Abstract
Cerebral microbleeds (CMBs) are tiny, round dark-signal lesions that are most often detected on gradient-echo MR images. CMBs consist of extravasations of blood components through fragile microvascular walls characterized by lipohyalinosis and surrounding macrophages. The prevalence of CMBs in elderly subjects with no history of cerebrovascular disease is around 5%, but is much higher in patients with ischemic or hemorrhagic stroke. Development of CMBs is closely related to various vascular risk factors; in particular, lobar CMBs are thought to be associated with cerebral amyloid angiopathy. The presence of CMBs has been hypothesized to reflect cerebral-hemorrhage-prone status in patients with hypertension or amyloid microangiopathy. Stroke survivors with CMBs have been consistently found to have an elevated risk of subsequent hemorrhagic stroke or an antithrombotic-related hemorrhagic complication, although studies have failed to establish a link between CMBs and hemorrhagic transformation after thrombolytic treatment. A large prospective study is required to clarify the clinical significance of CMBs and their utility in a decision-making index.
Keywords: Cerebral microbleed, Ischemic stroke, Intracerebral hemorrhage, Antithrombotics, Gradient-echo MRI
Introduction
The term cerebral microbleed (CMB) refers to small, round dark-signal lesions detected by T2*-weighted or gradient-echo (GRE) magnetic resonance imaging (MRI).1 CMBs were introduced to stroke physicians in the late 1990s and early 2000s after development of MRI techniques sensitive to paramagnetic effects.2 The clinical significance of CMBs has been actively investigated, especially in the stroke field and more recently in studies on cognitive impairment and aging.3 Histological investigation has shown that CMBs are tiny foci containing hemosiderin-laden macrophages and abnormal microvessels showing fibrohyalinosis.4,5 Clinical cases with frank symptoms caused by CMBs are uncommon. Because CMBs are manifestations of focal extravascular leakage of blood components, however, investigators have suggested that accumulation of CMBs reflects a bleeding-prone status in individuals with an elevated risk of cerebral hemorrhage. Clinical studies have found strong associations between CMBs and chronic hypertension and low cholesterol levels,6,7 and between the proximity and volume of CMBs and those of subsequent intracerebral hemorrhage (ICH).8,9 Longitudinal studies have found that CMBs are linked to subsequent hemorrhagic stroke in stroke survivors,10 and suggested that CMBs are related to antithrombotic-related hemorrhage.11,12 In this review, we discuss fundamental findings of CMBs, and the clinical implications of these observations for the field of cerebrovascular disease.
Visualization and detection of cerebral microbleeds
GRE sequences are more sensitive to susceptibility effects than are classical MRI sequences. Unlike classical T2-weighted imaging or echo-planar sequences, GRE sequences maximize the paramagnetic effects of blood components such as hemosiderin, deoxyhemoglobin, and ferritin.2,13 Due to the dephasing of MRI signals, GRE sequences tend to exaggerate lesion sizes. Therefore, sub-millimeter CMBs appear as signal-loss lesions of several millimeters, a phenomenon referred to as the blooming effect.
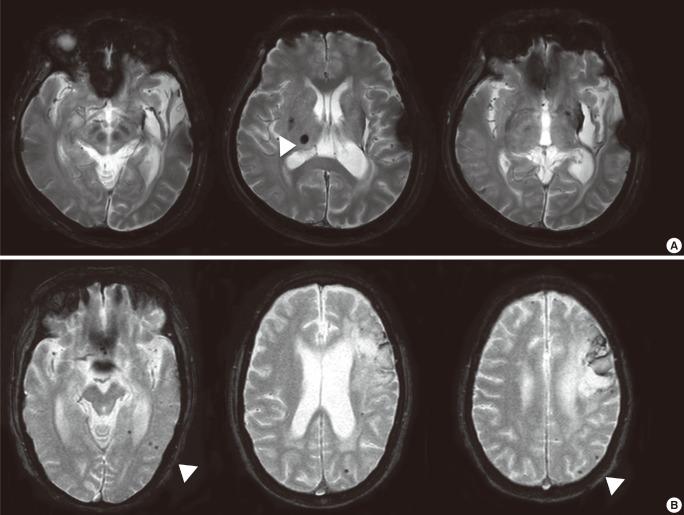
Susceptibility-weighted imaging (SWI) is a MRI sequence that maximizes sensitivity to magnetic susceptibility effects.14,15 SWI requires more time than does GRE, and also requires post-processing, but because SWI accentuates the magnetic properties of tissues, it enables visualization of areas such as CMBs containing deoxygenated blood substances.16 SWI permits visualization of a greater number of CMBs than can be seen with conventional GRE sequences, but the clinical implications of this increased sensitivity are not yet fully understood (Figure 1).17
Figure 1.
Cerebral microbleeds (CMBs) visualized on gradient-echo (GRE) images and susceptibility-weighted images (SWI). A lobar CMB on a SWI image (white arrow) is only faintly visible on the corresponding GRE image. Vessels located in the subarachnoid space could be mistakenly identified as CMBs on SWI sequence (hatched arrows).
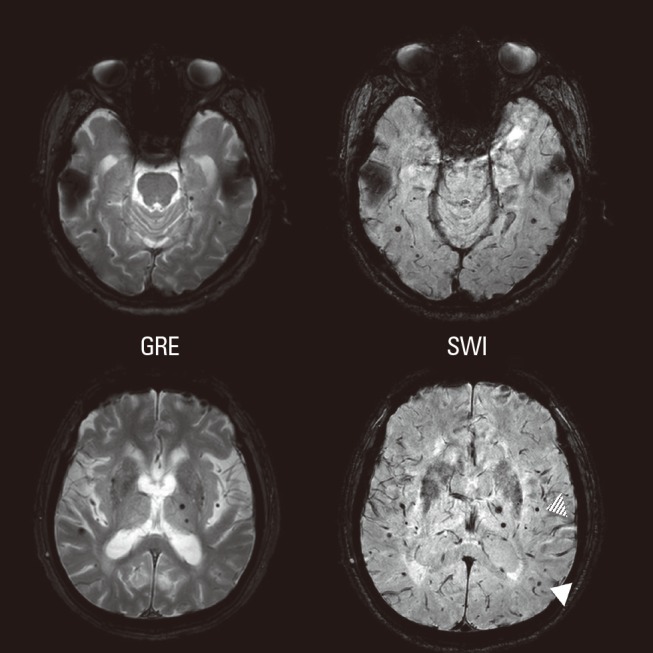
Since CMBs are primarily a radiologic concept, their sizes and shapes are dependent upon the parameters of the GRE sequence. Visualization of CMBs is thought to be influenced by pulse sequence, sequence parameters, spatial resolution, magnetic field strength, and post-processing of images.1 Thus, lesions due to old parenchymal hemorrhage, cortical or deep-seated mineralizations, or vascular malformations potentially all can be misinterpreted as CMBs. Small cortical vessels, partial volume effects of the cerebellar cortex, and cavernous hemangiomas also should be considered (Figure 2).21 Moreover, multifocal bleeding spots caused by diffuse axonal injury are virtually impossible to distinguish from cortico-subcortical CMBs.
