Abstract
The effect of ligand structure on the cytotoxicity of cationic CdSe/ZnS quantum dots (QDs) was systematically investigated using mono- and bidentate ligands. Monothiol-functionalized QDs are more cytotoxic than dithiol-functionalized QDs.
The cytotoxicity of QDs has become a major obstacle for their safe use in biomedical applications, 1 including imaging 2 and delivery3. QD toxicity strongly depends on QD physicochemical parameters including size,4 charge,5and stability. 6 The role of surface chemistry of QDs in determining toxicity is an important consideration since surface functionalization is required for biological applications. 7 A number of organic ligands, e.g. thiolate ligands, have been introduced on the surface of QDs to provide water solubility and colloidal stability. 8 Recently, multiple chelating groups have been employed to produce stronger affinity to the QD surface and concomitant increase of the stability.9 As an example, Mattoussi et al. have demonstrated that multidentate ligands provide enhanced stability for CdSe/ZnS QDs under extreme conditions.10 These studies focus on the stability of QDs, however, the effect of ligand structure on QD toxicity has not been systematically investigated.
Cationic QDs possess higher cellular permeability than uncharged (neutral) and negatively charged QDs, and also provide a complementary surface binding for negatively charged biomolecules (e.g., proteins11 and nucleic acids12) for biological applications.13 Cationic QDs, however, face challenges associated with toxicity compared to anionic and neutral QDs. 14 To investigate the cytotoxicity of cationic QDs with different surface ligand structures we used two types of cationic QDs featuring different anchoring groups (Fig. 1a). Our studies revealed that dithiol-functionalized QDs are substantially less toxic than monothiol-functionalized QDs. QD-induced cytotoxicity was systematically investigated via several determining factors, including the intracellular factors (i.e. cellular uptake and liberation of cadmium ions) and extracellular factor (i.e. cellular membrane damage), with acute toxicity primarily derived from membrane damage.
Fig. 1.
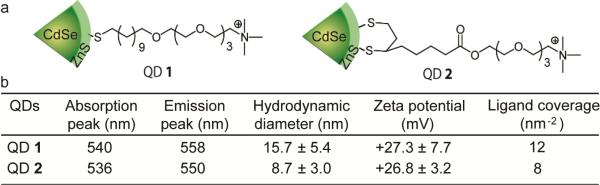
(a) Molecular structures of the cationic CdSe/ZnS QDs used in this work. (b) Physicochemical properties of the cationic QDs.
Quaternary ammonium ligands presenting monothiol and dithiol anchoring groups were used to investigate the effect of coordination number on the stability and cytotoxicity of QDs. The ligand design features a tetra(ethylene glycol) (TEG) spacer to minimize non-specific protein and cell interactions, 15 and dihydrolipoic acid16 or undecanethiol-based anchors17. (See ESI† for synthesis and characterization). Note that dithiolate ligands have 5 carbons in the hydrophobic alkane chain, while monothiolate ligands have 11 carbons. This is because the monothiolate ligand with shorter alkane chain (5 carbons) cannot stabilize QDs.18 Thus, monothiolate ligands with longer alkane chain were synthesized to optimize the ligand packing density and the colloidal stability of monothiolate QDs.
Green fluorescent CdSe/ZnS QDs (emission at 535 nm) were used to prepare the cationic QDs through a ligand exchange process. (See ESI† for preparation and characterization). The photophysical properties of QD 1 and QD 2 are shown in Fig. 1b. The absorption peak positions of the QDs were very similar but the emission peaks showed modest differences, as is commonly observed after surface modification.19 Moreover, the monothiol-functionalized QDs were less fluorescent compared to dithiol-functionalized QDs. This lower quantum yield of monothiol-functionalized QDs presumably arises from the higher density of thiolate ligands on the QD surface.20 Dynamic light scattering (DLS) data indicated that the hydrodynamic size of monothiol-functionalized QDs (16 nm) was slightly larger than dithiol-functionalized QDs (9 nm) while the zeta potentials of these two types of QDs were quite similar (+27 mV) (Fig. 1b).
