, et al., 2006), control subjects performed about equally well using shape and pigmentation cues. However, both the developmental prosopagnosics and the super-recognizers performed better using shape cues than pigmentation cues.
4. Experiments 2a & 2b: Face memory
In this study, we sought to characterize the relative utility of shape and pigmentation cues for recognizing previously viewed faces. In this way, we sought to investigate the ability of developmental prosopagnosics, controls, and super-recognizers to remember shape and pigmentation information in the face. To do this we used a two-alternative forced-choice (2AFC) memory test. In this test, novel faces are presented twice during a training phase which is followed by a test phase in which two images are presented side by side, the target face and a distractor face that has the same shape or the same pigmentation as the target. The subject’s task is to decide which of the two faces had been presented previously during the training phase. By presenting in any single trial a target and distractor that differed in terms of only shape or only pigmentation, we were able to test the ability of subjects to use shape or pigmentation for face recognition.
Because of the large difference in face memory ability between the super-recognizers and developmental prosopagnosics, we created two different versions of the test, a hard version for distinguishing normal from above normal performance, and an easy version for distinguishing normal from below normal performance. The hard version was given to the super-recognizers and one group of controls, and the easy version was given to the developmental prosopagnosics and a different group of controls. The two versions differed in the number of target faces and the length of the delay between training and test. The hard version had 30 target images, and a 20 minute delay between training and test. The easy version had 18 target images and no delay between training and test.
4.1 Experiment 2a results
The easy version of the 2AFC memory test was given to 12 control subjects and the ten developmental prosopagnosics. The results of the easy version are presented in Figure 3. A repeated measures analysis of variance (ANOVA) of performance on the easy version with cue type as a within subject factor and subject group as a between groups factor found significant main effects of cue type (F1,20 = 7.28, p <0.05, η2 = 0.267) and subject group (F1,20 = 10.74, p <0.01, η2 = 0.349). Performance was better using pigmentation information than shape information, and the control subjects performed better than the developmental prosopagnosics. However, there was not a significant interaction between cue type and subject group (F1,20 = 2.70, p > 0.1).
Figure 3.
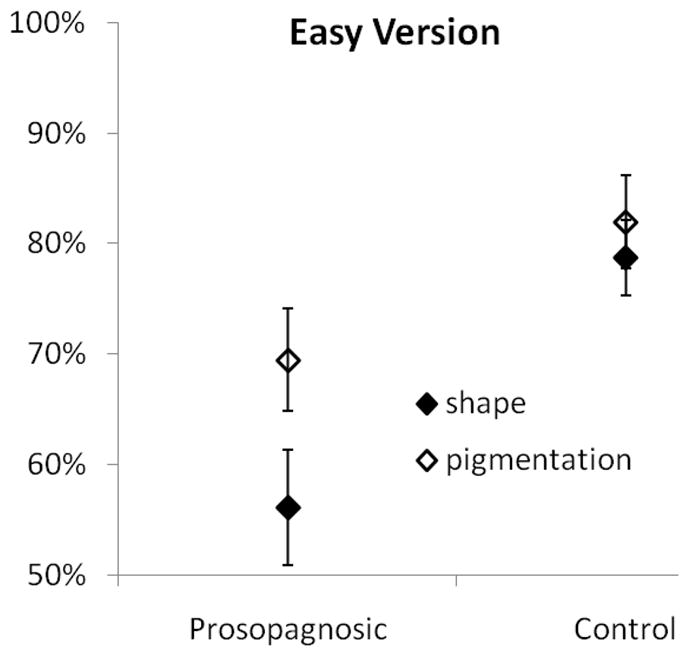
Performance by developmental prosopagnosic and control subjects on the easy version of the 2AFC memory test.
4.2 Experiment 2b results
The hard version of the 2AFC memory test was given to 11 control subjects and the six super-recognizers. The results of the hard version are presented in Figure 4. A repeated measures analysis of variance (ANOVA) of performance on the hard version with cue type as a within subject factor and subject group as a between groups factor found a significant main effect of subject group (F1,14= 23.83, p <0.001, η2 = 0.630). The super-recognizers performed better than the control subjects. However, there was not a significant main effect of cue type (F1,14 = 1.95, p > 0.1) or a significant interaction between cue type and subject group (F1,14 < 1).
Figure 4.