Figure 2.
Cerebellar microbleeds (CMBs; white arrow). Two vertebral arteries in the subarachnoid space look similar to microbleeds (dotted arrow). One vessel signal located inside of a sulcus could be mistakenly interpreted as a CMB (hatched arrow).
Comparison of magnetic field strength in detection of CMBs revealed 0.5 more microbleeds on average could be observed using a 3.0 Tesla MRI compared to a 1.5 Tesla unit (2.1 in 3.0 T and 1.6 in 1.5 T).18 No universal standard MR parameters exist for the detection of CMBs, and in addition, the definition of CMB size also varies. In published reports, the lower limit of CMB size is usually ≤2 mm, and the upper limit is usually between 5 and 10 mm.19 Greenberg et al. reported that the size distribution of microbleeds and macrobleeds is bimodal, and proposed that the most appropriate cut-off point between them is 5.7 mm.20 Those researchers suggested elsewhere that an upper size limitation is impractical, and that instead, a more desirable approach is to carefully exclude CMB-mimicking lesions such as those described above.1 Other investigators have proposed a specified rating scale for CMBs, the Microbleed Anatomical Rating Scale.21 In our view, setting an appropriate upper-size limitation, and determination of intra- and inter-rater agreement values, are necessary for clinical and radiologic research studies.
Pathology of cerebral microbleeds
Pathologic-radiologic correlation studies have revealed that CMBs are focal accumulations of hemosiderin adjacent to abnormal blood vessels demonstrating fibrolipohyalinosis or amyloid microangiopathy.4,5 CMBs usually develop adjacent to capillaries, small arteries, or arterioles; hemosiderin-laden macrophages are usually present.22 A recent pathological investigation of CMBs in patients with cerebral amyloid angiopathy demonstrated extravasation of blood and hemosiderin through vulnerable vascular walls, with β-amyloid pigmentation and surrounding inflammation.23 These findings suggest that CMBs are the radiologic correlates of extravasation of blood components through injured or fragile vascular walls, or of frank small hemorrhage spots. In the context of cerebral amyloid angiopathy, Greenberg et al. suggested that increased vascular wall thickness was indicative of microbleeds.20
Because blooming artifact on GRE images exaggerates the size of CMBs, it has been proposed that the actual size of hemosiderin deposition is too small to cause apparent neurological deficit.22 Selected cases, however, have suggested that CMBs can cause neurological symptoms and signs.24,25
Prevalence and associated factors for cerebral microbleeds
Cerebral microbleeds in healthy subjects
In subjects without a history of cerebrovascular disease, the prevalence of CMBs was reported to be between 3-7%.26-33 Significant associations have been consistently reported between CMBs and advanced age, as well as hypertension.33 In contrast, association between CMBs and diabetes has been inconsistent across published reports.19,34,35 The Rotterdam Scan Study described CMBs in 1,062 older subjects.39 In a group of patients with mean age 69.6 years and a hypertension prevalence of 71.9%, CMBs were detected in 17.8% of patients aged 60-69, in 31.3% of patients aged 70-79, and in 38.3% of patients aged 80-97. The prevalence of multiple CMBs was also found to increase significantly with age.37 A single-hospital-based cross-sectional study performed in Japan found no deep-seated CMBs in subject younger than 40 years old.38 The Rotterdam Scan Study also noted a strong association of very low serum cholesterol levels (<4.42 mmol/L versus higher values) with the presence of strictly lobar microbleeds,39 an observation consistent with our earlier findings.7
Cerebral microbleeds in ischemic stroke patients
The reported prevalence of CMBs in ischemic stroke patients varies significantly (35%-71%; Table 1).27,38,40-43 This variability may be due to the heterogeneity of ischemic stroke per se, or to differences in recruited populations, rating strategies, and MRI parameters. Two studies investigating CMB frequency in different ischemic stroke subtypes found that CMBs were less frequent in cardioembolic stroke than in atherothrombotic stroke or lacunar stroke.28,44 Accumulation of sublethal ischemic injuries in brain parenchyma is thought to differ in atherothrombotic stroke and cardioembolic stroke,45 suggesting that the relationship of CMBs and stroke subtype in turn may reflect a different degree of fragility of vascular walls. A recent study found that the absolute number of CMBs, as well as variability indices of blood pressure (including coefficient of variance and successive variation), were elevated in cases of ischemic stroke with CMBs.46
Table 1.
