Abstract
Objective. To evaluate the effect of eradication of Helicobacter pylori (H. pylori) on the progression of dementia in Alzheimer's disease (AD) patients with peptic ulcer. Methods. Participants with the diagnosis of AD and peptic ulcer were recruited between 2001 and 2008. We examined the association between eradication of H. pylori and the progression of AD using the multiple regression models. Medication shift from Donepezil, Rivastgmine, and Galantamine to Mematine is defined as progression of dementia according to the insurance of National Health Insurance (NHI) under expert review. Results. Among the 30142 AD patients with peptic ulcers, the ratio of medication shift in AD patients with peptic ulcers is 79.95%. There were significant lower incidence comorbidities (diabetes mellitus, hypertension, cerebrovascular disease, coronary artery disease, congestive heart failure and hyperlipidemia) in patients with H. pylori eradication as compared with no H. pylori eradication. Eradication of H. pylori was associated with a decreased risk of AD progression (odds ratio [OR] 0.35 [0.23–0.52]) as compared with no H. pylori eradication, which was not modified by comorbidities. Conclusions. Eradication of H. pylori was associated with a decreased progression of dementia as compared to no eradication of H. pylori in AD patients with peptic ulcers.
1. Introduction
Alzheimer's disease (AD) is a common neurodegenerative disorder for which causes are diverse, and it involves similar neuroinflammation cascade as prion disease [1]. Cerebral amyloid deposits are colocalized with a broad variety of inflammation-related proteins (complement factors, acute-phase protein, and proinflammatory cytokines) and clusters of activated microglia [2]. Currently, identified risk factors of AD include age, sex, plasma homocysteine level, and genetic factors like apolipoprotein E allele ε4 [3, 4]. Several studies have shown the association between infection and AD, including HSV-1, Chlamydia pneumonia, spirochetes, and Helicobacter pylori (H. pylori) [5–8]. As for H. pylori infection, previous case-control studies found an association between H. pylori and AD. An impressive intervention study has shown positive results that the H. pylori eradication may improve the cognitive functiona outcome within two years, but the sample size of case (28 patients) and controls (16 patients) might be small for application to general population [9]. Additionally, some agents like statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, have also shown to potentially attenuate neuroinflammatory processes and play a role in halting the degeneration process of AD [10–13]. A recent case-control study also showed preliminary results that AD patients with H. pylori infection may be more cognitively impaired. Roubaud-Baudron et al. found higher CSF cytokine (TNF-α, IL-8) and significantly positive correlation between H. pylori immunoglobulin level and homocysteine level and they concluded that the impact of H. pylori infection on AD course may be attributed to cerebrovascular lesions and neuroinflammation [14]. These observations led to the hypothesis that eradication of H. pylori infection modulating neuroinflammatory process may have a protective role for AD. Given that Taiwan's National Health Insurance Reimbursement Policy requested physicians to perform eradication of H. pylori infection depending on gastrointestinal endoscopy biopsy with or without 13C-urea breath test and neurologists to treat worsening of AD patients with anti-cholinesterase treatment according to repeated neuropsychological assessment and detailed expert review, we were allowed to observe the impact of eradication of H. pylori on the AD course.
2. Methods
2.1. Data Source
The database used in this study included one million randomly selected subjects from the 1996–2007 Taiwan National Health Insurance Research Database (NHIRD), which was developed for research purposes. The NHIRD is a research database developed at the National Health Research Institute, with linked data from the demographic and enrollment records, hospital claims, ambulatory care visits, and pharmacy dispensing claims from hospitals, outpatient clinics, and community pharmacies. Our source population comprised all beneficiaries from the Longitudinal Health Insurance Database 2005 who were at least 50 years of age on January 1, 2001. There were no statistically significant differences in age, gender, or average insured payroll-related amount between the sample group and all enrollees.