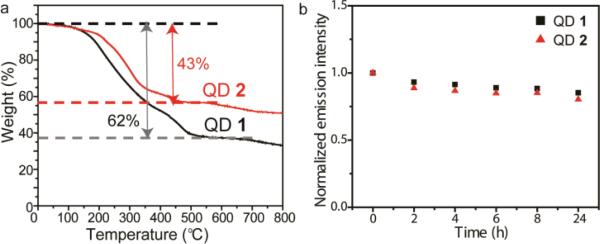
The coordination number of the monothiolate and dithiolate ligands can generate different ligand coating properties on particle surfaces, particularly the ligand density. The ligand amounts on monothiolate QD 1 and dithiolate QD 2 were measured using thermal gravimetric analysis (TGA). As shown in Fig. 2a, the weight loss in QD 1 was 62% while QD 2 was 43%, providing a calculated ligand amount for QD 1 of 320 and QD 3 of 220. Green QDs were 2.9 ± 0.5 nm in diameter based on the transmission electron microscopy (TEM) image.18 Therefore, the ligand packing densities on QD 1 and QD 2 were 12 and 8 nm−2, respectively (Fig. 1b and see ESI† for the calculation of ligand coverage), indicating monothiolate QD 1 presented a 1.5-fold increase in charge density compared to dithiolate QD 2. The higher packing density observed in QD 1 was presumably contributed from both of the smaller footprint of monothiols and stronger hydrophobic interactions between the 11-carbon alkyl chains.
Fig. 2.
(a) Thermal gravimetric analysis of QD 1 and QD 2.(b) Stability of QD 1 and QD 2 in 10% serum supplemented DMEM, as determined by the fluorescence change of each QD over time.
Colloidal stability in physiological media is a significant challenge for biological applications of QDs. QD 1 and QD 2 were incubated in low glucose Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% serum and their fluorescence was monitored over time. All QDs maintained their fluorescence and no aggregation were observed after 24 h, demonstrating their stable nature (Fig. 2b).
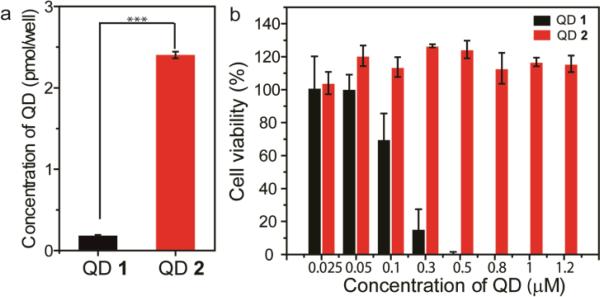
To probe the effect of ligand coordination on the cellular response, cationic QDs (25 nM) were incubated with HeLa cells and their subsequent cellular uptake was measured by inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. 3a, cellular uptake of QD 1 was significantly lower than QD 2. Cytotoxicity of the cationic QDs was determined through Alamar blue assays. QD 1 presented the cytotoxicity in a dose-dependent manner while no toxic effect was observed on QD 2 (up to 1.2 μM) (Fig. 3b). These results revealed that the dithiol-functionalized QDs were much less toxic than monothiol-functionalized QDs, in spite of their increased uptake efficiency. Similar uptake and toxicity results were obtained with analogous QDs featuring dimethylhexyl ammonium terminal functional group (Fig. S2†), indicating that these ligand-particle coordination effects are general within this particle family. Our findings are consistent with previous reports that indicate that reduction of surface charge decreases cytotoxicity, 21 however it is difficult to generalize our findings to uptake and toxicity of other particle core materials/sizes and monolayer structures.22.
Fig. 3.
(a) Cellular uptake of the cationic QDs after incubation with HeLa cells for 24 h. (a) Cell viability of the cationic QDs in HeLa cells at different concentrations after incubation for 24 h. (*** p≤ 0.001, one-way, ANOVA).