Prevalence of cerebral microbleeds in ischemic stroke patients
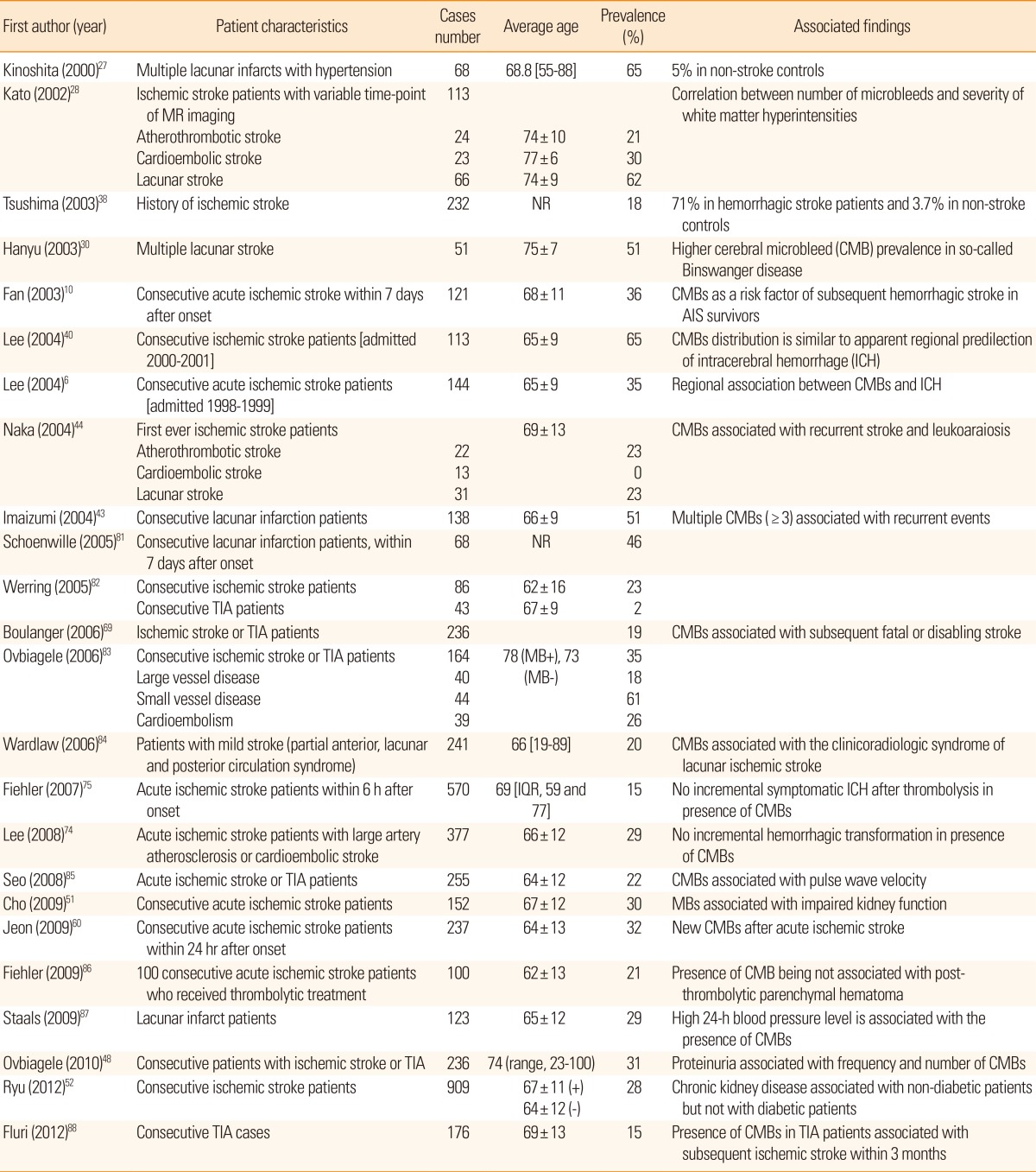
AIS, acute ischemic stroke; TIA, transient ischemic attack; MB, microbleeds; IQR, interquartile range; NR, not reported.
Another analysis of patients with ischemic stroke reported that serum uric acid level was associated in a dose-dependent manner with the presence of CMBs, but only in hypertensive patients.47 Complex interactions have been observed between chronic medical conditions and individual serologic markers in development of CMBs, such as in ischemic stroke patients with chronic kidney disease. Proteinuria and impaired kidney function has been linked to with small vessel disease in the brain and to CMBs.48-51 We further analyzed this issue, and found that chronic kidney disease is independently associated with cerebral microbleeds in patients without diabetes, but not in patients with diabetes.52
Cerebral microbleeds in hemorrhagic stroke patients
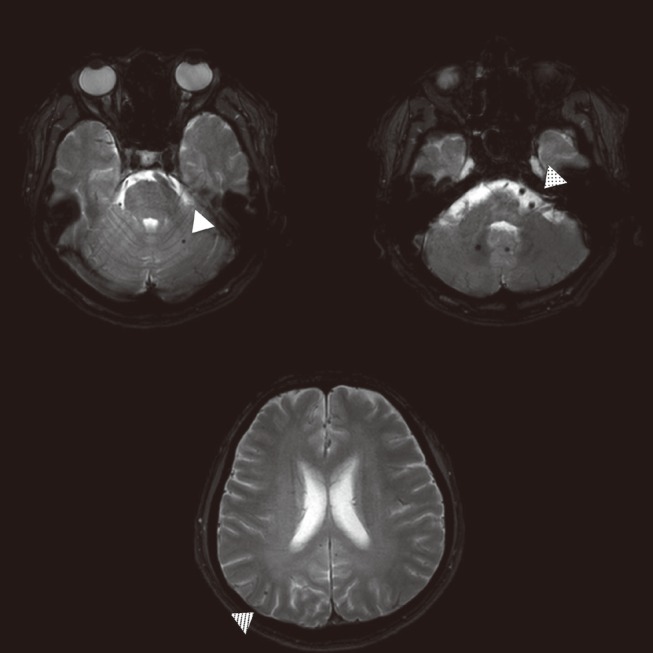
The frequency of CMBs in hemorrhagic stroke patients has been consistently higher than that that in ischemic stroke patients, reaching 50% to 80%.4,8,53-55 (Table 2; Figure 3) The detection rate of CMBs is higher in Asian populations, which may reflect a higher prevalence of the hemorrhagic stroke subtype in this ethnic group.56 CMBs also have been reported to be associated with hematoma volume, regardless of perihematomal edema volume.9 Patients with cerebral amyloid angiopathy have a higher CMB detection rate, with a preference for a lobar location,57,58 and the presence of the APOE e4 allele also has been reported to favor a lobar location (Figure 4).39,59
Table 2.