Study Population. From the NHID, AD patients who were collected from outpatient pharmacy database between January 1, 1997, and December 31, 2004, with a primary diagnosis of dementia (Classifications of Diseases-9 codes: 290.xx) and regularly taking anticholinesterase medications (include donepezil, rivasitgmine, or galantamine according to anatomical therapeutic chemical (ATC) classification system codes provided in Supplemental Table 1 in the supplementary material available online at http://dx.doi.org/10.1155/2013/175729) for more than 3 months. We then selected AD patients with the diagnosis of peptic ulcer (Classifications of Diseases-9 codes: 531–534, A-code: A534). Patients who received H. pylori eradication therapy and those who did not receive H. pylori eradication therapy were classified into two subgroups. Due to Taiwan's National Health Insurance Reimbursement Policy request, worsening of neuropsychological assessment including minimental status exam may shift anticholinesterase medications (donepezil, rivastgmine, or galantamine) to memantine and defined as worsening of dementia; AD patients who shift or did not shift anti-cholinesterase medications were analyzed separately. Comorbidities were defined as diseases diagnosed before the index outpatient clinic visit.
2.2. H. pylori Eradication Method
H. pylori eradication with triple or quadruple therapy was defined as proton pump inhibitor or H2 receptor blocker, plus clarithromycin or metronidazole, plus amoxicillin or tetracycline, with or without Bismuth (details for all eligible H. pylori eradication regimens are reported previously) [15]. These drug combinations were prescribed within the same prescription order, and the duration of therapy was between 7 and 14 days. One year was chosen as the cutoff value based on the distribution of H. pylori eradication date after index hospitalization.
2.3. Covariate Ascertainment and Adjustment
We used inpatient and outpatient diagnosis files and prescription files during the 12-month period before the index date to ascertain patients' history of hypertension diabetes mellitus, cerebrovascular disease, coronary artery disease, congestive heart failure, and hyperlipidemia (ICD-9-CM codes provided in Supplemental Table 2); we also collected patient information on age, sex, and resource utilization (number of outpatient visits, number of hospitalizations, number of laboratory test measurements) 12 months prior to the index date.
2.4. Statistical Analysis
Baseline characteristics, comorbidities, and medication use were presented. For all cohort members, we computed their person days of followup in each anti-cholinesterase medication category. We examined the effect of H. pylori eradication therapy on risk of shifting anticholinesterase medications by comparing the occurrence of shifting medications after the H. pylori eradication therapy among AD patients with peptic ulcers. Multiple regression model was used to calculate the odds ratios (ORs) and their 95% CIs.
3. Results
After excluding subjects who did not meet our study criteria, a total of 30142 AD patients were included in the analysis (Figure 1). A total of 1538 AD patients with peptic ulcer were then selected and classified into two groups: with H. pylori eradication (n = 675) and without H. pylori eradication (n = 863). Among these two groups enrolled in our study, several baseline characteristics, including diabetes mellitus, hypertension, cerebrovascular disease, congestive heart failure, coronary artery disease, and hyperlipidemia, were higher in frequency in patients without H. pylori eradication (Table 1). According to our previous study, the eradication rate of H. pylori was 89.4% to 90.5% [16]. Thus, it was estimated that 90% of our patients (n = 675) had successful eradication of H. pylori. Our results would underestimate the association between eradication therapy of H. pylori and the progression of AD.
Figure 1.
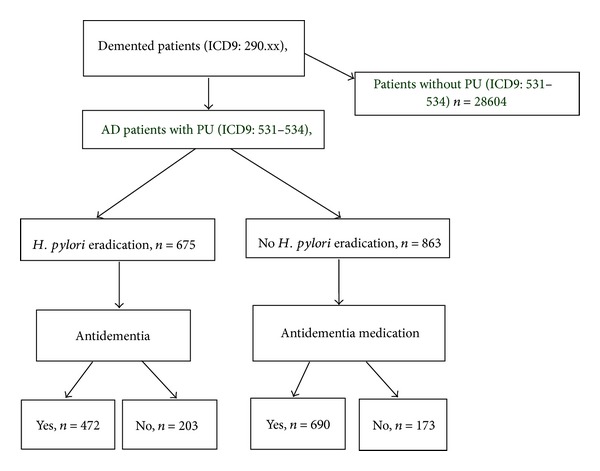
Flow chart of the study cohort assembly from prescriptions in Taiwan's National Health Insurance Research Database. Taiwan National Health Insurance Database, 1 million subtract from 23 million.
Table 1.