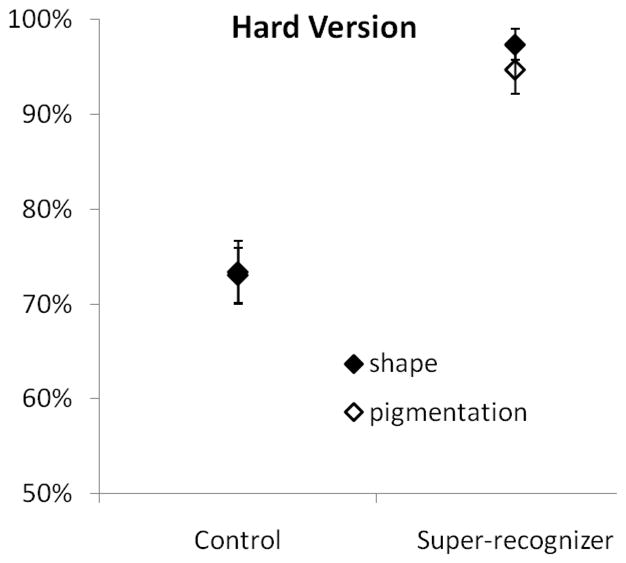
Performance by developmental prosopagnosic and control subjects on the hard version of the 2AFC memory test.
5. Discussion
Recognizing faces requires the perception of surface reflectance properties much more than recognizing most other kinds of objects. Because of this, developmental prosopagnosia and super-recognition could be caused by exceptionally bad or good surface reflectance perception, respectively. As such, prospagnosia would be more akin to amusia, with a relevant perceptual factor as the underlying deficit rather than face recognition per se. Our current experiments do not support this hypothesis. There was no relationship between face recognition ability and the relative use of shape and pigmentation information. In both experiments there was no significant interaction between the subject group and the kind of information (shape or pigmentation) used to perceive or recognize faces. Shape and pigmentation cues were used in roughly equal measure by people with very good and very bad face recognition ability. This supports the idea that ability to use both shape and pigmentation information in face perception and face recognition does not vary as a function of face recognition ability. People who are good at recognizing faces are good at using both shape and pigmentation cues to do so; people who are bad at recognizing faces are bad at using both shape and pigmentation cues to do so.
While these results indicate that prosopagnosia is not a condition caused by deficits in the perception of surface reflectance properties, we cannot rule out the possibility that prosopagnosia is a condition caused by deficits in some other perceptual competency that is face relevant but not face specific. However, two other accounts of developmental prosopagnosia as resulting from face relevant perceptual deficits, one account involving impaired perception of curved volumetric surfaces (Laeng & Caviness, 2001), and the other involving a greater local processing bias in perceiving hierarchically organized compound figures (Behrmann, Avidan, Marotta, & Kimchi, 2005), have not been supported by subsequent investigations (Duchaine, et al., 2004; Duchaine, Yovel, & Nakayama, 2007). Thus there remains little evidence for perceptual deficits that are not face-specific in developmental prosopagnosia, despite several investigations. This suggests that developmental prosopagnosia is not like amusia. Amusia is marked by deficits to a high level faculty (music perception and production), but is caused by a low level deficit (pitch perception). Developmental prosopagnosia is also marked by deficits to a high level faculty (face recognition) but unlike with amusia, it does not seem to be caused by a low level deficit. Rather, the weight of evidence suggests that developmental prosopagnosia is caused by a high level deficit. It remains to be seen whether the high level deficit is specific to face recognition itself, or to something more general (Chatterjee, et al., In preparation).
The knowledge that developmental prosopagnosia is not due to a perceptual deficit can aid in the search for neural anomalies in prosopagnosia and super-recognition, which in turn aids the quest to understand the neural mechanisms of face recognition. Neural circuits related to face recognition ability must use both shape and pigmentation information about equally. This supports the idea that these circuits represent facial appearance by pooling lower-level patterns of shape and reflectance into combinations that include both types of information (Jiang, et al., 2006). Further, this is consistent with the notion that the location of the Fusiform Face Area is midway along the shape–reflectance gradient in ventral cortex (Cant & Goodale, 2011) because the region integrates these two kinds of cues to visually process faces.
In Experiment 1, there was a trend toward an interaction between subject group and cue type (p = 0.058). While the control subjects performed equally well with shape and pigmentation cues, the developmental prosopaganosics and the super-recognizers performed better with shape cues than pigmentation cues. There are several possible explanations of this trend. It may be meaningless noise due to the small sample sizes among the developmental prosopganosics and particularly the super-recognizers. Another possibility is that it is a real effect with external validity, and that the two special populations actually do use shape cues more than pigmentation cues even outside the laboratory. This account would require multiple causes for the spectrum of face recognition ability, i.e. that the two special populations are not simply at opposite ends of the same distribution, but rather that what makes super-recognizers good at face recognition is qualitatively different from what makes developmental prosopagnosics bad. A piece of evidence arguing against the possibility that the special populations (or at least the developmental prosopagnosics) make relatively more use of shape cues than pigmentation cues is that the developmental prosopagnosics were somewhat better at recognizing the faces using pigmentation cues than shape cues in Experiment 2 (although the interaction was not significant even at the level of a trend). Another possible explanation of the trend toward an interaction in Experiment 1 is that it is an artifact of the different processes for subject selection between the groups. The different subject groups differed in motivation—the developmental prosopagnosics and super-recognizers are generally more interested in their performance than the controls. This difference in motivation may have resulted in the special populations more actively seeking strategies to perform the task, and that these strategies were more effective with the shape cues than the pigmentation cues.