Prevalence of cerebral microbleeds in hemorrhagic stroke patients
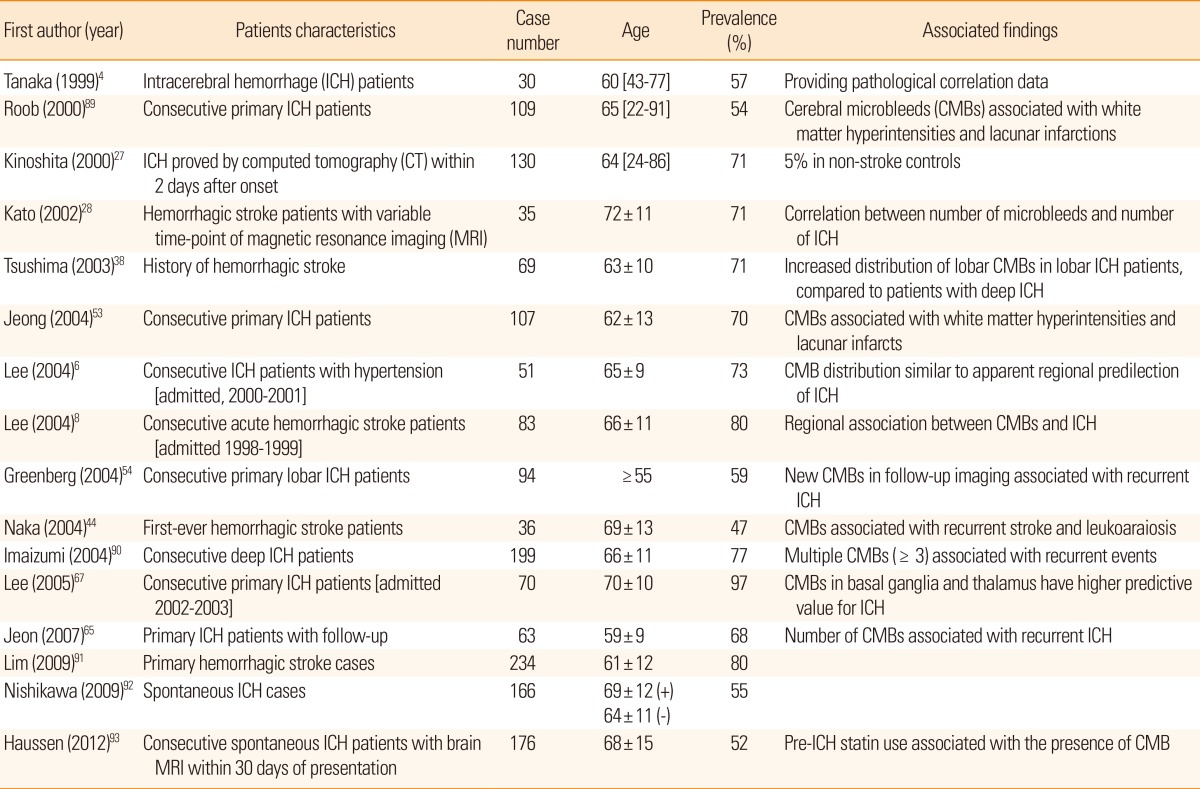
Figure 3.
Gradient-echo (GRE) images from a case of multiple lobar hemorrhage (white arrow). Multiple lobar cerebellar microbleeds (CMBs) are visible (dotted arrows).
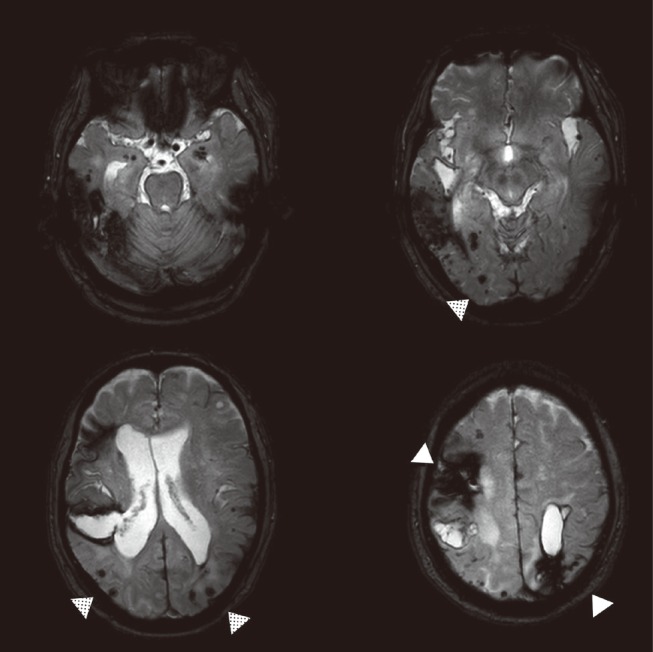
Figure 4.
Spatial distribution of cerebral microbleeds by location of hemorrhagic stroke. A case of basal ganglia cerebral hemorrhage with thalamic microbleed (A), compared to a case of lobar hemorrhage with multiple lobar microbleeds (B) in a patient with possible cerebral amyloid angiopathy.
Longitudinal changes in the number of cerebral microbleeds
The appearance and disappearance of CMBs over time have not been highlighted sufficiently. In an analysis of 237 acute ischemic stroke patients who underwent follow-up imaging, 13% had new CMBs (56 microbleeds in total) in the subsequent GRE images taken on average after 4 days.60 Long-term follow-up also revealed that 23% of cases demonstrated new CMBs on GRE imaging performed on average over 5.6 years.61 Development of new CMBs in this study was associated with high blood pressure and the presence of CMBs on baseline imaging. An additional follow-up study published in 2012 reported that the number of CMBs was increased in 54% of cases after 2.5 years.62 The annual change of CMBs significantly correlated with the number of CMBs on the baseline study. Another inter esting finding in that report was that in 15% of patients, CMBs disappeared on the follow-up GREs. Follow-up analyses of CMBs must be interpreted with caution, however, since spatial registration of baseline and follow-up images has not been performed in any published studies. Use of rigorous rating criteria21 is another important factor necessary for detailed evaluation of the natural history of CMBs, including their development and disappearance.
