Abstract
We have recently experienced an endometrial cancer 12 years after the diagnosis of colon cancer with Lynch syndrome. A 49-year-old Korean woman had a family history of colon cancer. Her mother had colon cancer at 56-year-old, and her brother had colon cancer at 48 years old. The patient received surgery for endometrial cancer at the same hospital 12 years after being treated for colon cancer. Immunohistochemistry showed that her endometrial tissue stained negative for MSH2. A microsatellite instability test was performed and showed the presence of instability high microsatellite instability. An hMLH2 gene mutation was detected at codon 629 codon of exon 12, in which a glutamine was replaced with an arginine (1886A>G [p.Gln629Arg]). To our knowledge, this is the first case of metachronous cancer in a Lynch syndrome family in Korea with a gap of more than ten years between cancer diagnoses.
Keywords: Colon cancer, Endometrial cancer, Lynch syndrome
Introduction
Lynch syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC), is a hereditary cancer syndrome associated with mutations in mismatch repair genes (MSH2, MLH1, MSH6, PMS2) [1]. Endometrial cancer is the second most common malignant disorder besides colon cancer and occurs in 2% to 3% of patients with Lynch syndrome [2].
Identification of individuals who have increased risk of developing hereditary cancers would allow screening and early cancer detection, which may decrease disease-specific mortality.
The lifetime risk of developing endometrial cancer is estimated to be 30% to 70% in female mutation carriers, which equals or exceeds the risk of developing colorectal cancer [3].
The diagnosis of Lynch syndrome is based on family history and genetic testing (Amsterdam [1999] [4] or Bethesda [2004] [5] criteria).
Current recommendations for gynecologic screening in patients with Lynch syndrome includes endometrial biopsy, transvaginal ultrasound, and CA-125 testing every 1 to 2 years starting at the age of 30 to 35 years old [6]. However, the benefit of screening for gynecological cancer in Lynch syndrome has been demonstrated. Further, there is no consensus regarding the optimal screening test.
Effective and invasive screening procedures are not widely performed. Hysterectomy with bilateral salpingo-oophorectomy is a preventive strategy for women with HNPCC. However, there are some obstacles to this treatment, including inadequate counseling, inadequate follow-up, and cost [3].
We present a case of endometrial cancer 12 years after the diagnosis of colon cancer in a patient with Lynch syndrome who was treated at a single institution. I would emphasize the valuable meaning of delayed development of endometrial cancer after colon cancer.
Case report
A 49-year-old Korean woman visited Samsung Changwon Hospital complaining of irregular menstruation and continuous vaginal spotting last three months accompanied with lower abdominal discomfort. Endometrial aspiration biopsy was performed because of suspicious endometrial pathology (em thickness was 14 mm), and the results revealed a well-differentiated adenocarcinoma.
She had been diagnosed with colon cancer 12 years earlier at the same institution and received a right hemicolectomy and chemotherapy. She had a family history of colon cancer. Her mother had colon cancer at the age of 56 years old, and her brother had colon cancer at the age of 48-year-old. Her family history was consistent with the clinical criteria for recommending genetic testing, i.e., at least three first-degree family members in two successive generations diagnosed with cancer before the age of 50-year-old. However, she was not provided genetic counseling at that time. According to the guidelines for genetic risk assessment of Lynch syndrome by the Society of Gynecologic Oncologists, patients have a 20% to 25% chance of developing cancer [7].
Preoperative cancer examinations were performed on biopsy samples, including genetic testing for microsatellite instability test (MSI) and mutations in MSH2 and MLH1.
Considering the age and family history of the patient, genetic counseling was provided, and genetic tests were performed. Two of her family members previously suffered from cancer (Fig. 1). Her mother had colon cancer at the age of 56 years old. Her older brother also had had colon cancer at the age of 48 years old.
Fig. 1.
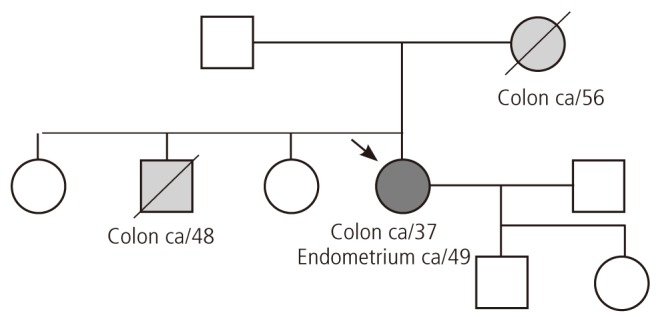
Family cancer history of patient. ca, cancer.
The carcinoembryonic antigen concentration for the patient was 1.32 ng/mL, and CA-125 was normal.
A 19-mm mass was observed in the endometrial cavity on a pelvic magnetic resonance imaging. There was no evidence of metastasis on the imaging test. In addition, washing cytology, total hysterectomy, bilateral salpingo-oophorectomy, and pelvic and paraaortic lymph node dissections were performed. Pathology results indicated a well-differentiated endometrioid adenocarcinoma (grade 1). Lymph vascular invasion was not observed (0/29).Washing cytology was negative.
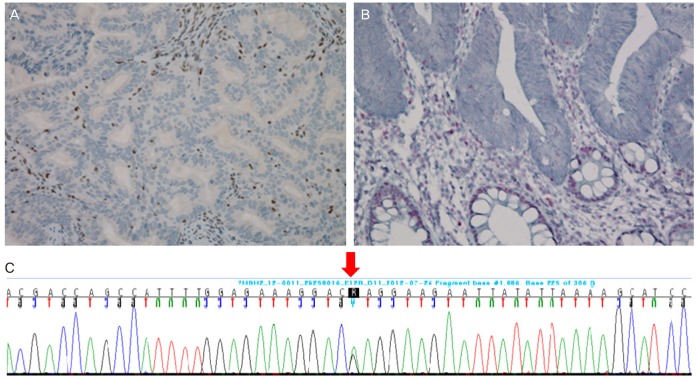
Immunohistochemistry (IHC) analysis showed that the endometrial tissue stained negative for MSH2 (Fig. 2). A MSI was performed and showed unstable final results (MSI-H; high microsatellite instability) (BAT25, BAT26, D2S123, and D5S346 were unstable, and D17S250 was stable). Loss of heterozygosity was not found in these markers.