Overall, the finding that there is no relationship between face recognition ability and the relative use of shape and pigmentation cues neither supports nor contradicts the notion that developmental prosopagnosia and super-recognition are at opposite ends of a unitary distribution. This idea remains speculative, and is based only on the findings that developmental prosopagnosics are at the opposite end of the spectrum of face recognition ability, and that their ability to perceive upright and inverted faces is correlated with their face recognition ability (Russell, et al., 2009).
The finding that people with very good and very bad face recognition use shape and pigmentation cues equally extends the literature comparing these kinds of cues. Previous work had looked only at subjects with typical face recognition. The current findings with subjects from the extremes of the spectrum of face recognition ability suggest that equivalent importance of shape and pigmentation is a general property of face recognition related to the underlying representations used for recognition (Jiang, et al., 2006), and that the neural representations used for face recognition integrate shape and pigmentation information.
Highlights.
We tested shape and surface reflectance processing in two special populations
Super-recognizers cannot be characterized by their perception of pigmentation
Developmental prosopagnosia is not caused by a general perceptual deficit
Face recognition ability is not correlated with surface reflectance perception
Acknowledgments
This study was supported by US National Eye Institute funding to Richard Russell (EY017245) and Ken Nakayama (EY13602). We thank Peng Sun for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayotte J, Peretz I, Hyde K. Congenital amusia: A group study of adults afflicted with a music-specific disorder. Brain. 2002;125:238–251. doi: 10.1093/brain/awf028. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. Congenital prosopagnosia: face-blind from birth. TRENDS in Cognitive Sciences. 2005;9(4):180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed Exploration of Face-related Processing in Congential Prosopagnosia: 1. Behavioral Findings. Journal of Cognitive Neuroscience. 2005;17(7):1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Biederman I, Ju G. Surface versus Edge-Based Determinants of Visual Recognition. Cognitive Psychology. 1988;20:38–64. doi: 10.1016/0010-0285(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Caharel S, Jiang F, Blanz V, Rossion B. Recognizing an individual face: 3D shape contributes earlier than 2D surface reflectance information. NeuroImage. 2009;47:1809–1818. doi: 10.1016/j.neuroimage.2009.05.065. [DOI] [PubMed] [Google Scholar]
- Cant JS, Arnott SR, Goodale MA. fMR-adaptation reveals separate processing regions for the perception of form and texture in the human ventral stream. Experimental Brain Research. 2009;192(3):391–405. doi: 10.1007/s00221-008-1573-8. [DOI] [PubMed] [Google Scholar]
- Cant JS, Goodale MA. Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cerebral Cortex. 2007;17:713–731. doi: 10.1093/cercor/bhk022. [DOI] [PubMed] [Google Scholar]
- Cant JS, Goodale MA. Scratching beneath the surface: new insights into the functional properties of the lateral occipital area and parahippocampal place area. The Journal of Neuroscience. 2011;31(22):8248–8258. doi: 10.1523/JNEUROSCI.6113-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee G, Russell R, Duchaine B, Nakayama K. Developmental prosopagnosia is typically accompanied by deficits in general visual memory but not verbal episodic memory. (In preparation) [Google Scholar]
- Debevec P, Hawkins T, Tchou C, Duiker HP, Sarokin W, Sagar M. Acquiring the Reflectance Field of a Human Face. Proceedings of SIGGRAPH. 2000;2000:145–156. [Google Scholar]
- Duchaine B, Dingle K, Butterworth E, Nakayama K. Normal greeble learning in a severe case of developmental prosopagnosia. Neuron. 2004;43(4):469–473. doi: 10.1016/j.neuron.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Germine L, Nakayama K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cognitive Neuropsychology. 2007;24(4):419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. Dissociations of Face and Object Recognition in Development Prosopagnosia. Journal of Cognitive Neuroscience. 2005;17(2):249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Normal performance and an investigation of its validity using inverted performance and prosopagnosic subjects. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Yovel G, Nakayama K. No global processing deficit in the Navon task in 14 developmental prosopagnosics. Social Cognitive and Affective Neuroscience. 2007;2:104–113. doi: 10.1093/scan/nsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain HM, Tanaka JR. The inverted face inversion effect in prosopagnosia: Evidence for mandatory, face-specific perceptual mechanisms. Vision Research. 1995;35:2089–2093. doi: 10.1016/0042-6989(94)00273-o. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Behrmann M, Tarr MJ. Can Face Recognition Really be Dissociated from Object Recognition? Journal of Cognitive Neuroscience. 1999;11(4):349–370. doi: 10.1162/089892999563472. [DOI] [PubMed] [Google Scholar]