Basic demographic data of demented patients with peptic ulcer.
| 1538 demented patients with PU (ICD-9:290.xx & 531–534) | P value | ||
|---|---|---|---|
| H. pylori eradication | Yes, n = 675 | No, n = 863 | |
| Age, yr | 79.41 ± 9.64 | 76.07 ± 11.59 | |
| Female, % | 338 (50.00) | 475 (55.77) | 0.0528 |
| Diabetes mellitus, % | 46 (6.81) | 161 (18.65) | <0.0001 |
| Hypertension, % | 85 (12.59) | 308 (35.56) | <0.0001 |
| Cerebrovascular disease and TIA, % | 57 (8.44) | 277 (32.09) | <0.0001 |
| Congestive heart failure, % | 32 (4.74) | 102 (11.81) | <0.0001 |
| Coronary artery disease, % | 42 (6.22) | 185 (21.43) | <0.0001 |
| Hyperlipidemia, % | 45 (6.66) | 159 (18.42) | <0.0001 |
PU: peptic ulcer. TIA: transient ischemic attack.
P value, by student's t-test.
As compared with no H. pylori eradication, H. pylori eradication was associated with a decreased risk of changing anticholinesterase medication with the OR of 0.58 (95% CI 0.46–0.74). The protective association of H. pylori eradication persisted with OR of 0.34 (95% CI 0.23–0.52) after adjustment of comorbidities (Table 2).
Table 2.
The effect of H. pylori eradication on the progression of dementia.
| 1538 demented people with PU (ICD-9:290.xx & ICD-9:531–534) | |||||
|---|---|---|---|---|---|
| H. pylori eradication | Percentage, % | OR, (95% CI) | Adjusted OR, (95% CI) | ||
| Yes | No | ||||
| Medication change (+) | 472 | 690 | 79.95 | 0.58 (0.46–0.74) | 0.34 (0.23–0.52) |
| Medication change (−) | 203 | 173 | 20.05 | 1 | 1 |
PU: peptic ulcer. OR: odds ratio. We estimated odds ratio of progression of dementia by χ 2 test.
We estimated adjusted odds ratio by multiple linear regression, adjusted with age, gender, diabetes mellitus, hypertension, cerebrovascular disease and TIA, congestive heart failure, coronary artery disease, and hyperlipidemia.
4. Discussion
Our study demonstrated that eradication therapy of H. pylori has a decreased association with AD progression as compared to no H. pylori eradication in AD patients with peptic ulcer, after adjusting for age, sex, comorbidities, and other potential confounder medications. We also observed a higher frequency of comorbidities in patients without eradication therapy of H. pylori. Previous reports also suggested the association between H. pylori infection and diabetes mellitus, hypertension, and cerebrovascular disease [17, 18]. In patients with diabetes mellitus, H. pylori infection or seropositivity not only increases the risk of atherosclerosis and cardiovascular disease but also contributed to promoting insulin resistance and increased microalbuminemia [18, 19].
Chronic H. pylori infection has shown to increase gastric pH level of gastric juice and thus leads to reduced folate absorption and increased blood homocysteine level, both of which would result in the damage of vascular endothelial cells and increased the risk of atherosclerosis [20–23]. However, the link between cerebrovascular disease and H. pylori infection still needs prospective studies [14, 24]. Our study may provide some support for the hypothesis of H. pylori infection causing atherosclerosis and risk of cerebrovascular disease.
Indeed, vascular risk factors, including adult-onset diabetes mellitus, hypertension, atherosclerosis disease and atrial fibrillation, are known to increase the risk of AD [25]. Cerebral vascular dysfunction may cause the accumulation of amyloid-beta (Abeta) protein and neuroinflammation in animal model, and reduction of fibrillar Abeta protein deposition may ameliorate the neuroinflammation [26, 27]. Neuroinflammation disrupting the blood brain barrier, together with fibrinogen, may be one of the contributing factors for familial cerebral amyloid angiopathy and Alzheimer's disease [28, 29]. It is plausible to propose that antibacterial treatment of chronic inflammation caused by H. pylori or other pathogens like spirochete may reduce neuroinflammation and thus prevent dementia [7].