Clinical implications of cerebral microbleeds
Further understanding of the characteristics of CMBs has generated interest in using detection of CMBs to enable hemorrhagic stroke risk stratification. A number of reports have described the hemorrhagic tendency of CMBs.24,63,64 A hemorrhagic transformation after multiple embolic infarctions occurred only in the site of the known CMB.63 A Hong Kong study followed 121 acute ischemic stroke patients, and observed that stroke survivors with CMBs on their initial MRI scans had a higher risk of subsequent hemorrhagic stroke.10 Hemorrhage counts in initial scans also were found to be pro portional to the elevated risk of a future hemorrhagic stroke.54 The increased risk of hemorrhagic stroke conferred by the presence of CMBs was also confirmed in a prospective study of 112 ICH survivors.65 Furthermore, association between CMBs and larger ICH volume has been suggested by two studies,9,66 and the predictive value of CMBs in ICH in patients with advanced white matter lesions also has been documented.67 The spot sign, an enhancing locus of contrast extravasation in a cerebral hematoma, suggesting ongoing bleeding, was reported to be negatively associated with the number of microbleeds; this result is not consistent with previous findings, and bears further investigation.68 Patients with CMBs also have been reported to be 2.8 times more likely to have a subsequent disabling or fatal stroke.69 A systematic review published in 2013 concluded that the presence of CMBs in patients with ischemic stroke was associated with greatly increased odds of a subsequent hemorrhagic stroke, but was only modestly linked to recurrence of ischemic stroke.56 This meta-analysis also noted that the strength of association between CMBs and subsequent ICH risk was modified by ethnic background, with a greater odds ratio in Asian cohorts than in Western cohorts (Figure 5).
Figure 5.
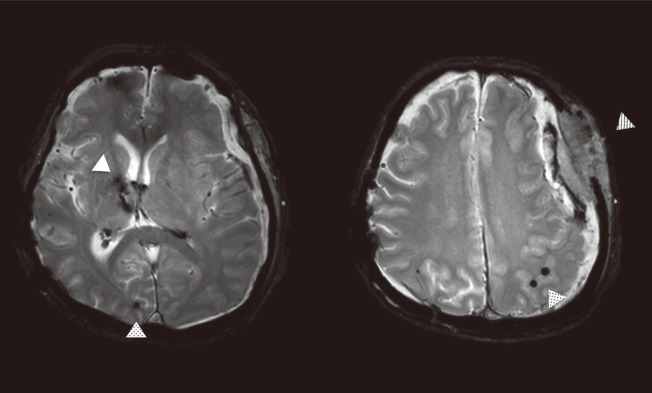
A basal ganglia intracerebral hemorrhage (white arrow) in a patient with a few lobar cerebral microbleeds (dotted arrow). The patient took an antiplatelet medication for several months and developed a subdural hemorrhage (hatched arrow).
The increased likelihood of cerebral hemorrhage associated with the presence of CMBs may allow prediction of hemorrhagic transformation after ischemic stroke. An earlier report suggested that hemorrhagic transformation after thrombolysis was associated with the presence of CMBs.70 In a case series of 100 acute ischemic stroke patients with imaging follow-up, the presence of CMBs was indicative of early hemorrhagic transformation.71 Embolic ischemic strokes occurring at the sites of previous CMBs were noted to become hemorrhagic.63 Contrary to these positive associations, however, a retrospective study of 279 acute ischemic stroke patients reported no association between CMB count and subsequent hemorrhagic transformation,72 and in an analysis of 70 stroke patients on thrombolytic treatment, CMBs failed to predict post-thrombolytic hemorrhagic transformation.73 No relationship between CMBs and prediction of hemorrhagic transformation was observed in a group of 1,034 acute ischemic stroke patients recruited in a single hospital.74 Finally, a pooled analysis of 570 acute ischemic stroke patients from 13 centers in Europe, North America, and Asia reported that symptomatic ICH after thrombolytic treatment developed regardless of the initial CMB frequency or extent.75 These studies suggest that for patients in need of thrombolysis, any increased risk of ICH attributable to CMBs is negligible, and unlikely to exceed the benefits from thrombolytic therapy. A subsequent meta-analysis indicated that the published analyses were vulnerable to publication bias and limited power, and identified a trend toward increased odds of symptomatic hemorrhage after thrombolysis (odds ratio, 1.98; 95% confidence interval 0.90-4.35).76 At present, identification of CMBs on baseline GRE images should not be considered a contraindication to thrombolysis treatment, but further investigation is warranted on this important issue.
Different findings regarding CMBs and the development of ICH or hemorrhagic transformation may be explained by the different pathological mechanisms of the two phenomena. Essentially, ICH involves rupture of a fragile microvascular wall affected by lipohyalinosis or microaneurysms under the chronic influence of hypertension.77 As CMBs have histological characteristics similar to those of vasculopathy, a correspondingly similar mechanism may underlie formation of CMBs and ICHs.22 In contrast, hemorrhagic transformation develops after acute lethal injury in relatively healthy microvasculature.
Considerable interest also exists in utilizing detection of CMBs to estimate the risks of hemorrhagic complications in patients on antithrombotic treatment. Two patients on warfarin were reported to have developed lobar hemorrhages right at the location of CMBs.64 I CMBs were found to be more frequent and extensive in patients with aspirin-associated ICH.11,78 In a cross-sectional study, CMBs were more common in patients taking antithrombotic agents, and aspirin use was found to be related to a lobar location.79 Results from our group demonstrated that patients with anticoagulation-associated hemorrhagic stroke complications are 3.6 times more likely to have CMBs than are age- and sex-matched controls.12 A recent pooled analysis involving 1,460 hemorrhagic strokes and 3,817 ischemic strokes concluded that the number of CMBs was greater in warfarin users who developed ICH.80 Given the strong association between CMBs and subsequent ICHs in stroke survivors, a prospective study is needed to assess the predictive power of CMBs in stroke patients on antithrombotic treatment.