The leaching of cadmium ions (Cd2+) from QD core can induce significant cell death. 23 To further investigate the role of intracellular liberation of Cd2+ on QD-induced cytotoxicity, cells were pretreated with N-acetylcysteine (NAC) for 2 h prior to QD addition and maintained continuously in the media. 24 After incubation for 24 h, cell viabilities did not show a significant change afterQD treatment in the absence and presence of NAC (Fig. S3†), indicating limited intracellular reactive oxygen species (ROS) generation by free Cd2+ release. The ZnS shell provided an effective barrier for the release of Cd2+ from the inner CdSe core, limiting cytotoxicity, which was consistent with reported studies.25
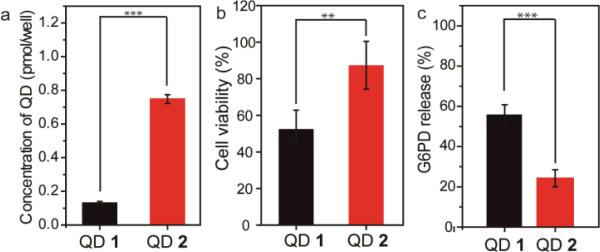
We further studied the QD toxicity at a short-term (3 h) incubation period to probe the acute cytotoxicity of QDs. As shown in Fig. 4a and 4b, cellular uptake of QD 1 was also lower than QD 2 after incubation for 3 h, and QD 1 still presented a higher toxicity compared to QD 2. To explore the potential determining factors in the short-term toxicity, we examined if these cationic QDs generated different degrees of cellular membrane damage. Cationic nanoparticles can induce bilayer disruption and form nanoscale holes on cell membranes,26 which is one of the significant factors in the mechanism of toxicity for QDs. 27 Cellular membrane damage induced from different cationic QDs was quantified by monitoring the leakage of the cytosolic enzyme glucose-6-phosphate dehydrogenase (G6PD) into the media. After 3 h of incubation, the G6PD release with QD 1 was ~2.5-fold higher than that of QD 2 (Fig. 4c), demonstrating monothiol-functionalized QDs damaged the cell membranes to a greater extent compared to dithiol-functionalized QDs.
Fig. 4.
(a) Cellular uptake amounts of the cationic QDs (25 nM) after incubation with HeLa cells for 3 h. (b) Cell viability of the cationic QDs (300 nM) after incubation with HeLa cells for 3 h. (b) Quantifications of membrane damage in HeLa cells determined by the G6PD assay. Membrane damage experiments were performed after incubation with HeLa cells for 3 h, and the concentration of QDs was 300 nM. (** p≤ 0.01, *** p≤ 0.001, one-way, ANOVA).
Cell membrane damage is a typical hallmark for necrosis and subsequent inflammation.28 We further confirmed the necrotic-mediated cell death by staining the cells with propidium iodide (PI) following QD treatment. QD 1 demonstrated a high amount of PI stained cells (fluorescent marker for necrotic mediated cell death) while QD 2 treated cells showed almost no PI positive cells (Fig. S4†). Therefore, the cytotoxicity caused by QD 1 is primarily due to physical cell membrane rupture/necrosis that can lead to inflammation and subsequent cell death.
Cellular toxicity of nanoparticles can arise from both extracellular interactions between nanoparticle surface molecules and cell membranes and intracellular mechanisms that would be dictated by the number of endocytosed nanoparticles. Here, our cellular uptake studies showed that monothiol-functionalized QDs were less internalized than dithiol-functionalized QDs, indicating that the number of endocytosed QDs is not proportional to their cytotoxicity. Our results suggest that the cationic ligands on the surface of QDs can disrupt the cell membranes and the degree of disruption is related to the number/density of ligands on the QD surface.29 This result is consistent to the reported studies that higher cationic charged nanoparticles or polymers frequently cause higher cytotoxities.29, 30
The fact that charge density determines cytotoxicity has been demonstrated in dendrimer systems; higher cytotoxicity of cationic dendrimers is correlated with higher number of primary amino groups.31 In our monothiolate and dithiolate ligand design, the major difference between these two ligands is the coordination number. Thus, the lower coordination number of ligand can increase the ligand packing density and subsequently enhance the cytotoxicity of the functionalized QDs, a similar finding to the higher generation of cationic dendrimers. Given the ligand exchange is a widely used method for nanomaterial functionalization, our study provides a straightforward approach to regulate the cytotoxic behaviour of nanomaterials through the design of ligand coordination number.