The strength of our study is the enrollment of a nationally representative cohort of a large sample size. The information regarding anticholinesterase medications and eradication therapy of H. pylori is obtained by linking to the NHI pharmacy database under the Reimbursement Policy request of NHI to reduce the possibility of duplication or misclassification. Furthermore, covariates including underlying diseases (especially diabetes mellitus), were taken into consideration. However, there are several limitations. First, although we analyzed health care records from a national representative dataset of 1 million people, there were still few AD cases to allow us to have a precise estimation. Second, we did not adjust the use of medications that potentially may affect AD risk such as statins, NSAID, antidiabetic agents, calcium channel blockers, and neuroleptic agents. Third, we were not able to assess the genotype of apolipoprotein E allele ε4, which cement the solid relevance in late-onset AD [30–32]. Fourth, our diagnosis of AD was based on the diagnosis code from the NHI database; therefore, we were not able to distinguish between AD and mixed type dementia. Nonetheless, a stringent policy from NHI validates our diagnosis by expert review before the use of anticholinesterase medications. However, given that all the medical information from the NHI database was deidentified due to ethical privacy concern, we could not recognize all the AD-diagnosed subjects in our study and therefore did not have the opportunity to review all their medical charts. Last, we could not exclude the possibility that the observed association was due to sick-stopper effect (nonadherence to medication due to higher risk) or protopathic bias (less AD symptoms may increase the awareness of the importance of eradication therapy). Further longitudinal study including measures of neuropsychiatric assessment over time is needed to clarify the interrelated roles of cognition, eradication therapy of H. pylori, and AD.
5. Conclusion
We observed that eradication therapy of H. pylori had a deceased association with AD progression compared with no eradication therapy among AD patients with peptic ulcer. Further long-term follow-up study is needed to confirm the potential beneficial role of antibacterial therapy of H. pylori in AD.
Supplementary Material
Table 1. Anatomical therapeutic chemical (ATC) for anticholinesterase medications.
Table 2. ICD codes for comorbid diseases.
Acknowledgments
The authors acknowledge the help of the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital, the supports from Excellence for Cancer Research Center Grant (DOH102-TD-C-111-002), and the Department of Health, Executive Yuan, Taiwan, Kaohsiung Medical University Hospital (KMUH100-0I01, KMUH100-0 M02, KMUH97-7G46, and KMUH99-9 M67).
References
- 1.Eikelenboom P, Bate C, van Gool WA, et al. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40(2):232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 4.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. The New England Journal of Medicine. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak MA, Itzhaki RF. Antiviral agents in Alzheimer’s disease: hope for the future? Therapeutic Advances in Neurological Disorders. 2010;3(3):141–152. doi: 10.1177/1756285610370069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb MB, Molloy DW, Smieja M, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. Journal of the American Geriatrics Society. 2004;52(3):381–387. doi: 10.1111/j.1532-5415.2004.52109.x. [DOI] [PubMed] [Google Scholar]
- 7.Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease—role of spirochetes. Journal of Alzheimer’s Disease. 2008;13(4):381–391. doi: 10.3233/jad-2008-13404. [DOI] [PubMed] [Google Scholar]
- 8.Figura N, Franceschi F, Santucci A, Bernardini G, Gasbarrini G, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2010;15(1):60–68. doi: 10.1111/j.1523-5378.2010.00778.x. [DOI] [PubMed] [Google Scholar]
- 9.Kountouras J, Boziki M, Gavalas E, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. Journal of Neurology. 2009;256(5):758–767. doi: 10.1007/s00415-009-5011-z. [DOI] [PubMed] [Google Scholar]
- 10.Pac-Soo C, Lloyd DG, Vizcaychipi MP, Ma D. Statins: the role in the treatment and prevention of Alzheimer’s neurodegeneration. Journal of Alzheimer’s Disease. 2011;27(1):1–10. doi: 10.3233/JAD-2011-110524. [DOI] [PubMed] [Google Scholar]