Conclusion
CMBs were first identified as tiny, round dark-signal lesions on GRE MRI, and are frequently detected in patients with ischemic or hemorrhagic strokes. Pathological analysis demonstrated that CMBs are extravasations of blood components through fragile microvascular walls, and therefore reflect a bleeding-prone vasculopathy in brain. Several clinical studies have concluded that CMBs are associated with hemorrhagic stroke and hemorrhagic complications following antithrombotic medications. The currently available data do not support the exclusion of thrombolytic treatment based solely on CMB presence or extent. Prospective studies are warranted to confirm the clinical implications of CMBs, and to establish their use for predictive models of hemorrhagic stroke in various situations.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. Am J Neuroradiol. 1996;17:573–578. [PMC free article] [PubMed] [Google Scholar]
- 3.Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke. 1999;30:1637–1642. doi: 10.1161/01.str.30.8.1637. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SH, Park JM, Kwon SJ, Kim H, Kim YH, Roh JK, et al. Left ventricular hypertrophy is associated with cerebral microbleeds in hypertensive patients. Neurology. 2004;63:16–21. doi: 10.1212/01.wnl.0000132525.36804.a1. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low Concentration of Serum Total Cholesterol Is Associated With Multifocal Signal Loss Lesions on Gradient-Echo Magnetic Resonance Imaging: Analysis of Risk Factors for Multifocal Signal Loss Lesions. Stroke. 2002;33:2845–2849. doi: 10.1161/01.str.0000036092.23649.2e. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004;62:72–76. doi: 10.1212/01.wnl.0000101463.50798.0d. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Kim BJ, Roh JK. Silent microbleeds are associated with volume of primary intracerebral hemorrhage. Neurology. 2006;66:430–432. doi: 10.1212/01.wnl.0000196471.04165.2b. [DOI] [PubMed] [Google Scholar]
- 10.Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34:2459–2462. doi: 10.1161/01.STR.0000090841.90286.81. [DOI] [PubMed] [Google Scholar]
- 11.Wong KS, Chan YL, Liu JY, Gao S, Lam WW. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology. 2003;60:511–513. doi: 10.1212/01.wnl.0000046583.40125.20. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72:171–176. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 13.Atlas SW, Mark AS, Grossman RI, Gomori JM. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology. 1988;168:803–807. doi: 10.1148/radiology.168.3.3406410. [DOI] [PubMed] [Google Scholar]
- 14.Reichenbach JR, Jonetz-Mentzel L, Fitzek C, Haacke EM, Kido DK, Lee BC, et al. High-resolution blood oxygen-level dependent MR venography (HRBV): a new technique. Neuroradiology. 2001;43:364–369. doi: 10.1007/s002340000503. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 16.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 17.Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging. 2010;31:142–148. doi: 10.1002/jmri.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehling C, Wersching H, Kloska SP, Kirchhof P, Ring J, Nassenstein I, et al. Detection of asymptomatic cerebral microbleeds: a comparative study at 1.5 and 3.0 T. Acad Radiol. 2008;15:895–900. doi: 10.1016/j.acra.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg SM, Nandigam RN, Delgado P, Betensky RA, Rosand J, Viswanathan A, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 22.Fisher M, French S, Ji P, Kim RC. Cerebral microbleeds in the elderly: a pathological analysis. Stroke. 2010;41:2782–2785. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119:291–302. doi: 10.1007/s00401-009-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YS, Lee J, Baek W, Lee YJ, Kim HY. Pontine hemorrhage at a microbleed site in a patient with central pontine myelinolysis. Neurol Sci. 2011;32:1251–1252. doi: 10.1007/s10072-011-0783-1. [DOI] [PubMed] [Google Scholar]
- 25.Simonsen CZ, Nielsen E. Hypertensive microbleed as a transient ischemic attack mimic. Case Rep Neurol. 2013;5:31–33. doi: 10.1159/000348400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991–994. doi: 10.1212/wnl.52.5.991. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Okudera T, Tamura H, Ogawa T, Hatazawa J. Assessment of Lacunar Hemorrhage Associated With Hypertensive Stroke by Echo-Planar Gradient-Echo T2*-Weighted MRI. Stroke. 2000;31:1646–1650. doi: 10.1161/01.str.31.7.1646. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536–1540. doi: 10.1161/01.str.0000018012.65108.86. [DOI] [PubMed] [Google Scholar]
- 29.Tsushima Y, Tanizaki Y, Aoki J, Endo K. MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology. 2002;44:31–36. doi: 10.1007/s002340100649. [DOI] [PubMed] [Google Scholar]
- 30.Hanyu H, Tanaka Y, Shimizu S, Takasaki M, Fujita H, Kaneko N, et al. Cerebral microbleeds in Binswanger's disease: a gradient-echo T2*-weighted magnetic resonance imaging study. Neurosci Lett. 2003;340:213–216. doi: 10.1016/s0304-3940(03)00121-6. [DOI] [PubMed] [Google Scholar]
- 31.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 32.Igase M, Tabara Y, Igase K, Nagai T, Ochi N, Kido T, et al. Asymptomatic cerebral microbleeds seen in healthy subjects have a strong association with asymptomatic lacunar infarction. Circ J. 2009;73:530–533. doi: 10.1253/circj.cj-08-0764. [DOI] [PubMed] [Google Scholar]
- 33.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 34.Kim BJ, Lee SH, Kang BS, Yoon BW, Roh JK. Diabetes increases large artery diseases, but not small artery diseases in the brain. J Neurol. 2008;255:1176–1181. doi: 10.1007/s00415-008-0864-0. [DOI] [PubMed] [Google Scholar]