- Henke K, Schwinbuerger SR, Grigo A, Klos T, Sommer W. Specificity of face recognition: Recognition of exemplars of non-face objects in prosopagnosia. Cortex. 1998;34:289–296. doi: 10.1016/s0010-9452(08)70756-1. [DOI] [PubMed] [Google Scholar]
- Jiang F, Blanz V, O’Toole AJ. Probing the Visual Representation of Faces With Adaptation. Psychological Science. 2006;17(6):493–500. doi: 10.1111/j.1467-9280.2006.01734.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerknecht I, Grueter T, Welling B, Wentzek S, Horst J, Edwards S, et al. First Report of Prevalence of Non-Syndromic Hereditary Prosopagnosia (HPA) American Journal of Medical Genetics Part A. 2006;140A:1617–1622. doi: 10.1002/ajmg.a.31343. [DOI] [PubMed] [Google Scholar]
- Lades M, Vortbruggen JC, Buhmann J, Lange J, von der Malsburg C, Wurtz RP, et al. Distortion invariant object recognition in the dynamic link architecture. IEEE Transactions on Computers. 1993;42:300–311. [Google Scholar]
- Laeng B, Caviness VS. Prosopagnosia as a Deficit in Encoding Curved Surface. Journal of Cognitive Neuroscience. 2001;13(5):556–576. doi: 10.1162/089892901750363163. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole AJ, Vetter T, Blanz V. Three-dimensional shape and two-dimensional surface reflectance contributions to face recognition: an application of three-dimensional morphing. Vision Research. 1999;39:3145–3155. doi: 10.1016/s0042-6989(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Peretz I. Musical Disorders. Current Directions in Psychological Science. 2008;17(5):329–333. [Google Scholar]
- Peretz I, Ayotte J, Zatorre RJ, Mehler J, Ahad P, Penhune VB, et al. Congenital Amusia: A Disorder of Fine-Grained Pitch Discrimination. Neuron. 2002;33:185–191. doi: 10.1016/s0896-6273(01)00580-3. [DOI] [PubMed] [Google Scholar]
- Peretz I, Coltheart M. Modularity of music processing. Nature Neuroscience. 2003;6(7):688–691. doi: 10.1038/nn1083. [DOI] [PubMed] [Google Scholar]
- Peretz I, Hyde KL. What is specific to music processing? Insights from congenital amusia. TRENDS in Cognitive Sciences. 2003;7(8):362–367. doi: 10.1016/s1364-6613(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Russell R, Biederman I, Nederhouser M, Sinha P. The utility of surface reflectance for the recognition of upright and inverted faces. Vision Research. 2007;47:157–165. doi: 10.1016/j.visres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychonomic Bulletin & Review. 2009;16(2):252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R, Sinha P. Real-world face recognition: The importance of surface reflectance properties. Perception. 2007;36:1368–1374. doi: 10.1068/p5779. [DOI] [PubMed] [Google Scholar]
- Russell R, Sinha P, Biederman I, Nederhouser M. Is pigmentation important for face recognition? Evidence from contrast negation. Perception. 2006;35:749–759. doi: 10.1068/p5490. [DOI] [PubMed] [Google Scholar]
- Siemionow M, Agaoglu G. The Issue of “Facial Appearance and Identity Transfer” after Mock Transplantation: A Cadaver Study in Preparation for Facial Allograft Transplantation in Humans. Journal of Reconstructive Microsurgery. 2006;22:239–334. doi: 10.1055/s-2006-946709. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Weiskopf D, Williams P. The role of color in high-level vision. Trends in Cognitive Sciences. 2001;5(5):211–215. doi: 10.1016/s1364-6613(00)01626-0. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proceeings of the National Academy of Sciences. 2008;105(49):19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman S. High-Level Vision: Object Recognition and Visual Cognition. Cambridge, Massachusetts: MIT Press; 1996. [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Williams M, Loken E, et al. Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):5238–5241. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. TRENDS in Cognitive Sciences. 2002;6(1):37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Song Y, Hu S, Li X, Tian M, Zhen Z, et al. Heritability of the Specific Cognitive Ability of Face Perception. Current Biology. 2010;20(2):137–142. doi: 10.1016/j.cub.2009.11.067. [DOI] [PubMed] [Google Scholar]