- 11.Kurata T, Miyazaki K, Kozuki M, et al. Atorvastatin and pitavastatin improve cognitive function and reduce senile plaque and phosphorylated tau in aged APP mice. Brain Research. 2011;1371:161–170. doi: 10.1016/j.brainres.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Infante-Duarte C, Waiczies S, Wuerfel J, Zipp F. New developments in understanding and treating neuroinflammation. Journal of Molecular Medicine. 2008;86(9):975–985. doi: 10.1007/s00109-007-0292-0. [DOI] [PubMed] [Google Scholar]
- 13.Farooqui AA, Ong W-Y, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Research Reviews. 2007;56(2):443–471. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiology of Aging. 2012;33(5):e11–e19. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Wu C-Y, Kuo KN, Wu M-S, Chen Y-J, Wang C-B, Lin J-T. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137(5):1641.e2–1648.e2. doi: 10.1053/j.gastro.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Wu I-C, Wu D-C, Hsu P-I, et al. Rabeprazole- versus esomeprazole-based eradication regimens for H. pylori infection. Helicobacter. 2007;12(6):633–637. doi: 10.1111/j.1523-5378.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 17.Eshraghian A. The continuous story of Helicobacter pylori infection and insulin resistance: this time in Japan. Helicobacter. 2010;15(2):p. 160. doi: 10.1111/j.1523-5378.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 18.Gunji T, Matsuhashi N, Sato H, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14(5):144–150. doi: 10.1111/j.1523-5378.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung GE, Heo NJ, Park MJ, Chung SJ, Kang HY, Kang SJ. Helicobacter pylori seropositivity in diabetic patients is associated with microalbuminuria. World Journal of Gastroenterology. 2013;19(1):97–102. doi: 10.3748/wjg.v19.i1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasceri V, Patti G, Cammarota G, Pristipino C, Richichi G, di Sciascio G. Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. American Heart Journal. 2006;151(6):1215–1222. doi: 10.1016/j.ahj.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Elkind MSV, Cole JW. Do common infections cause stroke? Seminars in Neurology. 2006;26(1):88–99. doi: 10.1055/s-2006-933312. [DOI] [PubMed] [Google Scholar]
- 22.Corrado E, Rizzo M, Tantillo R, et al. Markers of inflammation and infection influence the outcome of patients with baseline asymptomatic carotid lesions: a 5-year follow-up study. Stroke. 2006;37(2):482–486. doi: 10.1161/01.STR.0000198813.56398.14. [DOI] [PubMed] [Google Scholar]
- 23.Markus HS, Mendall MA. Helicobacter pylori infection: a risk factor for ischaemic cerebrovascular disease and carotid atheroma. Journal of Neurology Neurosurgery and Psychiatry. 1998;64(1):104–107. doi: 10.1136/jnnp.64.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimke K, Chubb SAP, Davis WA, Davis TME. Helicobacter pylori cytotoxin-associated gene-A antibodies do not predict complications or death in type 2 diabetes: the Fremantle Diabetes study. Atherosclerosis. 2010;212(1):321–326. doi: 10.1016/j.atherosclerosis.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert Review of Neurotherapeutics. 2008;8(5):743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 26.Miao J, Xu F, Davis J, Otte-Höller I, Verbeek MM, van Nostrand WE. Cerebral microvascular amyloid β protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid β precursor protein. American Journal of Pathology. 2005;167(2):505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao J, Vitek MP, Xu F, Previti ML, Davis J, van Nostrand WE. Reducing cerebral microvascular amyloid-β protein deposition diminishes regional neuroinflammation in vasculotropic mutant amyloid precursor protein transgenic mice. Journal of Neuroscience. 2005;25(27):6271–6277. doi: 10.1523/JNEUROSCI.1306-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Current Alzheimer Research. 2007;4(2):191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 29.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. Journal of Experimental Medicine. 2007;204(8):1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. The Lancet Neurology. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roses AD. Apolipoprotein E and Alzheimer’s disease: a rapidly expanding field with medical and epidemiological consequences. Annals of the New York Academy of Sciences. 1996;802:50–57. doi: 10.1111/j.1749-6632.1996.tb32598.x. [DOI] [PubMed] [Google Scholar]
- 32.Saunders AM, Schmader K, Breitner JCS, et al. Apolipoprotein E ε4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. The Lancet. 1993;342(8873):710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Anatomical therapeutic chemical (ATC) for anticholinesterase medications.
Table 2. ICD codes for comorbid diseases.