- 35.Naka H, Nomura E, Kitamura J, Imamura E, Wakabayashi S, Matsumoto M. Antiplatelet Therapy as a Risk Factor for Microbleeds in Intracerebral Hemorrhage Patients: Analysis Using Specific Antiplatelet Agents. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Vernooij MW, van der Lugt A, Breteler MM. Risk of thrombolysis-related hemorrhage associated with microbleed presence. Stroke. 2008;39:e115. doi: 10.1161/STROKEAHA.108.520197. [DOI] [PubMed] [Google Scholar]
- 37.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 38.Tsushima Y, Aoki J, Endo K. Brain Microhemorrhages Detected on T2*-Weighted Gradient-Echo MR Images. Am J Neuroradiol. 2003;24:88–96. [PMC free article] [PubMed] [Google Scholar]
- 39.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Cerebral microbleeds in patients with hypertensive stroke. Topographical distribution in the supratentorial area. J Neurol. 2004;251:1183–1189. doi: 10.1007/s00415-004-0500-6. [DOI] [PubMed] [Google Scholar]
- 41.Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry. 2004;75:423–427. doi: 10.1136/jnnp.2003.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Topographical distribution of pontocerebellar microbleeds. Am J Neuroradiol. 2004;25:1337–1341. [PMC free article] [PubMed] [Google Scholar]
- 43.Imaizumi T, Horita Y, Chiba M, Hashimoto Y, Honma T, Niwa J. Dot-like hemosiderin spots on gradient echo T2*-weighted magnetic resonance imaging are associated with past history of small vessel disease in patients with intracerebral hemorrhage. J Neuroimaging. 2004;14:251–257. doi: 10.1177/1051228404265714. [DOI] [PubMed] [Google Scholar]
- 44.Naka H, Nomura E, Wakabayashi S, Kajikawa H, Kohriyama T, Mimori Y, et al. Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: association with combination of stroke subtypes and leukoaraiosis. Am J Neuroradiol. 2004;25:714–719. [PMC free article] [PubMed] [Google Scholar]
- 45.Kim BJ, Lee SH, Ryu WS, Kang BS, Kim CK, Yoon BW. Low Level of Low-Density Lipoprotein Cholesterol Increases Hemorrhagic Transformation in Large Artery Atherothrombosis but Not in Cardioembolism. Stroke. 2009;40:1627–1632. doi: 10.1161/STROKEAHA.108.539643. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 47.Ryu WS, Kim CK, Kim BJ, Lee SH. Serum Uric Acid Levels and Cerebral Microbleeds in Patients with Acute Ischemic Stroke. PLoS ONE. 2013;8:e55210. doi: 10.1371/journal.pone.0055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovbiagele B, Liebeskind DS, Pineda S, Saver JL. Strong independent correlation of proteinuria with cerebral microbleeds in patients with stroke and transient ischemic attack. Arch Neurol. 2010;67:45–50. doi: 10.1001/archneurol.2009.310. [DOI] [PubMed] [Google Scholar]
- 49.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MMB. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 50.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho AH, Lee SB, Han SJ, Shon YM, Yang DW, Kim BS. Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology. 2009;73:1645–1648. doi: 10.1212/WNL.0b013e3181c1defa. [DOI] [PubMed] [Google Scholar]
- 52.Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. The relation between chronic kidney disease and cerebral microbleeds: difference between patients with and without diabetes. Int J Stroke. 2012;7:551–557. doi: 10.1111/j.1747-4949.2011.00732.x. [DOI] [PubMed] [Google Scholar]
- 53.Jeong SW, Jung KH, Chu K, Bae HJ, Lee SH, Roh JK. Clinical and radiologic differences between primary intracerebral hemorrhage with and without microbleeds on gradient-echo magnetic resonance images. Arch Neurol. 2004;61:905–909. doi: 10.1001/archneur.61.6.905. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage Burden Predicts Recurrent Intracerebral Hemorrhage After Lobar Hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 55.Imaizumi T, Honma T, Horita Y, Kawamura M, Kohama I, Miyata K, et al. The number of microbleeds on gradient T2*-weighted magnetic resonance image at the onset of intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2008;17:30–34. doi: 10.1016/j.jstrokecerebrovasdis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Kim SM, Kim N, Yoon BW, Roh JK. Cortico-subcortical distribution of microbleeds is different between hyperten sion and cerebral amyloid angiopathy. J Neurol Sci. 2007;258:111–114. doi: 10.1016/j.jns.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Rosand J, Muzikansky A, Kumar A, Wisco JJ, Smith EE, Betensky RA, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58:459–462. doi: 10.1002/ana.20596. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Bae HJ, Lee J, Kang L, Lee S, Kim S, et al. APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient-echo MRI. Neurology. 2005;65:1474–1475. doi: 10.1212/01.wnl.0000183311.48144.7f. [DOI] [PubMed] [Google Scholar]
- 60.Jeon SB, Kwon SU, Cho AH, Yun SC, Kim JS, Kang DW. Rapid appearance of new cerebral microbleeds after acute ischemic stroke. Neurology. 2009;73:1638–1644. doi: 10.1212/WNL.0b013e3181bd110f. [DOI] [PubMed] [Google Scholar]
- 61.Gregoire SM, Brown MM, Kallis C, Jäger HR, Yousry TA, Werring DJ. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke. 2010;41:184–186. doi: 10.1161/STROKEAHA.109.568469. [DOI] [PubMed] [Google Scholar]
- 62.Lee SH, Lee ST, Kim BJ, Park HK, Kim CK, Jung KH, et al. Dynamic Temporal Change of Cerebral Microbleeds: Long-Term Follow-Up MRI Study. PLoS ONE. 2011;6:e25930. doi: 10.1371/journal.pone.0025930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim BJ, Lee SH. Silent microbleeds and hemorrhagic conversion of an embolic infarction. J Clin Neurol. 2007;3:147–149. doi: 10.3988/jcn.2007.3.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee GH, Kwon SU, Kang DW. Warfarin-induced intracerebral hemorrhage associated with microbleeds. J Clin Neurol. 2008;4:131–133. doi: 10.3988/jcn.2008.4.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeon SB, Kang DW, Cho AH, Lee EM, Choi CG, Kwon SU, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254:508–512. doi: 10.1007/s00415-006-0406-6. [DOI] [PubMed] [Google Scholar]