In summary, we have investigated the toxicity of cationic QDs, focusing on the influence of surface ligand density in HeLa cells. Monothiol-functionalized QDs possess higher cytotoxicity compared to dithiol-functionalized QDs due to higher charge density and enhanced cellular membrane damage. Taken together, the modulation of the surface ligand density and cytotoxicity of nanomaterials by engineering their surface properties will enable the application-specific control and expand their biological utility.
Supplementary Material
Acknowledgements
This research was support by the NIH (EB014277) and the NSF-sponsored Center for Hierarchical Manufacturing (DMI-0531171) and MRSEC facilities (DMR-0820506). We thank Prof. Julian F. Tyson for access to the Elan 6100 ICP-MS instrumentation.
Footnotes
Electronic Supplementary Information (ESI) available: materials, instruments, preparation of cell culture, mass spectrametry, syntheses of surface ligands and cationic QDs, MALDI-MS spectra of QDs, cellular uptake and cytotoxicity of additional QDs after incubation with HeLa cells for 24 h, cell viability after QD incubation with HeLa cells for 24 h in the absence and presence of NAC, necrosis-mediated cell death experiments.
References
- 1.a Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WCW. Small. 2010;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]; b Kuo TR, Lee CF, Lin SJ, Dong CY, Chen CC, Tan HY. Chem. Res. Toxicol. 2011;24:253–261. doi: 10.1021/tx100376n. [DOI] [PubMed] [Google Scholar]
- 2.a Smith AM, Duan HW, Mohs AM, Nie SM. Adv. Drug. Deliv. Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang C, Gao X, Su XG. Anal.Bioanal.Chem. 2010;397:1397–1415. doi: 10.1007/s00216-010-3481-6. [DOI] [PubMed] [Google Scholar]; c De M, Ghosh PS, Rotello VM. Adv. Mater. 2008;20:4225–4241. [Google Scholar]; d Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Delehanty JB, Bradburne CE, Boeneman K, Susumu K, Farrell D, Mei BC, Blanco-Canosa JB, Dawson G, Dawson PE, Mattoussi H, Medintz IL. Integr. Biol. 2010;2:265–277. doi: 10.1039/c0ib00002g. [DOI] [PubMed] [Google Scholar]; b Medintz IL, Pons T, Delehanty JB, Susumu K, Brunel FM, Dawson PE, Mattoussi H. Bioconjugate Chem. 2008;19:1785–1795. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
- 4.a Lovric J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D. J. Mol. Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]; b Zhang Y, Chen W, Zhang J, Liu J, Chen G, Pope C. J. Nanosci. Nanotechnol. 2007;7:497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- 5.Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, Nemery B, Hoet PHM. Environ. Health Perspect. 2008;116:1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace HE, Lesher EK, Ranville JF. Environ. Toxicol. Chem. 2010;29:1338–1344. doi: 10.1002/etc.168. [DOI] [PubMed] [Google Scholar]
- 7.a Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K. Nano Lett. 2004;4:2163–2169. [Google Scholar]; b Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. J. Invest. Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]; c Hoshino A, Manabe N, Fujioka K, Suzuki K, Yasuhara M, Yamamoto K, Artif J. Organs. 2007;10:149–157. doi: 10.1007/s10047-007-0379-y. [DOI] [PubMed] [Google Scholar]; d Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier AM, Gaub HE, Stolzle S, Fertig N, Parak WJ. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 8.a Smith AM, Duan HW, Rhyner MN, Ruan G, Nie SM. Phys. Chem. Chem. Phys. 2006;8:3895–3903. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]; b Liu L, Guo XH, Li Y, Zhong XH. Inorg. Chem. 2010;49:3768–3775. doi: 10.1021/ic902469d. [DOI] [PubMed] [Google Scholar]; c Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Algar WR, Krull UJ. ChemPhysChem. 2007;8:561–568. doi: 10.1002/cphc.200600686. [DOI] [PubMed] [Google Scholar]; b Moody IS, Stonas AR, Lonergan MC. J. Phys. Chem. C. 2008;112:19383–19389. [Google Scholar]; c Fang Z, Liu L, Xu LL, Yin XG, Zhong XH. Nanotechnology. 2008;19:235603–235609. doi: 10.1088/0957-4484/19/23/235603. [DOI] [PubMed] [Google Scholar]; d Susumu K, Mei BC, Mattoussi H. Nat. Protocols. 2009;4:424–436. doi: 10.1038/nprot.2008.247. [DOI] [PubMed] [Google Scholar]; e Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. J. Am. Chem. Soc. 2005;127:3870–3878. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]; f Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. J. Am. Chem. Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]; g Mei BC, Susumu K, Medintz IL, Mattoussi H. Nat. Protocols. 2009;4:412–423. doi: 10.1038/nprot.2008.243. [DOI] [PubMed] [Google Scholar]
- 10.Stewart MH, Susumu K, Mei BC, Medintz IL, Delehanty JB, Blanco-Canosa JB, Dawson PE, Mattoussi H. J. Am. Chem. Soc. 2010;132:9804–9813. doi: 10.1021/ja102898d. [DOI] [PubMed] [Google Scholar]
- 11.a Manokaran S, Berg A, Zhang X, Chen W, Srivastava DK. J. Biomed. Nanotech. 2008;4:491–498. doi: 10.1166/jbn.2008.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Manokaran S, Zhang X, Chen W, Srivastava DK. Biochim. Biophys. Acta. 2010;1804:1376–1384. doi: 10.1016/j.bbapap.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Peng H, Zhang L, Kjällman THM, Soeller C, Travas-Sejdic J. J. Am. Chem. Soc. 2007;129:3048. doi: 10.1021/ja0685452. [DOI] [PubMed] [Google Scholar]; b Qiu T, Zhao D, Zhou GH, Liang YA, He ZK, Liu ZH, Peng XN, Zhou L. Analyst. 2010;135:2394–2399. doi: 10.1039/c0an00254b. [DOI] [PubMed] [Google Scholar]
- 13.a Yezhelyev MV, Qi LF, O'Regan RM, Nie S, Gao XH. J. Am. Chem. Soc. 2008;130:9006–9012. doi: 10.1021/ja800086u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee J, Choi Y, Kim J, Park E, Song R. Chemphyschem. 2009;10:806–811. doi: 10.1002/cphc.200800504. [DOI] [PubMed] [Google Scholar]
- 14.Tan SJ, Jana NR, Gao SJ, Patra PK, Ying JY. Chem. Mater. 2010;22:2239–2247. [Google Scholar]
- 15.a Hong R, Fischer NO, Verma A, Goodman CM, Emrick TS, Rotello VM. J. Am. Chem. Soc. 2004;126:739–743. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]; b You C-C, De M, Rotello VM. Org. Lett. 2005;7:5685–5687. doi: 10.1021/ol052367k. [DOI] [PubMed] [Google Scholar]; c Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh Y-C, Patra D, Yan B, Saha K, Miranda OR, Kim CK, Rotello VM. Chem. Commun. 2011;47:3069–3071. doi: 10.1039/c0cc04975a. [DOI] [PubMed] [Google Scholar]