Fig. 2.
Immunohisotchemistry. (A) Endometrium, ×400. (B) Colon, ×400. (C) Gene sequencing result of hMSH2.
A mutation was detected at codon 629 in exon12 of MLH2, in which a glutamine was replaced by an arginine (1886A>G [p.Gln629Arg]) (Fig. 2).
A colon biopsy obtained from the patient by colonoscopy was examined by IHC and found to be negative for MSH2 (Fig. 2).
The final diagnosis was stage IA endometrial cancer. No additional treatment was prescribed.
Discussion
This case demonstrates that a second cancer can occur in a patient with Lynch syndrome after a prolonged period of over 10 years. This is the longest follow-up of a patient with Lynch syndrome in Korea.
Lynch syndrome is associated with an early-onset of cancer and development of multiple types of cancer, particularly colon and endometrial cancers. The predisposition to developing cancers is inherited. Thus, early diagnosis and prevention may be possible for these patients.
About 230,000 new cancer cases are expected to occur in Korea in 2012 [8]. The numbers of female patients with colon and endometrial cancers are 12,546 and 2,085, respectively.
About five percent of endometrial cancers are due to inherited changes [9].
Prophylactic hysterectomy and bilateral salpingo-oophorectomy are known to prevent endometrial and ovarian cancers in patients with Lynch syndrome [10]. The proportion of cancers that are hereditary based on ethnicity has not been well-studied. The patient discussed in this case report was offered treatment but was not provided adequate genetic counseling or prophylactic surgery. In the future, large prospective studies are needed to determine the incidence of hereditary cancer in Korea.
Family histories are important for identifying individuals who may benefit from genetic counseling and predictive genetic testing. In addition, the presence of MSI in the tumors of patients with Lynch syndrome provides a useful adjunct for triaging patients who are at risk of having one of the germline DNA mismatch repair mutations [11,12].
Our case had a positive MSI result, indicating the presence of instability. MSI reflects genetic hypermutability due to mismatch repair gene defects. The original panel of mono- and di-nucleotide markers from 1997 remained the primary component of the Bethesda Guidelines for HNPCC [13]. However, MSI is also reported in as many as 90% to 95% of colorectal carcinomas and at least 75% of endometrial carcinomas associated with Lynch syndrome [14].
Women diagnosed with colon cancer who are mutation carriers or have a high likelihood for HNPCC syndrome can choose total abdominal hysterectomy and bilateral salpingo-oophorectomy at the time of surgery for their colon cancer. In one study, early-onset colorectal cancer, which is defined as occurring before the age of 45-year-old, was a stronger indicator of Lynch syndrome than late-onset disease [3,15]. Our patient was 37 years old at the time that she received surgery for colon cancer.
I would emphasize that this patient was diagnosed endometrial cancer after 12 years she was diagnosed colon cancer, which I describe 'delayed onset'. We should pay attention to this delayed onset of endometrial cancer after colon cancer of this patient, because it can be a good guidance when we decide how often, and how long we should do the screening ultrasound or endometrial biopsy for colon cancer patients with Lynch syndrome.
Concrete criteria must be established for gynecological oncologists and enterologists to triage patients for genetic counseling and testing. More information about appropriate genetic tests for counseling and prevention is also required.
In conclusion, gynecological oncologists should share information with enterologists to prevent the development of second cancers in patients with Lynch syndrome. Patients should be given appropriate genetic tests and counseled accordingly to reduce cancer mortality and treatment costs.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ladabaum U, Ford JM. Lynch syndrome in patients with colorectal cancer: finding the needle in the haystack. JAMA. 2012;308:1581–1583. doi: 10.1001/jama.2012.14171. [DOI] [PubMed] [Google Scholar]
- 2.Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009;16:14–22. doi: 10.1177/107327480901600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuckless S, Green J, Dawson L, Barrett B, Woods MO, Dicks E, et al. Impact of gynecological screening in Lynch syndrome carriers with an MSH2 mutation. Clin Genet. 2013;83:359–364. doi: 10.1111/j.1399-0004.2012.01929.x. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 5.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yurgelun MB, Mercado R, Rosenblatt M, Dandapani M, Kohlmann W, Conrad P, et al. Impact of genetic testing on endometrial cancer risk-reducing practices in women at risk for Lynch syndrome. Gynecol Oncol. 2012;127:544–551. doi: 10.1016/j.ygyno.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107:159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012;44:25–31. doi: 10.4143/crt.2012.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber SB, Thompson WD. A population-based study of endometrial cancer and familial risk in younger women: Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev. 1996;5:411–417. [PubMed] [Google Scholar]
- 10.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 11.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu KH, Broaddus RR. Gynecologic Cancers in Lynch Syndrome/HNPCC. Fam Cancer. 2005;4:249–254. doi: 10.1007/s10689-005-1838-3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KM, Zhang S, Geiger T, Hafez MJ, Bacher J, Berg KD, et al. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006;8:305–311. doi: 10.2353/jmoldx.2006.050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pino MS, Chung DC. Application of molecular diagnostics for the detection of Lynch syndrome. Expert Rev Mol Diagn. 2010;10:651–665. doi: 10.1586/erm.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]