- 66.Imaizumi T, Honma T, Horita Y, Kohama I, Miyata K, Kawamura M, et al. Hematoma size in deep intracerebral hemorrhage and its correlation with dot-like hemosiderin spots on gradient echo T2*-weighted MRI. J Neuroimaging. 2006;16:236–242. doi: 10.1111/j.1552-6569.2006.00042.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Heo JH, Yoon BW. Effects of microbleeds on hemorrhage development in leukoaraiosis patients. Hypertens Res. 2005;28:895–899. doi: 10.1291/hypres.28.895. [DOI] [PubMed] [Google Scholar]
- 68.Evans A, Demchuk A, Symons SP, Dowlatshahi D, Gladstone DJ, Zhang L, et al. The spot sign is more common in the absence of multiple prior microbleeds. Stroke. 2010;41:2210–2217. doi: 10.1161/STROKEAHA.110.593970. [DOI] [PubMed] [Google Scholar]
- 69.Boulanger JM, Coutts SB, Eliasziw M, Gagnon AJ, Simon JE, Subramaniam S, et al. Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke. 2006;37:911–914. doi: 10.1161/01.STR.0000204237.66466.5f. [DOI] [PubMed] [Google Scholar]
- 70.Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke. 2002;33:95–98. doi: 10.1161/hs0102.101792. [DOI] [PubMed] [Google Scholar]
- 71.Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke. 2002;33:735–742. doi: 10.1161/hs0302.104615. [DOI] [PubMed] [Google Scholar]
- 72.Kim HS, Lee DH, Ryu CW, Lee JH, Choi CG, Kim SJ, et al. Multiple cerebral microbleeds in hyperacute ischemic stroke: impact on prevalence and severity of early hemorrhagic transformation after thrombolytic treatment. AJR Am J Roentgenol. 2006;186:1443–1449. doi: 10.2214/AJR.04.1933. [DOI] [PubMed] [Google Scholar]
- 73.Kakuda W, Thijs VN, Lansberg MG, Bammer R, Wechsler L, Kemp S, et al. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology. 2005;65:1175–1178. doi: 10.1212/01.wnl.0000180519.27680.0f. [DOI] [PubMed] [Google Scholar]
- 74.Lee SH, Kang BS, Kim N, Roh JK. Does microbleed predict haemorrhagic transformation after acute atherothrombotic or cardioembolic stroke? J Neurol Neurosurg Psychiatry. 2008;79:913–916. doi: 10.1136/jnnp.2007.133876. [DOI] [PubMed] [Google Scholar]
- 75.Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38:2738–2744. doi: 10.1161/STROKEAHA.106.480848. [DOI] [PubMed] [Google Scholar]
- 76.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke. 2013;8:348–356. doi: 10.1111/j.1747-4949.2012.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 78.Wong KS, Mok V, Lam WW, Kay R, Tang A, Chan YL, et al. Aspirin-associated intracerebral hemorrhage: Clinical and radiologic features. Neurology. 2000;54:2298–2301. doi: 10.1212/wnl.54.12.2298. [DOI] [PubMed] [Google Scholar]
- 79.Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 80.Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, et al. Group TESS. Antithrombotic Drug Use, Cerebral Microbleeds, and Intracerebral Hemorrhage: A Systematic Review of Published and Unpublished Studies. Stroke. 2010;41:1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 81.Schonewille WJ, Singer MB, Atlas SW, Tuhrim S. The prevalence of microhemorrhage on gradient-echo magnetic resonance imaging in acute lacunar infarction. J Stroke Cerebrovasc Dis. 2005;14:141–144. doi: 10.1016/j.jstrokecerebrovasdis.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Werring DJ, Coward LJ, Losseff NA, Jäger HR, Brown MM. Cerebral microbleeds are common in ischemic stroke but rare in TIA. Neurology. 2005;65:1914–1918. doi: 10.1212/01.wnl.0000188874.48592.f7. [DOI] [PubMed] [Google Scholar]
- 83.Ovbiagele B, Saver JL, Sanossian N, Salamon N, Villablanca P, Alger JR, et al. Predictors of cerebral microbleeds in acute ischemic stroke and TIA patients. Cerebrovasc Dis. 2006;22:378–383. doi: 10.1159/000094855. [DOI] [PubMed] [Google Scholar]
- 84.Wardlaw JM, Lewis SC, Keir SL, Dennis MS, Shenkin S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37:2633–2636. doi: 10.1161/01.STR.0000240513.00579.bf. [DOI] [PubMed] [Google Scholar]
- 85.Seo WK, Lee JM, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovasc Dis. 2008;26:618–623. doi: 10.1159/000166837. [DOI] [PubMed] [Google Scholar]
- 86.Fiehler J, Siemonsen S, Thomalla G, Illies T, Kucinski T. Combination of T2*W and FLAIR abnormalities for the prediction of parenchymal hematoma following thrombolytic therapy in 100 stroke patients. J Neuroimaging. 2009;19:311–316. doi: 10.1111/j.1552-6569.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 87.Staals J, van Oostenbrugge RJ, Knottnerus IL, Rouhl RP, Henskens LH, Lodder J. Brain microbleeds relate to higher ambulatory blood pressure levels in first-ever lacunar stroke patients. Stroke. 2009;40:3264–3268. doi: 10.1161/STROKEAHA.109.558049. [DOI] [PubMed] [Google Scholar]
- 88.Fluri F, Jax F, Amort M, Wetzel SG, Lyrer PA, Katan M, et al. Significance of microbleeds in patients with transient ischaemic attack. Eur J Neurol. 2012;19:522–524. doi: 10.1111/j.1468-1331.2011.03522.x. [DOI] [PubMed] [Google Scholar]
- 89.Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2000;31:2665–2669. doi: 10.1161/01.str.31.11.2665. [DOI] [PubMed] [Google Scholar]
- 90.Imaizumi T, Horita Y, Hashimoto Y, Niwa J. Dotlike hemosiderin spots on T2*-weighted magnetic resonance imaging as a predictor of stroke recurrence: a prospective study. J Neurosurg. 2004;101:915–920. doi: 10.3171/jns.2004.101.6.0915. [DOI] [PubMed] [Google Scholar]
- 91.Lim JB, Kim E. Silent microbleeds and old hematomas in spontaneous cerebral hemorrhages. J Korean Neurosurg Soc. 2009;46:38–44. doi: 10.3340/jkns.2009.46.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishikawa T, Ueba T, Kajiwara M, Fujisawa I, Miyamatsu N, Yamashita K. Cerebral microbleeds predict first-ever symptomatic cerebrovascular events. Clin Neurol Neurosurg. 2009;111:825–828. doi: 10.1016/j.clineuro.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43:2677–2681. doi: 10.1161/STROKEAHA.112.657486. [DOI] [PubMed] [Google Scholar]