- 17.a You C-C, Miranda OR, Basar Gider B, Ghosh PS, Kim IK-B, Erdogan B, Krovi SA, Bunz UHF, Rotello VM. Nat. Nanotech. 2007;2:318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]; b Miranda OR, Chen H-T, You C-C, Mortenson DE, Yang X-C, Bunz UHF, Rotello VM. J. Am. Chem. Soc. 2010;132:5285–5289. doi: 10.1021/ja1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z-J, Yeh Y-C, Tang R, Yan B, Tamayo J, Vachet RW, Rotello VM. Nat. Chem. 2011;3:963–968. doi: 10.1038/nchem.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a Thuy UTD, Liem NQ, Thanh DX, Protière M, Reiss P. Appl. Phys. Lett. 2008;91:241908–241911. [Google Scholar]; b Leatherdale CA, Bawendi MG. Phys.Rev. B. 2001;63:165315. [Google Scholar]; c Jin T, Fujii F, Yamada E, Nodasaka Y, Kinjo M. J. Am. Chem.Soc. 2006;128:9288–9289. doi: 10.1021/ja062021k. [DOI] [PubMed] [Google Scholar]
- 20.Breus VV, Heyes CD, Nienhaus GU. J. Phys. Chem. C. 2007;111:18589–18594. [Google Scholar]
- 21.Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Nano Lett. 2010;10:2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F. Nat. Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu ZJ, Arcaro KF, Rotello VM. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Su G, Zhou H, Mu Q, Zhang Y, Li L, Jiao P, Jiang G, Yan B. J. Phys. Chem. C. 2012;116:4993–4998. [Google Scholar]; d Verma A, Stellacci F. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 23.a Rzigalinski BA, Strobl JS. Toxic. Appl. Pharmacol. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang L, Nagesha DK, Selvarasah S, Dokmeci MR, Carrier RL. J. Nanobiotech. 2008;6:1. doi: 10.1186/1477-3155-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a Li KG, Chen JT, Bai SS, Wen X, Song SY, Yu Q, Li J, Wang YQ. Toxicol. in Vitro. 2009;23:1007–1013. doi: 10.1016/j.tiv.2009.06.020. [DOI] [PubMed] [Google Scholar]; b Choi AO, Cho SJ, Desbarats J, Lovric J, Maysinger D. J. Nanobiotech. 2007;5:1. doi: 10.1186/1477-3155-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Poliandri AHB, Cabilla JP, Velardez MO, Bodo CCA, Duvilanski BH. Toxic. Appl. Pharmacol. 2003;190:17–24. doi: 10.1016/s0041-008x(03)00191-1. [DOI] [PubMed] [Google Scholar]; d Yan CY, Ferrari G, Greene LA. J. Biol. Chem. 1995;270:26827–26832. doi: 10.1074/jbc.270.45.26827. [DOI] [PubMed] [Google Scholar]; e Wispriyono B, Matsuoka M, Igisu H, Matsuno K. J. Pharmacol. Exp. Ther. 1998;287:344–351. [PubMed] [Google Scholar]
- 25.Su YY, He Y, Lu HT, Sai LM, Li QN, Li WX, Wang LH, Shen PP, Huang Q, Fan CH. Biomaterials. 2009;30:19–25. doi: 10.1016/j.biomaterials.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 26.a Chen JM, Hessler JA, Putchakayala K, Panama BK, Khan DP, Hong S, Mullen DG, DiMaggio SC, Som A, Tew GN, Lopatin AN, Baker JR, Holl MMB, Orr BG. J. Phys. Chem. B. 2009;113:11179–11185. doi: 10.1021/jp9033936. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Leroueil PR, Berry SA, Duthie K, Han G, Rotello VM, McNerny DQ, Baker JR, Orr BG, Holl MMB. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 27.Choi SJ, Oh JM, Choy JH. J. Inorg. Biochem. 2009;103:463–471. doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 28.a Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Scaffidi P, Misteli T, Bianchi ME. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 29.Lin JQ, Zhang HW, Chen Z, Zheng YG. ACS Nano. 2010;4:5421–5429. doi: 10.1021/nn1010792. [DOI] [PubMed] [Google Scholar]
- 30.Lin C, Engbersen JFJ. J. Control. Rel. 2008;132:267–272. doi: 10.1016/j.jconrel.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Naha PC, Davoren M, Lyng FM, Byrne HJ. Toxicol. Appl. Pharmacol. 2010;246:91–99. doi: 10.1016/j.taap.2010.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.